Abstract
Introduction
Gamma knife radiosurgery (GKR) has shown promising results in the treatment of intraocular uveal melanoma (UM) in terms of local tumor control. However, GKR is not free from potentially sight-threatening side effects, including cataract, dry eye disease, vitreous hemorrhage, radiation retinopathy (RR), radiation maculopathy (RM), optic neuropathy, and neovascular glaucoma. The aim of this paper is to report our 20-year experience in UM management with GKR focusing on the rate of clinical treatment-induced complications.
Methods
Single-center, retrospective, observational study, including all patients with UM treated at the Ocular Oncology and Uveitis Service, in the Department of Ophthalmology of the San Raffaele Scientific Institute, Milan from September 1993 to September 2018. Clinical charts comprised complete ophthalmological examination with measurement of best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure measurement, gonioscopy, and indirect ophthalmoscopy at each visit. B-scan ultrasound (Aviso S, 10 MHz probe; Paris, France), optical coherence tomography (Heidelberg Spectralis; Heidelberg Engineering, Heidelberg, Germany), retinography, and fundus fluorescein angiography (standard or ultra-widefield [UWF; California, Optos, Dunfermline, Scotland, UK]) were performed aiding in the diagnosis of complications.
Results
Overall, 194 patients (100 males, 51.6%) were reviewed. The median age at the time of the treatment was 65 years (range 27–89) and all participants were Caucasian. In 185 eyes (95.4%), the tumor was primarily located at the choroid. The median follow-up was 57.6 months; radiation-induced complications were found in 145 eyes (74.7%). Radiation-induced cataract and RR were the most frequent events, with a relative incidence of 41.2 and 34.5%, respectively, followed by neovascular glaucoma (27.3%), optic neuropathy (18.6%), RM (11.4%), vitreous hemorrhage (14.4%), phthisis bulbi (7.7%), hyphema (0.5%), and corneal melting (0.5%). The shorter onset of side effects involved the optic nerve (median 14.9 months) and the macula (median 13.7 months).
Conclusion
Despite modern and advanced strategies introduced to limit GKR side effects, cataract and RR still represent a serious limitation of this treatment. Incidence of RR was higher in our cohort compared to other reports, probably due to increased diagnosis rate permitted by UWF retinal imaging.
Keywords: Choroidal melanoma, Gamma knife radiosurgery, Ultra-widefield imaging, Radiation retinopathy
Introduction
Uveal melanoma (UM) is the most commonly diagnosed primary intraocular malignancy in adults, with an annual incidence of about 2–11 per 1,000,000 persons in the USA and in Europe [1, 2, 3, 4]. Despite recent advances in diagnosis and treatment, UM remains life-threatening, with an overall mortality rate of 40–50% in 15 years, mainly due to liver metastasis [5].
Since the Collaborative Ocular Melanoma Treatment Study (COMS) revealed that conservative treatments had the same survival outcome as mutilating surgical treatment as enucleation, many sight- and/or globe-sparing strategies have been proposed [6, 7]. At present, several options are available, including transpupillary thermotherapy, plaque brachytherapy (iodine-125 or ruthenium-106)[8], proton beam radiotherapy [9], stereotactic radiotherapy [10], and stereotactic radiosurgery[11, 12]. The selected approach depends on different clinical factors, namely the tumor size, location (choroid, ciliary body, or iris) and the extension of the tumor, as well as the status of the fellow eye, the age, the general health, and the psychological status of the patient.
Gamma knife radiosurgery (GKR), initially developed for the treatment of intracranial lesions, has shown promising results in the treatment of orbital and intraocular UM in terms of local tumor control[13]. The primary advantage of GKR is preservation of the globe, with complete or relative sparing of the visual function [14]. GKR also represents a precious resource in cases where brachytherapy is contraindicated, as large tumors (greater than 15 mm in diameter or 10 mm in height) or lesions within 2 mm from the optic disc[15]. However, GKR is not free from the risk of damage to the healthy adjacent eye structures[13], leading to potentially sight-threatening side effects, including cataract, dry eye disease, vitreous hemorrhage, radiation retinopathy (RR), radiation maculopathy (RM), optic neuropathy, and neovascular glaucoma [14]. The optic nerve pathway, as well as the lacrimal glands, have been shown to be particularly radiosensitive, and damage of these structures often leads to irreversible visual impairment which significantly reduces the quality of life[16, 17].
Adequate selection of the patient, record of the baseline characteristics, and regular follow-up are crucial for early identification of GKR-related side effects. Moreover, modern imaging techniques, including ultra-widefield (UWF) imaging instruments, capturing up to 200° of the retina in a single shot, aid in the recognition of these complications and serve as a guide for the treatment of each condition. The aim of the paper is to report our 20-year experience in UM management with GKR focusing on the rate of clinical treatment-induced complications.
Material and Methods
The present study was a single-center, retrospective, observational study, including all patients with UM consecutively treated at the Ocular Oncology and Uveitis Service, in the Department of Ophthalmology of the San Raffaele Scientific Institute, Milan from September 1993 to September 2018. Each subject signed a written consent to participate in observational studies, which gained the approval of the Ethics Committee of San Raffaele Hospital. This study adhered to the tenets of the Declaration of Helsinki for research involving human subjects.
Inclusion criteria were: diagnosis of UM based on clinical evaluation by a retina specialist (i.e., biopsy was not required); age older than 18 years; treatment with GKR; charts and imaging studies available at our Department. Technical details of the GKR treatment have been published elsewhere [11]. Exclusion criteria were: inability to obtain the informed consent; other ocular tumors different from UM (namely, choroidal metastasis, choroidal hemangioma, optic nerve glioma, etc.); treatment with other modalities other than or in concomitance with GKR (transpupillary thermotherapy, brachytherapy, proton beam radiotherapy, etc.).
We reviewed charts and imaging studies of each patient up to April 2019. Clinical charts comprised a complete ophthalmological examination with measurement of best-corrected visual acuity (BCVA, measured in Snellen units), color vision, pupillary reaction to light, slit-lamp biomicroscopy and photography, intraocular pressure measurement, gonioscopy, and indirect ophthalmoscopy at each visit. Accurate drawings and/or fundus photography were used to catalogue location (choroid, ciliary body, cilio-choroidal), position (macular, peripapillary, mid- or far-periphery), and quadrant (superior, inferior, nasal, temporal or diffuse). For all patients, diagnostic investigations available at the time of each visit were revised, including ophthalmic B-scan ultrasound (Aviso S, 10 MHz probe; Paris, France), optical coherence tomography (Heidelberg Spectralis; Heidelberg Engineering, Heidelberg, Germany), and standard (Topcon Trc-50Dx Retinal Camera) or UWF retinography (California; Optos, Dunfermline, Scotland, UK). Diagnosis of RR or RM was made from ancillary fundus fluorescein angiography (FFA, standard or UWF, if available). FFA was performed at the first detection of signs of RR to quantify the extent of peripheral capillary nonperfusion and to distinguish between the proliferative and nonproliferative form of the disease. Suggestive angiographic features were peripheral capillary dropout, choroidopathy, ischemic maculopathy, epiretinal neovascularization, retinal branch occlusion, and rupture of the blood-retinal barrier.
Parameters included in the analysis were patients' gender, age at the time of the treatment, ethnicity, mean follow-up, rate of both anterior and posterior segment complications, interval between GKR and each event, and specific treatment. Quantitative variables were expressed as mean ± SD, median and/or range; absolute frequencies were expressed as absolute values and percentage. GraphPad Prism 6.0 (GraphPad software, Inc., San Diego, CA, USA) was used for statistical analysis.
Results
Clinical records of 194 patients (100 males, 51.6%) were reviewed. The median age at the time of the treatment was 65 years (range 27–89) and all participants were Caucasian. All cases were unilateral. Overall, in 185 eyes (95.4%), the tumor was primarily located at the choroidal level, in 3 eyes (1.5%) at the ciliary body level, and in 6 eyes (3.1%) the tumor involved both the choroid and the ciliary body. Ophthalmoscopy revealed that the majority of tumors were pigmented, and only 14 eyes featured an amelanotic UM (7.2%). UWF fundus photography, allowing a 3-dimensional virtual reconstruction of the globe, helped to realistically replicate the clinical features of the tumor, revealing as an ancillary technique to B-scan ultrasound in assessing the exact position of the lesion.
Patients were treated with a single-fraction marginal dose of 35.8 ± 4.7 Gy at the 50% isodose line with a maximum dose of 63.5 ± 9.1 Gy. The median lesion volume was 475 ± 428 mm3.
The median follow-up was 57.6 months (range 2–296); in 168 eyes (93.3%), local control of the tumor was achieved, while in 12 eyes (6.2%) a recurrence was noted, after a median time of 29.43 months (range 7.1–74.7). Nine patients were enucleated (75%), 1 eye with a small tumor underwent transpupillary thermotherapy (8.3%), 1 proton beam radiotherapy (8.3%), and 1 refused any treatment (8.3%). Enucleation was performed in 18 eyes (9.3%), including all 9 eyes with recurrence, plus 1 eye which developed an extraocular extension despite treatment, and 8 blind eyes which experienced phthisis bulbi and/or untreatable pain.
During the follow-up, radiation-induced complications were found in 145 eyes (74.7%; Table 1).
Table 1.
Rate and interval of the different clinical complications of gamma knife radiosurgery in 194 patients with uveal melanoma
| Complication | n (rate, %) | Interval, months |
|---|---|---|
| Cataract | 80 (41.2) | 18 (median) 23.4±22.5 (mean±SD) 2.7–143.8 (range) 18.4–28.4 (95% CI) |
| NVG | 53 (27.3) | 28.4 (median) 30.7±22.3 (mean±SD) 1–113.1 (range) 24.6–36.9 (95% CI) |
| Radiation retinopathy | 67 (34.5) | 23.9 (median) 25.2±16.3 (mean±SD) 3–78.9 (range) 21.2–29.2 (95% CI) |
| Optic neuropathy | 36 (18.6) | 14.9 (median) 21.7±14.7 (mean±SD) 3–58.4 (range) 16.5–26.9 (95% CI) |
| Radiation maculopathy | 23 (11.4) | 13.7 (median) 24.2±23.2 (mean±SD) 1.4–87.5 (range) 13.9–34.5 (95% CI) |
| Vitreous hemorrhage | 28 (14.4) | 26.8 (median) 29.5±26.5 (mean±SD) 1.1–113.1 (range) 19–40 (95% CI) |
| Phthisis bulbi | 15 (7.7) | 53.3 (median) 66.3±39.9 (mean±SD) 18.2–150.2 (range) 43.3–89.3 (95% CI) |
| Hyphema | 1 (0.5) | 43.1 |
| Melting | 1 (0.5) | 52.1 |
NVG, neovascular glaucoma.
Anterior segment complications included a new-onset cataract or a significant worsening of previously known lens opacification in 80 eyes (41.2%), of which 26 eyes (32.5%) were treated with phacoemulsification (Fig. 1a–c). Neovascular glaucoma was documented in 53 eyes (27.3%; Fig. 1d); it was managed with simple observation in 1 eye (1.9%), topical medications in 38 eyes (71.7%), retinal laser photocoagulation in 2 eyes (3.8%), intravitreal injections of anti-vascular endothelial growth factors (anti-VEGF) in 2 (3.8%), combination of the 2 in 8 (13.2%), cyclophotocoagulation in 1 (1.9%), and enucleation in 2 eye (3.8%). One eye (0.5%) developed complete corneal pannus and then corneal melting (Fig. 1e), while 1 eye (0.5%) presented with complete hyphema (Fig. 1f).
Fig. 1.
Spectrum of anterior segment complications of gamma knife radiosurgery. a–c Cataract, recognizable as a whitish hue of the lens behind the iris. d Neovascular glaucoma, defined as the presence of abnormal blood vessel along the pupillary border. e Corneal pannus, with complete loss of corneal transparency. f Blood in the anterior chamber and in the vitreous cavity.
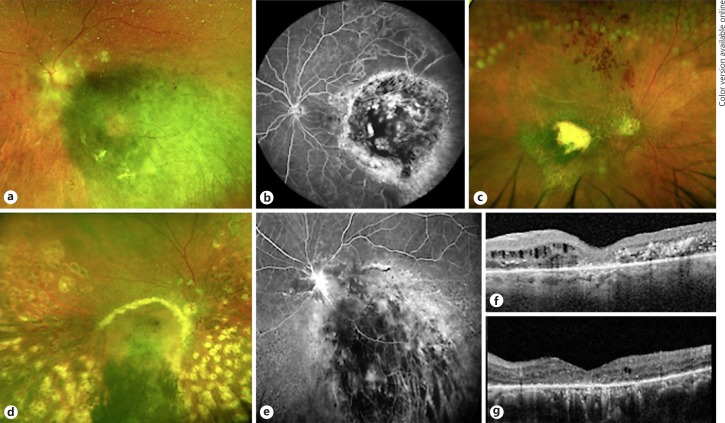
The most frequent posterior segment complication was radiation retinopathy, documented in 67 eyes (34.5%) and presenting as peripheral capillary nonperfusion, epiretinal neovascularization or vascular occlusion (Fig. 2a–e); 3 eyes featured epiretinal neovascularization (4.5%). This condition was treated with observation in 28 eyes (41.8%), retinal laser photocoagulation in 13 eyes (19.4%), intravitreal anti-VEGF in 8 eyes (11.9%), or combination of both in 9 eyes (13.4%). The remaining cases were treated with local steroids plus laser (8 eyes, 11.9%) or transconjunctival anterior cryotherapy (1 eye, 1.5%). Radiation maculopathy, featuring either macular ischemia/atrophy or intraretinal edema and exudation, was observed in 23 eyes (11.4%), of which 5 eyes presented without any sign of RR. This condition was managed with intravitreal anti-VEGF in 9 eyes (40.9%), local steroids in 4 eyes (18.2%), or combination of both in 1 eye (4.5%); the rest of the cases were observed.
Fig. 2.
Spectrum of posterior segment complications of gamma knife radiosurgery. a Fundus photography of the posterior pole, revealing exudates around the optic nerve head and cotton-wool spots. b Fluorescein angiography showing temporal capillary nonperfusion. c Fundus photography showing hemorrhages in the superior retina, an indirect sign of branch retinal vein occlusion. In the periphery, laser treatment is noticeable. d, e Radiation maculopathy, with extensive exudation involving the macula (yellow halo around the pigmented lesion) and leakage of fluorescein. f, g Optical coherence tomography revealing retinal thinning, atrophy of the external retinal layers, and intraretinal edema and exudates.
Optic neuropathy was diagnosed in 36 eyes (18.6%), ranging from acute papillopathy with disc edema and hemorrhages to pale optic disc atrophy (Fig. 2a, d). Vitreous hemorrhage took place in 28 eyes (14.4%) and was treated with observation in 22 eyes (78.6%), vitrectomy plus endolaser in 4 eyes (14.3%), or only laser photocoagulation in 2 eyes (7.1%; Fig. 2c, d). At the end of follow-up, 15 eyes experienced phthisis bulbi (7.7%), managed with globe removal in 7 eyes (46.7%).
Discussion/Conclusion
In essentially every patient with UM treated with radiotherapy, normal cells neighboring tumors inevitably receive a considerable dose of ionizing radiation, causing damage to healthy tissues, which may appear immediately or years after the initial treatment. As the number of GKR treatments have been increasing in the past decades for both intracranial and intraocular or orbital lesions[18, 19], the adverse effects related to local irradiation should elicit serious concern.
We found that radiation-induced cataract and RR were the most frequent events, with a relative incidence of 41.2 and 34.5%, respectively. Conversely, the shorter onset of side effects involved the optic nerve (median onset of 14.9 months) and the macula (median onset of 13.7 months). This shorter interval might be related to the timely detection of symptoms (such as vision loss and metamorphopsia) connected to alteration to the normal function of these 2 ocular structures, or to an earlier occurrence of these complications with respect to RR[20].
The response of normal tissue to radiation is complex and involves different molecular pathways, including DNA damage repair, cell death, inflammation, angiogenesis, and matrix remodeling, depending on the dose and time course of the exposure[21]. The lens is thought to be one of the most sensitive organs to radiation injuries, because of its superficial location and direct contact with the radiation beam during therapy for intraocular tumors. The first dose-effect quantification of the relationship between cataract formation and cumulative doses of ionizing radiation was documented in epidemiologic studies from atomic bomb survivors in Japan[22]. Recently, the occurrence of cataract was specifically linked to GKR [23] as an independent risk factor, with a global hazard ratio of 1.5; this risk was even higher in subjects between 30 and 50 years old (up to a hazard ratio of 3.5).
Although specific data on the outcome of phacoemulsification surgery after GKR are missing, several studies have shown that cataract extraction with or without intraocular lens placement in patients who have previously received radiation treatment is generally safe and well-tolerated[24]. When intraocular malignancy is present, the surgery has not been associated with tumor recurrence or spread [25, 26]. On the other hand, the functional outcome of cataract extraction might be severely limited by ocular posterior segment complications of GKR.
RR and optic nerve disease are the major causes of irreversible vision loss in irradiated patients. RR comprises a wide spectrum of retinal manifestations secondary to radiation-induced damage[27]. One of the first clinical signs of the disease is capillary nonperfusion, which leads to retinal ischemia, upregulation of the VEGF, and formation of epiretinal neovascularization and macular edema, that is an additional cause of vision loss in these patients[28]. Macular involvement can present even independently from RR, as our series demonstrated.
The relative prevalence of RR in our cohort seems to be slightly higher compared with the previously published literature (Table 2). This could be related to the longer follow-up of our study, a higher rate of diagnosis, or the tendency of underreporting of clinically insignificant RR (i.e., exam changes without a decline in best-corrected visual acuity or constriction of the visual field). While slit-lamp examination and indirect ophthalmoscopy have been the standard of care for many years, these methods require cooperation (many patients might be limited by intense photophobia) and adequate pupil dilation to explore the retinal periphery (response to mydriatic drops might be suboptimal, especially if laser or intraocular surgery have been performed). FFA was particularly useful in eyes with media opacity, such as cataract or vitreous hemorrhage, increasing the detection rate of peripheral changes with respect to indirect ophthalmoscopy or fundus photography. UWF-FFA was of undeniable aid in the clinical practice; while traditional fundus imaging devices only include 45–50° of the posterior pole, which is optimal for imaging the optic nerve and of the macula, they are inadequate for the visualization of the medium-far and of the very-far retinal periphery. On the contrary, UWF allows to photograph very peripheral retina and to identify early signs of RR in patients who are otherwise judged healthy by traditional methods; consequently, the rate of RR incidence invariably surges.
Table 2.
Relative prevalence of radiation retinopathy in the published literature
| Author | Year | Dose of radiation, Gy | Isodose, % | Follow-up, months | n | Incidence of relative risk, % |
|---|---|---|---|---|---|---|
| Marchini et al. [29] | 1996 | 55 (surface) | 60–90 | 6 (median) | 36 | 8.3 |
| Mueller et al. [30] | 2000 | 25 (average) | 50 | 12 (median) | 35 | 2.9 |
| Haas et al. [31] | 2002 | 50 (marginal) | 50 | 38 (mean) | 32 | 84 |
| Modorati et al. [11] | 2009 | 35 (marginal) | 50 | 31.3 (median) | 78 | 13.5 |
| Schirmer et al. [32] | 2009 | 22.2 (marginal) | 50 | 20.9 (median) | 14 | 14 |
| Chan et al. [33] | 2011 | 25 (marginal) | 50 | 24 (median) | 6 | 16.7 |
| Kang et al. [13] | 2012 | 45.6 (marginal) | 50 | 67 (median) | 22 | 22.7 |
| Dinca et al. [34] | 2012 | 35–70 (marginal) | 50 | 63.5 (median) | 170 | 25.8–41.7 |
| Sarici and Pazarli [35] | 2013 | 30 (marginal) | 50 | 40 (median) | 30 | 24 |
| Joye et al. [36] | 2014 | 21.7 (average) | 50 | 41.5 (median) | 23 | 8.7 |
| Reynolds et al. [37] | 2017 | 25 (marginal) | 50 | 19.74 (median) | 12 | 18.2 |
| Modorati et al. | Present study | 35.8 (marginal) | 50 | 57.6 (median) | 194 | 34.5 |
Another explanation for the higher rate of RR in our cohort could be related to a higher marginal dose with respect to other series. Although the comparison could be affected by the heterogeneity in dose reporting (Table 2), a higher dosage of radiation is associated with a greater rate of cataract and ischemic complications[31, 32, 34, 38]. A median marginal dose as low as 22 Gy has been proven to provide a favorable local tumor control (93%) with a lower rate of RR (50%) and neovascular glaucoma (7%), compared with previous series using higher doses. Similarly, a dramatic decrease in blindness and postradiosurgery enucleation has been described shifting from 45 to 35 Gy marginal dose [34]. However, the threshold for radiation retinopathy depends on other factors as well, as the fractionation scheme, the radiation delivery system, the dose received by 1% of the optic nerve, and the presence of systemic risk factors like diabetes mellitus[14].
Diverse studies have highlighted the importance of preoperative planning of the radiation, especially for critical structures, such as the optic nerve and the lacrimal gland. One of the first strategies that have been successfully introduced to limit GKR side effects relied either on fractionation [39] or on reduction of the global dose of irradiation [14]. According to Schirmer et al. [32], a marginal tumor dose of <25 Gy resulted in excellent local tumor control with limited local and regional damage. Recently, de-escalation strategies have been applied also with brachytherapy, showing a significant reduction in the dose-dependent ocular radiotoxicity and rate of side effects[40].
Modern imaging techniques, along with traditional ones (namely ultrasound, computed tomography, and magnetic resonance imaging) may play a pivotal role in increasing the safety and the tolerability of the treatment. Integrated data from all the aforementioned imaging techniques can aid in the accurate delineation of the tumor and healthy anatomical structures, which can help further spare critical normal tissue (i.e., optic nerve and chiasma, brain stem, lacrimal gland, and lens). We have started to routinely use UWF imaging system in patients with UM who underwent GKR, finding relevant support in their management [Cicinelli et al. 3D WrapTM ultra-widefield reconstruction in stereotactic radiosurgery for choroidal melanoma. Ocul Oncol Pathol., in press]. The device gives a consistent appreciation of the location and size of the lesion and the pseudo-color retinography acquired pre-treatment works well as baseline documentation for comparing reference during the follow-up [41]. Recently, new grading systems for RR with UWF have also been proposed [42].
Reviewing the classification proposed by McCannel et al. [42], 4 degrees of RR have been described: grade 1, identified by late foveal leakage; grade 2, peripheral vascular leakage in addition to foveal leakage; grade 3, associated with nonperfusion, peripheral and foveal leakage; and grade 4, demonstrating retinal neovascularization. In our series, RM, angiographically corresponding to McCannel's grade 1, was observed in 23 eyes (11.4%); 5 eyes out of 23 presented without any sign of RR, while in the remaining cases maculopathy was associated with retinopathy. Of the 67 eyes diagnosed with RR, the great majority was distributed between grades 2 and 3. Only 3 eyes featured epiretinal neovascularization, corresponding to grade 4. However, a coded treatment scheme has not been proposed yet for the different stages of the disease; further studies are needed to clarify the therapeutic management.
In conclusion, this paper represents one of the largest experiences with GKR for UM with long-term follow-up assessing the rate of complications found in an Italian tertiary referral center. Despite modern and advanced strategies introduced to limit GKR side effects, cataract and RR still represent a serious limitation of this treatment. Nevertheless, we remain hopeful that continued advancement in imaging and radiation delivery techniques combined with refined dose/fractionation schemes will provide valuable advantages regarding the management of patients undergoing GKR.
Statement of Ethics
The research complied with the guidelines for human studies and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Patients gave their written consent to participate in the study.
Disclosure Statement
The authors have no competing interest in publishing the present work.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
All the authors contributed to the conception or design of the work, the acquisition, analysis and interpretation of data, drafting the work, revising it critically for important intellectual content and gave final approval of the version to be published.
References
- 1.Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005 Mar;18((1)):75–84. doi: 10.1016/j.ohc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Teikari JM, Raivio I. Incidence of choroidal malignant melanoma in Finland in the years 1973-1980. Acta Ophthalmol (Copenh) 1985 Dec;63((6)):661–5. doi: 10.1111/j.1755-3768.1985.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 3.Huerta C, Rodríguez LA. Incidence of ocular melanoma in the general population and in glaucoma patients. J Epidemiol Community Health. 2001 May;55((5)):338–9. doi: 10.1136/jech.55.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isager P, Østerlind A, Engholm G, Heegaard S, Lindegaard J, Overgaard J, et al. Uveal and conjunctival malignant melanoma in Denmark, 1943-97: incidence and validation study. Ophthalmic Epidemiol. 2005 Aug;12((4)):223–32. doi: 10.1080/09286580591000836. [DOI] [PubMed] [Google Scholar]
- 5.Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond) 2017 Feb;31((2)):241–57. doi: 10.1038/eye.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol. 2018 Feb;10:1758834018757175. doi: 10.1177/1758834018757175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jampol LM, Moy CS, Murray TG, Reynolds SM, Albert DM, Schachat AP, et al. Collaborative Ocular Melanoma Study Group (COMS Group) The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology. 2002 Dec;109((12)):2197–206. doi: 10.1016/s0161-6420(02)01277-0. [DOI] [PubMed] [Google Scholar]
- 8.AlMahmoud T, Quinlan-Davidson S, Pond GR, Deschênes J. Outcome Analysis of Visual Acuity and Side Effect after Ruthenium-106 Plaque Brachytherapy for Medium-sized Choroidal Melanoma. Middle East Afr J Ophthalmol. 2018 Apr-Jun;25((2)):103–7. doi: 10.4103/meajo.MEAJO_198_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seibel I, Riechardt AI, Erb-Eigner K, Böker A, Cordini D, Heufelder J, et al. Proton Beam Irradiation: A Safe Procedure in Postequatorial Extraocular Extension From Uveal Melanoma. Am J Ophthalmol. 2018 Jul;191:49–53. doi: 10.1016/j.ajo.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Dunavoelgyi R, Dieckmann K, Gleiss A, Sacu S, Kircher K, Georgopoulos M, et al. Local tumor control, visual acuity, and survival after hypofractionated stereotactic photon radiotherapy of choroidal melanoma in 212 patients treated between 1997 and 2007. Int J Radiat Oncol Biol Phys. 2011 Sep;81((1)):199–205. doi: 10.1016/j.ijrobp.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Modorati G, Miserocchi E, Galli L, Picozzi P, Rama P. Gamma knife radiosurgery for uveal melanoma: 12 years of experience. Br J Ophthalmol. 2009 Jan;93((1)):40–4. doi: 10.1136/bjo.2008.142208. [DOI] [PubMed] [Google Scholar]
- 12.Arnett AL, Reynolds MM, Pulido JS, Parney IF, Laack NN. Gamma Knife Stereotactic Radiosurgery for the Treatment of Primary and Metastatic Ocular Malignancies. Stereotact Funct Neurosurg. 2017;95((6)):363–8. doi: 10.1159/000478271. [DOI] [PubMed] [Google Scholar]
- 13.Kang DW, Lee SC, Park YG, Chang JH. Long-term results of Gamma Knife surgery for uveal melanomas. J Neurosurg. 2012 Dec;117((Special_Suppl Suppl)):108–14. doi: 10.3171/2012.8.GKS121002. [DOI] [PubMed] [Google Scholar]
- 14.Gigliotti CR, Modorati G, Di Nicola M, Fiorino C, Perna LA, Miserocchi E, et al. Predictors of radio-induced visual impairment after radiosurgery for uveal melanoma. Br J Ophthalmol. 2018 Jun;102((6)):833–9. doi: 10.1136/bjophthalmol-2017-310801. [DOI] [PubMed] [Google Scholar]
- 15.Emara K, Weisbrod DJ, Sahgal A, McGowan H, Jaywant S, Michaels H, et al. Stereotactic radiotherapy in the treatment of juxtapapillary choroidal melanoma: preliminary results. Int J Radiat Oncol Biol Phys. 2004 May;59((1)):94–100. doi: 10.1016/j.ijrobp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Wackernagel W, Holl E, Tarmann L, Avian A, Schneider MR, Kapp K, et al. Visual acuity after Gamma-Knife radiosurgery of choroidal melanomas. Br J Ophthalmol. 2013 Feb;97((2)):153–8. doi: 10.1136/bjophthalmol-2012-302399. [DOI] [PubMed] [Google Scholar]
- 17.Horwath-Winter J, Schneider MR, Wackernagel W, Rabensteiner D, Boldin I, Haller-Schober EM, et al. Influence of single-fraction Gamma-Knife radiosurgery on ocular surface and tear function in choroidal melanoma patients. Br J Ophthalmol. 2013 Apr;97((4)):466–70. doi: 10.1136/bjophthalmol-2012-302402. [DOI] [PubMed] [Google Scholar]
- 18.Park SH, Kano H, Niranjan A, Monaco E, 3rd, Flickinger JC, Lunsford LD. Gamma Knife radiosurgery for meningiomas arising from the tentorium: a 22-year experience. J Neurooncol. 2015 Jan;121((1)):129–34. doi: 10.1007/s11060-014-1605-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim MS, Park K, Kim JH, Kim YD, Lee JI. Gamma knife radiosurgery for orbital tumors. Clin Neurol Neurosurg. 2008 Dec;110((10)):1003–7. doi: 10.1016/j.clineuro.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Kim IK, Lane AM, Egan KM, Munzenrider J, Gragoudas ES. Natural history of radiation papillopathy after proton beam irradiation of parapapillary melanoma. Ophthalmology. 2010 Aug;117((8)):1617–22. doi: 10.1016/j.ophtha.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006 Sep;6((9)):702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 22.Otake M, Schull WJ. A review of forty-five years study of Hiroshima and Nagasaki atomic bomb survivors. Radiation cataract. J Radiat Res (Tokyo) 1991 Mar;32(Suppl):283–93. doi: 10.1269/jrr.32.supplement_283. [DOI] [PubMed] [Google Scholar]
- 23.Liang CL, Liliang PC, Chen TB, Hsu HC, Chuang FC, Wang KW, et al. The risk of cataractogenesis after gamma knife radiosurgery: a nationwide population based case-control study. BMC Ophthalmol. 2017 Apr;17((1)):40. doi: 10.1186/s12886-017-0435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osman IM, Abouzeid H, Balmer A, Gaillard MC, Othenin-Girard P, Pica A, et al. Modern cataract surgery for radiation-induced cataracts in retinoblastoma. Br J Ophthalmol. 2011 Feb;95((2)):227–30. doi: 10.1136/bjo.2009.173401. [DOI] [PubMed] [Google Scholar]
- 25.Payne JF, Hutchinson AK, Hubbard GB, 3rd, Lambert SR. Outcomes of cataract surgery following radiation treatment for retinoblastoma. J AAPOS. 2009;13((5)):454–58 e3. doi: 10.1016/j.jaapos.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velásquez-Aguilar M, Matiz-Moreno H, Amato-Almanza M, Chen-López CY, Márquez-García G, Ramírez-Ortiz MA. Outcomes and complications after phacoemulsification in retinoblastoma patients with cataract after radiation treatment. Arch Soc Esp Oftalmol. 2017 Apr;92((4)):160–5. doi: 10.1016/j.oftal.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology. 1982 Dec;89((12)):1494–501. doi: 10.1016/s0161-6420(82)34611-4. [DOI] [PubMed] [Google Scholar]
- 28.Kinyoun JL, Lawrence BS, Barlow WE. Proliferative radiation retinopathy. Arch Ophthalmol. 1996 Sep;114((9)):1097–100. doi: 10.1001/archopht.1996.01100140299007. [DOI] [PubMed] [Google Scholar]
- 29.Marchini G, Gerosa M, Piovan E, Pasoli A, Babighian S, Rigotti M, et al. Gamma Knife stereotactic radiosurgery for uveal melanoma: clinical results after 2 years. Stereotact Funct Neurosurg. 1996;66(Suppl 1):208–13. doi: 10.1159/000099812. [DOI] [PubMed] [Google Scholar]
- 30.Mueller AJ, Talies S, Schaller UC, Horstmann G, Wowra B, Kampik A. Stereotactic radiosurgery of large uveal melanomas with the gamma-knife. Ophthalmology. 2000 Jul;107((7)):1381–7. doi: 10.1016/s0161-6420(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 31.Haas A, Pinter O, Papaefthymiou G, Weger M, Berghold A, Schröttner O, et al. Incidence of radiation retinopathy after high-dosage single-fraction gamma knife radiosurgery for choroidal melanoma. Ophthalmology. 2002 May;109((5)):909–13. doi: 10.1016/s0161-6420(02)01011-4. [DOI] [PubMed] [Google Scholar]
- 32.Schirmer CM, Chan M, Mignano J, Duker J, Melhus CS, Williams LB, et al. Dose de-escalation with gamma knife radiosurgery in the treatment of choroidal melanoma. Int J Radiat Oncol Biol Phys. 2009 Sep;75((1)):170–6. doi: 10.1016/j.ijrobp.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 33.Chan MD, Melhus CS, Mignano JE, Do-Dai D, Duker JS, Yao KC. Analysis of visual toxicity after gamma knife radiosurgery for treatment of choroidal melanoma: identification of multiple targets and mechanisms of toxicity. Am J Clin Oncol. 2011 Oct;34((5)):517–23. [PubMed] [Google Scholar]
- 34.Dinca EB, Yianni J, Rowe J, Radatz MW, Preotiuc-Pietro D, Rundle P, et al. Survival and complications following γ knife radiosurgery or enucleation for ocular melanoma: a 20-year experience. Acta Neurochir (Wien) 2012 Apr;154((4)):605–10. doi: 10.1007/s00701-011-1252-6. [DOI] [PubMed] [Google Scholar]
- 35.Sarici AM, Pazarli H. Gamma-knife-based stereotactic radiosurgery for medium- and large-sized posterior uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2013 Jan;251((1)):285–94. doi: 10.1007/s00417-012-2144-z. [DOI] [PubMed] [Google Scholar]
- 36.Joye RP, Williams LB, Chan MD, Witkin AJ, Schirmer CM, Mignano JE, et al. Local control and results of Leksell Gamma Knife therapy for the treatment of uveal melanoma. Ophthalmic Surg Lasers Imaging Retina. 2014 Mar-Apr;45((2)):125–31. doi: 10.3928/23258160-20140306-05. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds MM, Arnett AL, Parney IF, Kumar R, Laack NN, Maloney PR, et al. Gamma knife radiosurgery for the treatment of uveal melanoma and uveal metastases. Int J Retina Vitreous. 2017 May;3((1)):17. doi: 10.1186/s40942-017-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langmann G, Pendl G, Müllner K, Feichtinger KH, Papaefthymiouaf G. High-compared with low-dose radiosurgery for uveal melanomas. J Neurosurg. 2002 Dec;97((5 Suppl)):640–3. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 39.Zehetmayer M, Kitz K, Menapace R, Ertl A, Heinzl H, Ruhswurm I, et al. Local tumor control and morbidity after one to three fractions of stereotactic external beam irradiation for uveal melanoma. Radiother Oncol. 2000 May;55((2)):135–44. doi: 10.1016/s0167-8140(00)00164-x. [DOI] [PubMed] [Google Scholar]
- 40.Le BA, Patel R, Jennelle R, Berry JL, Le BhA. Patel R, Jennelle R, Berry JL. Evidence for Dose De-escalation in Brachytherapy for Choroidal Melanoma. Adv Ophthalmol Optom. 2018 Aug;3((1)):139–53. [Google Scholar]
- 41.Kernt M, Schaller UC, Stumpf C, Ulbig MW, Kampik A, Neubauer AS. Choroidal pigmented lesions imaged by ultra-wide-field scanning laser ophthalmoscopy with two laser wavelengths (Optomap) Clin Ophthalmol. 2010 Jul;4:829–36. doi: 10.2147/opth.s11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCannel TA, Kim E, Kamrava M, Lamb J, Caprioli J, Yang D, et al. New Ultra-Wide-Field Angiographic Grading Scheme for Radiation Retinopathy after Iodine-125 Brachytherapy for Uveal Melanoma. Retina. 2018 Dec;38((12)):2415–21. doi: 10.1097/IAE.0000000000001874. [DOI] [PubMed] [Google Scholar]