Summary
Adaptive CD8+ T cells were observed to contribute to the initiation and progression of obesity-induced visceral adipose tissue (VAT) chronic inflammation that is critically linked to metabolic disorders. Numerous peptides presented by the major histocompatibility complex (MHC) class I molecules at the cell surface are collectively termed as MHC I-associated immunopeptidome (MIP) for the interaction with CD8+ T cells. We conducted the in-depth mapping of MIP of VAT from lean and obese mice using large-scale high-resolution mass spectrometry and observed that obesity significantly alters the landscape of VAT MIPs. Additionally, the obese VAT-exclusive MIP source proteome reflected a distinct obesity-associated signature. A peptide derived from lactate dehydrogenase A (LDHA) or B chain, named LDHA237-244, was identified as an obese VAT-exclusive immunogenic peptide that was capable of eliciting pro-inflammatory CD8+ T cells responses. Our findings suggest that certain immunogenic peptides generated by obesity may trigger CD8+ T cell-mediated VAT inflammation.
Subject Areas: Diabetology, Immunology, Proteomics
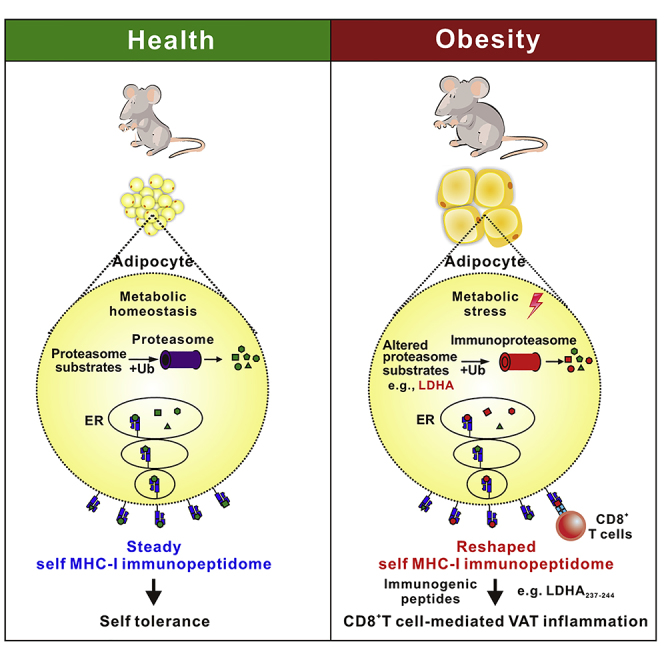
Graphical Abstract
Highlights
-
•
Obesity reshapes the landscape of VAT-derived MIP
-
•
The obese VAT-exclusive MIP reflects an obesity-associated signature
-
•
An obese VAT-exclusive peptide LDHA237-244 can stimulate CD8+ T cell responses
-
•
LDHA237-244-reactive CD8+ T cells were present in obese mice but not lean mice
Diabetology; Immunology; Proteomics
Introduction
The prevalence of obesity and its associated metabolic abnormalities, including insulin resistance, type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), and non-alcoholic fatty liver disease (NAFLD), has attained epidemic proportions, severely affecting health and global mortality rates. Obesity-induced visceral adipose tissue (VAT) chronic inflammation is now considered to portray a pivotal role in the development of metabolic diseases. Increasing data indicate alterations in multiple immune cells in obese VAT, including alterations in macrophages, mast cells, eosinophils, as well as in adaptive immune cells (Sun et al., 2012). T lymphocytes have been regarded as unexpected contributors to obesity-induced VAT inflammation and insulin resistance (Wu et al., 2007). The inflammatory reaction within obese VAT has been characterized by a striking influx of CD8+ T cells (Rausch et al., 2008). Similarly, the ratio of CD8+ to CD4+ T cells increased in obese VAT weeks before macrophage infiltration (Feuerer et al., 2009, Winer et al., 2009). The infiltration of significant number of CD8+ T cells preceded macrophage recruitment in obese VAT of mice after short-term feeding of a high-fat diet (HFD). Depletion of CD8+ T cells lowered macrophage infiltration and adipose tissue inflammation, and ameliorated systemic insulin resistance, whereas adoptive transfer of CD8+ T cells into CD8-deficient mice reverted these effects and increased the numbers of pro-inflammatory macrophages in VAT (Nishimura et al., 2009). These evidences indicate that CD8+ T cells may portray a significant role in the initiation of obesity-induced VAT inflammation. Obese VAT-infiltrated T cells have a restricted TCR-Vβ repertoire, suggesting that expansion of these T cells in progressive obesity is possibly driven by VAT-specific antigens as a result of obesity (McDonnell et al., 2018, Nishimura et al., 2009, Yang et al., 2010). It has been reported that obese adipose tissue possesses the ability to activate CD8+ T cells, whereas lean fat does not (Nishimura et al., 2009). However, the antigenic mechanisms underlying the activation, proliferation, and pro-inflammatory responses of CD8+ T cells in obese VAT remain unknown.
Numerous peptides presented on the cell surface of major histocompatibility complex (MHC) class I molecules are collectively referred to as the MHC class I-associated immunopeptidome (MIP) interacting with adaptive CD8+ T cells (Granados et al., 2015). Recent studies have adopted large-scale mass spectrometry (MS) as the sole direct approach for analyzing the global composition of MIP. The molecular composition of MIP is complex and varies from one cell/tissue type to another; it is intertwined with protein metabolism and is ultimately shaped by two processes: protein translation and degradation (Adamopoulou et al., 2013, de Verteuil et al., 2010). Under certain pathological conditions, selected intrinsic and extrinsic cellular factors, including neoplastic transformation, infection, as well as metabolic perturbations, can restructure self-MIPs, possibly resulting in the generation of immunogenic peptides (de Verteuil et al., 2012). Malignant transformation has a profound impact on the MIPs. Numerous tumor-associated MIPs have been described to encompass immunogenic peptides that are recognized by CD8+ T cells (Bassani-Sternberg et al., 2016, Boon et al., 2006, Dutoit et al., 2012, Kowalewski et al., 2015, Loffler et al., 2018). Alteration of cellular metabolism via the inhibition of the mammalian target of rapamycin results in dynamic alterations in the cell surface MIP landscape as well as generation of immunogenic peptides (Caron et al., 2011). Additionally, inflammatory cytokines induce the alteration of human β cell MIPs and yield conventional and neo-antigenic peptides recognized by CD8+ T cells in type 1 diabetes and healthy donors (Gonzalez-Duque et al., 2018). However, it remains to be determined whether obesity reshapes VAT-derived MIP and generates immunogenic peptides for driving CD8+ T cell responses. We performed large-scale high-resolution MS to analyze VAT-derived MIPs isolated from lean and obese mice fed a normal chow diet (NCD) and HFD, respectively, and observed that HFD-induced obesity led to significant alterations in the VAT MIP landscape. Moreover, abnormal adipocyte metabolism under obese conditions generated obese VAT-exclusive immunogenic peptides that elicited the pro-inflammatory responses of CD8+ T cells, representing a mechanism underlying the obesity-induced VAT inflammation.
Results
Characterization of MHC Class I-Associated Immunopeptidome Derived from the Visceral Adipose Tissue of NCD-Fed (Lean) and HFD-Fed (Obese) Mice
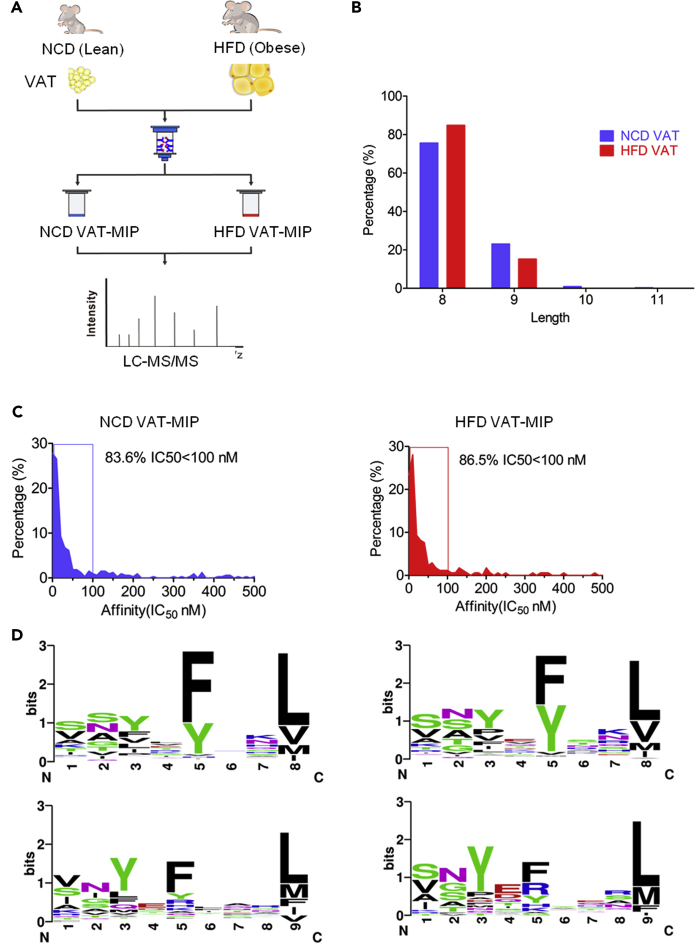
First, the HFD-induced obese mouse model was well established. HFD-fed obese mice exhibited significantly increased weight and fat mass, along with impaired glucose tolerance and insulin sensitivity (Figures S1A–S1E) at 8 weeks post HFD administration. The epididymal fat pads (VAT samples) freshly harvested from HFD-fed obese mice and NCD-fed lean mice, respectively, were lysed to immunopurify H2-Kb-peptide complexes. Although the mRNA expression level of H2-Kb was lower in obese VAT from HFD-fed mice compared with that from NCD-fed mice, there was no significant difference in H2-Kb protein expression levels in VAT between lean and obese mice (Figures S1F and S1G). The quantity of H2-Kb protein purified from obese VAT was marginally lower than that obtained from lean VAT (Figure S1G). H2-Kb-bound peptides were acid-eluted from the H2-Kb molecules and analyzed using reverse-phase HPLC-tandem mass spectrometry (LC-MS/MS) (Figure 1A). By combining the Mascot and Sequest search results from three technical repetitions, we identified 913 unique peptides from NCD VAT and 404 unique peptides from HFD VAT, which met the following criteria: FDR<5%, either Ionscore >20 in Mascot search or q values < 0.05 in Sequest search, and 8–12 amino acids in length. When applying a more stringent filter using the predicted H2-Kb binding affinity with IC50 <500 nM (netMHCpan4.0), we eventually discovered 324 (Table S1. The identified unique high confidence H2-Kb-bound peptides from visceral adipose tissues of NCD-fed mice, related to Figure 1) and 171 (Table S2. The identified unique high confidence H2-Kb-bound peptides from visceral adipose tissues of HFD-fed mice, related to Figure 1) unique high confidence H2-Kb-bound peptides from NCD and HFD VAT, respectively. The two MIP datasets indicated the characteristic length distribution for H2-Kb-restricted peptides, predominantly 8 amino acids in length, with a small number of nonameric peptides and very few of 10- to 11-mer peptides. Notably, the proportion of octapeptides in HFD VAT MIP was higher than that in NCD VAT MIP (Figure 1B). Most of these MIPs (>80%) were predicted to elicit strong H2-Kb binding affinity with IC50 < 100 nM (Figure 1C). Similarly, both octapeptides and nonapeptides from the two MIPs exhibited the typical anchor motifs (P5 and P8/9) for H2-Kb molecules binding (Figure 1D). The results confirm the predominant presence of tyrosine and phenylalanine in position P5 and that of leucine, valine, and methionine in the C-terminal positions of peptides. No significant alterations were noted in the amino acid preferences within the MIPs purified from NCD- and HFD VAT. In conjunction, these data indicated that our established VAT MIP datasets were highly reliable. HFD-induced obesity did not change the H2-Kb-binding affinity and the amino acid preferences in MIPs derived from VAT observably; however, it resulted in an increase in the proportion of H2-Kb-bound octameric peptides.
Figure 1.
Characterization of MHC Class I-Associated Immunopeptidome (MIP) Derived from the Visceral Adipose Tissue (VAT) of NCD-Fed (Lean) and HFD-Fed (Obese) Mice
(A)Schematic representation of the experimental workflow used to generate and identify VAT MIPs. The epididymal fat pad (VATs) from NCD and HFD-fed C57BL/6 mice were extracted. H2-Kb-associated peptides were isolated independently by immunoaffinity purification using the monoclonal antibodies Y-3, and the eluted peptides were identified using LC-MS/MS.
(B) The column diagram illustrating the length distribution of NCD- and HFD VAT MIPs (8–11 amino acids).
(C) The graphs indicating the distribution of the predicted affinity values of NCD- and HFD VAT MIPs by netMHCpan4.0.
(D) The binding motifs for octameric and nonameric peptides identified in NCD- and HFD VAT MIPs, respectively, were illustrated. The x-axis represents the residue position within octameric and nonameric peptide sequences. The y axis represents the information content, with the size of each amino acid symbol proportional to its frequency.
Obesity Reshapes Visceral Adipose Tissue-Derived-MHC Class I-Associated Immunopeptidome
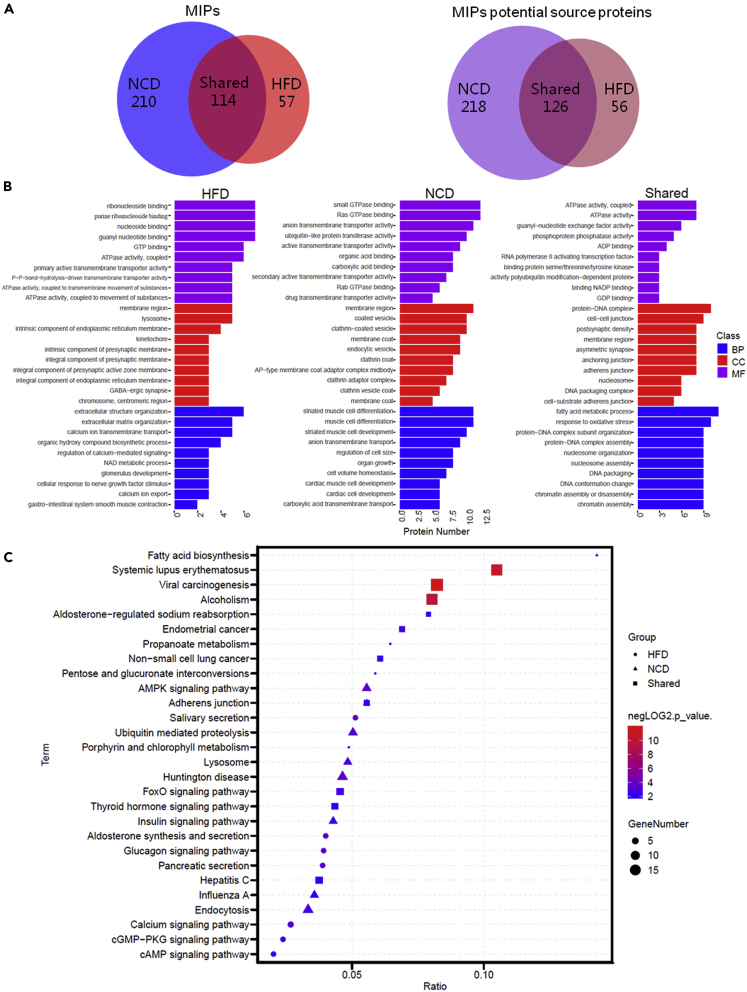
To investigate whether obesity affects the composition of VAT-derived MIP, we overlapped and compared the VAT MIP landscape of NCD- (lean) and HFD-fed (obese) mice (Figure 2A). There were 114 H2-Kb-associated peptides shared by both VAT MIPs. HFD VAT MIP had 57 unique peptides (33.33%, Table S3) that were undetected in NCD VAT MIP. The majority (64.81%) of peptides identified in NCD VAT MIP dataset did not exist in the HFD VAT MIP dataset. The NCD VAT MIP and HFD VAT MIP represented 344 and 182 potential source proteins, respectively. Overlaps between the two VAT MIP source proteomes revealed that 126 potential source proteins were shared by MIPs from NCD VAT (36.63%) and HFD VAT (69.23%) samples. Two hundred and eighteen proteins (63.37% of the mapped NCD VAT potential source proteome) were represented exclusively in the NCD VAT MIP. Fifty-six proteins (30.77% of the mapped HFD VAT potential source proteome) and the 52 corresponding peptides were represented exclusively in the HFD VAT MIP (Table S4).
Figure 2.
High-Fat Diet-Induced Obesity Reshapes Visceral Adipose Tissue Derived-MHC Class I-Associated Immunopeptidome (MIP)
(A)The Venn diagrams represent the overlap analysis of VAT MIPs and their potential source proteins comparing NCD-fed (lean) and HFD-fed (obese) mice, respectively. Numbers indicate the number of identified peptides or their potential source proteins that were shared between or unique to each MIP.
(B) The top 10 GO enrichment terms of shared or exclusive MIP potential source proteins identified in HFD and NCD VAT. The three GO categories include biological process (BP), molecular function (MF), and cellular component (CC). The x- and y axes represent the protein number of corresponding GO terms and the GO terms, respectively.
(C) KEGG pathway enrichment analysis of shared or exclusive MIP potential source proteins identified in HFD and NCD VAT. The horizontal axis indicates the ratio of the number of identified proteins versus the total number of proteins in the same KEGG pathway, and the vertical ordinates represent the terms of the KEGG pathways. The bubble size indicates the number of proteins matched in the KEGG pathway. The color represents −log2 (p value): Logarithmic conversion of Fisher exact test p value.
Next, we mapped the exclusive or shared MIP source proteins from NCD VAT and HFD VAT to the GO enrichment and KEGG pathway enrichment analysis using clusterProfiler. The top 10 statistically significant GO term classification from each group of proteins revealed visibly different clusters in biological process, molecule function, and cellular components among exclusive or shared MIP source proteins from NCD and HFD VAT. Notably, at the biological process level, the shared MIP source protein content was enriched most significantly in fatty acid metabolic processes and response to oxidative stress and nucleosome assembly, whereas the NCD VAT-exclusive MIP source protein content was enriched most significantly in cell volume homeostasis, cardiac muscle cell development, and anion transmembrane transport. The HFD VAT-exclusive MIP source protein content was enriched in extracellular structure organization, NAD metabolic process, calcium ion transmembrane transport, and regulation of calcium-mediated signaling (Figure 2B). The KEGG pathway enrichment analysis further indicated that the NCD and HFD VAT exclusive or shared MIP source protein content was enriched in almost entirely different signaling pathways. Notably, the shared MIP source protein content was significantly enriched in some diseases-related pathways, FoxO signaling pathway, and thyroid hormone signaling pathway; the NCD VAT-exclusive MIP source protein content was enriched in AMPK signaling pathway, ubiquitin-mediated proteolysis, lysosome, endocytosis, and insulin signaling pathway. Both the shared and NCD VAT-exclusive MIP source protein contents were significantly enriched in the adherens junction-related pathways, whereas aldosterone synthesis and secretion, glucagon signaling, calcium signaling, cGMP-PKG signaling, cAMP signaling pathway, pentose and glucuronate interconversions, and pancreatic secretion-related pathway were observed to be enriched in the HFD VAT-exclusive MIP source proteins (Figure 2C). Notably, among the HFD VAT-exclusive MIP source proteins, we observed that a series of proteins, including the lipopolysaccharide-binding protein (LBP), melatonin related receptor (GPR50), and phospholipid transfer protein (PLTP), have been previously reported to be associated with obesity-related chronic inflammation and metabolic disorders (Table S5). These data revealed that obesity reshapes VAT-derived-MIP and the obese VAT-exclusive MIP source proteome reflects a distinct obesity-associated signature.
Selection of Potential Candidate Peptides for Exemplified Immunogenicity Evaluation
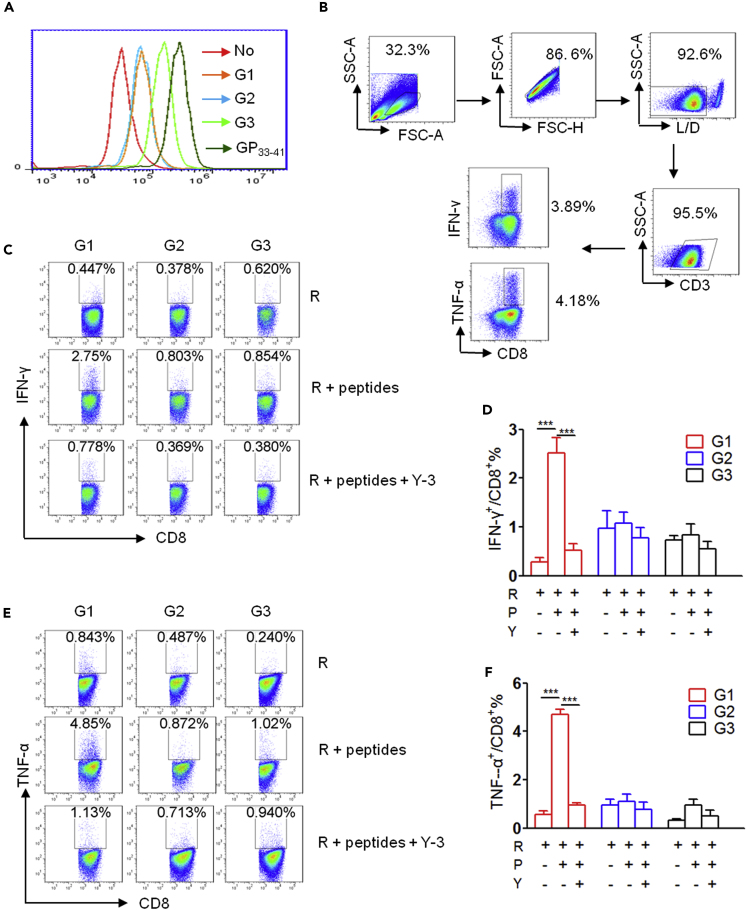
We further demonstrated that the increase in CD8+ T cell levels in VAT occurred early and was sustained during the development of HFD-induced obesity (Figures S2A–S2E); hence, we considered whether HFD-induced obesity generates obese VAT-exclusive MHC class I-associated antigenic peptides to drive the pro-inflammatory response of CD8+ T cells. Therefore, we selected several peptides for immunogenicity analysis based on the following selection criteria: obese VAT MIP specificity (on source protein level), significant H2-Kb-affinity, sufficient abundance, and reproducibility (Table S6, G1 and G2). The peptides that were shared by MIP datasets from both HFD VAT and NCD VAT, although these were more abundant in HFD VAT, were selected for the immunogenicity analysis as well (Table S6, G3). First, the H2-Kb molecule binding affinity of the three groups of peptides was determined using the transporter associated with Ag processing (TAP)-deficient RMAS cell-based binding assay. The LCMV GP33-41 peptide, a positive control, elicited the highest binding affinity for H2-Kb. Peptides in G1 and G2 exhibited similar medium binding ability, and peptides in G3 exhibited relatively high binding affinity with H2-Kb (Figure 3A). This observation indicated that all groups of peptides have detectable H2-Kb binding abilities. Following this, we evaluated the immunogenicity of these H2-Kb-bound peptides in vitro by stimulating splenocytes isolated from C57BL/6 mice with each group of peptide individually and measured CD8+ T cell responses against peptide pool-loaded RMAS cells using intracellular IFN-γ and TNF-α staining (Figure 3B). We observed that peptides in G1 induced pro-inflammatory CD8+ T cell response when restimulated with the corresponding peptides pool-loaded RMAS cells, whereas those in G2 and G3 did not. Moreover, RMAS cells in isolation could not stimulate IFN-γ and TNF-α production by G1 peptide-primed CD8+ T cells, and the CD8+ T cell responses against G1 peptides-loaded RMAS cells were almost completely blocked by anti-H2-Kb antibody Y-3 (Figures 3C–3F).
Figure 3.
Selection of Potential Candidate Peptides for Exemplified Immunogenicity Evaluation
(A) Representative histogram of FACS analysis of the surface H2-Kb molecules on TAP-deficient RMAS (R) cells incubated with or without the indicated peptide groups.
(B–F) (B) Gating strategy for analysis of intracellular cytokine production by CD8+ T cells. Representative FACS plots indicating intracellular IFN-γ (C) and TNF-α (E) staining of CD8+ T cells restimulated with R alone, or R loaded with the indicated peptide groups, or in the presence of Y-3 antibody. Summary graph for FACS analysis of the frequency of IFN-γ (D) and TNF-α (F)-producing cells among CD8+ T cells stimulated with the indicated peptide group. Each bar represents the mean ± SEM of three independent experiments. ∗∗∗p < 0.001 determined by Student's t test.
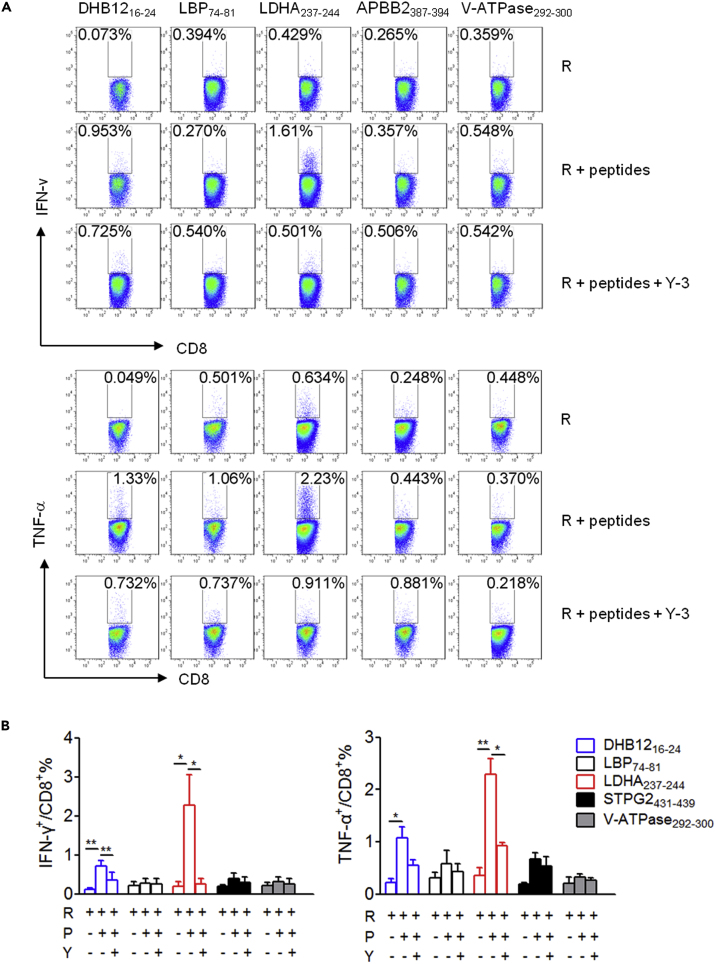
Furthermore, the immunogenicity of peptides in G1 was assessed in detail by priming the splenocytes with the respective single peptide, and the splenocyte cultures were then restimulated with peptide-pulsed RMAS cells or RMAS cells alone to determine peptide-specific CD8+ T cell responses using intracellular IFN-γ and TNF-α staining (Figure 4). Among peptides in G1, DHB1216-24, with 17-beta-hydroxys-teroid dehydrogenase 12 (DHB12 or Hsd17b12) as its source protein, stimulated a weak peptide-specific CD8+ T cell response. Notably, a peptide derived from lactate dehydrogenase (LDH) A or B chain, named LDHA237-244, induced a strong peptide-specific CD8+ T cell pro-inflammatory response. Similarly, Y-3 almost eliminated the CD8+ T cell response primed by these two peptides. The other peptides did not prime any apparent CD8+ T cell response (Figures 4A and 4B). As negative controls, CATA384-392 and PLIN2213-221 in G3 remained non-immunogenic (Figures S3A and S3B). These data indicate that HFD-induced obesity results in generation of exclusive immunogenic peptides in obese VAT for driving CD8+ T cell pro-inflammatory responses.
Figure 4.
Immunogenicity Characterization of Single Peptide in Group 1
(A) Representative FACS plots depicting intracellular IFN-γ and TNF-α staining of CD8+ T cells restimulated with RMAS cells (R) alone, or R loaded with the indicated peptide, or in the presence of Y-3 antibody.
(B) Summary graph for FACS analysis in (A). Each bar represents the mean ± SEM of three independent experiments. ∗p < 0.05; ∗∗p < 0.01 determined by Student's t test.
LDHA237-244-Specific CD8+ T Cells Are Present in HFD-Induced Obese Mice
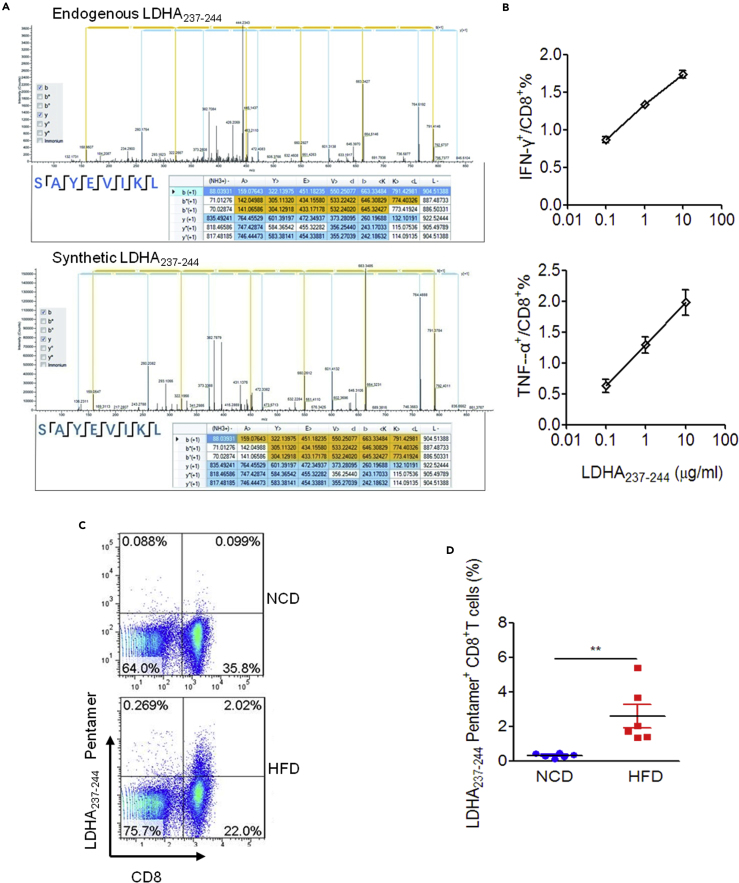
Since the LDHA237-244 peptide is immunogenic, and exists exclusively in the MIP derived from obese VAT, we considered whether LDHA237-244-specific CD8+ T cells are associated with obesity in mice. First, in order to exclude the potential error due to mass spectrometry, we compared the secondary tandem mass spectra of identified endogenous LDHA237-244 and its synthetic counterpart. Although there remained mild deviations between the two secondary mass spectrum that may have been induced by the difference in the fragmentation form of these two peptides during detection, we observed that the sequences identified for these two peptides were identical and the molecular weights were almost identical. This basically confirmed the accuracy of mass spectrum identification of endogenous LDHA237-244 peptides (Figure 5A). The immunogenicity of synthetic LDHA237-244 peptide was further verified by peptide dose-dependent stimulation of CD8+ T cells to produce IFN-γ and TNF-α (Figure 5B). Next, the presence of peripheral LDHA237-244-specific CD8+ T cells in HFD-induced obese mice was confirmed by phycoerythrin (PE)-labeled H2-Kb/LDHA237-244 pentamer staining (Figure 5C). As expected, significantly higher levels of peripheral H2-Kb/LDHA237-244 pentamer positive CD8+ T cells were detected in HFD-induced obese mice as compared with lean control mice (Figure 5D). To further confirm the presence of LDHA237-244 peptide-reactive Th1-like CD8+ T cells in VAT from obese mice, we stimulated VAT-infiltrated lymphocytes collected from obese mice and lean mice with RMAS cells loaded with or without LDHA237-244 peptide, respectively, and determined the intracellular IFN-γ secretion in VAT CD8+ T cells. Although the LDHA237-244 peptide-pulsed RMAS cells significantly stimulated the secretion of IFN-γ by VAT-infiltrated CD8+ T cells in HFD-fed obese mice, the same was not observed in lean mice (Figures S4A). These data indicated that the LDHA237-244 peptide represents an obesity-associated target recognized by peripheral and VAT-infiltrated CD8+ T cells in HFD-induced obese mice. Therefore, we wanted to investigate whether HFD-induced obesity and metabolic disorders can be alleviated by induction of LDHA237-244 peptide-specific CD8+ T cell tolerance. Unfortunately, both intranasal and intraperitoneal administration of a single LDHA237-244 peptide repeatedly failed to affect weight gain, glucose intolerance, and insulin resistance in HFD-fed mice significantly (Figures S4B and S4C).
Figure 5.
Peripheral CD8+ T Cells Recognize LDHA237-244 Peptide in HFD-Induced Obese Mice
(A) Tandem mass spectra of endogenous LDHA237-244 (upper: m/z 461.76538Da) and its corresponding synthetic peptide (lower: m/z 461.76566Da).
(B) Statistical graph of the frequency of IFN-γ- and TNF-α-producing cells among the CD8+ T cells primed by various concentrations of LDHA237-244 peptide.
(C) Exemplified FACS plots of the lymphocytes from the mesenteric lymph nodes of HFD-fed (obese) mice and NCD-fed (lean) mice stained with H2-Kb/LDHA237-244 pentamer (Proimmune) and anti-mouse CD8 antibody.
(D) Summary graph of FACS analysis of the frequency of H2-Kb/LDHA237-244 pentamer positive CD8+ T cells in the mesenteric lymph nodes from NCD and HFD-fed mice after 8-week HFD or NCD administration (n = 6 per group). The results are representative of one of two independent experiments. Data are presented as scatterplots with minimum and maximum values for data distribution. ∗∗p < 0.01.
See also Figure S4.
Source Proteins of LDHA237-244 Peptide Are Downregulated in Obese Mice
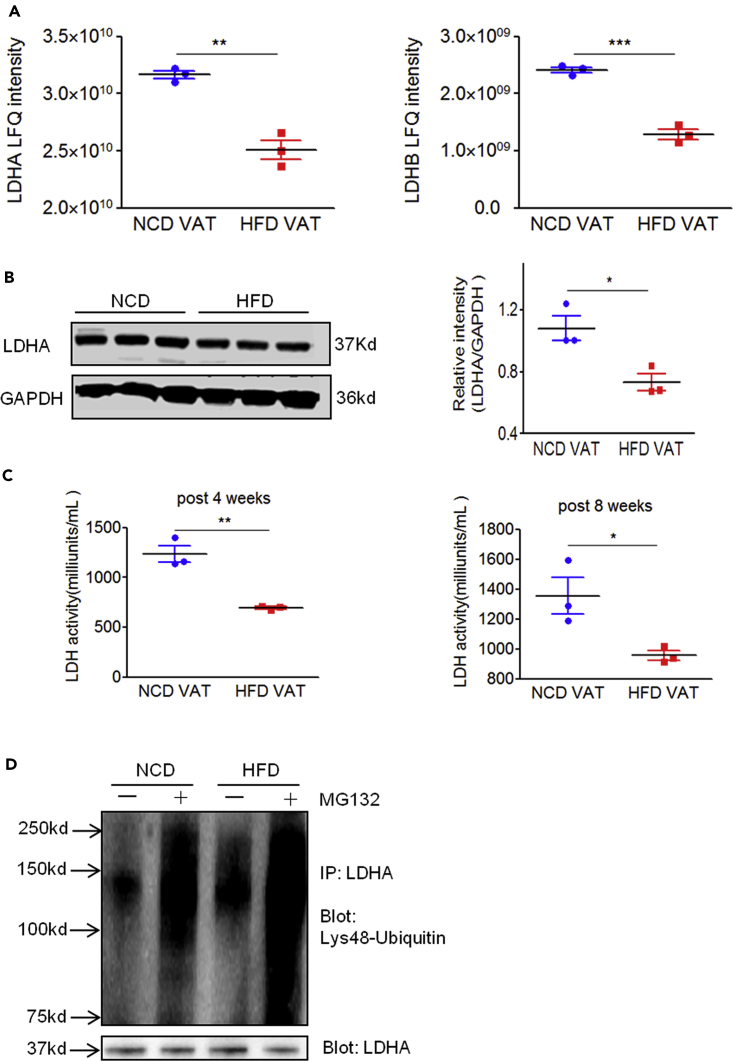
MHC class I-associated peptides are derived from source proteins degraded through the ubiquitin-proteasome pathway. There are two types of proteasomes, constitutive proteasome and immunoproteasome, responsible for generating peptides presented by MHC class I molecules. The immunoproteasome is believed to elicit greater competence at producing immunodominant peptides. We observed that the mRNA levels of constitutive proteasome, particularly that of immunoproteasome catalytic subunit genes, were significantly higher in obese VAT than in lean VAT (Figure S5), suggesting a hyperactive antigen processing machinery in obesity. Furthermore, we wanted to investigate whether the H2-Kb-presented LDHA237-244 peptide is generated in obese VAT due to the altered expression of its source proteins. In general, higher gene expression can favor peptide presentation. However, we observed that the mRNA expression of both Ldha and Ldhb did not alter significantly between NCD VAT and HFD VAT (data not shown). On the contrary, our mass spectrometry analysis of VAT proteome indicated that both LDHA and LDHB protein levels in obese VAT were significantly lower when compared with that in lean VAT (Figure 6A). We observed that LDHA expression was dominant in VAT (Figure 6A); western blotting analysis confirmed that the LDHA protein levels were significantly lower in obese VAT (Figure 6B). Consistent with these results, the enzyme activity of LDH in obese VAT decreased significantly when compared with that in lean VAT (Figure 6C). However, the LDH enzyme activity in skeletal muscle tissue did not differ between lean and obese mice (data not shown). Therefore, these results indicate that LDHA/LDHB stability might decrease in obese VAT. Since Lys48-linked ubiquitins act as a universal signal for proteins targeted for proteosomal degradation, we further investigated whether LDHA underwent increased Lys48-linked ubiquitination in obese VAT and whether proteasome was involved in the degradation of Lys48-ubiquitinated LDHA in VAT. Total protein was extracted from HFD VAT and NCD VAT in the presence or absence of MG132 (a proteasome inhibitor), after which protein extract was immunoprecipitated using anti-LDHA antibodies. Immunoblotting using anti-Lys48-linked ubiquitin antibody revealed that the basal level of Lys48-linked ubiquitination of LDHA in HFD VAT was higher than that in NCD VAT. Moreover, inhibition of the proteasome by MG132 markedly enhanced the accumulation of Lys48-linked ubiquitinated LDHA in VAT (Figure 6D). Considered in conjunction, we inferred that the generation of H2-Kb-restricted LDHA237-244 peptide might be attributed to abnormal ubiquitin-proteasome degradation of LDHA/LDHB in obese VAT.
Figure 6.
The Source Proteins of LDHA237-244 Peptide Are Downregulated in Obese Mice
(A) Comparison of LDHA and LDHB peptide levels in NCD VAT and HFD VAT samples measured by LFQ ion intensities revealed a significant decrease in both LDHA and LDHB peptide levels in obese VAT. Error bars represent ±SD (each data point consists of VAT samples pooled from 3–4 mice).
(B) Quantitative western blot analysis of LDHA in VAT of C57BL/6 mice administered NCD or HFD for 4 weeks (n = 3 per group). Error bars represent ±SD.
(C) Summary graph of the LDH activity levels in VAT isolated from C57BL/6 mice administered NCD or HFD for 4 and 8 weeks (n = 3 per group) determined using an LDH activity assay kit. The results are representative of one of two independent experiments. Error bars represent ±SD.
(D) The epididymal fat pads (VAT samples) were collected from three or four obese or lean mice fed with HFD or NCD for 8 weeks, respectively, and total protein was extracted from HFD or NCD VAT in the presence or absence of MG132 (10 μM). The VAT protein solution was immunoprecipitated with anti-LDHA antibodies. Eluted proteins were subjected to western blot analysis and probed with specific antibodies against Lys48-specific ubiquitin and LDHA. Data are representative of two experiments. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p<0.001, determined by Student’s t test.
See also Figure S5.
Discussion
Obesity-induced VAT chronic inflammation is currently considered to play a pivotal role in the progression of obesity-related clinical comorbidities, such as insulin resistance, T2DM, atherosclerosis, neurodegenerative disorders, and some forms of cancer. The involvement of CD8+ T cells in the initiation and propagation of obesity-related VAT chronic inflammation has been reported previously (Nishimura et al., 2009), and several studies suggest that the activation and proliferation of CD8+ T cells in obese VAT may be driven by specific antigens enriched in obese VAT (Feuerer et al., 2009, Nishimura et al., 2009, Winer et al., 2009, Yang et al., 2010). However, the antigenic mechanisms driving CD8+ T cell pro-inflammatory responses in obese VAT remain unknown. We adopted a large-scale mass spectrometry approach and presented an in-depth analysis of VAT-derived MIPs, comparing obese and non-obese states. We observed that HFD-induced obesity led to significant alteration in the VAT MIP landscape and obese VAT presents a unique set of MIP source proteins. Additionally, we demonstrated that some obesity-exclusive MHC class I-presented antigenic peptides were immunogenic and might contribute to the pro-inflammatory CD8+ T cell responses.
When compared with the analysis of MIPs derived from cells, analysis of MIPs derived from mouse primary tissue is reported with limited success, considering the high number of mice needed to perform an experiment (Caron et al., 2015). Recently, MIPs from 19 normal tissue samples from C57BL/6 mice, excluding adipose tissue, were investigated, and the number of peptides identified in different primary tissues ranged from over hundred to thousands (Schuster et al., 2018). Considering the MIP variation in primary tissue between mice within the same treatment group and the sufficient coverage and depth of MIP identified by LC/MS, we pooled VAT samples from a large number of lean mice or obese mice from three different batches to isolate MIP for one experiment. To establish VAT MIP datasets, we performed technical triplicates for the analysis of each MIP sample and combined all high confidence peptides identified by three repeated Mascot and Sequest searches of each MIP sample. This may help in increasing the coverage and depth of identified peptides, as well as in reducing sampling errors in the comparison of different biological replicates. Although lean VAT and obese VAT expressed similar levels of H2-Kb protein, and comparable quantity of H2-Kb proteins was immunoaffinity-purified from the two samples, the number of H2-Kb-associated peptides identified in HFD VAT was close to half of the number of peptides identified in NCD VAT. We inferred that HFD VAT MIP might comprise more unconventional peptides (e.g., spliced peptides) that cannot be identified in a conventional database search.
MIP is not a reflection of the proteome and presents a small fraction of the proteome (Pearson et al., 2016). Comprehensive analysis of MIP is significant for identification of novel antigenic targets and potential biomarker signatures in diseases. It has been reported that the defined unique tumor MIP reflects tumor-associated pathways implicated in oncogenesis and complex alterations in tumor cell metabolism result in non-mutated tumor-specific antigenic targets for immunotherapeutic applications (Loffler et al., 2018). By comparing the two MIP datasets, we observed that HFD-induced obesity significantly altered the VAT MIP landscape. We screened out HFD VAT-exclusive peptides at the source protein level and observed that HFD VAT-exclusive MIP source proteome conceals a distinct obesity-associated signature. The KEGG pathway enrichment analysis revealed that the HFD VAT-exclusive MIP source protein content was significantly enriched in some pathways, such as aldosterone synthesis and secretion (Dinh Cat et al., 2016), glucagon signaling (Charron and Vuguin, 2015), calcium signaling (Bravo-Sagua et al., 2017), cGMP-PKG signaling (Kovacs et al., 2016), propanoate metabolism (Chen et al., 2015), and cAMP signaling pathway (Madsen and Kristiansen, 2010), and abnormalities in these pathways are closely associated with obesity-related metabolic diseases. Notably, previously identified obesity-related proteins or biomarkers, such as LBP (Kim et al., 2016), GPR50 (Ivanova et al., 2008), PLTP (Qin et al., 2014) (Tzotzas et al., 2009), and mitochondrial glycerol 3-phosphate dehydrogenase (Zheng et al., 2019), were detected as obese VAT-exclusive MIP source proteins (Table S2). We proposed that these proteins are likely to be expressed or modified abnormally and, hence, degraded into peptides and enter the MHC class I presentation pathway in obese VAT. Therefore, discovery of MIP source proteins using large-scale analyses might represent a feasible strategy to detect the key molecular targets involved in pathogenesis for diagnosis and prevention of obesity and its related metabolic disorders.
In this study, we hypothesized that certain MHC class I-presented antigenic peptides derived exclusively from aberrant proteins on overloaded adipocytes might be recognized specifically by CD8+ T cells that initiate obesity-induced inflammation. As expected, certain MHC I-restricted peptides, such as LDHA237-244, present exclusively in obese VAT MIPs and undetectable in normal VAT MIPs, exhibited dose-dependent immunogenicity to induce CD8+ T cell responses. Furthermore, we demonstrated that the LDHA237-244-specific CD8+ T cells were present in obese mice, whereas they were absent in lean mice. The results suggested that the alterations in VAT MIP landscape in obesity may be accompanied by the generation of obesity-exclusive antigenic peptides that might not be presented at the T cell negative selection stage or at the steady state and, thus, are immunogenic for CD8+ T cell activation and consequent initiation of adipose tissue inflammation. Therefore, these obesity-exclusive antigenic peptides may be potential targets for the prevention of obesity-related chronic inflammation. However, we observed that repeated intranasal or intraperitoneal administration of a single LDHA237-244 peptide failed to affect HFD-induced obesity and abnormal glucose metabolism significantly. This failure might be attributed to the existence of multiple antigenic peptides in obese VAT that are responsible for triggering and maintaining multiantigen-specific CD8+ T cell-mediated chronic inflammatory response in obese VAT. Therefore, different obese VAT-relevant antigens/peptides need to be identified in future. The early activation and amplification of tissue-resident CD8+ T cells is reportedly involved in the progression of other inflammatory diseases, such as hypertension-related cardiovascular inflammation (Ma et al., 2014) and non-alcoholic fatty liver disease (Ghazarian et al., 2017, Wolf et al., 2014). Considering that MHC class I molecules are widely expressed in nucleated somatic cells, we hypothesized that the functions of CD8+ T cells are not just limited to monitoring of infected host cells or malignant transformed cells; it extends to monitoring of abnormal host cells in which MHC I molecules present “abnormal-antigenic peptides” under certain pathological conditions as well, thus initiating early inflammation or cascading autoimmune responses. Additional evidence is necessary to support this perspective.
The MIP repertoire originates primarily from ubiquitin-proteasome-dependent degradation of source proteins that include miscoded/misfolded or mismodified proteins, premature translation-termination products, intrinsically disordered proteins, and some regulatory proteins among others that temporarily regulate the cellular activities (de Verteuil et al., 2012, Sijts and Kloetzel, 2011). The MIPs generated by the constitutive proteasome and the immunoproteasome are proposed to differ quantitatively and qualitatively (Van den Eynde and Morel, 2001). We suggest that the obese VAT MIP landscape differs significantly from that of lean fat MIP, partly due to significant upregulation of immunoproteasome in obese VAT. The constitutive proteasome contains three catalytic subunits, namely, β1 (PSMB1), β2 (PSMB1), and β5, and plays a critical role in structuring MHC class I-presenting self-peptide under steady conditions in non-lymphoid tissues. The immunoproteasome, including LMP2 (PSMB8), LMP7 (PSMB9), and MECL1 (PSMB10), is constitutively expressed by mature dendritic cells and upregulated when cells are stimulated by inflammatory stimuli. Studies on tumor immunology have reported production of tumor antigenic peptides by the immunoproteasome and the intermediate conformations, whereas the same was not reported for constitutive proteasome (Vigneron and Van den Eynde, 2014). It has been reported that an immunoproteasome subunit LMP7 deficiency in both immune cells and non-immune cells attenuated adipose tissue inflammation and prevented the development of obesity and metabolic disorders (Kimura et al., 2015), providing further support to our perspective on the probable involvement of immunoproteasomes in generation of obesity-associated antigenic peptides for priming pro-inflammatory CD8+ T cell responses.
Lys48-linked polyubiquitin molecules are key regulators of protein degradation mediated by the proteasome (Wilkinson et al., 1995). We observed that the protein levels of the candidate source proteins of LDHA237-244 peptide, LDHA/LDHB, were reduced in obese VAT; moreover, the Lys48-linked ubiquitination of LDHA was upregulated in obese VAT compared with lean VAT, and inhibition of the proteasome markedly enhanced the accumulation of Lys48-linked ubiquitinated LDHA in VAT, suggesting an increased ubiquitin-proteasome degradation and MHC class I-associated presentation of LDHA or LDHB occurring in obese VAT. The LDHA or LDHB subunit can assemble into homo- or hetero-tetramers that catalyze the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+ to maintain normal metabolic flux. However, the limited studies on the correlation between LDH in VAT and obesity remain controversial so far. Selected studies have demonstrated that obese individuals exhibit high LDH activity in correlation with high lactate accumulation in multiple tissues (Jha and Mitra Mazumder, 2019, Piantedosi et al., 2016). Conversely, LDHA was reported to be downregulated in obese adipose tissue, resulting in the direction of pyruvate into acetyl-CoA production, which in turn promotes fatty acid biosynthesis and triglyceride storage (Lopez et al., 2004). Given lactate has been indicated to induce browning of white adipose cells in mice and humans by enhancing thermogenic gene expression (Carriere et al., 2014), it has been suggested that increasing the LDHA protein levels in adipose tissue might prevent obesity, which in turn would increase conversion of pyruvate to lactate (Parray and Yun, 2015). Our results suggest that the stability of LDHA in obese VAT is reduced due to its excessive ubiquitin-proteasome degradation, which may further disrupt metabolic flux and promote obesity. The exact role of VAT LDH in obesity-related metabolic diseases and the underlying mechanisms need to be investigated extensively.
To summarize, this study confirmed the molecular composition of obese VAT MIP determined by high-throughput and high-resolution MS, reflecting a distinctive obesity-associated signature that is affected by the metabolic status of adipocytes. The obese VAT-exclusive antigenic peptides generated from abnormal protein degradation may function as the antigenic factors that drive the pro-inflammatory responses of CD8+ T cells in VAT, representing a mechanism underlying obesity-induced VAT inflammation. Identification of obesity-exclusive adipose tissue antigenic targets should pave the way for the development of antigen-specific strategies for prevention of obesity-related chronic VAT inflammation and its related diseases.
Limitations of the Study
One of the major limitations of our study was the lack of biological replicates for VAT-derived MIP identification, which is more helpful in demonstrating HFD VAT specificity of certain peptides definitively. The current limitations of mass spectrometry analysis and database search strategies for MIP make it difficult to identify extremely low-abundance peptides and unconventional peptides in the present study. Although adipocytes are the primary components of adipose tissue, we cannot completely rule out the contribution of obesity-related immunogenicity peptides from other stromal cells in adipose tissue. Utilization of normal or hypertrophic mature adipocytes differentiated from 3T3-L1 for MIP analysis is a topic worthy of further study.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Key Project for Research & Development of China (Grant no. 2016YFA0502204) and the National Natural Science Foundation of China (No. 81273213, No. 31570931, and No. 81871301).
Author Contributions
X.C. and S.W. performed ex vivo, in vitro, and in vivo experiments, analyzed data, and wrote the manuscript. Y.H. and S.W. performed the mass spectrometry experiments and analyzed the MIP data. X.Z. conducted bioinformatics analysis. X.J. and G.M. supported in vitro functional analyses of peptide-specific T cell response. Q.Z. and M.J. performed in vitro qPCR and WB analyses of adipose tissue. Y.W. contributed substantially to the conceptualization and discussion of the project and contributed to writing of the manuscript. L.W. conceptualized and designed the study, analyzed and interpreted data, and wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100977.
Contributor Information
Yuzhang Wu, Email: wuyuzhang@tmmu.edu.cn.
Li Wang, Email: liwang118@icloud.com.
Data and Code Availability
Raw mass spectrometry files are available at http://www.immunoinformatics.net/upload/MS/. The mzXML format of raw mass spectrometry files converted by msConvert (Chambers et al., 2012) are provided as well.
Supplemental Information
References
- Adamopoulou E., Tenzer S., Hillen N., Klug P., Rota I.A., Tietz S., Gebhardt M., Stevanovic S., Schild H., Tolosa E. Exploring the MHC-peptide matrix of central tolerance in the human thymus. Nat. Commun. 2013;4:2039. doi: 10.1038/ncomms3039. [DOI] [PubMed] [Google Scholar]
- Bassani-Sternberg M., Braunlein E., Klar R., Engleitner T., Sinitcyn P., Audehm S., Straub M., Weber J., Slotta-Huspenina J., Specht K. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 2016;7:13404. doi: 10.1038/ncomms13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T., Coulie P.G., Van den Eynde B.J., van der Bruggen P. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- Bravo-Sagua R., Parra V., Lopez-Crisosto C., Diaz P., Quest A.F., Lavandero S. Calcium transport and signaling in mitochondria. Compr. Physiol. 2017;7:623–634. doi: 10.1002/cphy.c160013. [DOI] [PubMed] [Google Scholar]
- Caron E., Kowalewski D.J., Chiek Koh C., Sturm T., Schuster H., Aebersold R. Analysis of major histocompatibility complex (MHC) immunopeptidomes using mass spectrometry. Mol. Cell. Proteomics. 2015;14:3105–3117. doi: 10.1074/mcp.O115.052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E., Vincent K., Fortier M.H., Laverdure J.P., Bramoulle A., Hardy M.P., Voisin G., Roux P.P., Lemieux S., Thibault P. The MHC I immunopeptidome conveys to the cell surface an integrative view of cellular regulation. Mol. Syst. Biol. 2011;7:533. doi: 10.1038/msb.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere A., Jeanson Y., Berger-Muller S., Andre M., Chenouard V., Arnaud E., Barreau C., Walther R., Galinier A., Wdziekonski B. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63:3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- Chambers M.C., Maclean B., Burke R., Amodei D., Ruderman D.L., Neumann S., Gatto L., Fischer B., Pratt B., Egertson J. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012;30:918–920. doi: 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M.J., Vuguin P.M. Lack of glucagon receptor signaling and its implications beyond glucose homeostasis. J. Endocrinol. 2015;224:R123–R130. doi: 10.1530/JOE-14-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.H., Tseng Y.J., Wang S.Y., Tsai Y.S., Chang C.S., Kuo T.C., Yao W.J., Shieh C.C., Wu C.H., Kuo P.H. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int. J. Obes. (Lond.) 2015;39:1241–1248. doi: 10.1038/ijo.2015.65. [DOI] [PubMed] [Google Scholar]
- de Verteuil D., Granados D.P., Thibault P., Perreault C. Origin and plasticity of MHC I-associated self peptides. Autoimmun. Rev. 2012;11:627–635. doi: 10.1016/j.autrev.2011.11.003. [DOI] [PubMed] [Google Scholar]
- de Verteuil D., Muratore-Schroeder T.L., Granados D.P., Fortier M.H., Hardy M.P., Bramoulle A., Caron E., Vincent K., Mader S., Lemieux S. Deletion of immunoproteasome subunits imprints on the transcriptome and has a broad impact on peptides presented by major histocompatibility complex I molecules. Mol. Cell Proteomics. 2010;9:2034–2047. doi: 10.1074/mcp.M900566-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh Cat A.N., Friederich-Persson M., White A., Touyz R.M. Adipocytes, aldosterone and obesity-related hypertension. J. Mol. Endocrinol. 2016;57:F7–F21. doi: 10.1530/JME-16-0025. [DOI] [PubMed] [Google Scholar]
- Dutoit V., Herold-Mende C., Hilf N., Schoor O., Beckhove P., Bucher J., Dorsch K., Flohr S., Fritsche J., Lewandrowski P. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135:1042–1054. doi: 10.1093/brain/aws042. [DOI] [PubMed] [Google Scholar]
- Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A.B., Benoist C., Shoelson S. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazarian M., Revelo X.S., Nohr M.K., Luck H., Zeng K., Lei H., Tsai S., Schroer S.A., Park Y.J., Chng M.H.Y. Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci. Immunol. 2017;2 doi: 10.1126/sciimmunol.aai7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Duque S., Azoury M.E., Colli M.L., Afonso G., Turatsinze J.V., Nigi L., Lalanne A.I., Sebastiani G., Carre A., Pinto S. Conventional and neo-antigenic peptides presented by beta cells are targeted by circulating naive CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab. 2018;28:946–960.e6. doi: 10.1016/j.cmet.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Granados D.P., Laumont C.M., Thibault P., Perreault C. The nature of self for T cells-a systems-level perspective. Curr. Opin. Immunol. 2015;34:1–8. doi: 10.1016/j.coi.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Ivanova E.A., Bechtold D.A., Dupre S.M., Brennand J., Barrett P., Luckman S.M., Loudon A.S. Altered metabolism in the melatonin-related receptor (GPR50) knockout mouse. Am. J. Physiol. Endocrinol. Metab. 2008;294:E176–E182. doi: 10.1152/ajpendo.00199.2007. [DOI] [PubMed] [Google Scholar]
- Jha D., Mitra Mazumder P. High fat diet administration leads to the mitochondrial dysfunction and selectively alters the expression of class 1 GLUT protein in mice. Mol. Biol. Rep. 2019;46:1727–1736. doi: 10.1007/s11033-019-04623-y. [DOI] [PubMed] [Google Scholar]
- Kim K.E., Cho Y.S., Baek K.S., Li L., Baek K.H., Kim J.H., Kim H.S., Sheen Y.H. Lipopolysaccharide-binding protein plasma levels as a biomarker of obesity-related insulin resistance in adolescents. Korean J. Pediatr. 2016;59:231–238. doi: 10.3345/kjp.2016.59.5.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Usui F., Karasawa T., Kawashima A., Shirasuna K., Inoue Y., Komada T., Kobayashi M., Mizushina Y., Kasahara T. Immunoproteasome subunit LMP7 deficiency improves obesity and metabolic disorders. Sci. Rep. 2015;5:15883. doi: 10.1038/srep15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs A., Alogna A., Post H., Hamdani N. Is enhancing cGMP-PKG signalling a promising therapeutic target for heart failure with preserved ejection fraction? Neth. Heart J. 2016;24:268–274. doi: 10.1007/s12471-016-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewski D.J., Schuster H., Backert L., Berlin C., Kahn S., Kanz L., Salih H.R., Rammensee H.G., Stevanovic S., Stickel J.S. HLA ligandome analysis identifies the underlying specificities of spontaneous antileukemia immune responses in chronic lymphocytic leukemia (CLL) Proc. Natl. Acad. Sci. U S A. 2015;112:E166–E175. doi: 10.1073/pnas.1416389112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler M.W., Kowalewski D.J., Backert L., Bernhardt J., Adam P., Schuster H., Dengler F., Backes D., Kopp H.G., Beckert S. Mapping the HLA ligandome of colorectal cancer reveals an imprint of malignant cell transformation. Cancer Res. 2018;78:4627–4641. doi: 10.1158/0008-5472.CAN-17-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez I.P., Milagro F.I., Marti A., Moreno-Aliaga M.J., Martinez J.A., De Miguel C. Gene expression changes in rat white adipose tissue after a high-fat diet determined by differential display. Biochem. Biophys. Res. Commun. 2004;318:234–239. doi: 10.1016/j.bbrc.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Ma F., Feng J., Zhang C., Li Y., Qi G., Li H., Wu Y., Fu Y., Zhao Y., Chen H. The requirement of CD8+ T cells to initiate and augment acute cardiac inflammatory response to high blood pressure. J. Immunol. 2014;192:3365–3373. doi: 10.4049/jimmunol.1301522. [DOI] [PubMed] [Google Scholar]
- Madsen L., Kristiansen K. The importance of dietary modulation of cAMP and insulin signaling in adipose tissue and the development of obesity. Ann. N. Y Acad. Sci. 2010;1190:1–14. doi: 10.1111/j.1749-6632.2009.05262.x. [DOI] [PubMed] [Google Scholar]
- McDonnell W.J., Koethe J.R., Mallal S.A., Pilkinton M.A., Kirabo A., Ameka M.K., Cottam M.A., Hasty A.H., Kennedy A.J. High CD8 T-cell receptor clonality and altered CDR3 properties are associated with elevated isolevuglandins in adipose tissue during diet-induced obesity. Diabetes. 2018;67:2361–2376. doi: 10.2337/db18-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Parray H.A., Yun J.W. Proteomic identification of target proteins of thiodigalactoside in white adipose tissue from diet-induced obese rats. Int. J. Mol. Sci. 2015;16:14441–14463. doi: 10.3390/ijms160714441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H., Daouda T., Granados D.P., Durette C., Bonneil E., Courcelles M., Rodenbrock A., Laverdure J.P., Cote C., Mader S. MHC class I-associated peptides derive from selective regions of the human genome. J. Clin. Invest. 2016;126:4690–4701. doi: 10.1172/JCI88590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantedosi D., Di Loria A., Guccione J., De Rosa A., Fabbri S., Cortese L., Carta S., Ciaramella P. Serum biochemistry profile, inflammatory cytokines, adipokines and cardiovascular findings in obese dogs. Vet. J. 2016;216:72–78. doi: 10.1016/j.tvjl.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Qin S., Song G., Yu Y. Phospholipid transfer protein in diabetes, metabolic syndrome and obesity. Cardiovasc. Hematol. Disord. Drug Targets. 2014;14:149–153. doi: 10.2174/1871529x1402140807144435. [DOI] [PubMed] [Google Scholar]
- Rausch M.E., Weisberg S., Vardhana P., Tortoriello D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond.) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- Schuster H., Shao W., Weiss T., Pedrioli P.G.A., Roth P., Weller M., Campbell D.S., Deutsch E.W., Moritz R.L., Planz O. A tissue-based draft map of the murine MHC class I immunopeptidome. Sci. Data. 2018;5:180157. doi: 10.1038/sdata.2018.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijts E.J., Kloetzel P.M. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol. Life Sci. 2011;68:1491–1502. doi: 10.1007/s00018-011-0657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Ji Y., Kersten S., Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu. Rev. Nutr. 2012;32:261–286. doi: 10.1146/annurev-nutr-071811-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzotzas T., Desrumaux C., Lagrost L. Plasma phospholipid transfer protein (PLTP): review of an emerging cardiometabolic risk factor. Obes. Rev. 2009;10:403–411. doi: 10.1111/j.1467-789X.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- Van den Eynde B.J., Morel S. Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome. Curr. Opin. Immunol. 2001;13:147–153. doi: 10.1016/s0952-7915(00)00197-7. [DOI] [PubMed] [Google Scholar]
- Vigneron N., Van den Eynde B.J. Proteasome subtypes and regulators in the processing of antigenic peptides presented by class I molecules of the major histocompatibility complex. Biomolecules. 2014;4:994–1025. doi: 10.3390/biom4040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K.D., Tashayev V.L., O'Connor L.B., Larsen C.N., Kasperek E., Pickart C.M. Metabolism of the polyubiquitin degradation signal: structure, mechanism, and role of isopeptidase T. Biochemistry. 1995;34:14535–14546. doi: 10.1021/bi00044a032. [DOI] [PubMed] [Google Scholar]
- Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J., Dorfman R., Wang Y., Zielenski J., Mastronardi F. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M.J., Adili A., Piotrowitz K., Abdullah Z., Boege Y., Stemmer K., Ringelhan M., Simonavicius N., Egger M., Wohlleber D. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Wu H., Ghosh S., Perrard X.D., Feng L., Garcia G.E., Perrard J.L., Sweeney J.F., Peterson L.E., Chan L., Smith C.W. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- Yang H., Youm Y.H., Vandanmagsar B., Ravussin A., Gimble J.M., Greenway F., Stephens J.M., Mynatt R.L., Dixit V.D. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Qu H., Xiong X., Wang Y., Liu X., Zhang L., Liao X., Liao Q., Sun Z., Ouyang Q. Deficiency of mitochondrial glycerol 3-phosphate dehydrogenase contributes to hepatic steatosis. Hepatology. 2019;70:84–97. doi: 10.1002/hep.30507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw mass spectrometry files are available at http://www.immunoinformatics.net/upload/MS/. The mzXML format of raw mass spectrometry files converted by msConvert (Chambers et al., 2012) are provided as well.