Introduction
The incidence of cutaneous squamous cell carcinoma in situ (SCCIS) is high and increasing. Surgery, considered the gold standard, may not always be an option because of the size or location, associated comorbidities, or cost. Solid organ transplant recipients (SOTRs) have an increased risk of skin cancer, especially SCCs, often have multiple lesions simultaneously, and have significant medical comorbidities. We present an 87-year-old SOTR with a large SCCIS on the left hand successfully treated with combined systemic and intratumoral administration of the 9-valent human papillomavirus (HPV) vaccine (Gardasil 9, Merck & Co Inc, Miami Lakes, FL).
Case report
An 87-year-old Hispanic man who had received a deceased donor renal transplant 15 years prior, was taking mycophenolic acid, 180 mg, and tacrolimus, 2 mg, both twice daily and methylprednisolone, 1 mg/d, presented with a painful, enlarging, scaly plaque from the palm to the dorsum on the left hand (Fig 1, A and B). Biopsy confirmed SCCIS (Fig 2, A and B). The orthopedic surgical oncologist consultant considered him a poor surgical candidate given the location of the tumor, its size, and the fact that he was dependent on a walker to ambulate. The patient refused radiation therapy.
Fig 1.
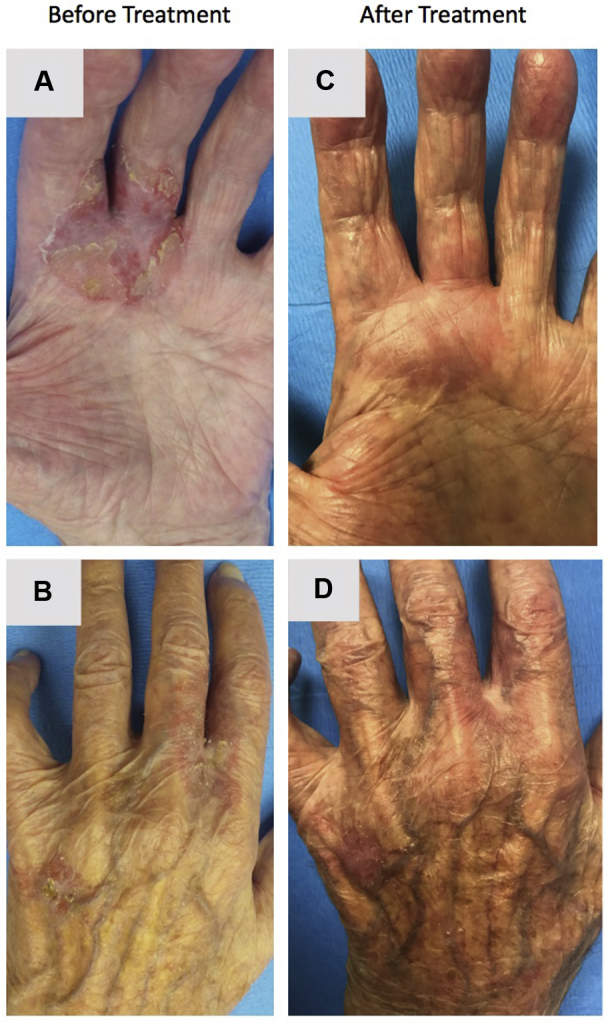
Complete clinical regression of a large SCCIS in a deceased donor renal transplant recipient after combined systemic and intratumoral administration of the 9-valent HPV vaccine. A and B, A deceased donor renal transplant recipient initially presented with a painful, enlarging, scaly plaque on the left hand extending from the palm to the dorsum. C and D, Nine months after combined systemic and intratumoral delivery of the 9-valent HPV vaccine, there was clinical resolution of the lesion.
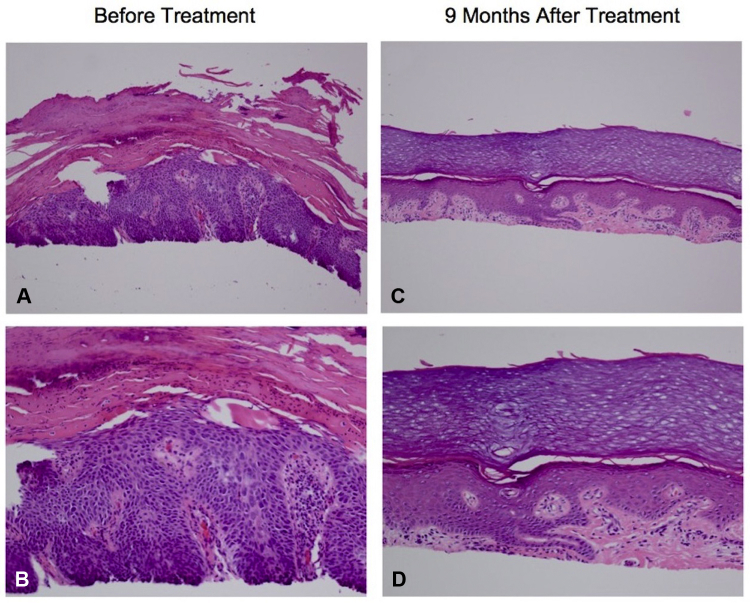
Fig 2.
Histopathologic analysis before and after combined systemic and intratumoral administration of the 9-valent HPV vaccine. A and B, On initial presentation, biopsy found full-thickness atypia of the epidermis with scattered mitotic figures. There is a “windblown” appearance of the cells without epidermal maturation. There is also overlying parakeratosis, consistent with a diagnosis of squamous cell carcinoma. C and D, Nine months after combined systemic and intratumoral administration of the 9-valent HPV vaccine, biopsy found hyperkeratosis and focal hypergranulosis of the acanthotic epidermis. Within the dermis is a band-like infiltrate with scattered Civatte bodies without evidence of residual carcinoma. (A-D, Hematoxylin-eosin stain; original magnifications: A and C, ×20; B and D, ×40.)
Given our experience using combined systemic and intratumoral administration of the 9-valent HPV vaccine for SCCs, we offered the same to this patient.1 After informed consent, the patient received 2 intramuscular (IM) vaccine injections at 0 and 6 weeks. Two weeks after the second IM dose, the vaccine (0.5 mL) was diluted in 2 mL of sterile normal saline and 0.5 mL of 2% lidocaine and was injected intratumorally. Two months after the first intratumoral injection, a second intratumoral dose was administered. Cumulatively, the patient received 2 IM and 2 intratumoral doses. Nine months after the first IM injection of the HPV 9-valent vaccine, the lesion resolved (Fig 1, C and D), and biopsy confirmed histologic cure (Fig 2, C and D). The treatments were well tolerated without adverse events.
Discussion
Nonmelanoma skin cancer is the most commonly diagnosed cancer among whites with an increased incidence after solid organ transplantation.2 The relative cancer mortality rate for nonmelanoma skin cancer after transplant surgery is also increased (standardized mortality ratio 2.9; 95% confidence interval, 2.7-3.1).3 This increased risk is thought to be secondary to decreased immune surveillance, which may allow for proliferation of cancer-related viruses like HPV, normally suppressed by a healthy immune system.3 Gene products from skin-trophic β-HPV subtypes can be found in premalignant actinic keratoses and SCCs in SOTRs implying a role for HPV in both the early and later stages of carcinogenesis. HPV is thought to act synergistically with ultraviolet radiation for tumor progression and maintenance.4
Surgical excision, typically used for SCCIS, is not always feasible. Current nonsurgical options include radiation therapy or intratumoral injections of 5-fluorouracil, methotrexate, or bleomycin. We were the first to report the use of combined systemic and intratumoral administration of the 9-valent HPV vaccine (subtypes 6, 11, 16, 18, 31, 33, 45, 52, and 58) to successfully treat inoperable SCCs in an immunocompetent woman.1 Here, we report the first use of combined systemic and intratumoral administration of the 9-valent HPV vaccine for a large SCCIS in an immunosuppressed SOTR. This treatment resulted in complete clinical remission and histologic cure without residual pain and with return of full function of the hand. The observed benefit may be owing to a combination of antiviral, antitumor, and immunologic effects. Future studies will help further evaluate the utility and underlying mechanism of the 9-valent HPV vaccine as therapy for SCCs and other cutaneous malignancies in SOTRs.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Tim Ioannides has a patient issued for this application of the 9-valent HPV vaccine. The rest of the authors have no conflicts to disclose.
References
- 1.Nichols A.J., Gonzalez A., Clark E.S. Combined Systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154(8):927–930. doi: 10.1001/jamadermatol.2018.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madeleine M.M., Patel N.S., Plasmeijer E.I. Epidemiology of keratinocyte carcinomas after organ transplantation. Br J Dermatol. 2017;177(5):1208–1216. doi: 10.1111/bjd.15931. [DOI] [PubMed] [Google Scholar]
- 3.Rosales B.M., De La Mata N., Vajdic C.M., Kelly P.J., Wyburn K., Webster A.C. Cancer mortality in kidney transplant recipients: an Australian and New Zealand population-based cohort study, 1980-2013. Int J Cancer. 2019 doi: 10.1002/ijc.32585. [DOI] [PubMed] [Google Scholar]
- 4.Borgogna C., Lanfredini S., Peretti A. Improved detection reveals active beta-papillomavirus infection in skin lesions from kidney transplant recipients. Mod Pathol. 2014;27(8):1101–1115. doi: 10.1038/modpathol.2013.240. [DOI] [PubMed] [Google Scholar]