Abstract
Objective
The purpose of this study was to determine whether estrogen supplementation primarily from oral contraceptive pills compared to no estrogen supplementation is associated with differences in mean bone mineral density (BMD) measured by DXA in a cross-sectional study of women with cystic fibrosis (CF).
Methods
In this cross-sectional study of women with CF followed at a single center, we analyzed 49 women with CF ages 18–50 years with a documented DXA. BMD of women with CF taking estrogen supplementation was compared to BMD of women with CF not taking estrogen supplementation.
Results
Twelve women with CF were taking estrogen supplementation with mean dose of 23.3 mcg/day (SD 6.9 mcg/day) of ethinyl estradiol. There were no statistically significant differences between demographics of the 12 women with CF taking estrogen supplementation compared to the 37 women with CF not taking estrogen supplementation. Women taking estrogen had lower mean lumbar spine Z-score: −0.7 ± 0.7, compared to women not taking estrogen, Z-score: −0.04 ± 1.0 (p-value 0.046). Women taking estrogen had lower mean BMD at the lumbar spine: 0.952 ± 0.086 g/cm2, compared to women not taking estrogen: 1.023 ± 0.105 g/cm2 (p-value 0.038). Similar trends were seen at the total hip and femoral neck.
Conclusion
Low-dose estrogen supplementation in premenopausal women with CF was associated with lower BMD compared to no estrogen supplementation in a similar group of premenopausal young women with CF. Future studies are needed to investigate the optimal formulation, route of administration, and dose to accrue and preserve bone mass in premenopausal women with CF.
Keywords: Osteoporosis, Hypogonadism, Cystic fibrosis-related bone disease, Estrogen, Ethinyl estradiol, Lumbar spine
Abbreviations: CF, Cystic fibrosis; BMD, Bone mineral density; CFBD, Cystic fibrosis-related bone disease; BMI, Body mass index; DXA, Dual X-ray absorptiometry; POI, Primary ovarian insufficiency; FEV1, Forced expiratory volume in 1 second; FVC, Forced vital capacity; CFTR, Cystic fibrosis transmembrane conductance regulator
Introduction
Over 24% of adults with cystic fibrosis (CF) have low bone mineral density (BMD) [1]. As the median expected survival of patients with CF, now 44 years [1], increases with advancements in CF therapies, so too will the prevalence of CF-related bone disease (CFBD). CFBD increases the risk for low-impact [2] and vertebral fractures [3]. Vertebral fractures limit the ability to perform daily therapies necessary to maintain optimal lung health [3]. Primary prevention for osteoporosis includes treatment of hypogonadism, treatment of vitamin D deficiency, optimizing calcium and vitamin K intake, optimizing body mass index (BMI), and increasing weight-bearing activities [4]. These are all recommended in the current CF Foundation guidelines for CFBD [3], [4]. Few studies have investigated the impact of estrogen supplementation on bone mass in women with CF.
Some women with CF have delayed menarche [5], [6], [7], irregular menses and fertility problems, in addition to decreased quality of life [8], which can be related to untreated hypogonadism. Condoms and oral contraceptives are the most common forms of contraception used by patients with CF [9]; recent cross-sectional studies found 17 – 30% of women with CF use oral contraceptives [10], [11], [12]. Recent studies of healthy adolescents and young adults have raised concerns that doses of oral ethinyl estradiol commonly found in oral contraceptives are inadequate for attaining peak bone mass [13], [14].
The purpose of this study was to determine whether estrogen supplementation primarily from oral contraceptive pills compared to no estrogen supplementation is associated with differences in the mean indices of BMD as assessed by dual X-ray absorptiometry (DXA) in a cross-sectional study of women with CF. We hypothesized that estrogen therapy in women with CF results in differences in lumbar spine BMD compared to no estrogen therapy in women with CF.
Material and methods
Human subjects
The parent study was approved by the IRB. Health information of women with CF presenting for follow-up at a single CF center over a 12-week period was collected to screen for potential subjects of the parent study. Adults with CF are recommended to have follow-up approximately every 3 months; thus, the study was designed to capture most of the women actively seen in our center. Women aged 18–50 years were included if they had a diagnosis of CF confirmed by genotype or sweat chloride testing. Women older than 50 years were excluded as they were assumed to be post-menopausal. As BMD by DXA was the primary outcome of interest in this secondary data analysis of screened subjects, women with CF without a documented DXA result were excluded. The current CF Foundation and European guidelines for CF care recommend screening for CFBD in all subjects with CF older than 18 years at least once every five years [2], [3].
Data collection
The subjects’ health information was extracted from the electronic medical record including their most recent DXA report. DXAs had been performed as routine screening for patients with CF on both Hologic and Lunar machines. As the majority of subjects had their most recent DXA performed on a Hologic machine, the BMD was converted to Hologic equivalent according to industry-accepted formulas [15]. Women were classified as currently taking estrogen or not according to the medication list in their clinic note. The medication list is typically updated by the medical assistant or nurse rooming the patient at each clinic visit and verified by the pulmonology healthcare provider.
Statistical analysis
The mean BMD and BMD Z-score at the lumbar spine, femoral neck and total hip were compared via 2-tailed t-tests according to estrogen supplementation status. The normal distribution of the dependent variables was confirmed using Shapiro-Wilk, Kolmogorov-Smirnov, Cramer-von Mises and Anderson-Darling tests and inspection of probability plots for normality. Hemoglobin A1C was not normally distributed and was compared with Wilcoxon rank sum test between exposure groups. Categorical variables were compared by chi-squared test or Fisher’s exact test if sparse data. Sensitivity analyses excluding the three subjects exposed to progesterone without estrogen and the two subjects on systemic steroids were performed. All calculations were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Subject demographics
Of the 127 women seen in the CF clinic during the study period, 49 women met inclusion criteria. Of these 49 subjects included in this this study, 12 women were documented as taking estrogen and progesterone, 3 women were using progesterone only, 34 women were taking neither estrogen nor progesterone and no subjects were taking estrogen without progesterone. Of the 49 subjects, two subjects were documented as having living children, and no subjects were pregnant during the screening period. As the diagnosis of osteoporosis in premenopausal women requires a Z-score < −2 in addition to a significant trauma history [16], none of the subjects could be categorized as having osteoporosis because fracture history was not available for these subjects.
Demographics of women taking an estrogen supplement
Of the 12 women taking an estrogen supplement, the mean age was 31.4 years (SD 8.1 years), 33% were F508del homozygous, and 75% were Caucasian. Of women taking estrogen, 25% had CFRD, 83% had exocrine pancreatic insufficiency, and one subject (8.3%) had a previous transplant (Table 1). The mean dose of the estrogen supplement taken by the subjects was 23.3 ± 6.9 mcg/day of ethinyl estradiol, range 10–30 mcg/day. Of the 12 subjects taking estrogen, 11 were taking oral ethinyl estradiol and one was taking transvaginal ethinyl estradiol.
Table 1.
Baseline characteristics of subjects by estrogen supplementation status.
| Women taking estrogen N = 12 |
Women not taking estrogen N = 37 |
p-value | |
|---|---|---|---|
| Age at clinic visit (years) | 31.4 (8.1) | 30.4 (7.1) | 0.69 |
| F508del homozygous (%) | 33.3% | 56.8% | 0.16 |
| At least one copy of F508del (%) | 83.3% | 89.2% | 0.63 |
| Caucasian (%) | 75.0% | 89.2% | 0.34 |
| Not caucasian (%) | 25.0% | 10.8% | |
| CF-related diabetes (%) | 25.0% | 37.8% | 0.50 |
| No CF-related diabetes (%) | 75.0% | 62.2% | |
| Hemoglobin A1C (%) | 6.1 (1.4) | 5.7 (0.8) | 0.89 |
| Exocrine pancreatic insufficiency (%) | 83.3% | 97.3% | 0.14 |
| No exocrine pancreatic insufficiency (%) | 16.7% | 2.7% | |
| Vitamin D (25-hydroxyvitamin D) (ng/mL) | 31.9 (17.6) | 27.1 (11.2) | 0.41 |
| Using anti-osteoporosis medication (anti-resorptive or anabolic agents) | 0.0% | 0.0% | |
| Systemic glucocorticoid use (%) | 8.3% | 3.7% | 0.43 |
| Not on systemic glucocorticoid (%) | 91.7% | 97.3% | |
| History of lung or liver transplant (%) | 8.3% | 0.0% | 0.24 |
| Number of hospitalizations in previous year | 0.8 (1.1) | 1.4 (1.8) | 0.27 |
| Height (cm) | 160.1 (5.4) | 160.2 (5.3) | 0.99 |
| Weight (kg) | 56.2 (12.3) | 58.4 (14.4) | 0.63 |
| BMI (kg/m2) | 21.9 (4.5) | 22.8 (5.7) | 0.64 |
| FEV1 % predicted (%) | 61.9 (28.3) | 62.6 (25.0) | 0.95 |
| FVC % predicted (%) | 76.1 (22.7) | 81.3 (22.1) | 0.51 |
| CFTR modulator use (%) | 33.3% | 48.6% | 0.35 |
| No CFTR modulator use (%) | 66.7% | 51.4% |
Mean (SD) reported for continuous variables, and percentage reported for categorical variables.
Demographics of women not taking an estrogen supplement
Of the 37 women not taking an estrogen supplement, the mean age was 30.4 years (SD 7.1 years), 57% were F508del homozygous and 89% were Caucasian. Of women not taking estrogen, 38% had CFRD, 97% had exocrine pancreatic insufficiency, and no subjects had a previous lung or liver transplant. Three of the subjects not taking an estrogen supplement were using progesterone: one woman had a progesterone implant and two women were taking progesterone-only pill. Subjects taking estrogen supplement compared to subjects not taking estrogen supplement did not differ significantly in baseline characteristics (Table 1).
BMD among women with CF taking estrogen vs not taking estrogen
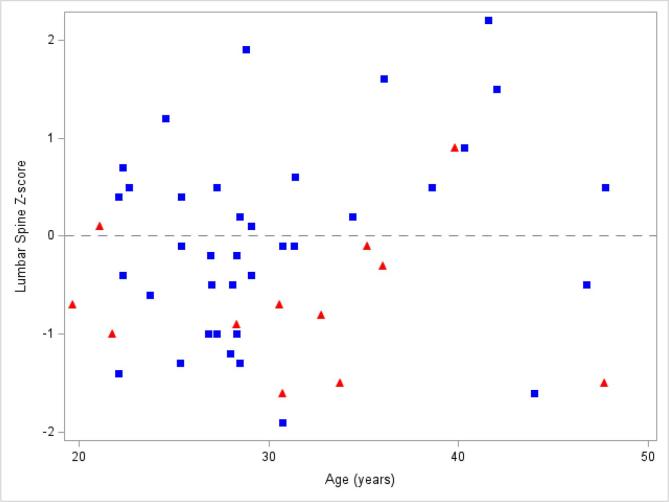
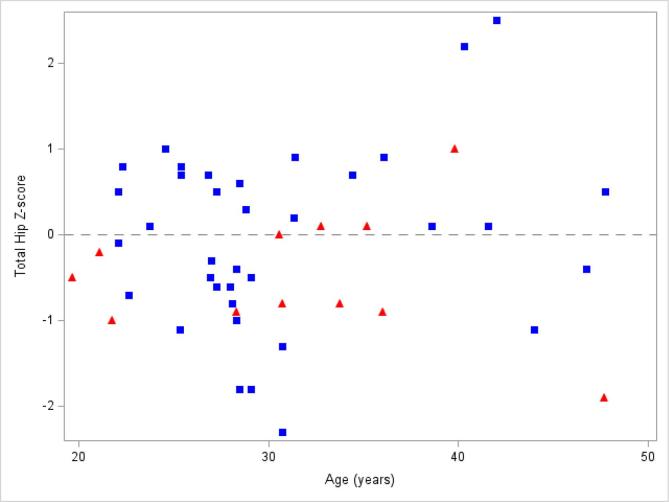
Comparisons of BMD Z-score and age by estrogen supplementation status are shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4. As detailed in Table 2 and shown in Fig. 5, women taking estrogen had lower lumbar spine and femoral neck BMD Z-scores compared to women not taking estrogen (p-value 0.046 and 0.049, respectively). Women taking estrogen also had lower BMD at the lumbar spine compared to women not taking estrogen (p-value 0.038). Similar but non-significant trends were seen at the total hip and femoral neck (Table 2, Fig. 5).
Fig. 1.
Lumbar spine Z-score vs age by estrogen exposure: Bone mineral density Z-scores at the lumbar spine from each subject taking estrogen (red triangles) compared to each subject not taking estrogen (blue squares). The dashed line represents Z-score of 0. Only 2 (16.7%) women with CF taking estrogen had a Z-score > 0; whereas, 17 (45.9%) women with CF not taking estrogen had a Z-score > 0. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Femoral neck Z-score vs age by estrogen exposure: Bone mineral density Z-scores at the femoral neck from each subject taking estrogen (red triangles) compared to each subject not taking estrogen (blue squares). The dashed line represents Z-score of 0. Of the women with CF taking estrogen, 3 of 12 (25%) subjects had Z-score > 0; whereas, 20 of 36 (55.6%) subjects with CF not taking estrogen had Z-score > 0. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Total hip Z-score vs age by estrogen exposure: Bone mineral density Z-scores at the total hip from each subject taking estrogen (red triangles) compared to each subject not taking estrogen (blue squares). The dashed line represents Z-score of 0. Of the women with CF taking estrogen, 4 of 12 (33.3%) subjects had Z-score > 0; whereas, 19 of 36 (52.8%) subjects with CF not taking estrogen had Z-score > 0. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.

Box plots of BMD Z-score by estrogen exposure: Women exposed to estrogen compared to women not exposed to estrogen had lower BMD Z-score at lumbar spine (p-value 0.046), femoral neck (0.049), and total hip (>0.05).
Table 2.
BMD of subjects by estrogen supplementation status:
| Women taking estrogen N = 12 |
Women not taking estrogen N = 37 |
p-value | |
|---|---|---|---|
| Age at DXA (years) | 29.0 (8.5) | 26.7 (7.2) | 0.419 |
| Time since DXA performed (months) | 29.2 (23.6) | 43.6 (25.3) | 0.088 |
| Hologic DXA scanner (%) | 75% | 67.6% | 1.000 |
| GE Lunar DXA scanner (%) | 25% | 29.7% | |
| Missing data regarding scanner system (%) | 0% | 2.7% | |
| Lumbar spine BMD Z-score (SD) | −0.7 (0.7) | 0.0 (1.0) | 0.046 |
| Lumbar spine BMD (g/cm2) | 0.952 (0.086) | 1.023 (0.105) | 0.038 |
| Femoral neck BMD Z-score (SD) | −0.8 (0.8) | −0.1 (1.1) | 0.049 |
| Femoral neck BMD (g/cm2) | 0.744 (0.088) | 0.816 (0.124) | 0.067 |
| Total hip BMD Z-score (SD) | −0.5 (0.7) | 0.0 (1.0) | 0.171 |
| Total hip BMD (g/cm2) | 0.857 (0.077) | 0.928 (0.124) | 0.068 |
Mean (SD) reported for continuous variables, and percentage reported for categorical variables. P-values < 0.05 highlighted in bold.
Fig. 5.
Lumbar spine BMD Z-score by ethinyl estradiol dose: The lumbar spine BMD Z-score of subjects exposed to estrogen (red triangles) are plotted against the average daily dose of ethinyl estradiol in their prescribed estrogen supplement. Subjects exposed to progesterone only without estrogen (purple circle) have been plotted adjacent to subjects exposed to neither estrogen not progesterone (blue square). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Sensitivity analysis
Results were similar when excluding the three subjects who were exposed to progesterone without estrogen. Estrogen exposed subjects compared to unexposed subjects had lower lumbar spine BMD (p-value 0.048), lower lumbar spine Z-score (p-value 0.055), and lower femoral neck Z-score (p-value 0.052).
The two subjects taking systemic steroids had been on chronic steroids for allergic bronchopulmonary aspergillosis (ABPA) and immunosuppression after transplant. When excluding these two subjects, the results were similar as well. Estrogen exposed subjects compared to unexposed subjects had lower lumbar spine BMD (p-value 0.041), lower lumbar spine Z-score (p-value 0.051) and lower femoral neck Z-score (p-value 0.063).
Discussion
In this cross-sectional study of young adult women with CF, the use of estrogen supplementation compared to no estrogen supplementation was associated with lower bone mineral density at the lumbar spine, femoral neck and total hip. These findings were significant at the lumbar spine. The women taking estrogen supplementation were primarily taking combined oral contraceptives containing ethinyl estradiol. These findings raise concern that the estrogen supplements being taken by these women were inadequate for attaining peak potential bone mass or maintaining bone mass, possibly due to the dose of ethinyl estradiol (10–30 mcg per day).
It is well established that estrogen deficiency causes loss of bone mass, primarily trabecular bone, and increases fracture risk. Estrogens inhibit osteoclasts and stimulate osteoblasts [17]. Within one year of menopause, loss of trabecular bone and increased bone resorption without compensatory increase in bone formation is apparent [18]. As the DXA site with most trabecular bone, the lumbar spine is the most sensitive to sex hormone status. Similar changes have been shown in conditions affecting younger women including eating disorders and female athletes [19], [20].
Recent studies of healthy adolescents have raised concerns that oral ethinyl estradiol dosing of less than 20 or 30 mcg per day is inadequate for optimal accrual of bone. In a 4-year observational study of healthy Finnish adolescents ages 12–19 years who were estrogen naïve, subjects who did not take any estrogen for contraception had a greater increase in lumbar spine bone mineral content compared to those who were exposed to estrogen for contraception for 1–2 years [13]. The adolescents in this study who took contraception for more than 2 years had the lowest increase in the lumbar spine bone mineral content compared to those who were exposed to contraception for 1–2 years and no exposure [13]. In this observational study, the maximum dose of ethinyl estradiol taken was 35 mcg, and 81% of the subjects took 30 mcg or less of ethinyl estradiol daily [13]. In another study of 67 healthy Brazilian adolescents ages 12–19 years who were naïve to hormonal contraception, subjects who took combined oral contraceptive containing 20 mcg ethinyl estradiol for one year had lower increases in lumbar spine bone mineral density and content than those who did not take hormonal contraception [14]. These two studies in healthy adolescents and young women suggest that estrogen supplementation from oral conceptive pills may adversely impact the accrual of bone mineral content. These findings raise concern that oral estrogen supplementation in women with CF may also be inadequate to protect against CFBD.
The optimal dose and route for estrogen supplementation to optimize bone health in adolescent girls and young women is unclear. The most recent guidelines for management of primary ovarian insufficiency (POI) by the European Society of Human Reproduction and Embryology [21] favor transdermal estrogen for optimization of bone health. In an open-label randomized crossover trial, 18 women ages 18–39 years with POI due to Turner Syndrome, oophorectomy, or idiopathic took one year of combined oral contraceptive containing 30 mcg ethinyl estradiol or one year of transdermal estradiol with transvaginal progesterone before crossing over to the other treatment arm for another year. During treatment with transdermal estradiol, subjects accrued more lumbar spine BMD and had increased markers of bone formation than during treatment with combined oral contraceptive, suggesting that transdermal estrogen may be more beneficial on bone accrual than oral estrogen [22]. Similarly, in a study of oligo-amenorrheic athletes ages 14–25 years, the subjects randomized to 100 mcg of daily transdermal estradiol had higher BMD at the lumbar spine and femoral neck after one year compared to those randomized to a daily combined oral contraceptive with 30 mcg ethinyl estradiol or no treatment [23]. These findings in hypogonadal adolescents and young women suggest that transdermal estradiol may be superior to oral estradiol for accruing and maintaining bone density. However, there are no studies in girls or women with CF comparing the effects of transdermal estradiol to oral estradiol on bone health.
This cross-sectional study relied on DXA performed as routine screening for CFBD. Only 57% of women ages 18 – 50 years followed in the CF clinic had a DXA documented which is consistent with national screening rates for CFBD with 54% of individuals with CF (range 0–94.7%) having had a DXA in the prior 5 years [1]. Also, DXA scan was not necessarily performed at the same time as the clinic visit. Because DXA scans were obtained for clinical purposes, it is possible that there may have been unintentional bias in terms of which patients had DXA scans and those who did not. Another potential limitation is the relatively small number of subjects evaluated. However, despite a relatively low number of subjects, we were still able to detect significant differences in BMD at the lumbar spine and femoral neck.
Limitations of this study include potential misclassification bias regarding estrogen supplementation status due to reliance on documentation within the electronic medical record. The duration of estrogen use was unknown. Similarly, the reason why subjects were taking oral contraceptives was unknown. The rate of contraception use, as defined by prescription of a product that can be used for contraception, in this study was 24.5%. This is similar to rates of hormonal contraception use reported in other cross-sectional studies of women with CF (17–30%) [10], [11], [12]. Some subjects may have been taking oral contraceptives to optimize their bone health and for primary prevention of osteoporosis. Some women may have been taking estrogen supplementation for other indications such as regulation of menses, acne, or contraception. Due to the cross-sectional nature of this study, we cannot conclude that low-dose contraceptive pills are the cause of lower BMD in these women with CF, but these findings underscore the importance of future prospective longitudinal studies and clinical trials in this field.
Conclusion
In summary, we found that low-dose estrogen supplementation in premenopausal young women with CF was associated with lower bone mineral density compared to no estrogen supplementation in a similar group of premenopausal young women. Future studies are needed to investigate the optimal formulation, route of administration, and dose to accrue and preserve bone mass in premenopausal women with CF. Healthcare providers should be wary that standard oral contraceptives that contain estrogen may not be adequate for skeletal health in CF and should monitor bone mineral density of their patients according to CF Foundation guidelines.
Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002382 and UL1TR002378. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors have no financial disclosures.
CRediT authorship contribution statement
Malinda Wu: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. William R. Hunt:: Writing - review & editing. Melissa S. Putman: Conceptualization, Writing - review & editing. Vin Tangpricha: Conceptualization, Methodology, Data curation, Writing - original draft, Writing - review & editing, Supervision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2020.100223.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cystic Fibrosis Foundation Patient Registry 2018 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2019.
- 2.Sermet-Gaudelus I., Bianchi M.L., Garabedian M. European cystic fibrosis bone mineralisation guidelines. J Cyst Fibros. 2011;10(Suppl 2):S16–S23. doi: 10.1016/S1569-1993(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 3.Aris R.M., Merkel P.A., Bachrach L.K. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90:1888–1896. doi: 10.1210/jc.2004-1629. [DOI] [PubMed] [Google Scholar]
- 4.Putman M.S., Anabtawi A., Le T., Tangpricha V., Sermet-Gaudelus I. Cystic fibrosis bone disease treatment: current knowledge and future directions. J Cyst Fibros. 2019;18:S56–S65. doi: 10.1016/j.jcf.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Umlawska W., Sands D., Zielinska A. Age of menarche in girls with cystic fibrosis. Folia Histochem Cytobiol. 2010;48:185–190. doi: 10.2478/v10042-010-0051-x. [DOI] [PubMed] [Google Scholar]
- 6.Johannesson M., Gottlieb C., Hjelte L. Delayed puberty in girls with cystic fibrosis despite good clinical status. Pediatrics. 1997;99:29–34. doi: 10.1542/peds.99.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Stead R.J., Hodson M.E., Batten J.C., Adams J., Jacobs H.S. Amenorrhoea in cystic fibrosis. Clin Endocrinol. 1987;26:187–195. doi: 10.1111/j.1365-2265.1987.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 8.Kazmerski T.M., Sawicki G.S., Miller E. Sexual and reproductive health behaviors and experiences reported by young women with cystic fibrosis. J Cyst Fibros. 2018;17:57–63. doi: 10.1016/j.jcf.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Roe A.H., Traxler S., Schreiber C.A. Contraception in women with cystic fibrosis: a systematic review of the literature. Contraception. 2016;93:3–10. doi: 10.1016/j.contraception.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Rousset Jablonski C., Reynaud Q., Perceval M. Contraceptive practices and cervical screening in women with cystic fibrosis. Hum Reprod. 2015;30:2547–2551. doi: 10.1093/humrep/dev217. [DOI] [PubMed] [Google Scholar]
- 11.Korzeniewska A., Grzelewski T., Jerzynska J. Sexual and reproductive health knowledge in cystic fibrosis female patients and their parents. J Sex Med. 2009;6:770–776. doi: 10.1111/j.1743-6109.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 12.Plant B.J., Goss C.H., Tonelli M.R., McDonald G., Black R.A., Aitken M.L. Contraceptive practices in women with cystic fibrosis. J Cyst Fibros. 2008;7:412–414. doi: 10.1016/j.jcf.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Pikkarainen E., Lehtonen-Veromaa M., Mottonen T., Kautiainen H., Viikari J. Estrogen-progestin contraceptive use during adolescence prevents bone mass acquisition: a 4-year follow-up study. Contraception. 2008;78:226–231. doi: 10.1016/j.contraception.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Biason T.P., Goldberg T.B., Kurokawa C.S., Moretto M.R., Teixeira A.S., Nunes H.R. Low-dose combined oral contraceptive use is associated with lower bone mineral content variation in adolescents over a 1-year period. BMC Endocr Disorders. 2015;15:15. doi: 10.1186/s12902-015-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson KE. Practical Considerations When Replacing a DXA System: Hologic; 2011.
- 16.Anabtawi A., Le T., Putman M., Tangpricha V., Bianchi M.L. Cystic fibrosis bone disease: pathophysiology, assessment and prognostic implications. J Cyst Fibros. 2019;18:S48–S55. doi: 10.1016/j.jcf.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Manolagas S.C., O'Brien C.A., Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsay R. Hormones and bone health in postmenopausal women. Endocrine. 2004;24:223–230. doi: 10.1385/ENDO:24:3:223. [DOI] [PubMed] [Google Scholar]
- 19.Kandemir N., Slattery M., Ackerman K.E. Bone parameters in anorexia nervosa and athletic amenorrhea: comparison of two hypothalamic amenorrhea states. J Clin Endocrinol Metab. 2018;103:2392–2402. doi: 10.1210/jc.2018-00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra M., Katzman D., Miller K.K. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webber L., Davies M., Anderson R. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 22.Crofton P.M., Evans N., Bath L.E. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf) 2010;73:707–714. doi: 10.1111/j.1365-2265.2010.03868.x. [DOI] [PubMed] [Google Scholar]
- 23.Ackerman K.E., Singhal V., Baskaran C. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53:229–236. doi: 10.1136/bjsports-2018-099723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.