Abstract
Background
Androgenetic alopecia (AGA) affects up to 80% of men and 50% of women throughout their lifetime, causing significant discomfort. Minoxidil, finasteride, and low-level laser light therapy are the only Food and Drug Administration-approved treatments for AGA, and they have shown positive results in randomized controlled trials and meta-analyses. However, their efficacy is limited, and new therapies are needed. Injection of platelet-rich plasma (PRP), a minimally invasive technique, has been described by several authors as a promising treatment for AGA. Although many studies report beneficial effects of PRP on AGA, there is no standardized practice for PRP preparation and administration or a standard method to evaluate results.
Objective
The aim of this study was to evaluate the efficacy of manually prepared PRP in the treatment of male AGA.
Materials and Methods
We treated 20 male patients with AGA with 3 monthly injections of PRP and analyzed results by TrichoScan®.
Results
In this study, there was no statistically significant improvement in hair count or proportion of anagen hairs.
Conclusions
This lack of response could be related to any of the variables during PRP preparation described above and also to the limited number of patients in the study.
Keywords: Alopecia, Androgenetic alopecia, Baldness, Hair disorder, Platelet-rich plasma
Introduction
Androgenetic alopecia (AGA) affects up to 80% of men and 50% of women throughout their lifetime, causing significant discomfort related to appearance which may lead to anxiety symptoms, depression, and daily implications [1].
Minoxidil, finasteride, and low-level laser light therapy are the only Food and Drug Administration-approved treatments for AGA, and they have shown positive results in randomized controlled trials and meta-analyses. However, their efficacy is limited, and new therapies are needed [2].
Injection of platelet-rich plasma (PRP), a minimally invasive technique, has been described by several authors as a promising treatment for AGA [3, 4, 5, 6]. PRP is prepared from autologous venous blood. Its platelets contain several growth factors (GF), chemokines, and cytokines that facilitate the healing process in hard and soft tissues [7, 8, 9]. Some of these GF stimulate hair regrowth: vascular endothelial growth factor helps promote microcirculation, transforming growth factor-B activates the dermal papilla and inhibits apoptosis during the cell cycle, platelet-derived growth factor stimulates stem cell mitosis, epidermal growth factor, fibroblast growth factor, connective tissue growth factor, and insulin-like growth factor-1 [10, 11, 12].
Although many studies report beneficial effects of PRP on AGA, there is no standardized practice for PRP preparation and administration or a standard method to evaluate results. The aim of this study was to evaluate the efficacy of manually prepared PRP in the treatment of male AGA.
Materials and Methods
Patients
From July 2018 to September 2018, 24 male patients were selected for the study. Of these, 20 healthy male patients between 18 and 45 years old with a clinical diagnosis of AGA were selected according to the following exclusion criteria. Patients should not have received any treatment for AGA for the last 6 months and should not take any continuous drugs for chronic diseases. Also, patients with blood disorders, such as anemia or blood clots, bleeding disorders, such as hemophilia, and blood cancers, such as leukemia, lymphoma, or myeloma, were excluded.
All patients provided written informed consent, and the study was approved by the ethics committee on human research of the University of Mogi das Cruzes.
Treatment Protocol
We selected the area most affected by AGA (vertex or frontotemporal) on each patient and treated the area with 3 monthly injections of PRP using a 3-mL syringe and a 30.5-gauge needle. Injections of 0.1 mL per cm2 were given subdermally. We treated the rest of the scalp affected by AGA when there was more PRP left after treating selected areas.
We did not use any anesthesia prior to application. Patients were instructed not to wash their hair for 12 h.
PRP Preparation
We collected 24 mL of blood from each patient in 8 tubes of 3-mL sodium citrate solution (BD Vacutainer, BD Biosciences, San Jose, CA, USA). Tubes were centrifuged once for 5 min with 200 gravitational force (G) on a Thermo Electron LED centrifuge (Thermo Fisher, Germany).
We tested several protocols with different G forces and spin durations as well as single versus double centrifugation, comparing platelet concentration in peripheral blood and PRP collected. Platelet concentration was double or more than double at 200 G for 5 min.
After centrifugation, there was a yellow top layer (plasma); a thin middle layer, the buffy coat (platelets and white blood cells); and a red bottom layer (red blood cells [RBC]).
We collected the supernatant (plasma and buffy coat layer with a fraction of the RBCs) in a 10-mL syringe and added calcium gluconate in a 1:9 ratio (0.1 mL per 0.9 mL of PRP) in order to activate platelets and release GF based on the work of Gkini et al. [13].
A volume of 3–9 mL of PRP was obtained from patients after centrifugation. All materials used were sterile.
Outcomes
The primary goal was to achieve a change in hair density in the target area. Participants received a small tattoo before the start of treatment. A circular template of 2.2 cm2 was centered over the tattoo. Hairs inside the template were clipped to approximately 0.5 mm in length. A 10-fold enlargement dermatoscopic digital image was performed 48 h after. This procedure was performed before treatment (D0), before the third injection (D1), and 30 days after the third injection (D2). Secondary outcomes measured were change in terminal hair density, proportion of anagen hairs, and terminal/vellus ratio. The hair counts were performed by the TrichoScan® software
Statistics
Statistical analyses used the ANOVA test to assess whether there were statistically significant differences between results measured at the 3 times D0, D1, and D2. Next, the Bonferroni multiple comparisons test (post hoc) was used to compare times by pairs (D0 to D1, D0 to D2, and D1 to D2).
Results
One patient missed the last session and was excluded from the study. The main complaint was pain during injections. There were no other adverse effects, such as infection, telogen effluvium, scars, or others.
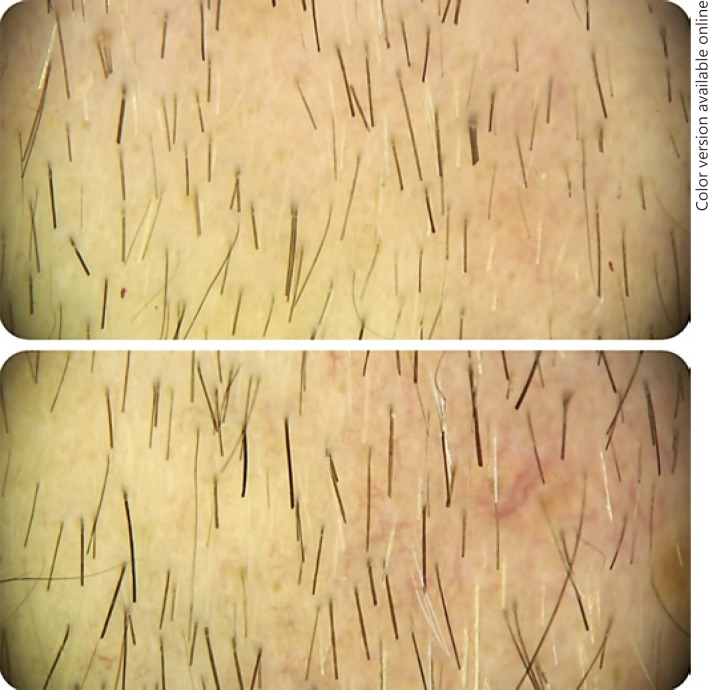
ANOVA tests were carried out for each parameter: total hair density, terminal hair density, anagen/telogen ratio, and terminal/vellus ratio, and no statistically significant differences were found. Nonetheless, the Bonferroni test was conducted, and it also indicated no statistically significant differences. Clinical comparative results are exemplified in Figure 1.
Fig. 1.
Before and after 3 sessions of therapy with PRP.
Discussion
PRP can be obtained in commercial kits or prepared manually by collecting blood samples and separating platelets by centrifugation. All systems of PRP preparation follow a similar method: blood collection involving use of an anticoagulant, such as sodium citrate or EDTA, to prevent blood clotting and consequent platelet activation, centrifugation to separate RBC and platelets, and activation with calcium chloride or calcium gluconate to release GF.
However, the final composition of PRP may vary due to inherent differences in the concentration of platelets and GF between patients and variability during PRP preparation in factors such as blood volume collected, anticoagulant method used, centrifuge device used and its parameters, and whether a platelet activator was used. Also, clinical results may vary due to the method used to inject PRP, the number of sessions, and the interval between them [14].
The centrifuge device, spin rate or G force, duration of each spin cycle, and the number of cycles used for PRP preparation vary significantly between studies. Some authors do not even report this information. Cervantes et al. [15] reviewed 12 treatment protocols with different methods of PRP preparation. Ten of them had positive results and 2 had negative results. Objective end point measures also differed in some cases. Most analyzed hair density and hair count. One of the studies with negative results was done by Puig et al. [16]. They measured hair count and hair mass index in a double-blind, randomized, placebo-controlled trial with 1 PRP or placebo treatment that did not show statistically significant improvement. The second study with negative results was a half-head comparative pilot study with 2 monthly sessions. This study reported a mean decrease in the number of terminal and vellus hairs after 6 months of PRP treatment [17].
In the meta-analysis by Giordano et al. [18], all 16 studies used different centrifugation methods. Five reported a significant difference in the number of hairs per cm2, favoring the PRP group.
Calcium gluconate and calcium chloride are the most frequently reported activation methods used [14]. However, this last step is controversial as platelets can be activated spontaneously after injection due to exposure to dermal collagen and thrombin even without prior activation [19]. Gentile et al. [20] evaluated GF concentrations in activated versus nonactivated PRP and did not find any significant difference with calcium activation.
In the review by Kramer and Keaney [14], only 32% of studies reported initial and final platelet concentrations. Among these, the average increase in platelet count was 3.8-fold. There is no consensus regarding which platelet concentration is best for PRP treatment. Some studies consider 1–3 times above baseline more therapeutically effective than 3–8 times above baseline. However, other studies suggest that platelet concentrations should be more than 1 million platelets per microliter [21, 22, 23]. Giusti et al. [24] consider 1.5 × 106 platelets per microliter the optimal concentration to induce angiogenesis in endothelial cells and suggest that higher concentrations would decrease this stimulus.
In our study, we assessed different spin cycle times and forces and single versus double cycle and found a higher platelet concentration at 200 G for 5 min with a 2-fold increase in platelet concentration. Although it is reasonable to think that the double cycle would result in a higher concentration, the second spin could impact the integrity and viability of the platelets. Also, higher forces or excessive spin duration could do the same.
We manually collected the supernatant plasma with a pipette, and the final product was orange-yellow because some RBCs were also collected. Kushida et al. [25] analyzed different commercial kits for PRP preparation and found significant differences in platelet recovery and the amounts of RBCs and granulocytes in the PRP. RBCs and white blood cells can induce inflammation locally, which could reduce the treatment effect for AGA and could also be related to burning pain at the injection site.
The volume of PRP obtained from patients varied from 3 to 9 mL. This can be explained by differences in patients' hydration status, lipemia, and hematocrit, which affect the final PRP characteristics [26].
PRP can be injected intra- or subdermally. In the review by Stevens and Khetarpal [27], subdermal bolus injections were recommended, which are less painful and were the more efficient technique with 3 monthly sessions at least. On the other hand, Cervantes et al. [15] suggest intradermal injections of approximately 0.1 mL/cm2 using the nappage technique based on analyzed studies with positive results. In our study, we subdermally injected 0.1 mL/cm2 without anesthesia.
Lastly, there are many quantitative and qualitative measures defined in studies' objectives. Since there is no standard recommended method of assessment, it is difficult to assess which is the best. Most studies use hair count, hair density, and hair thickness; some use anagen/telogen ratio, histology and immunohistochemistry analysis, global photographs, and phototrichograms. We decided to compare only TrichoScan® hair counts as global photographs could confuse final analysis due to hair length.
In this study, there was no statistically significant improvement in hair count or proportion of anagen hairs. This lack of response could be related to any of the variables during PRP preparation described above and also to the limited number of patients in the study.
Conclusion
Although some patients experienced positive results after PRP treatment, statistical analyses did not show significance. This could be related to the small number of patients or PRP protocol variables. Further studies are needed to establish a standardized and effective PRP protocol.
Statement of Ethics
All patients provided written informed consent, and the study was approved by the ethics committee on human research of the University of Mogi das Cruzes.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Tabolli S, Sampogna F, di Pietro C, Mannooranparampil TJ, Ribuffo M, Abeni D. Health status, coping strategies, and alexithymia in subjects with androgenetic alopecia: a questionnaire study. Am J Clin Dermatol. 2013 Apr;14((2)):139–45. doi: 10.1007/s40257-013-0010-3. [DOI] [PubMed] [Google Scholar]
- 2.Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017 Jul;77((1)):136–141.e5. doi: 10.1016/j.jaad.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Takikawa M, Nakamura S, Nakamura S, Ishirara M, Kishimoto S, Sasaki K, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011 Dec;37((12)):1721–9. doi: 10.1111/j.1524-4725.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 4.Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. BioMed Res Int. 2014;2014:760709. doi: 10.1155/2014/760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez V, Vaya A, Bautista D, Ricart JM. Autologous platelet-rich plasma as a potential therapeutic tool in androgenetic alopecia. J Am Acad Dermatol. 2013 Apr;68((4)):AB103. [Google Scholar]
- 6.Park KY, Kim HK, Kim BJ, Kim MN. Letter: platelet-rich plasma for treating male pattern baldness. Dermatol Surg. 2012 Dec;38((12)):2042–4. doi: 10.1111/dsu.12037. [DOI] [PubMed] [Google Scholar]
- 7.Petrungaro PS. Using platelet-rich plasma to accelerate soft tissue maturation in esthetic periodontal surgery. Compend Contin Educ Dent. 2001 Sep;22((9)):729–32. [PubMed] [Google Scholar]
- 8.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006 Oct;77((5)):806–12. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 9.Hammond JW, Hinton RY, Curl LA, Muriel JM, Lovering RM. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009 Jun;37((6)):1135–42. doi: 10.1177/0363546508330974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama M, Smith LT, Holbrook KA. Growth factor and growth factor receptor localization in the hair follicle bulge and associated tissue in human fetus. J Invest Dermatol. 1996 Mar;106((3)):391–6. doi: 10.1111/1523-1747.ep12343381. [DOI] [PubMed] [Google Scholar]
- 11.McElwee K, Hoffmann R. Growth factors in early hair follicle morphogenesis. Eur J Dermatol. 2000 Jul-Aug;10((5)):341–50. [PubMed] [Google Scholar]
- 12.Perez-Meza D. Wound healing and revascularization of the hair transplant graft: the role of growth factors. Hair Transpl; 2004. pp. pp. 287–98. [Google Scholar]
- 13.Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D, Kouskoukis K. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014 Oct-Dec;7((4)):213–9. doi: 10.4103/0974-2077.150743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer ME, Keaney TC. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J Cosmet Dermatol. 2018 Oct;17((5)):666–71. doi: 10.1111/jocd.12679. [DOI] [PubMed] [Google Scholar]
- 15.Cervantes J, Perper M, Wong LL, Eber AE, Villasante Fricke AC, Wikramanayake TC, et al. Effectiveness of Platelet-Rich Plasma for Androgenetic Alopecia: A Review of the Literature. Skin Appendage Disord. 2018 Jan;4((1)):1–11. doi: 10.1159/000477671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puig CJ, Reese R, Peters M. Double-Blind, Placebo-Controlled Pilot Study on the Use of Platelet-Rich Plasma in Women With Female Androgenetic Alopecia. Dermatol Surg. 2016 Nov;42((11)):1243–7. doi: 10.1097/DSS.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 17.Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: A pilot randomized controlled trial. J Cosmet Laser Ther. 2016 Dec;18((8)):452–5. doi: 10.1080/14764172.2016.1225963. [DOI] [PubMed] [Google Scholar]
- 18.Giordano S, Romeo M, Lankinen P. Platelet-rich plasma for androgenetic alopecia: does it work? Evidence from meta analysis. J Cosmet Dermatol. 2017 Sep;16((3)):374–81. doi: 10.1111/jocd.12331. [DOI] [PubMed] [Google Scholar]
- 19.Cavallo C, Roffi A, Grigolo B, Mariani E, Pratelli L, Merli G, et al. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. BioMed Res Int. 2016;2016:6591717. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentile P, Cole JP, Cole MA, Garcovich S, Bielli A, Scioli MG, et al. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int J Mol Sci. 2017 Feb;18((2)):E408. doi: 10.3390/ijms18020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xian LJ, Chowdhury SR, Bin Saim A, Idrus RB. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy. 2015 Mar;17((3)):293–300. doi: 10.1016/j.jcyt.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Ayatollahi A, Hosseini H, Shahdi M, AhmadNasrollahi S, NassiriKashani M, Yadangi S, et al. Platelet-rich Plasma by Single Spin Process in Male Pattern Androgenetic Alopecia: Is it an Effective Treatment? Indian Dermatol Online J. 2017 Nov-Dec;8((6)):460–4. doi: 10.4103/idoj.IDOJ_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rappl LM. Effect of platelet rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord injury. Int Wound J. 2011 Apr;8((2)):187–95. doi: 10.1111/j.1742-481X.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giusti I, Rughetti A, D'Ascenzo S, Millimaggi D, Pavan A, Dell'Orso L, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009 Apr;49((4)):771–8. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 25.Kushida S, Kakudo N, Morimoto N, Hara T, Ogawa T, Mitsui T, et al. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. J Artif Organs. 2014 Jun;17((2)):186–92. doi: 10.1007/s10047-014-0761-5. [DOI] [PubMed] [Google Scholar]
- 26.Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012 Mar;28((3)):429–39. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Stevens J, Khetarpal S. Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2018 Sep;5((1)):46–51. doi: 10.1016/j.ijwd.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]