Abstract
Purpose
The aim of this study was to assess whether mucoepidermoid carcinoma of the lacrimal sac is a counterpart of CRTC1/3-MAML2 gene fusion-related salivary gland mucoepidermoid carcinoma.
Methods
In this retrospective observational case series, pathology records were searched for all cases of lacrimal sac mucoepidermoid carcinoma diagnosed between 1990 and 2018. Data collected included demographics, clinical findings, management, and follow-up. Pathologic parameters assessed included tumor morphology, immunohistochemistry, and MAML2 and EGFR fluorescence in situ hybridization (FISH) studies.
Results
Six patients with mucoepidermoid carcinoma of the lacrimal sac, 5 males and 1 female, with a median age of 63 years (range 24–66) were identified. Five tumors were managed with radical resection and 1 patient underwent orbital exenteration. None of the patients developed recurrence or metastases with an average follow-up of 18 months (range 13–23). All tumors had morphologic and immunohistochemical features of mucoepidermoid carcinoma and overexpressed EGFR. MAML2 FISH was negative for MAML2 rearrangement in all tumors. EGFR FISH demonstrated EGFR amplification in 1 tumor.
Conclusions
Mucoepidermoid carcinoma of the lacrimal sac is not a lacrimal sac counterpart of CRTC1/3-MAML2 gene fusion-related salivary gland mucoepidermoid carcinoma. EGFR pathway activation and EGFR amplification in a subset of these neoplasms suggest the potential role for anti-EGFR agents.
Keywords: Lacrimal sac mucoepidermoid carcinoma, Mucoepidermoid carcinoma, Lacrimal sac carcinoma, MAML2, CRTC1/3-MAML2, EGFR, Adenosquamous carcinoma
Introduction
Mucoepidermoid carcinoma of the lacrimal sac is a rare locally aggressive malignancy, with <20 cases reported in the literature [1, 2, 3, 4, 5, 6, 7, 8]. Analogous to its salivary gland counterpart, mucoepidermoid carcinoma of the lacrimal sac, nasal cavity, and paranasal sinuses is composed of epidermoid, mucus-secreting, and intermediate cells and is presumed to originate predominantly from the mucosal mucoserous glands and less frequently from the overlying epithelium [1, 2, 9, 10, 11]. Recent studies from the salivary gland literature have documented recurrent translocations involving mastermind-like protein 2 (MAML2) and CREB-regulated transcription coactivator 1 (CRTC1) or CRTC3 genes, resulting in CRTC1/3-MAML2 gene fusion in 34–81% of mucoepidermoid carcinomas [12, 13, 14, 15]. The MAML2 translocation status has gained recognition for its diagnostic utility as an objective confirmation of mucoepidermoid carcinoma diagnosis, particularly in tumors with overlapping histology [12, 13, 14]. Additionally, MAML2 translocation in salivary gland carcinomas has been traditionally associated with a lower histologic grade, less advanced clinical stage, and longer disease-free and overall survival [15, 16]. With increasing emphasis on targetable therapy, identification of the CRTC1/3-MAML2 fusion product may emerge as a potential therapeutic target. Finally, mucoepidermoid carcinomas have been shown to demonstrate epidermal growth factor receptor/extracellular receptor kinase (EGFR/ERK) pathway activation, which may be influenced by CRTC1/3-MAML2 fusion and EGFR gene copy number alterations, suggesting the role for EGFR and mitogen-activated protein kinase pathway inhibitors [13, 16, 17].
The role of MAML2 gene rearrangement, EGFR gene copy number alterations, and EGFR pathway activation in the mucoepidermoid carcinoma of the lacrimal sac has not been systematically explored. Herein, we describe clinical-pathologic characteristics of 6 lacrimal sac mucoepidermoid carcinomas, with a focus on MAML2 and EGFR status.
Materials and Methods
The Wills Eye Hospital Institutional Review Board approved this study. The study was performed in compliance with HIPAA guidelines and with the tenets of the Declaration of Helsinki.
Patient Selection
Wills Eye Hospital Pathology records were searched for all cases of mucoepidermoid carcinoma of the lacrimal sac diagnosed between 1990 and 2018. Medical records of identified patients were reviewed. Data collected included patients' age, sex, presenting symptoms and their duration, clinical and imaging findings at presentation, management, and follow-up information. Because lacrimal sac carcinoma does not have specific American Joint Committee on Cancer (AJCC) staging guidelines, the tumors were staged based on the AJCC 8th edition guidelines for carcinoma of the nasal cavity and paranasal sinuses [18].
Histopathology
Routine sections stained with hematoxylin-eosin, periodic acid-Schiff (PAS), Alcian blue, and Hale's colloidal iron were prepared from paraffin-embedded, formalin-fixed tissues. Morphologic parameters assessed included the presence of an intraepithelial or intraglandular in situ component, constituent cell types (epidermoid, intermediate, and mucocytes), perineural invasion, local extent of disease, pathologic staging (based on the AJCC 8th edition, carcinoma of the nasal cavity and paranasal sinuses guidelines) [18], and tumor grading in accordance with the AFIP grading system for mucoepidermoid carcinoma of the salivary glands (low-grade = score 0–4, intermediate grade = score 5–6, high-grade = score >6; score components: intracystic component <20% = score 2, neural invasion = score 2, mitosis ≥4/10 high power fields = score 3, necrosis = score 3, anaplasia = score 4) [19].
Immunohistochemistry
The immunostaining was performed with the following primary antibodies: monoclonal mouse anti-human cytokeratin 7 (CK7; prediluted; DAKO, CA, USA), monoclonal mouse anti-human CK20 (prediluted; Biocare, CA, USA), monoclonal mouse anti-human p63 (prediluted; Biocare), and monoclonal mouse anti-human EGFR (Clone H11, 1:50; DAKO). Peroxidase activity was visualized by applying diaminobenzidine solution containing 0.05% H2O2. Sections were counterstained with a modified Mayer's hematoxylin, dehydrated, cleared, and mounted. Appropriate positive and negative controls were run with each batch. All immunohistochemical stains were prepared on Leica autostainer BOND III in accordance with the manufacturer's instructions.
Immunohistochemical stains for CK7, CK20, and p63 were scored semi-quantitatively based on the strength of cytoplasmic (CK7 and CK20) and nuclear (p63) expression (0 = no staining, 1+ = weak staining, 2+ = moderate staining, 3+ = strong staining) and based on the percentage of immunoreactive cells (0% = 0, 1–25% = 1+, 26–50% = 2+, 51–75% = 3+, 76–100% = 4+). Because of heterogeneity in methodology for EGFR expression assessment, we evaluated EGFR expression by 3 methods: (1) as percentage of immunoreactive cells with membranous and cytoplasmic staining, (2) semi-quantitatively (0, no positive cells; 1+, low discontinuous membrane staining; 2+, unequivocal membrane staining with moderate intensity; and 3+, strong and complete membrane staining), as described by Lujan et al. [17], and (3) by an H-score (defined as a continuous variable with a scale ranging from 0 to 300 and calculated using the following formula: 1 × [percentage of weakly stained cells, 1+] + 2 × [percentage of moderately stained cells staining, 2+] + 3 × [percentage of strongly stained cells, 3+] [range 0–300]), as described by Avilés-Salas et al. [20]. EGFR overexpression was defined as 2+ (moderate membranous staining) and 3+ (strong and complete membranous staining) in >10% of neoplastic cells [17] or H-score of >100 [20].
Fluorescence in situ Hybridization Studies
After the areas for cell counting were determined by a hematoxylin-eosin slide review, a 4-μm-thick section of formalin-fixed paraffin-embedded tissue was placed onto a positively charged slide.
Fluorescence in situ Hybridization for Detection of MAML2 Gene Rearrangement
The MAML2 fluorescence in situ hybridization (FISH) assay was performed with the probe Zytolight SPEC MAML2 Dual Color Break Apart Probe (11q21; ZytoVision, Bremerhaven, Germany) and the Histology FISH Accessory Kit (ZytoVision) in accordance with the manufacturer's protocols. At least 40 randomly selected nonoverlapping tumor cell nuclei were evaluated for the presence of yellow (normal) or green and red (chromosomal break-apart) fluorescent signals at ×1,000 magnification. The sample was considered positive for rearrangement when >20% of nuclei showed break-apart signals. CRTC1/3-MAML2 fusion gene-positive salivary gland mucoepidermoid carcinoma cases were used as a positive control. The MAML2 FISH assay was internally validated on 70 salivary gland mucoepidermoid carcinomas and 40 other (nonmucoepidermoid carcinoma) epithelial salivary gland tumors.
FISH for Detection of EGFR Gene Copy Number Alteration
The EGFR FISH assay was carried out with the EGFR (11p11.2) SpectrumOrange/Con 7 (11q11.1) SpectrumGreen probe (Empire Genomics, NY, USA) in accordance with the manufacturer's protocols. Four physically distant tumor areas were selected and the EGFR and Con 7 (centromeric control) signals were counted in 40 tumor nuclei at ×1,000 magnification, and the proportion of EGFR/Con 7 signal number was calculated. The cases were considered normal if 2 blue and 2 red signals were visualized in each nucleus. Polysomy was considered where 3 or more blue and red signals (in equal number) were seen in each nucleus. The EGFR amplification was defined as EGFR/Con 7 ratio of >2.0. The EGFR FISH methodology was adopted from the FDA-approved FISH assay for the Her2 family and validated internally.
Results
Clinical Characteristics
Review of pathology medical records identified 6 patients with mucoepidermoid carcinoma of the lacrimal sac, 5 males and 1 female, with a median age of 63 years (average 54 months; range 24–66). All patients presented with unilateral right-sided (4/6, 67%) and left-sided (2/6, 33%) epiphora, associated with a palpable mass in the region of the lacrimal sac (4/6, 67%), eye pain or irritation (4/6, 67%), recurrent dacryocystitis (2/6, 33%), and nasal congestion (1/6, 17%; Fig. 1, 2). Duration of symptoms ranged from 4 to 16 months (average 9 months). Two patients had undergone prior dacryocystorhinostomy for nasolacrimal duct obstruction. Imaging demonstrated a soft tissue mass centered in the lacrimal sac, ranging in size from 1.5 to 2.5 cm (average 2.0 cm; Fig. 1, 2). Five patients (83%) were managed with radical resection of the lacrimal sac and nasolacrimal duct system and varying portions of the adjacent orbital tissue, maxillary sinus, and ethmoid sinus. One patient (17%) with extensive orbital soft tissue involvement underwent orbital exenteration. All resections were performed with frozen section control of margins. Postoperative radiation therapy was delivered to 4 (67%) patients. Follow-up information was available on 4 of 6 patients (67%). None of the patients developed recurrence or metastases with an average follow-up of 18 months (range 13–23). The clinical characteristics of the patients are summarized in Table 1.
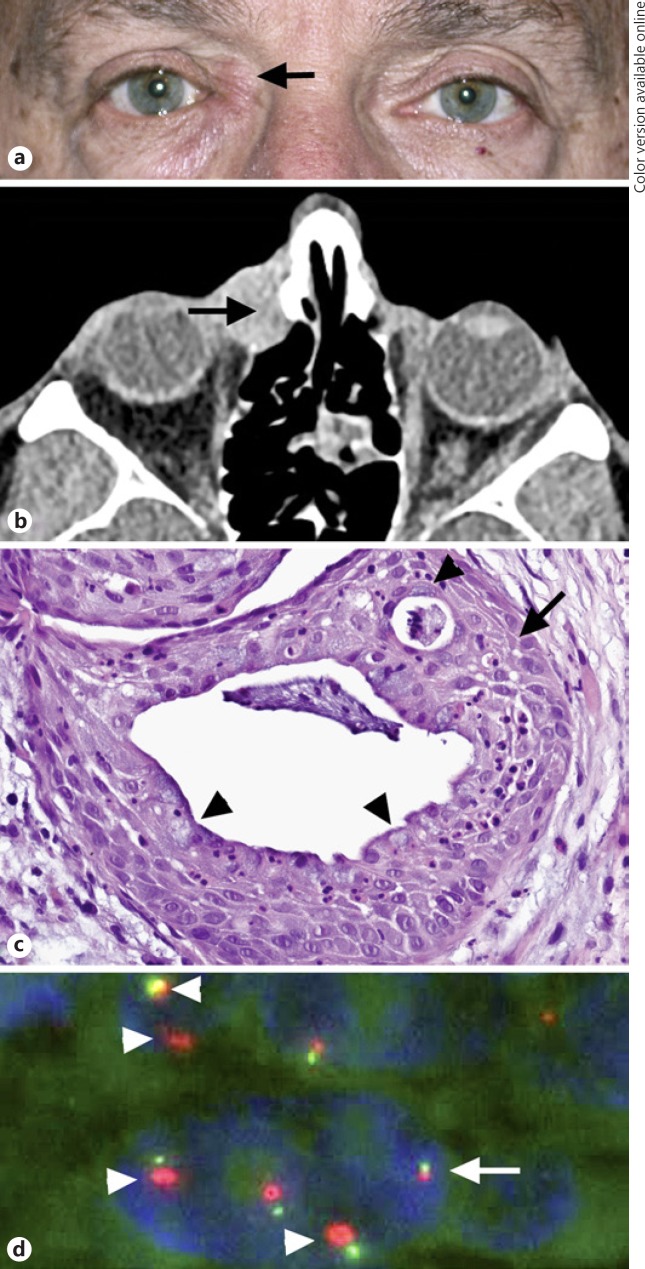
Fig. 1.
Clinical and pathologic characteristics of lacrimal sac tumor in patient 3. a Right medial canthal mass, focally situated above the medial canthal tendon (arrow). b Axial post-contrast computed tomography scan demonstrates a mass in the region of the lacrimal sac (arrow), with focal adjacent bony changes and with extension into the medial orbit. c Well-differentiated mucoepidermoid carcinoma is composed of epidermoid cells with intercellular bridges (arrow), mucocytes (arrowheads), and cells with intermediate morphology without appreciable nuclear atypia or mitotic activity, forming mucin-filled cysts (stain, hematoxylin-eosin; original magnification, ×100). dEGFR fluorescence in situ hybridization studies demonstrate amplification of red EGFR signal (arrowheads), which is 2–4 times the size of the green centromeric (Con 7) signal in neoplastic cell nuclei, compatible with an EGFR/Con 7 ratio of >2. In contrast, the cell nucleus without EGFR amplification features EGFR and centromeric signals of similar size (arrow).
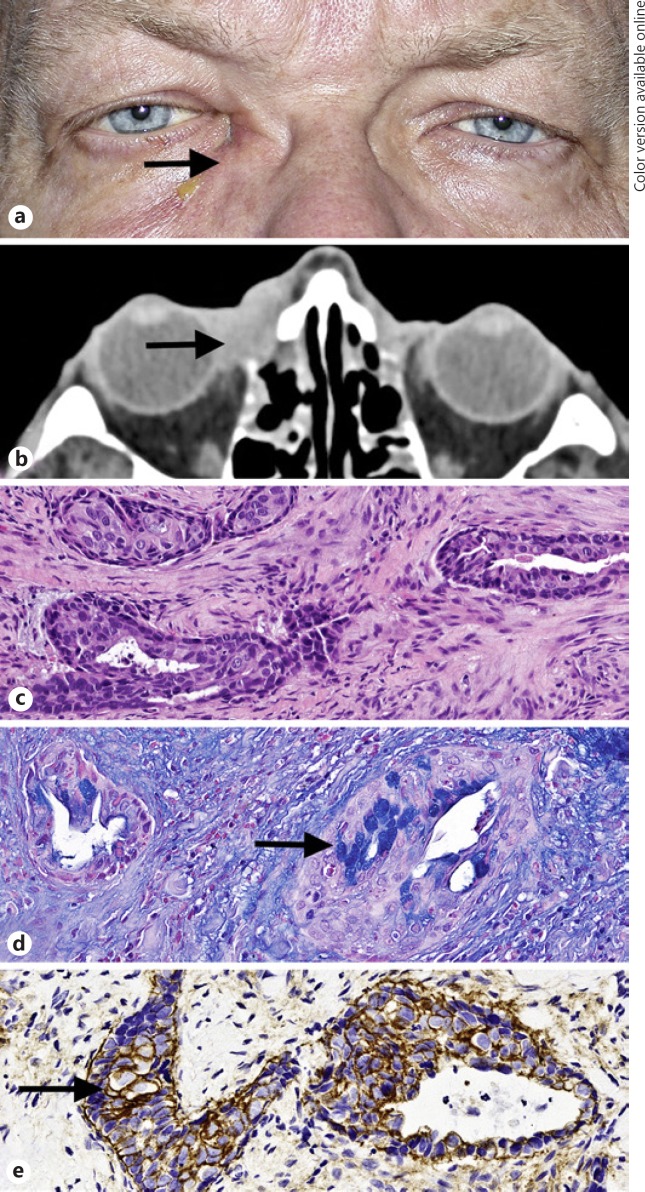
Fig. 2.
Clinical and pathologic characteristics of lacrimal sac tumor in patient 5. a Right medial canthal mass (arrow). b Axial post-contrast computed tomography scan demonstrates a mass in the region of the lacrimal sac (arrow), with focal bone destruction and with extension into the medial orbit. c Invasive neoplasm, composed predominantly of nonkeratinizing epidermoid cells and intermediate cells, without readily identifiable mucocytes forms solid nests and cysts in a background of markedly desmoplastic stroma. d Alcian blue highlights the intracytoplasmic mucin in mucocytes (arrow). e Intense membranous staining with EGFR (arrow) is present in most neoplastic cells. (stains: a, hematoxylin-eosin; b, Alcian blue; c, EGFR; a–d, original magnification, ×50).
Table 1.
Clinical features and staging of patients with lacrimal sac mucoepidermoid carcinoma
| Patient | Age, years/sex | Laterality | Presenting symptoms (duration in months) | Imaging findings (CT scan) | Secondary sites of involvementa | pTb | cNb | cMb | Stageb | Surgical management | Adjuvant therapy | Recurrence or metastases, follow-up in months | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/M | Left | Epiphora and recurrent dacryocystitis (12 months) | 2.5-cm soft tissue mass at medial canthal area, associated with globe displacement | Inferomedial orbit, nasal cavity, ethmoid sinus | 3 | x | x | At least III | LS and NLD resection, lateral rhinotomy, ethmoidectomy, medial maxillectomy, orbital exenteration | XRT | N/A | |
| 2 | 24/M | Right | Ephiphora (9 months), LS mass (4 months) | N/A | Nasal cavity, medial orbit | 3 | x | x | At least III | LS and NLD resection, medial orbital tissue resection | None | N/A | |
| 3 | 62/M | Right | Epiphora, recurrent dacyocystitis, LS mass, pain (16 months) | 1.9-cm LS mass with erosion of lacrimal bone and bony lacrimal duct | Maxilla, medial orbit | 3 | 0 | 0 | III | LS and NLD resection, ethmoidectomy, medial maxillectomy, middle and inferior turbinectomy | None | No, 23 months | |
| 4 | 66/F | Left | Epiphora, LS mass, eye irritation, nasal congestion (10 months) | 1.6-cm soft tissue mass in LS without bony erosion | Nasal cavity | 1 | 0 | 0 | I | LS and NLD resection, medial maxillectomy, ethmoidectomy, anterior turbinectomy | XRT | No, 20 months | |
| 5 | 65/M | Right | Epiphora, eye pain (6 months) | 2.5-cm soft tissue mass in LS | Maxilla, medial orbit | 3 | 0 | 0 | III | LS and NLD resection, medial maxillectomy | XRT | No, 13 months | |
| 6 | 63/M | Right | LS mass, pain (6 months) | 1.5-cm soft tissue mass in LS with bony changes | Maxilla, medial orbit | 3 | 0 | 0 | III | LS resection, medial maxillectomy | XRT | No, 17 months | |
CT, computed tomography; NLD, nasolacrimal duct; LS, lacrimal sac; x, not available; N/A, not available; pT, pathology tumor grouping by size; cN, clinical documentation of nodal metastasis; cM, clinical documentation of distal metastasis; XRT, external beam radiotherapy.
Based on imaging studies with pathologic confirmation.
TNM classification and stage groupings are adopted from the AJCC classification of carcinoma of the nasal cavity and ethmoid sinus (patients 1, 2, and 4) and maxillary sinus (patients 3, 5, and 6), 8th edition [18].
Histopathology and Immunohistochemistry
Histopathologic and immunohistochemical features of the 6 lacrimal sac mucoepidermoid carcinomas are summarized in Table 2. All tumors were composed of varying proportions of epidermoid (nonkeratinizing squamous) cells, mucocytes (goblet-like cells), and intermediate cells (basal or cuboidal cells with morphology in-between mucocytes and squamous cells), forming cystic structures filled with mucin (Fig. 1c). Solid tumor nests were conspicuous in higher grade tumors (Fig. 2c). The intracytoplasmic mucin in mucocytes and extracellular mucin was highlighted with PAS, Alcian blue, and Hale's colloidal iron stains (Fig. 2d). Notably, no keratinization (squamous pearl formation) was identified. An association with the overlying dysplastic lacrimal sac epithelium was observed in 2 (33%) tumors. No definitive in situ component was identified in the other 4 (67%) tumors. Perineural invasion was documented in 2 (33%) tumors. Four (67%) tumors were of intermediate histologic grade (grade 2), and 2 tumors (33%) were low-grade (grade 1).
Table 2.
Pathology features of lacrimal sac mucoepidermoid carcinoma
| Patient | In situ component | Grade (AFIP)a | Perineural invasion | CK7 | CK20 | p63 | EGFR IHCb %, S, H-score | EGFR FISH | MAML2 FISH |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | G2 | No | + | − | + | 80, 2+, 190 | Negative | Negative |
| 2 | Yes | G2 | No | + | − | + | 80, 3+, 210 | N/A | Negative |
| 3 | N/A | G1 | No | + | − | + | 50, 2+, 120 | Positive | Negative |
| 4 | N/A | G1 | No | + | − | + | 98, 3+, 290 | Negative | Negative |
| 5 | N/A | G2 | Yes | + | + | + | 90, 3+, 240 | Negative | Negative |
| 6 | N/A | G2 | Yes | + | − | + | 90, 3+, 280 | Negative | Negative |
CK, cytokeratin; EGFR, Epidermal Growth Factor Receptor; FISH, fluorescence in situ hybridization; MAML2, Mastermind Like Transcriptional Coactivator 2 gene; N/A, not available or unable to assess; (+), positive; (–), negative.
AFIP grading system for mucoepidermoid carcinoma of salivary glands [19].
EGFR IHC, immunohistochemical expression of EGFR staining is scored by 3 methods: (1) %, percentage of immunoreactive cells with membranous and cytoplasmic staining; (2) S, semi-quantitative assessment (0, no positive cells; 1+, low discontinuous membrane staining; 2+, unequivocal membrane staining with moderate intensity; and 3+, strong and complete membrane staining; only cases with 2+ and 3+ staining patterns in >10% of tumor cells were considered positive) as described by Lujan et al. [17]; (3) H-score (EGFR H-score was defined as a continuous variable with a scale ranging from 0 to 300 and was calculated using the following formula: 1 × [percentage of weakly stained cells, 1+] + 2 × [percentage of moderately stained cells staining, 2+] + 3 × [percentage of strongly stained cells, 3+] [range 0–300]) as described by Avilés-Salas et al. [20].
Immunohistochemical studies showed that all tumors moderately or strongly expressed CK7 (25–100% of cells) and strongly expressed p63 (75–100% of cells). CK20 was focally weakly to moderately expressed in 2 of 6 (33%) tumors. EGFR was overexpressed in all neoplasms. EGFR expression appeared as moderate-to-strong, membranous, circumferential staining in 50% or more cells in all tumors and in 80% or more cells in 5 of 6 (83%) tumors (Fig. 2e). H-score was ≥100 in all tumors (median 225; average 222; range 120–290).
MAML2 and EGFR FISH
MAML2 FISH was negative for rearrangement in the MAML2 gene in all tumors. EGFR FISH demonstrated amplification in EGFR gene (3.8 EGFR signals/cell; EGFR/Con 7 ratio 2.02) in 1 tumor and no evidence of polysomy in any of the tumors.
Discussion
Mucoepidermoid carcinoma is the most common salivary gland malignancy, composed of 3 cell types: goblet cell-like mucocytes, epidermoid cells, and intermediate cells [12, 14]. Mucoepidermoid carcinoma can infrequently involve the lacrimal sac, where this neoplasm affects predominantly males between the 4th and 6th decades, presenting with signs and symptoms of nasolacrimal duct obstruction and a mass in the lacrimal sac region − a pattern that was also seen in our patients[1, 2, 3, 4, 5, 6, 7, 8].
During the past decade, a translocation involving the CRTC1 gene at 19p13 and the MAML2 gene at 11q21 t(11;19)(q21;p13) has gained recognition for its diagnostic utility as an objective confirmation of the diagnosis of salivary gland mucoepidermoid carcinoma [12, 13, 14, 15]. Later studies revealed that a small subset of mucoepidermoid carcinoma instead harbors a t(11;15)(q21;q26) translocation generating a molecularly similar CRTC3-MAML2 fusion[12]. In addition to its diagnostic value, the CRTC1/3-MAML2 gene fusion initially has been associated with a lower histologic grade, a less advanced clinical stage, and longer disease-free and overall survival, suggesting a more indolent subset of mucoepidermoid carcinoma [14, 15]. More recent reappraisal of the prognostic value of the CRTC1/3-MAML2 gene fusion questioned its prognostic utility, possibly reflecting the use of stricter diagnostic criteria for mucoepidermoid carcinoma and more rigorous exclusion of mucoepidermoid carcinoma mimics, including adenosquamous carcinoma [14, 21].
The evaluation for MAML2 rearrangement has been incorporated into the diagnostic workup of mucoepidermoid carcinoma of other body sites, such as the lacrimal gland, pulmonary tree, pancreas, and cervix, leading to reappraisal of these tumors [22, 23, 24, 25]. MAML2 status has been found to be helpful in distinguishing mucoepidermoid carcinoma from morphologically similar neoplasms and has led to the suggestion that CRTC1/3-MAML2-negative tumors may be more appropriately termed adenocarcinomas or adenosquamous carcinomas with mucoepidermoid carcinoma-like features, a distinction that may have therapeutic and prognostic implications [22, 23, 24, 25].
There are limited data on the molecular genetic landscape of mucoepidermoid carcinoma of the lacrimal sac, nasal cavity, and paranasal sinuses. In a study evaluating mucoepidermoid carcinomas from various body sites for MAML2 translocation, Chiosea et al. [14] documented a MAML2 rearrangement in 2 of 4 sinonasal tumors. Interestingly, MAML2 translocation was not identified in 1 recently described lacrimal sac mucoepidermoid carcinoma arising in an inverted Schneiderian papilloma [8]. We similarly did not observe a MAML2 translocation in any of the tumors in our study. Although all tumors included in this study had the characteristic morphologic features of mucoepidermoid carcinoma and an immunohistochemical profile similar to that previously documented in the sinonasal mucoepidermoid carcinoma (CK7 focal-to-diffuse positive, CK20 negative-to-focal positive, p63 diffuse positive), the absence of MAML2 rearrangement raises a question regarding the nature of these lacrimal sac tumors [10]. It is possible that, analogous to MAML2-negative tumors in other body sites, the term adenosquamous carcinoma with mucoepidermoid carcinoma-like features may be more appropriate for these lesions. This hypothesis is strengthened by the documentation of the lacrimal sac epithelial origin of a subset of previously reported mucoepidermoid carcinomas and of 2 tumors described in this study [2, 8]. The overall rarity of lacrimal sac adenocarcinoma, adenosquamous carcinoma, and mucoepidermoid carcinoma precludes a meaningful comparison of biologic behavior of these tumors.
In addition to MAML2 translocation, salivary gland mucoepidermoid carcinomas have been shown to demonstrate upregulation of the EGFR/ERK signaling pathway. This phenomenon has been attributed to the downstream effect of the CRTC1/3-MAML2 gene fusion product and to EGFR gene copy number alterations [13, 16, 17]. We identified EFGR gene amplification in 1 tumor in our study and increased EGFR protein expression in all tumors, suggestive of EGFR pathway activation. Interestingly, 1 recently described lacrimal sac mucoepidermoid carcinoma similarly demonstrated increased EGFR protein expression [7]. However, these events are not specific to mucoepidermoid carcinoma and have been documented in the sinonasal adenocarcinoma [26, 27].
Mucoepidermoid carcinoma of the lacrimal sac has a low risk for metastases and tends to be locally aggressive, with a potential for involvement of vital structures [1, 2, 3, 4, 5, 6, 7, 8]. In recent years, orbital exenteration has been largely supplanted by globe-sparing multimodal therapies, including en-block resection of the nasolacrimal system and adjacent sinuses followed by adjuvant radiotherapy and, in some cases, chemotherapy [4, 5, 28]. This management approach is also evident in the care of our patients. The role of targeted therapies for locally aggressive and metastatic lacrimal sac tumors remains to be explored. While our findings suggest that targeted therapy toward CRTC1/3-MAML2 gene fusion may not be effective for lacrimal sac mucoepidermoid carcinoma, it is possible that anti-EGFR agents might be effective.
Statement of Ethics
This study was approved by the Wills Eye Hospital Institutional Review Board and was performed in compliance with the tenets of the Declaration of Helsinki.
Disclosure Statement
The authors have no conflicts of interest to declare. None of the authors have relevant financial relationships with commercial interests.
Funding Sources
This work was supported by the Filkins Family Foundation, Council Bluffs, IA, USA.
Author Contributions
T.M. and P.J.L.Z.: study design. K.A.G., R.B.P., M.A.S., R.C.E., and T.M.: data collection. K.A.G., P.J.L.Z., R.B.P., M.A.S., R.C.E., R.P., and T.M.: data analysis and interpretation. K.A.G., P.J.L.Z., R.B.P., M.A.S., R.C.E., R.P., and T.M.: manuscript drafting and final review. R.C.E. and T.M.: grant and laboratory support.
References
- 1.Stefanyszyn MA, Hidayat AA, Pe'er JJ, Flanagan JC. Lacrimal sac tumors. Ophthal Plast Reconstr Surg. 1994 Sep;10((3)):169–84. doi: 10.1097/00002341-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fliss DM, Freeman JL, Hurwitz JJ, Heathcote JG. Mucoepidermoid carcinoma of the lacrimal sac: a report of two cases, with observations on the histogenesis. Can J Ophthalmol. 1993 Aug;28((5)):228–35. [PubMed] [Google Scholar]
- 3.Lee SB, Kim KN, Lee SR, Bernardino CR. Mucoepidermoid carcinoma of the lacrimal sac after dacryocystectomy for squamous papilloma. Ophthal Plast Reconstr Surg. 2011 Mar-Apr;27((2)):e44–6. doi: 10.1097/IOP.0b013e3181eea4e4. [DOI] [PubMed] [Google Scholar]
- 4.Brar ST, Meyer D. Diagnosis and management of mucoepidermoid carcinoma of the lacrimal duct. Orbit. 2011 Jan;30((1)):34–6. doi: 10.3109/01676830.2010.542873. [DOI] [PubMed] [Google Scholar]
- 5.Yuksel D, Kosker M, Saribas F, Simsek S. Surgical treatment of mucoepidermoid carcinoma of the lacrimal sac. Semin Ophthalmol. 2014 Mar;29((2)):70–2. doi: 10.3109/08820538.2013.771192. [DOI] [PubMed] [Google Scholar]
- 6.Roos JC, Beigi B. Lacrimal Sac Mucoepidermoid Carcinoma with Metastases to the Cavernous Sinus Following Dacryocystorhinostomy Treated with Stereotactic Radiotherapy. Case Rep Ophthalmol. 2016 May;7((1)):274–8. doi: 10.1159/000446152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janakiram TN, Sagar S, Sharma SB, Subramaniam V. Primary Mucoepidermoid Carcinoma of the Lacrimal Sac - a Case Report and Literature Review. Klin Onkol. 2016;29((4)):291–4. [PubMed] [Google Scholar]
- 8.Hyrcza MD, Gilbert RW, Yu E, Perez-Ordõnez B. Mucoepidermoid carcinoma ex-inverted papilloma. Diagn Histopathol. 2015;21((5)):212–5. [Google Scholar]
- 9.Subramaniam V, Kumar P, Thahir M. Mucoepidermoid carcinoma of a nasal cavity—a rare tumour. Klin Onkol. 2010;23((5)):354–7. [PubMed] [Google Scholar]
- 10.Wolfish EB, Nelson BL, Thompson LD. Sinonasal tract mucoepidermoid carcinoma: a clinicopathologic and immunophenotypic study of 19 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2012 Jun;6((2)):191–207. doi: 10.1007/s12105-011-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leivo I. Sinonasal Adenocarcinoma: Update on Classification, Immunophenotype and Molecular Features. Head Neck Pathol. 2016 Mar;10((1)):68–74. doi: 10.1007/s12105-016-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skálová A, Stenman G, Simpson RH, Hellquist H, Slouka D, Svoboda T, et al. The Role of Molecular Testing in the Differential Diagnosis of Salivary Gland Carcinomas. Am J Surg Pathol. 2018 Feb;42((2)):e11–27. doi: 10.1097/PAS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 13.Yan K, Yesensky J, Hasina R, Agrawal N. Genomics of mucoepidermoid and adenoid cystic carcinomas. Laryngoscope Investig Otolaryngol. 2018 Feb;3((1)):56–61. doi: 10.1002/lio2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiosea SI, Dacic S, Nikiforova MN, Seethala RR. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope. 2012 Aug;122((8)):1690–4. doi: 10.1002/lary.22419. [DOI] [PubMed] [Google Scholar]
- 15.Noda H, Okumura Y, Nakayama T, Miyabe S, Fujiyoshi Y, Hattori H, et al. Clinicopathological significance of MAML2 gene split in mucoepidermoid carcinoma. Cancer Sci. 2013 Jan;104((1)):85–92. doi: 10.1111/cas.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell D, El-Naggar AK. Molecular heterogeneity in mucoepidermoid carcinoma: conceptual and practical implications. Head Neck Pathol. 2013 Mar;7((1)):23–7. doi: 10.1007/s12105-013-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lujan B, Hakim S, Moyano S, Nadal A, Caballero M, Diaz A, et al. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br J Cancer. 2010 Aug;103((4)):510–6. doi: 10.1038/sj.bjc.6605788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin MB, Greene FL, Byrd DR, et al. AJCC Cancer Staging Manual. 8th ed. NYC: Springer International Publishing; 2017. pp. pp. 137–47. [Google Scholar]
- 19.Qannam A, Bello IO. Comparison of histological grading methods in mucoepidermoid carcinoma of minor salivary glands. Indian J Pathol Microbiol. 2016 Oct-Dec;59((4)):457–62. doi: 10.4103/0377-4929.191765. [DOI] [PubMed] [Google Scholar]
- 20.Avilés-Salas A, Muñiz-Hernández S, Maldonado-Martínez HA, Chanona-Vilchis JG, Ramírez-Tirado LA, HernáNdez-Pedro N, et al. Reproducibility of the EGFR immunohistochemistry scores for tumor samples from patients with advanced non-small cell lung cancer. Oncol Lett. 2017 Feb;13((2)):912–20. doi: 10.3892/ol.2016.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkeland AC, Foltin SK, Michmerhuizen NL, Hoesli RC, Rosko AJ, Byrd S, et al. Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncol. 2017 May;68:5–8. doi: 10.1016/j.oraloncology.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Holstein SL, Fehr A, Heegaard S, Therkildsen MH, Stenman G. CRTC1-MAML2 gene fusion in mucoepidermoid carcinoma of the lacrimal gland. Oncol Rep. 2012 May;27((5)):1413–6. doi: 10.3892/or.2012.1676. [DOI] [PubMed] [Google Scholar]
- 23.Lennerz JK, Perry A, Mills JC, Huettner PC, Pfeifer JD. Mucoepidermoid carcinoma of the cervix: another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion. Am J Surg Pathol. 2009 Jun;33((6)):835–43. doi: 10.1097/PAS.0b013e318190cf5b. [DOI] [PubMed] [Google Scholar]
- 24.Saeki K, Ohishi Y, Matsuda R, Mochidome N, Miyasaka Y, Yamamoto H, et al. “Pancreatic Mucoepidermoid Carcinoma” Is not a Pancreatic Counterpart of CRTC1/3-MAML2 Fusion Gene-related Mucoepidermoid Carcinoma of the Salivary Gland, and May More Appropriately be Termed Pancreatic Adenosquamous Carcinoma With Mucoepidermoid Carcinoma-like Features. Am J Surg Pathol. 2018 Nov;42((11)):1419–28. doi: 10.1097/PAS.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 25.Huo Z, Wu H, Li J, Li S, Wu S, Liu Y, et al. Primary Pulmonary Mucoepidermoid Carcinoma: Histopathological and Moleculargenetic Studies of 26 Cases. PLoS One. 2015 Nov;10((11)):e0143169. doi: 10.1371/journal.pone.0143169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franchi A, Innocenti DR, Palomba A, Miligi L, Paiar F, Franzese C, et al. Low prevalence of K-RAS, EGF-R and BRAF mutations in sinonasal adenocarcinomas. Implications for anti-EGFR treatments. Pathol Oncol Res. 2014 Jul;20((3)):571–9. doi: 10.1007/s12253-013-9730-1. [DOI] [PubMed] [Google Scholar]
- 27.Rampinelli V, Ferrari M, Nicolai P. Intestinal-type adenocarcinoma of the sinonasal tract: an update. Curr Opin Otolaryngol Head Neck Surg. 2018 Apr;26((2)):115–21. doi: 10.1097/MOO.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 28.El-Sawy T, Frank SJ, Hanna E, Sniegowski M, Lai SY, Nasser QJ, et al. Multidisciplinary management of lacrimal sac/nasolacrimal duct carcinomas. Ophthal Plast Reconstr Surg. 2013 Nov-Dec;29((6)):454–7. doi: 10.1097/IOP.0b013e31829f3a73. [DOI] [PMC free article] [PubMed] [Google Scholar]