Highlights
-
•
Both males and females sniff ‘insunko’ herb, 3 g per day.
-
•
Females also insert ‘insunko’ herb vaginally, 6 g prior for sexual intercourse.
-
•
High concentration of toxic heavy metals have been detected in ‘insunko’ herb.
-
•
Hazard Indexes for arsenic, cadmium, chromium, manganese, and copper were greater than one.
Keywords: Carcinogens, Health hazards, Toxic trace metals, ‘Insunko’ herb
Abstract
There has emerged a herb in Zambia called ‘insunko’ which has unknown chemical composition. The use of ‘insunko’ herb with unknown chemical composition has brought mixed feelings among many Zambians. This study, therefore, aimed to assess the toxic and carcinogenic substances in‘insunko’ herb. ‘Insunko’ herb was purchased from Chipata, Lusaka, Mpika, Mwense, Kitwe, and Solwezi. 5 samples were collected from each of these districts and were thoroughly mixed to give 6 consolidated samples (n = 6). Nicotine and, nitrosamines were analysed using UV spectrometer lambda 35 Perkin Elmer while trace metals were analysed using ICP-MS Inductively Coupled Plasma Mass Spectroscopy (Agilent Technologies, Santa Clara, CA, USA). Nicotine, nitrosamines, and trace metals were detected in high concentrations. The concentrations ranged from 3.87 to 9.83 mg/kg for nitrosamines and 10.94–34.01 mg/kg for nicotine. Hazard Indexes for arsenic, cadmium, chromium, manganese, and copper were greater than one (HI > 1). ‘Insunko’ herb is a potentially toxic and carcinogenic substance because it contains toxic and carcinogenic constituents in high concentrations. These toxic and carcinogenic constituents have been confirmed to cause gastrointestinal disorders, cancers, degenerative, cardiovascular, hematopoietic, neurologic and cognitive problems as well as male infertility.
1. Introduction
There has emerged a herb in Zambia called ‘insunko’ which is composed of powdered tobacco, ash, soda and other ingredients. ‘Insunko’ herb is widely used in Zambia for sniffing while females also use it vaginally to make them tight and warm during sexual intercourse, thus increasing the sexual pleasure of the male partner [1]. There have been reports of vaginal drying agents in South Africa [2], Senegal [3], Malawi, Zimbabwe, Saudi Arabia, Democratic Republic of Congo (DRC), Haiti, Costa Rica, Cameroon, and Kenya [4].
Although ‘insunko’ herb is widely used in Zambia, its chemical composition is not clearly understood. However, its main constituent is smashed tobacco and ash, therefore, can be classified as Smokeless Tobacco Product (STP). STPs ingredients have been associated with various health problems [5] like hypertension, cardiovascular diseases, diabetes, compromised platelet function and oxidative stress as well as oral and oesophageal cancers [[6], [7], [8], [9], [10], [11], [12], [13]]. The World Health Organization (WHO) International Agency for Research on Cancer (IARC) has classified STPs as Group One carcinogens because they are confirmed human carcinogenic substances [14]. Group Two A substances are classified as probably carcinogenic to humans while those in Group Two B are possibly carcinogenic [14].
The main groups of carcinogens in STPs include N-nitrosamine acids, non-volatile tobacco-specific nitrosamines (TSNA) and some trace metals while nicotine remains the addictive constituent [[15], [16], [17]]. Trace metals are found in tobacco leaves and in processed tobacco products such as STPs and cigarettes due to the fact that ash is the binding factor for the other agents while lime is used to alkalinize the product [18]. Other trace metals that are detected in polluted air are absorbed from the soil [19,20]. The major health concerns in STPs come from Arsenic (As+3), Cadmium (Cd+2), Chromium (Cr+2) and Nickel (Ni+2) which are Group One carcinogens and Lead (Pb+2) which is a Group Two A probable human carcinogen according to the declaration by IARC [21].
Consuming ‘insunko’ herb, which has unknown chemical composition has brought mixed feelings among many Zambians more especially health service providers who highly suspect it to be carcinogenic while consumers are enjoying the traditional myths behind its ability to make the vagina tight and warm during sexual intercourse. Cancers have been on the increase in Zambia more especially cervical cancer. This study, therefore, aimed to assess toxic and carcinogenic substances in ‘insunko’ herb.
2. Materials and methods
2.1. Study design
This study was conducted in Zambia between 4th December 2017 and 24th June 2019 after ethical clearance from the National Health Research Authority of Zambia. Five (5) samples were collected from each of the six provinces of Zambia namely; Eastern, Lusaka, Luapula, Muchinga, Copperbelt and North Western. One representative district from each province was selected namely: - Chipata, Lusaka, Mpika, Kitwe, Solwezi, and Mwense. A total of 30 samples of ‘Insunko’ herb was purchased and thoroughly mixed to have 6 consolidated samples (n = 6) according to the districts. Sellers were blinded whilst buying ‘insunko’ herb to ensure that only what was being consumed by the general public was bought. ‘Insunko’ herb is a dark grey substance made by way of roasting the powdered ingredients on a pan. Fig. 1 shows the physical appearance of ‘insunko’ herb.
Fig. 1.
Physical appearance of ‘insunko’ herb.
After thorough mixing, each of the six samples was divided into two parts. Two experiments were conducted to assess the presence of carcinogenic substances in ‘insunko’ herb. Nitrosamines and Nicotine were assessed using UV spectrometer lambda 35 Perkin Elmer. Trace metals were assessed using ICP-MS Inductively Coupled Plasma Mass Spectroscopy (Agilent Technologies, Santa Clara, CA, USA).
2.2. Sample preparation for nicotine and nitrosamines
Prior to use, all the containers were soaked in 10 % (v/v) HNO3 and rinsed with ultra-pure water. 10 g of ‘insunko’ herb sample from each district was weighed and put in the containers where a 100 ml NaOH solution was added. The mixture was stirred for 15 min followed by filtration. 30 ml of distilled water was then added to the filtrate to ensure the removal of all impurities during the second stage filtration. The filtrate was transferred to the separating funnel and extract by 25 ml of ether. The extraction procedure was repeated 3 times and filtrates were gathered in 4 conical flasks followed by drying using 1.0 teaspoon anhydrous potassium carbonate and filtering again. Diethyl ether was evaporated on the water bath and 4 ml of methanol was added to dissolve the resulting oil. Avoid direct heating as nicotine is hydrolyzed by extreme heating. 14 ml of 1.0 Mol/L HCL was then added and made up to 100 ml using distilled water [22].
2.3. Working standard and instrument calibration
A series of Quality Control (QC) samples were prepared to contain the standard N'-nitrosonornicotine (NNN) and 4-methylnitrosamino (NNK). The sequentially diluted quality control samples and standards solution were run to get a calibration. All known standard calculated values were within ± 3% of their true values or 0.003 g/dl. The prepared internal standard solution was evaluated by preparing and analyzing a blank sample at 206 nm for nicotine and 258 for nitrosamines. That was in demonstrating the absence of any interfering compounds and an area count within ± 20 % of the reference value.
The function group of nitrosamines is the amine group. Since nitrosamines are formed from nicotine, an acidic media was provided in order to detect the amines in ‘insunko’ herb. The media provided better quantitation accuracy and precision. The matrix effect was observed by comparing the slope of the samples and the calibration graphs obtained for the QC and standards [23].
2.4. Sample preparation for trace metals
Before use, all the containers were soaked in 10 % (v/v) HNO3 overnight and repeatedly rinsed with ultra-pure water, obtained from a Milli-Q system (Millipore Corporation, Billerica, MA, USA). We weighed all the samples and digested them overnight with 1 ml GR grade 65 % (v/v) HNO3 (CNW Corporation, Shanghai, China), and then the following day with 1 ml GR grade 30 % (v/v) H2O2 (Sinopharm Chemical Reagent Co., Ltd, Beijing, China). The samples were then mixed, sealed in Teflon microwave digestion tubes, and digested in an accelerated microwave digestion system (Mars CEM, CEM Corporation, Matthews NC, USA) at 800 W, 120 C for 10 min and then 800 W, 170 C for 30 min. All digested samples were filtered by using 0.22 lm nylon membrane and finally, the volume was raised to 10 ml using ultra-pure water [24].
2.5. Analytical aspects
Trace metals (As+3, Cd+2, Cu+2, Co+2, Cr+2, Ni+2, Mn+2, and Pb+2) were analysed using ICP-MS Inductively Coupled Plasma Mass Spectroscopy (Agilent Technologies, Santa Clara, CA, USA). Operating parameters were set as RF power 1510 W, carrier gas 1.1 L min1, makeup gas 0.10 L min1, helium gas 3.5 ml min1 and nebulizer pump 0.1 reps. We obtained standard stock solution mixed with Cr+2, Ni+2, Cd+2, As+3, Cu+2, Co+2, Mn+2 and Pb+2 (100 lg mL1, GSB 04-1767-2004) from the National Centre of Analysis and Testing for Nonferrous Metals and Electronic Materials (NCATN), China. Quality Control (QC) sample was then prepared by mixing aliquots of each sample in a composite broadly representative of the whole sample set. The mixture of internal standards (Sc+3, Ge+4, Rh+3, Tb+3, Lu+3) was used in order to check the stability and sensitivity of the instrument, and the mean and %RSD values of these elements were also calculated. The working solutions were prepared on a daily basis by appropriate dilutions of standard stock solution using a mixture of 65 % (v/v) HNO3, 30 % (v/v) H2O2 and H2O (v/v/v = 1:1:3). We used a randomized fashion to run all the samples in order to reduce the possible uncertainty from the artifacts related to the injection order and instrumental sensitivity change. The sampler probe was washed between two samples injections, in three steps: rinsing by Milli-Q water for 30 s; washing with 5% HNO3 for 30 s; washing again by Milli-Q water for 30 s. After the three washing steps, the instrument would run the next sample [25,26].
3. Results
3.1. Observed levels of nicotine and nitrosamines in ‘insunko’ herb
High levels of nicotine and nitrosamines were detected in ‘insunko’ herb. All the samples indicated that ‘insunko’ herb has high levels of nicotine more especially the herb from Mpika, Mwense and Solwezi districts. Nitrosamines were more concentrated in ‘insunko’ herb from Mwense and Chipata districts. Table 1 shows the details.
Table 1.
Concentration of Nitrosamines and Nicotine in ‘Insunko’ herb.
| District | Nitrosamines Concentration (mg/kg) & SD | Nicotine Concentration (mg/kg) & SD |
|---|---|---|
| Chipata | 9.58 ± 1.46 | 19.29 ± 0.62 |
| Solwezi | 6.39 ± 3.89 | 22.22 ± 0.16 |
| Lusaka | 3.87 ± 0.32 | 10.94 ± 0.29 |
| Kitwe | 6.93 ± 1.65 | 17.27 ± 0.19 |
| Mpika | 5.81 ± 0.78 | 34.01 ± 0.27 |
| Mwense | 9.83 ± 0.95 | 23.18 ± 0.23 |
In this study, we compared the observed levels of nicotine and nitrosamines in ‘insunko’ herb with the concentration ranges of Nicotine- derived nitrosamine ketone (NNK) and N-Nitrosonornicotine (NNN) in smokeless tobacco products according to IARC Monograph volume 89. The concentration of nicotine and nitrosamines in ‘insunko’ herb was much higher than the concentration of NNK and NNN in smokeless tobacco products from European Countries, North America, and India as shown in Table 2.
Table 2.
International comparison of the concentration ranges of NNN and NNK in smokeless tobacco products.
| Concentration (mg/kg) & SD |
||||
|---|---|---|---|---|
| Country | Product | Reported as | NNN | NNK |
| Belgium | CT | Dry | 7.38 ± 1.25 | 0.13 ± 0.01 |
| Canada | CT | Dry | 2.09 ± 0.05 | 0.24 ± 0.02 |
| Denmark | CT | Wet | 0.84 ± 0.76 | 0.96 ± 0.94 |
| Germany | CT | Dry | 1.85 ± 0.45 | 0.03 ± 0.001 |
| DS | Dry | 10.6 ± 8.2 | 3.47 ± 2.93 | |
| India | MS | Wet | 0.56 ± 0.01 | 0.24 ± 0.01 |
| CT | Dry | 0.66 ± 0.19 | 0.37 ± 0.24 | |
| Cutka | Wet | 0.59 ± 0.52 | 0.24 ± 0.2 | |
| Mishri | Dry | 3.65 ± 3.35 | 0.7 ± 0.41 | |
| Sweden | MS | Wet | 2.45 ± 1.96 | 0.75 ± 0.56 |
| UK | CT | Dry | 0.9 ± 0.01 | 0.3 ± 0.01 |
| DS | Wet | 1.8 ± 0.01 | 0.26 ± 0.01 | |
| USA | CT | Dry | 3.59 ± 2.92 | 1.05 ± 0.01 |
CT- chewing tobacco, MS-moist snuff, DS- dry snuff.
3.2. Observed levels of trace metals in ‘insunko’ herb
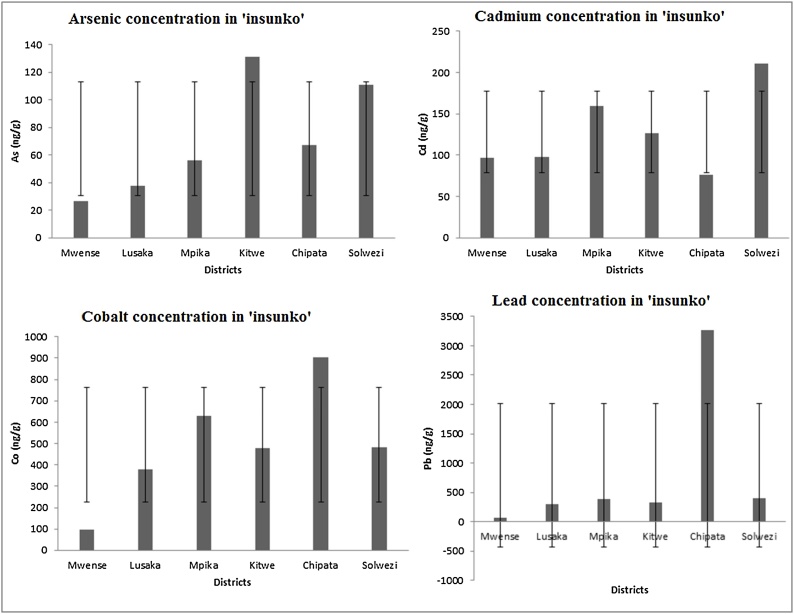
Eight trace metals were detected in ‘insunko’ herb namely, arsenic, cadmium, cobalt, chromium, copper, lead, manganese, and nickel. Fig. 2 shows the levels of arsenic, lead, cobalt, and cadmium in ‘insunko’ herb in milligrams per kilogram (mg/kg) by district. ‘Insunko’ herb from Kitwe and Solwezi had high levels of arsenic followed by Chipata and Mpika. The detected level of Lead in ‘insunko’ herb from Chipata was very high compared with other districts.
Fig. 2.
Concentrations of arsenic, cadmium, cobalt and lead in ‘insunko’ herb.
‘Insunko’ herb from Chipata, Mpika, Kitwe, Solwezi, and Lusaka had high levels of cobalt in that order while ‘insunko’ herb from Mwense had a moderate level. High level of cadmium was observed in ‘insunko’ herb from Solwezi followed by Mpika, Kitwe, Mwense, Lusaka, and Chipata in that order.
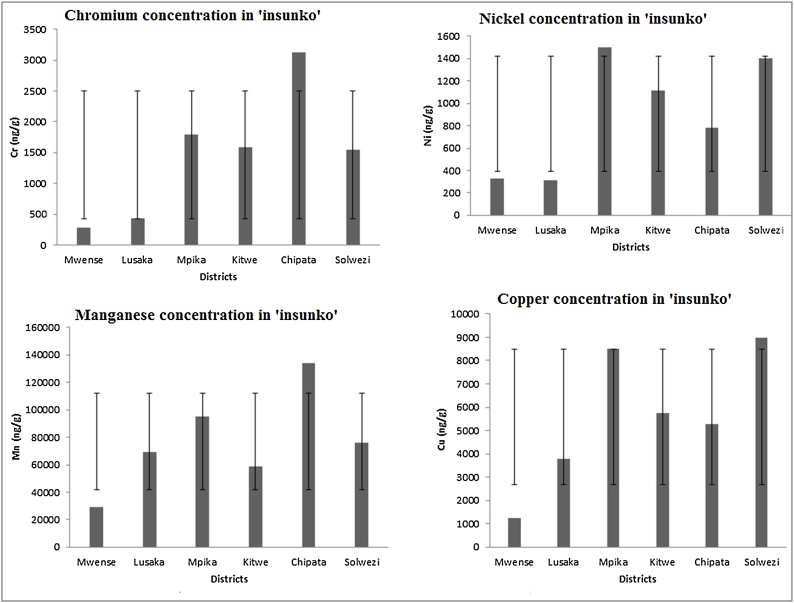
Fig. 3 shows the levels of chromium, nickel, copper, and manganese in ‘insunko’ herb. The herb from Chipata had a very high level of chromium compared with other districts. Other districts with high levels of chromium in ‘insunko’ herb were Mpika, Kitwe, and Solwezi in that order. Nickel was highly concentrated in ‘insunko’ herb from Mpika followed by Solwezi and Kitwe in that order.
Fig. 3.
Concentrations of chromium, nickel, manganese and copper in ‘insunko’ herb.
Copper was the second most concentrated trace metal in ‘insunko’ herb after manganese. By district however, copper was highly concentrated in ‘insunko’ herb from Solwezi followed by Mpika, Kitwe, and Chipata in that order. The most concentrated trace metal in ‘insunko’ herb was manganese. ‘Insunko’ herb from Chipata District had the most concentrated manganese followed by Mpika and Solwezi. Generally, ‘insunko’ herb from Chipata had high concentration of all the trace metals compared with other districts.
3.3. Potential carcinogenicity and toxicity of ‘insunko’ herb
After weighing the daily intake of ‘insunko’ herb as guided by the users, the average daily use was 6 g vaginally and 3 g via sniffing and chewing. ‘Insunko’ herb is placed under the tongue where it is mixed with saliva which is then swallowed. Women who use ‘insunko’ herb vaginally do so in order to give maximum sexual pleasure to the male partner. ‘Insunko’ herb is believed to tighten the vagina and increase vaginal temperature. Therefore, toxic substances from ‘insunko’ herb can enter the human body through ingestion, inhalation, and vaginal route.
Daily intake of nicotine and nitrosamines in ‘insunko’ herb is shown in Table 3.
Table 3.
Daily intake/ use of nicotine and nitrosamines in ‘insunko’ herb.
| Nitrosamines | Nicotine | |||
|---|---|---|---|---|
| District | Vagina (mg/use) & SD | Oral/Sniff (mg/day) & SD | Vagina (mg/use) & SD | Oral/Sniff (mg/use) & SD |
| Chipata | 5.75E-2 ± 3E-4 | 2.87E-2 ± 3E-4 | 1.16E-1 ± 2E-3 | 5.79E-2 ± 2E-4 |
| Solwezi | 3.83E-2 ± 2E-4 | 1.92E-2 ± 2E-4 | 1.33E-1 ± 3E-3 | 6.67E-2 ± 3E-4 |
| Lusaka | 2.32E-2 ± 2E-4 | 1.16E-2 ± 2E-4 | 6.56E-2 ± 2E-4 | 3.28E-2 ± 2E-4 |
| Kitwe | 4.16E-2 ± 3E-4 | 2.08E-2 ± 3E-4 | 1.04E-1 ± 2E-3 | 5.18E-2 ± 2E-4 |
| Mpika | 3.49E-2 ± 2E-4 | 1.74E-2 ± 2E-4 | 2.04E-1 ± 3E-3 | 1.02E-1 ± 3E-3 |
| Mwense | 5.9E-2 ± 3E-4 | 2.95E-2 ± 3E-4 | 1.39E-1 ± 3E-3 | 6.95E-2 ± 3E-4 |
Table 4 shows the intake of trace metals in ‘insunko’ herb in mg per day/use. We further compared the daily intake of trace metals in ‘insunko’ herb to their reference dose (RfD). The United States Environmental Protection Agency (US EPA), Risk Assessment Information System (RAIS) and the Agency for Toxic Substances and Disease Registry (ATSDR) have set a reference dose as a threshold for daily consumption of substances beyond which would be detrimental to human health. Hazard Index (HI) for cadmium, arsenic, chromium, copper, and manganese in insunko’ herb is greater than 1 which means that insunko’ herb is potentially carcinogenic to consumers.
Table 4.
Vaginal/ Oral intake of trace metals in 'insunko' herb (mg/day/use).
| Intake of trace metals in 'insunko' (mg/day/use) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| District | Route | Cr | Mn | Cu | Ni | Co | As | Cd | Pb |
| Mwense | Vagina | 1.66E-3 ± 3E-5 | 1.76E-1 ± 2E-3 | 7.43E-3 ± 2E-5 | 1.99E-3 ± 3E-5 | 5.88E-4 ± 2E-6 | 1.61E-4 ± 1E-6 | 5.83E-4 ± 3E-6 | 4.42E-4 ± 1E-6 |
| Oral/Sniff | 8.32E-4 ± 3E-6 | 8.8E-2 ± 2E-4 | 3.72E-3 ± 2E-5 | 9.94E-4 ± 3E-6 | 2.94E-4 ± 2E-6 | 8.06E-5 ± 1E-6 | 2.92E-3 ± 3E-5 | 2.21E-3 ± 1E-6 | |
| Lusaka | Vagina | 2.29E-3 ± 2E-5 | 4.17E-1 ± 2E-3 | 2.26E-3 ± 2E-5 | 1.99E-3 ± 2E-5 | 3.78E-3 ± 3E-5 | 2.27E-4 ± 1E-6 | 5.89E-4 ± 2E-6 | 1.84E-3 ± 2E-5 |
| Oral/Sniff | 1.29E-3 ± 2E-5 | 2.08E-1 ± 2E-3 | 1.13E-2 ± 2E-4 | 9.45E-4 ± 2E-6 | 1.34E-3 ± 3E-5 | 1.14E-4 ± 1E-6 | 2.94E-4 ± 2E-6 | 9.22E-4 ± 2E-6 | |
| Mpika | Vagina | 1.08E-2 ± 2E-4 | 5.7E-1 ± 3E-3 | 5.1E-2 ± 2E-4 | 8.98E-3 ± 3E-5 | 3.78E-3 ± 3E-5 | 3.37E-4 ± 1E-6 | 9.59E-4 ± 3E-6 | 2.28E-3 ± 3E-5 |
| Oral/Sniff | 5.4E-3 ± 3E-5 | 2.85E-1 ± 3E-3 | 2.55E-2 ± 2E-4 | 4.49E-3 ± 3E-5 | 1.89E-3 ± 2E-5 | 1.69E-4 ± 1E-6 | 4.8E-4 ± 3E-6 | 1.14E-3 ± 3E-5 | |
| Kitwe | Vagina | 9.52E-3 ± 2E-5 | 3.51E-1 ± 3E-3 | 3.44E-2 ± 2E-4 | 6.68E-3 ± 3E-5 | 2.87E-3 ± 2E-5 | 7.86E-4 ± 2E-6 | 7.57E-4 ± 2E-6 | 1.96E-3 ± 2E-5 |
| Oral/Sniff | 4.76E-3 ± 2E-5 | 1.75E-1 ± 2E-3 | 1.72E-2 ± 2E-4 | 3.34E-3 ± 2E-5 | 1.43E-3 ± 3E-5 | 3.93E-4 ± 2E-6 | 3.79E-4 ± 2E-6 | 9.79E-4 ± 2E-5 | |
| Chipata | Vagina | 1.88E-3 ± 2E-4 | 8.03E-1 ± 4E-3 | 3.17E-2 ± 2E-4 | 4.67E-3 ± 2E-5 | 5.42E-3 ± 3E-5 | 4.03E-4 ± 2E-6 | 4.58E-4 ± 3E-6 | 1.96E-2 ± 2E-4 |
| Oral/Sniff | 9.38E-3 ± 3E-5 | 4.02E-1 ± 4E-3 | 1.58E-2 ± 2E-4 | 2.34E-3 ± 2E-5 | 2.71E-3 ± 2E-5 | 2.02E-4 ± 2E-6 | 2.29E-4 ± 3E-6 | 9.79E-3 ± 2E-5 | |
| Solwezi | Vagina | 9.28E-3 ± 3E-5 | 4.57E-1 ± 3E-3 | 5.39E-2 ± 2E-4 | 8.4E-3 ± 2E-5 | 2.88E-3 ± 2E-5 | 6.65E-4 ± 2E-6 | 1.26E-3 ± 2E-5 | 2.39E-3 ± 3E-5 |
| Oral/Sniff | 4.64E-3 ± 2E-5 | 2.29E-1 ± 3E-3 | 2.7E-2 ± 2E-4 | 4.2E-3 ± 2E-5 | 1.44E-3 ± 2E-5 | 3.33E-4 ± 2E-5 | 6.31E-4 ± 2E-6 | 1.2E-3 ± 3E-6 | |
Table 5 shows reference dose for the eight trace metals detected in ‘insunko’ herb.
Table 5.
Reference dose for trace metals.
| Heavy metal | Reference dose (RfD) |
REFERENCE | |
|---|---|---|---|
| Inhalation (mg/m3) | Oral (mg/kg/ day) | ||
| Chromium | 1.00E-4 | 3.00E-3 | EPA |
| Manganese | 5.00E-5 | 1.40E-1 | EPA |
| Copper | 1.43E-1 ± 2.25E-2 | 4.00E-2 | RAIS |
| Nickel | 9.00E-5 | 2.00E-2 | EPA |
| Cobalt | ND by EPA | 3.00E-2 | RTP |
| Arsenic | 4.3E-6 | 3.00E-4 | EPA |
| Cadmium | 1.00E-5 | 1.00E-3 | EPA/ATSDR |
| Lead | ND by EPA | 3.5E-3 | ND by EPA |
*ND = Not Determined. RTP (see ref [31]).
4. Discussion
‘Insunko’ herb is a complex substance with complex chemical composition. This study has established that ‘insunko’ herb has a high concentration of nicotine and nitrosamines. Nicotine is a very addictive substance mostly found in both cigarettes and smokeless tobacco products [[15], [16], [17]]. Previous studies have shown that the uptake of approximately 2 mg of nicotine gives rise to average arterial plasma concentrations of about 0.03 mg/l equivalent to 30 ng/ml [27] and bioavailability of nicotine is 20 % orally [28]. The high rate of leukoplakia has also been observed at a place in the mouth where Smokeless Tobacco users place their chew or dip. Approximately 75 % of daily users of moist snuff and chewing tobacco had noncancerous or precancerous lesions in the mouth [[29], [30], [31]]. The prolonged use of ‘insunko’ herb in the vagina could have similar adverse effects.
Nitrosamines pose the most dangerous health hazard and prove to be the most harmful carcinogens which are present in smokeless tobacco. An increasing body of knowledge about the carcinogenicity of nitrosamines indicate that most nitrosamines pertain to structure-activity relationships rather than to dose-response. Nitrosamines are responsible for liver, lung and kidney tumours when administered orally and through sniffing [32]. They also act as a trans-placental carcinogen when administered to pregnant rats, mice, and Syrian golden hamsters by several routes [33].
Our study has also detected eight trace metals in ‘insunko’ herb namely, arsenic, cadmium, cobalt, chromium, copper, lead, manganese, and nickel. These trace metals are highly concentrated in ‘insunko’ herb hence posing high health risk to consumers.
In assessing the potential health risk associated with the use of ‘insunko’ herb, we considered parameters set by key global health bodies such as the United States Environmental Protection Agency (US EPA), Risk Assessment Information System (RAIS) and the Agency for Toxic Substances and Disease Registry (ATSDR) [[34], [35], [36], [37]]. Based on this approach, this study has identified five toxic trace metals namely, arsenic, cadmium, chromium, copper, and manganese with Hazard Index greater than one (HI > 1) indicating high health risk to consumers of ‘insunko’ herb. ‘Insunko’ herb constituents can cause cancers, gastrointestinal disorders, degenerative, hematopoietic, cardiovascular, neurologic and cognitive problems. They can also cause prenatal neurological and developmental disorders as well as male infertility [38]. Arsenic, cadmium and chromium belong to Group 1 carcinogen to humans [39].
‘Insunko’ herb can cause tumors in many tissues including skin, liver, lung, kidney, and bladder, as well as prostate and uterus due to its arsenic constituent [40,41]. Acute exposure to insunko’ herb can lead to gastrointestinal tract disorders [42], whereas chronic exposure can result in degenerative, inflammatory, and neoplastic changes of the respiratory, hematopoietic, cardiovascular, and the central nervous system [38]. ‘Insunko’ herb can also damage testicular cells which can lead to male infertility [[43], [44], [45]] while exposure in utero can lead to spontaneous abortion and miscarriage as well as decreased birth weights [[46], [47], [48]].
The cadmium and chromium constituents in insunko’ herb can lead to lung, liver, pancreas and stomach cancers and can also damage the male reproductive system [[49], [50], [51], [52], [53], [54], [55]] while the highly neurotoxic constituent of lead in the herb can lead to raised systemic blood pressure and reduced glomerular filtration rate among elderly people [56,57].
‘Insunko’ herb has also been found to contain high concentration of copper which can cause liver damage, gastrointestinal and renal diseases and can also affect the respiratory pathways leading to alveolar migration of macrophages, eosinophilia, formation of histiocytic and noncaseating granulomas among others [58].
Last but not the least; excess manganese in the herb can cause serious side effects, including symptoms resembling Parkinson's disease, such as shaking or tremors. Neurotoxicity symptoms do not manifest immediately but usually become clinically detectable with long-term exposure [59]. Compounds of manganese are suspected to induce or exacerbate asthma [60]. This is of great concern as ‘insunko’ herb has been found to contain a very high concentration of manganese.
This study had some limitations; firstly, we could not detect specific nitrosamines such as non-volatile tobacco-specific nitrosamines (TSNA) and N-nitrosamine acids in ‘insunko’ herb. Secondly, the study only assessed carcinogenic and toxic substances in ‘insunko’ herb and did not involve animal model to detect carcinogenicity and toxicity of the herb. Future studies should, therefore, consider in vivo research on ‘insunko’ herb to determine its carcinogenicity and toxicity.
In conclusion, this study has established that ‘insunko’ herb is potentially carcinogenic and toxic as in contains nicotine, nitrosamines and toxic and carcinogenic trace metals (As+3, Cd+2, Cr+2, Cu+2, and Mn+2) in high concentrations. ‘Insunko’ herb constituents can cause cancers, gastrointestinal disorders, degenerative, hematopoietic, cardiovascular, neurologic and cognitive problems. They can also cause prenatal neurological and developmental disorders as well as male infertility. Consumers of the herb both vaginally and sniffing should, therefore, be wary of the consequences of using ‘insunko’ herb.
Authors’ contributions
MK designed the study, developed and programmed the model and drafted the manuscript. HS gave technical support and statistical analysis while TK did the chemical analysis. All authors read and approved the manuscript.
CRediT authorship contribution statement
Maybin Kalubula: Conceptualization, Methodology, Software, Data curation, Writing - original draft. Heqing Shen: Supervision, Validation, Writing - review & editing. Tasawar Khanam: Visualization, Investigation.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
Compliments go to the National Health Research Authority of the Republic of Zambia, University of Zambia Biomedical Research Ethics Committee and the Research Ethics Committee of the Institute of Urban Environment, Chinese Academy of Sciences for allowing the researchers to conduct a study in Zambia. The principal researcher is a PhD candidate at the Institute of Urban Environment, University of Chinese Academy of Sciences, 1799 Jimei Road, Xiamen, 361021, PR China under the scholarship of UCAS.
Contributor Information
Maybin Kalubula, Email: mkalubula@gmail.com.
Heqing Shen, Email: hqshen@iue.ac.cn.
References
- 1.Phiri P. Zambian women practice dry sex to avoid accusations of infidelity. Global Press J. 2016;(April) [Google Scholar]
- 2.Karim Q.A. Women and AIDS Research Program, International Center for Research on Women; Washington, DC: 1994. Women and AIDS in Natal/KwaZulu, South Africa: Determinants of the Adoption of HIV Protective Behavior, Report in Brief. [Google Scholar]
- 3.Niang C.I. Women and AIDS Research Program, International Center for Research on Women; Washington, DC: 1994. Sociocultural Factors Favoring HIV Infection and the Integration of Traditional Women’s Associations in AIDS Prevention Strategies in Kolda, Senegal, Report in Brief. [Google Scholar]
- 4.Brown J.E., Ayowa O.B., Brown R.C. Dry and tight: sexual practices and potential AIDS risk in Zaire. Soc. Sci. Med. 1993;37(8):989–994. doi: 10.1016/0277-9536(93)90433-5. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization; 2002. Reducing Risks, Promoting Healthy Life, the World Health Report. [From: www.who.int/whr/2002/en/ Accessed May 2014] [DOI] [PubMed] [Google Scholar]
- 6.Wasnik K.S., Ughade S.N., Zodpey S.P., Inqole D.L. Tobacco consumption practices and risk of oropharyngeal cancer: a case-control study in central India. Southeast Asian J. Trop. Med. Public Health. 1998;29:827–834. [PubMed] [Google Scholar]
- 7.Merchant A., Husain S.S., Hosain M., Fikree F.F., Pitiphat W., Siddiqui A.R. Paan without tobacco: an independent risk factor for oral cancer. Int. J. Cancer. 2000;86:128–131. doi: 10.1002/(sici)1097-0215(20000401)86:1<128::aid-ijc20>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Boffetta P., Hecht S., Gray N., Gupta P., Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667–675. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 9.Khan A.M., Khan M.S., ul Haq N. Oral snuff and carcinoma oesophagus. Gomal J. Med. Sci. 2009;7:58–61. [Google Scholar]
- 10.Bolinder G., de Faire U. Ambulatory 24-h blood pressure monitoring in healthy, middle-aged smokeless tobacco users, smokers, and nontobacco users. Am. J. Hypertens. 1998;11:1153–1163. doi: 10.1016/s0895-7061(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 11.Persson P.G., Carlsson S., Svanström L., Ostenson C.G., Efendic S., Grill V. Cigarette smoking, oral moist snuff use and glucose intolerance. J. Intern. Med. 2000;248:103–110. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz D., Liu Y.S., Ercal N., Armstrong D.W. Comparison of pure nicotine and smokeless tobacco extract-induced toxicities and oxidative stress. Arch. Environ. Contam. Toxicol. 1999;37:434–439. doi: 10.1007/s002449900537. [DOI] [PubMed] [Google Scholar]
- 13.Stegmayr B., Johansson I., Huhtasaari F., Moser U., Asplund K. Use of smokeless tobacco and cigarettes: effects on plasma levels of antioxidant vitamins. Int. J. Vitam. Nutr. Res. 1993;63:195–200. [PubMed] [Google Scholar]
- 14.Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines; 2013. World Health Organization International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 89. Available at: www.monographs.iarc.fr/ENG/recentpub/mono89.pdf Accessed November 2013. [PMC free article] [PubMed] [Google Scholar]
- 15.Shaikh A.N., Khandekar, Anand J.S., Mishra U.C. Determination of some toxic trace elements in Indian tobacco and its smoke. J. Radioanal. Nucl. Chem. 1992;163:349–353. [Google Scholar]
- 16.Rickert W.S., Kaiserman M.J. Level of lead, cadmium, and mercury in Canadian cigarette tobacco as indicators of environmental change: result from a 21-year study. Environ. Sci. Technol. 1994;28:924–927. doi: 10.1021/es00054a025. [DOI] [PubMed] [Google Scholar]
- 17.Golia E.E., Dimirkou A., Mitsios I.K. Accumulation of metal on tobacco leaves (primings) grown in agriculture area in relation to soil. Bull. Environ. Contam. Toxicol. 2007;79:158–162. doi: 10.1007/s00128-007-9111-0. [DOI] [PubMed] [Google Scholar]
- 18.Zakiullah U., Saeed M., Muhammad N., Khan S.A., Gul F., Khuda F. Assessment of potential toxicity of a smokeless tobacco product (naswar) available on the Pakistani market. Tob. Control. 2012;21:396–401. doi: 10.1136/tc.2010.042630. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar A., Kazi T.G., Afridi H.I. Application of modified cloud point extraction method for the chromium speciation in artificial saliva extracts of different snuff products. J. Ind. Eng. Chem. 2018;59:320–327. DOI10.1016/j.jiec.2017.10.038. [Google Scholar]
- 20.Kazi T.G., Arain S.S., Afridi H.I. Analysis of cadmium, nickel, and lead in commercial moist and dry snuff used in Pakistan. Environ. Monit. Assess. 2013;185:5199–5208. doi: 10.1007/s10661-012-2936-y. [DOI] [PubMed] [Google Scholar]
- 21.2014. World Health Organization International Agency for Research on Cancer Agents Classified by the IARC Monographs, Vols. 1-109. Available at: www.monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf (Accessed May 2014) [Google Scholar]
- 22.Taheri S., Jalali F., Fattahi N. Sensitive determination of methadone in human serum and urine by dispersive liquid-liquid microextraction based on the solidification of a floating organic droplet followed by HPLC-UV. J. Sep. Sci. 2015;38:3545–3551. doi: 10.1002/jssc.201500636. PMID: 26289536. [DOI] [PubMed] [Google Scholar]
- 23.Pirsaheb M., Fattahi N. Development of a liquid-phase microextraction based on the freezing of a deep eutectic solvent followed by HPLC-UV for sensitive determination of common pesticides in environmental water samples. RSC Adv. 2018;8:11412–11418. doi: 10.1039/c8ra00912k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadeghi M., Nematifar Z., Irandoust M. Efficient and selective extraction and determination of ultra trace amounts of Hg2+ using solid phase extraction combined with ion pair based surfactant-assisted dispersive liquid–liquid microextraction. RSC Adv. 2015;5:100511–100521. [Google Scholar]
- 25.Rezaee M., Khalilian F., Mashayekhi H.A. A novel method for the high preconcentration of trace amounts of the aflatoxins in pistachios by dispersive liquid–liquid microextraction after solid-phase extraction. Anal. Methods. 2014;6:3456–3461. [Google Scholar]
- 26.Karimaei M., Sharafi K., Moradi M. Optimization of a methodology for simultaneous determination of twelve chlorophenols in environmental water samples using in situ derivatization and continuous sample drop flow microextraction combined with gas chromatography-electron-capture detection. Anal. Methods. 2017;9:2865–2872. [Google Scholar]
- 27.Gourlay S.G., Benowitz N.L. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin. Pharmacol. Ther. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- 28.Hukkanen J., Jacob P., Benowitz N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Jacob B.J., Straif K., Thomas G. Betel quid without tobacco as a risk factor for oral precancers. Oral Oncol. 2004;40:697–704. doi: 10.1016/j.oraloncology.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Fisher M.A., Bouquot J.E., Shelton B.J. Assessment of risk factors for oral leukoplakia in West Virginia. Community Dent. Oral Epidemiol. 2005;33:45–52. doi: 10.1111/j.1600-0528.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 31.Shulman J.D., Beach M.M., Rivera-Hidalgo F. The prevalence of oral mucosal lesions in U.S. adults: data from the Third National Health and Nutrition Examination Survey, 1988–1994. J. Am. Dent. Assoc. 2004;135:1279–1286. doi: 10.14219/jada.archive.2004.0403. [DOI] [PubMed] [Google Scholar]
- 32.Druckrey H., Preussmann R., Ivankovic S., Schmaehl D. Organotropism and c carcinogenic effects of 65 different N-nitroso compounds in BD-rats. Z. Kerbsforsch. 1967;69(2):103–201. [PubMed] [Google Scholar]
- 33.Terracini B., Magee O.N., Barnes J.M. Hepatic pathology in rats on low dietary levels of dimethylnitrosamine. Br. J. Cancer. 1967;21:559–565. doi: 10.1038/bjc.1967.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US EPA . Office of Solid Waste and Emergency Response, U.S. Environmental Protection Agency; Washington, DC: 2007. Estimation of Relative Bioavailability of Lead in Soil and Soil-Like Materials Using in Vivo and in Vitro Methods. [Google Scholar]
- 35.US EPA . US Environmental Protection Agency; Washington, D.C: 2001. Risk Assessment Guidance for Superfund: Volume III Part A, Process for Conducting Probabilistic Risk Assessment. [Google Scholar]
- 36.Finley B.L., Monnot A.D., Paustenbach D.J., Gaffney S.H. Derivation of a chronic oral reference dose for cobalt. Regul. Toxicol. Pharmacol. 2012;64(3):491–503. doi: 10.1016/j.yrtph.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 37.EPA . EPA 120/R-07/001; 2007. Framework for Metals Risk Assessment.https://www.epa.gov/sites/production/files/2013-09/documents/metals-risk-assessment-final.pdf March Available at: [Google Scholar]
- 38.Papadopoulou P., Marouli C. Food Addit. Contam. Part A. 2015;32:1140–1147. [Google Scholar]
- 39.Vols. 1–42. International Agency for Research on Cancer; Lyon: 1987. pp. 100–103. (Overall Evaluations of Carcinogenicity: an Updating of IARC Monographs). [Google Scholar]
- 40.Agency for Toxic Substances and Disease Registry; Atlanta, Georgia: 2007. Toxicological Profile for Arsenic; pp. 20–375. [PubMed] [Google Scholar]
- 41.National Academy Press; Washington DC: 1999. NRC (National Research Council), Arsenic in Drinking Water; pp. 1–310. Arsenic in Drinking Water. [Google Scholar]
- 42.Goebl H.H., Schmidt P.F., Bohl J., Teltenborn B., Kramer G., Gutman L. Polyneuropathy due to arsenic intoxication: biopsy studies. J. Neurol. 1990;49:137–149. doi: 10.1097/00005072-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Naiger R.D., Osweiler G.D. Effect of sub-acute low level dietary sodium arsenite on dogs. Fundam. Appl. Toxicol. 1989;13:439–451. doi: 10.1016/0272-0590(89)90281-9. [DOI] [PubMed] [Google Scholar]
- 44.Lindberg A.L., Sohel N. Impact of smoking and chewing tobacco on arsenic induced skin lesions. Environ. Health Perspect. 2010;118:533–538. doi: 10.1289/ehp.0900728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y.J., Kim J.M. Arsenic toxicity in male reproduction and development. Dev. Reprod. 2015;19(December(4)):167–180. doi: 10.12717/DR.2015.19.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad S.A., Sayed M.H., Barua S., Khan M.H., Faruquee M.H., Jalil A., Hadi S.A., Talukder H.K. Arsenic in drinking water and pregnancy outcomes. Environ. Health Perspect. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihrig M.M., Shalat S.L., Baynes C. A hospital-based case-control study of stillbirths and environmental exposure to arsenic using an atmospheric dispersion model linked to a geographical information system. Epidemiology. 1998;9:290–294. [PubMed] [Google Scholar]
- 48.Milton A.H., Smith W., Rahman B., Hasan Z., Kulsum U., Dear K., Rakibuddin M., Ali A. Chronic arsenic exposure and adverse pregnancy outcomes in Bangladesh. Epidemiology. 2005;16:82–86. doi: 10.1097/01.ede.0000147105.94041.e6. [DOI] [PubMed] [Google Scholar]
- 49.International Agency for Research on Cancer; Lyon: 2009. Overall Evaluations of Carcinogenicity to Humans.http://monographs.iarc.fr/ENG/Classification/crthgr01.php (Accessed 6 May 2010) [Google Scholar]
- 50.Moller D.R. Delayed anaphylactoid reaction in a worker exposed to chromium. J. Allergy Clin. Immunol. 1986;77:451–456. doi: 10.1016/0091-6749(86)90179-x. [DOI] [PubMed] [Google Scholar]
- 51.Rüegger M. Lung diseases due to metals. Schweizerische Medizinizche Wochenschrift. 1995;125:467–474. [PubMed] [Google Scholar]
- 52.Leroyer C. Occupational asthma due to chromium. Respiration. 1998;65:403–405. doi: 10.1159/000029303. [DOI] [PubMed] [Google Scholar]
- 53.Hansen M.B., Johansen J.D., Menné T. Chromium allergy: signifi cance of both Cr (III) and Cr (VI) Contact Derm. 2003;49:206–212. doi: 10.1111/j.0105-1873.2003.0230.x. [DOI] [PubMed] [Google Scholar]
- 54.Linneberg A., Nielsen N.H. Smoking might be a risk factor for contact allergy. J. Allergy Clin. Immunol. 2003;111:980–984. doi: 10.1067/mai.2003.1394. [DOI] [PubMed] [Google Scholar]
- 55.Sockanathan S., Setterfi eld J., Wakelin S. Oral lichenoid reaction due to chromate/cobalt in dental prosthesis. Contact Dermatol. 2003;48:342–343. doi: 10.1034/j.1600-0536.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 56.International Agency for Research on Cancer; 2006. Inorganic and Organic Lead Compounds. Lyon; p. 378. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 87) [Google Scholar]
- 57.Agency for Toxic Substances and Disease Registry; Atlanta, Georgia: 2007. Toxicological Profile for Lead; pp. 21–169.http://www.atsdr.cdc.gov/ToxProfiles/tp13.pdf (Accessed 3 January 2012) [PubMed] [Google Scholar]
- 58.Agency for Toxic Substances and Disease Registry; Atlanta, Georgia: 2004. Toxicological Profile for Copper; pp. 22–23.http://www.atsdr.cdc.gov/ToxProfiles/tp132-c3.pdf (Accessed 3 January 2012) [Google Scholar]
- 59.Agency for Toxic Substances and Disease Registry; Atlanta, Georgia: 2008. Toxicological Profile for Manganese; pp. 38–86.http://www.atsdr.cdc.gov/ToxProfiles/tp151-c3.pdf (Accessed 3 January 2012) [PubMed] [Google Scholar]
- 60.Leikauff G.D. Hazardous air pollutants and asthma. Environ. Health Perspect. 2002;110(Suppl. 4):505–526. doi: 10.1289/ehp.02110s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]