Abstract
We recently reported the dose-dependent therapeutic effect of 211At-NaAt in differentiated thyroid cancer xenograft models. In the present study, we evaluated the radiation-induced toxicity of 211At-NaAt using detailed hematological, biochemical, and histological analyses. Biodistribution of 211At-NaAt was measured in normal ICR mice (n = 12), absorbed doses in the major organs were calculated. Groups of ICR mice (n = 60) were injected with 0.1 MBq or 1 MBq of 211At-NaAt, using saline as the control group (n = 30). Body weight and food intake were followed up for 60 days. Blood cell counts and serum level of biochemical parameters were measured 3, 7, 15, 29, 60 days after injection. Histological analyses of the major organs with hematoxylin and eosin staining were performed. Biodistribution study revealed a high-absorbed dose in the thyroid gland, stomach, bladder, heart, lungs, spleen, kidneys, and testis. The 0.1 MBq group showed no abnormalities. The 1 MBq group showed decreased body weight and food intake. Histological analysis showed atrophy and fibrosis in the thyroid gland, a transient hypospermatogenesis in the testis on day 29 was found in one mouse. Hematological toxicity was mild and transient. The total cholesterol, albumin, and total protein increased with no signs of recovery, which was considered to be caused by hypothyroidism. High-dose administration of 211At-NaAt showed transient toxicity in the white blood cells and testis without severe hematological or renal toxicity, suggesting its tolerable safety as targeted alpha-therapy for differentiated thyroid cancer in the 1 MBq group.
Introduction
Radioactive iodine (RAI) therapy is used for the treatment of patients with differentiated thyroid cancer [1,2]. However, a percentage of patients show insufficient 131I accumulation [3] or low therapeutic effect even with enough 131I accumulation, and some patients suffer from recurrence or metastases and become RAI-refractory during follow-up [4,5]. For these patients, a more effective treatment is needed.
Astatine (211At) is a halogen with chemical properties similar to those of iodine, which emits alpha particles that have a shorter range in tissue but higher linear energy transfer (LET) compared with beta particles [6,7]. 211At has been reported to yield a better therapeutic effect by inducing a more clustered DNA double-strand break and highly reactive hydroxyl radicals [8]. Targeted alpha therapy, using 225Ac-PSMA-617 in metastatic prostate cancer patients with resistance to 177Lu-PSMA-617, has also proved the potential beneficial effect of targeted alpha therapy over beta-particle radiation [9]. Meanwhile, it has been noted that side effects, such as bone marrow toxicity are critical aspects of radionuclide therapy [10]. In addition, although severe and irreversible xerostomia has been reported to be caused by the physiological accumulation of 225Ac-PSMA-617, alpha-radiation therapy in prostate cancer and a low rate of xerostomia is observed when 177Lu-PSMA-617 is used [9,11,12].
We have found that the radiochemical purity of astatide dramatically improves upon treatment with 1% ascorbic acid and the uptake of 211At increases in the thyroid gland and thyroid cancer cells. Since the treatment effect of 211At is dose-dependent, as shown in our previous study, a better therapeutic effect was expected with the increased tumor uptake of astatide in the clinical application [13]. However, radiation-induced toxicity has not been thoroughly evaluated using the 211At-NaAt solution with ascorbic acid.
In the present study, we aimed to evaluate the radiation-induced toxicity of different doses of 211At-NaAt solution at different time points to estimate the time-dependent toxicity. Since we used ascorbic acid to enhance the radiochemical purity and change the biodistribution of 211At-NaAt, this preclinical safety study is necessary to prevent unexpected side effects.
Materials and Methods
Preparation of the 211At Solution
211At was procured from the Research Center for Nuclear Physics at Osaka University and RIKEN via the short-lived RI supply platform. After the 209Bi(α, 2n)211At reaction and isolation with a dry-distillation method, 211At was dissolved in water at a concentration of 10 MBq/ml [13]. Ascorbic acid and sodium bicarbonate were added to the 211At solution at a final concentration of 2.1% (w/v) at pH 8.0, and the solution was allowed to stand for 1 hour at 23 ± 2 °C.
Preparation and Observation of Animals
The experiment protocol was approved by the Animal Care and Use Committee of the Osaka University Graduate School of Medicine. Animals were purchased from Japan SLC Inc. (Hamamatsu, Japan) and housed in a 12 h light/12 h dark cycle, allowed to get food and water freely. Twelve male ICR mice (9 weeks old, body weight = 38.0 ± 1.2 g) were injected with 132.4 ± 6.3 kBq of 211At-NaAt solution through the tail vein for biodistribution study. Three groups of male ICR mice (n = 90, 10 weeks old, body weight = 38.3 ± 1.9 g) were fed with a one-week low-iodine diet before injection and injected with saline (n = 30) or 211At-NaAt solution (0.10 ± 0.02 MBq and 1.00 ± 0.11 MBq, n = 30 for each dose) through the tail vein for toxicity evaluation. Body weight and food intake were measured three times a week for 60 days after administration.
Biodistribution Study and Calculation of Absorbed Dose
For the biodistribution experiment, mice were euthanized and dissected 1, 3, 6 and 24 h after the injection of a 211At-NaAt solution (0.13 MBq, n = 3 at each time point). Blood and major organs were removed and weighed. The radioactivity of tissues was measured using the gamma counter (2480 Wizard2 Gamma Counter, Perkin Elmer, US). The residence time (h) was calculated from the area under the curve of the percentage of injected dose (%ID) using the trapezoid method, and the absorbed doses in mice were calculated by OLINDA/EXM version2 (HERMES Medical Solutions, Stockholm, Sweden). The decay correction was performed using %ID calculation, while it was not applied in the calculation of residence time and absorbed dose. The absorbed doses in human males were estimated using IDAC-Dose 2.1 software [14].
Hematological and Biochemical Analysis
For each group, six mice per time point were deeply anesthetized with 5% isoflurane 3, 7, 15, 29 and 60 days after the injection. Heart blood was collected by heparinized syringes from the left ventricle, and then the mice were euthanized. Complete blood cell counts, including white blood cells (WBC), red blood cells (RBC) and platelets (PLT) were measured by chemistry analyzer (SPOTCHEM D-Concept, Arkray, Japan). The rest of the blood samples were centrifuged (112 g/30 min) to get the serum. Biochemical parameters including total cholesterol (TC), albumin (ALB), total protein (TP), total bilirubin (T-BIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LD), blood urea nitrogen (BUN), creatine (Cr), creatine kinase (CK), amylase (AMY) and blood glucose (GLU) were measured by an automatic blood cell counter (Thinka CB-1010, Arkray, Japan). Since repeated blood sampling from the same mouse is not feasible in the present study due to the required volume for all biomarkers, we performed validation tests for reproducibility before the main experiments.
Histological Analysis
Mice were dissected after blood collection and euthanasia at each time point. The thyroid gland, salivary glands, stomach, spleen, kidneys, and testis, were removed and embedded in Optimal Cutting Temperature (OCT) compound to make tissue blocks. Lungs, liver, small intestine, and large intestine were removed on days 3, 7 and 15 in the 1 MBq group. Three blocks of each group at each time point were sliced for histological analysis. In addition, additional analysis was also performed in the organ at risk, such as the thyroid gland, kidneys and testis. Sections (10 μm) were obtained using the cryostat (CryoStar NX70, Thermo Fisher Scientific, USA) from each block and stained with hematoxylin and eosin (HE) for histological microscopy analysis using BZ-9000 Fluorescence Microscope (Keyence Corp, Japan). The results of the histological analysis were evaluated and confirmed by two researchers in consensus and reviewed by the experts specialized in the evaluation of toxicity tests, unblinded or unscored.
Statistical Analysis
Bodyweight, weighed food intake, blood cell counts, and the value of biochemical parameters was expressed as mean value ± standard deviation (SD) and compared using unpaired t-test with Bonferroni correction among three groups. P < .05 was considered statistically significant differences.
Results
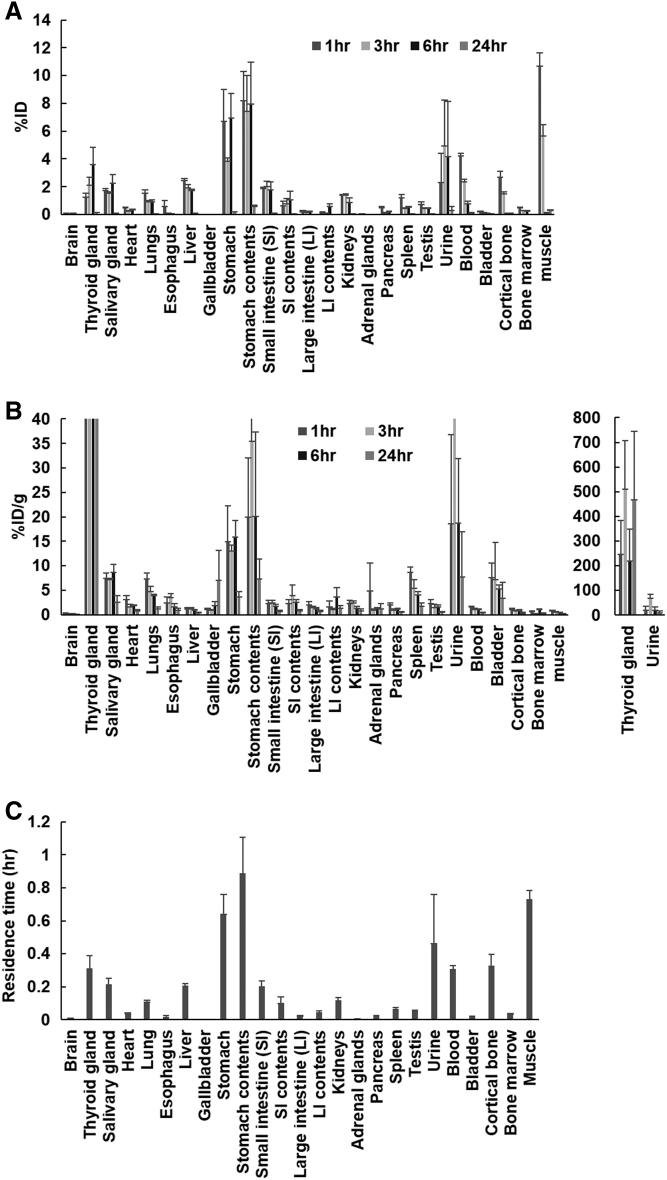
Biodistribution of 211At-NaAt is shown in Figure 1A and Figure 1B. The residence time per organ is given in Figure 1C. The highest value was observed in the stomach, followed by the muscle, urine, bone, thyroid gland and blood. The absorbed doses in mice and estimated dose in humans were calculated and are shown in Table 1. The highest absorbed dose in humans was estimated in the thyroid gland, followed by the stomach, salivary glands, and testis.
Figure 1.
The %ID (A), %ID/g (B) and residence time (C) of the main organs in normal ICR mice administered with the 211At-NaAt solution.
Table 1.
Absorbed doses (mGy/MBq) of main organs in mice and estimated numbers for humans
| Organ | Absorbed doses in mice | Absorbed doses in humans |
|---|---|---|
| Brain | 4.62 | 0.0108 |
| Thyroid gland | 5691 | 19.1 |
| Salivary gland | N.A. | 3.43 |
| Heart | 374 | 0.150 |
| Lungs | 297 | 0.0264 |
| Liver | 29.6 | 0.145 |
| Stomach | 3941 | 4.79 |
| Small intestine | 80.8 | 0.352 |
| Colon | 70.8 | 0.199 |
| Kidneys | 97.4 | 0.417 |
| Pancreas | 20.2 | 0.219 |
| Spleen | 148 | 0.433 |
| Testis | 83.3 | 2.01 |
| Bladder | 1955 | 0.459 |
| Bone marrow | N.A. | 0.0901 |
N.A.: not available.
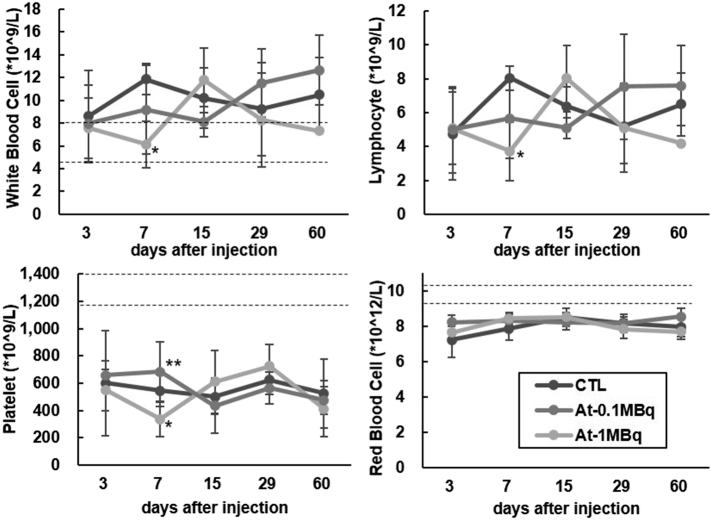
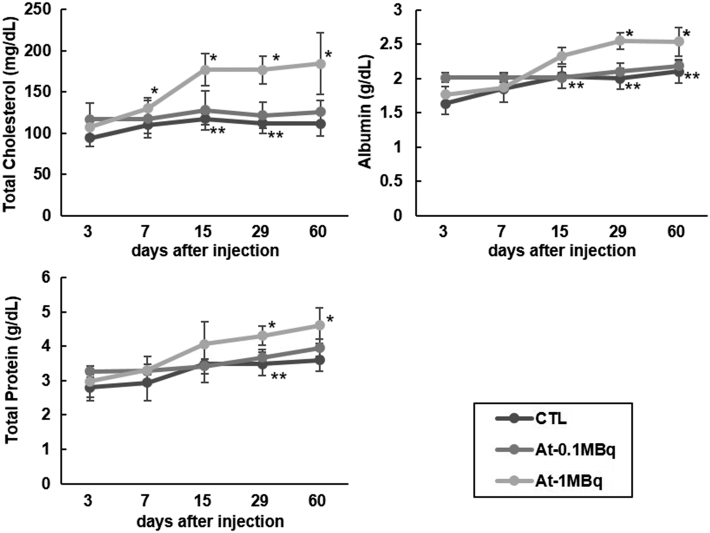
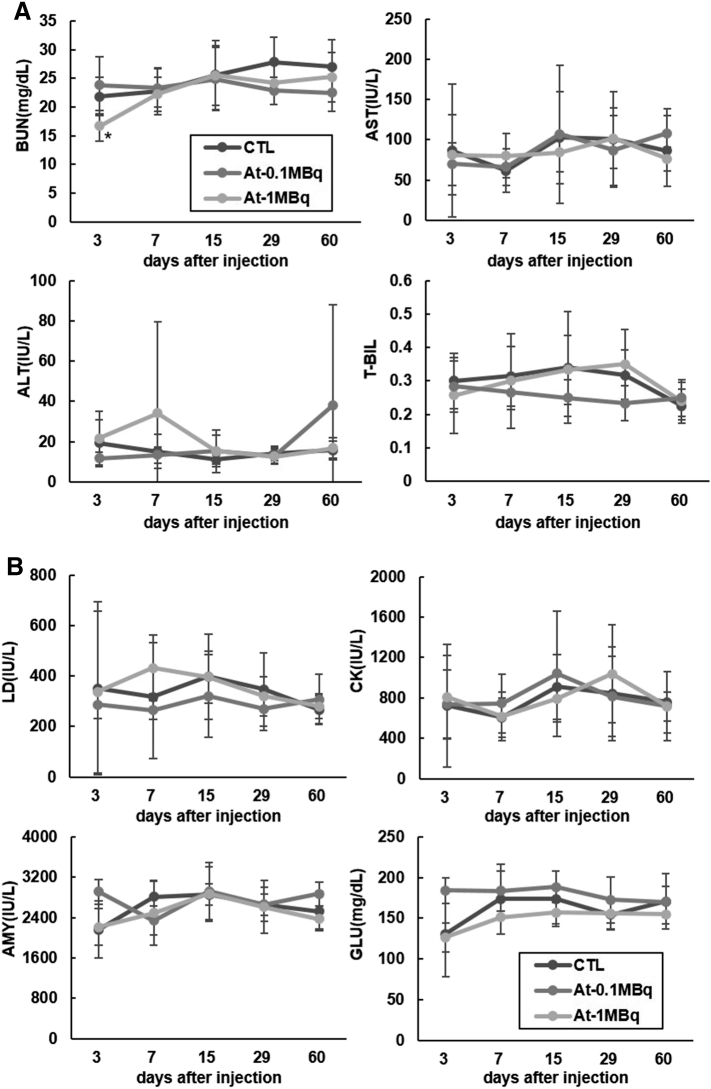
The body weight, as well as the food intake of the 211At-NaAt group, showed a decreasing trend compared to the control (Figure 2, A and B). No hematological changes were observed between 0.1 MBq and control groups. The mean count of WBC, lymphocytes and PLT in the 1 MBq group decreased to 6.1 ± 2.1*10^9/L, 3.7 ± 1.7*10^9/L and 335 ± 128*10^9/L on day 7, respectively, but returned to normal after day 15 (Figure 3). TC levels of the 1 MBq group increased from 130 mg/dl on day 7 (P = .026) and remained above the normal range until day 60 (P = .027) without recovery (Figure 4). ALB levels increased to 2.55 g/dl on day 29 (P = .0002), and to 2.54 g/dl on day 60 compared with 2.10 g/dl in the control group (P = .028); TP started to elevate from 4.3 g/dl on day 29 (P = .04) to 4.6 g/dl on day 60 (P = .029) in the 1 MBq group with no signs of recovery (Figure 4). BUN levels of the 1 MBq group showed a sudden decrease on day 3 (P = .039) but returned to normal levels on day 7 (Figure 5A). No significant increase was observed in Cr since all measurements were within <0.2 mg/dl. No alterations were observed in the levels of ALT, AST, T-BIL, LD, CK, AMY, and GLU in the 1 MBq group (Figure 5, A and B). No changes were observed in all the biochemical parameters of the 0.1 MBq group when compared with the control group.
Figure 2.
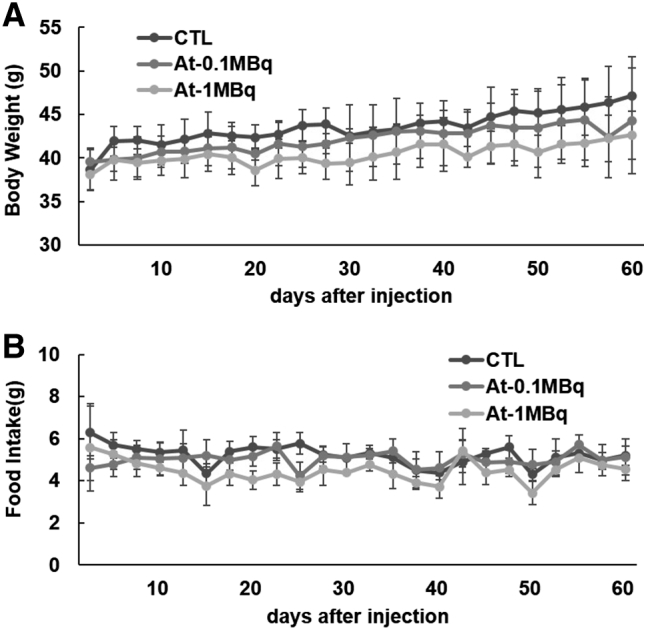
Change in the body weight (A) and weighed food intake (B) after administration of 211At-NaAt solution shown as mean ± SD.
Figure 3.
Evaluation of hematological toxicity in the control, 0.1 MBq, and 1 MBq groups. The mean counts of WBC, lymphocytes, PLT, and RBC, are expressed as mean ± SD (*: P < .05 between the control group and 1 MBq group. **: P < .05 between 0.1 MBq group and 1 MBq group by unpaired t-test with Bonferroni correction). The normal count of WBC (6.38 ± 1.80 *10^9/L), PLT (1241 ± 154 *10^9/L) and RBC (9.82 ± 0.5 *10^12/L) from the supplier are shown as dotted lines.
Figure 4.
Temporal changes in serum levels of TC, ALB and TP (*: P < .05 between control group and 1 MBq group and **: P < .05 between 0.1 MBq group and 1 MBq group by unpaired t-test with Bonferroni correction).
Figure 5.
Temporal changes in serum levels of (A) BUN, AST, ALT and T-BIL for renal and liver toxicity assessment and (B) serum levels of LD, CK, AMY and GLU for myocardium and pancreas function assessment (*: P < .05 between control group and 1 MBq group by unpaired t-test with Bonferroni correction).
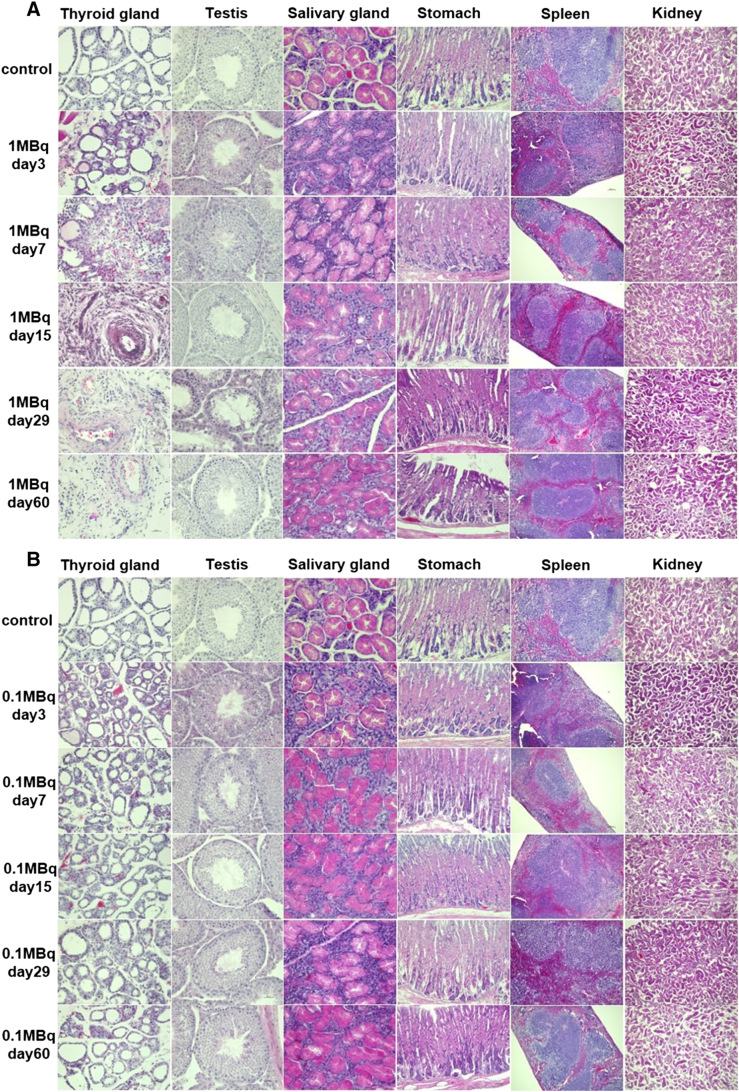
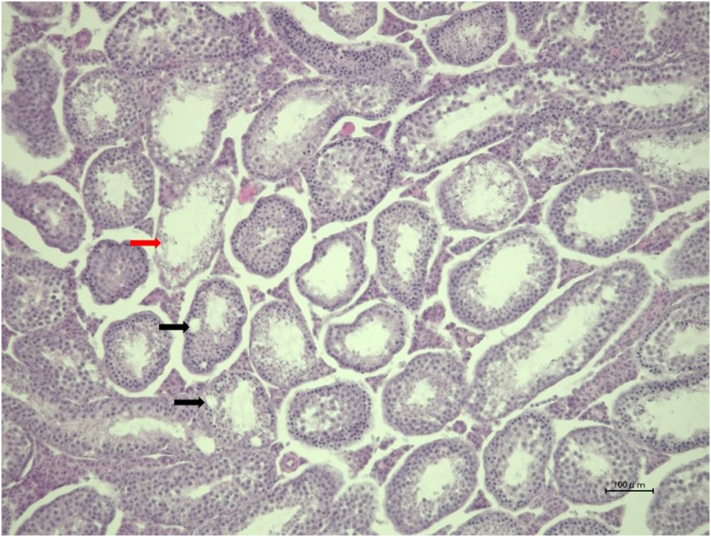
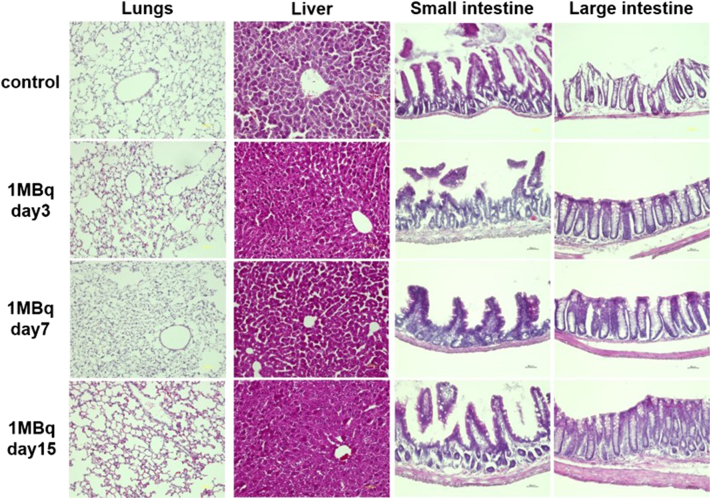
Upon histological examination of the thyroid, no histological changes were seen in the 0.1 MBq group (n = 6). On the other hand, a time-dependent disappearance of follicles was observed from day 3 and fibrosis was detected from day 7 in the 1 MBq group with no signs of recovery (Figure 6A). Histological changes were not observed in the testis of 0.1 MBq, while the loss of spermatozoa and atrophy and vacuolation of seminiferous tubules were found in one mouse (n = 6) on day 29, but no abnormality was observed on day 60 in the 1 MBq group (Figure 6A, Supplemental Figure 1). No damages in the salivary glands, stomach, kidneys or spleen were evident histologically, both in 1 MBq and 0.1 MBq 211At groups (Figure 6). No histological abnormalities were found in the lungs, liver, small intestine and large intestine on day 3, 7 and 15 in the 1 MBq 211At group (Figure 7).
Figure 6.
Histological changes evaluated by HE staining in the 1 MBq group (A) and 0.1 MBq group (B).
Supplementary Fig 1.
Histological changes in the testis on day 29 in one mouse in the 1 MBq group, showing loss of germ cells and atrophy of seminiferous tubules (red arrow) and seminiferous tubules vacuolation (black arrows).
Figure 7.
Histological changes in the lungs, liver, small intestine, and large intestine evaluated by HE staining in the 1 MBq group on day 3, 7, and 15.
Discussion
We evaluated radiation-induced toxicity of 211At-NaAt by measuring the body weight, food intake, as well as hematological, biochemical and histological parameters. Body weight and weighed food intake showed a decrease in the 1 MBq group compared to the control group or 0.1 MBq group. In addition, no significant adverse effects were observed in the 0.1 MBq group compared to the control group, and a transient decrease in the mean count of WBC and lymphocytes, as well as atrophy of thyroid gland and a loss of spermatozoa, were observed in the 1 MBq group.
Relatively high-absorbed doses were estimated in the thyroid gland, stomach, salivary gland, testis, bladder, spleen and kidneys. The thyroid gland started to show atrophy extensively on day 7 and turned to fibrosis. Like 131I, 211At was reported to be transported into the thyroid gland by the sodium iodide symporter in our previous study [13]. Since 131I therapy is performed for ablation of remnant thyroid gland tissue after thyroidectomy, the ablation effect of 211At-NaAt was an expected phenomenon like the 131I-NaI therapy. Furthermore, 211At-NaAt therapy will also be used for patients with thyroid cancer after thyroidectomy. Thus, these effects are not adverse effects, but one of the expected therapeutic effects. Meanwhile, Cobb et al. reported that when treated with 28 kBq/g of 211At-NaAt, thyroid gland shows significant atrophy, which is similar to our results when we used 1 MBq (about 26 kBq/g) 211At-NaAt solution [15].
Despite the high absorbed dose in the stomach, we did not find any histological changes. 131I is absorbed by parietal cells of the gastric mucosa, then excreted into stomach contents in the same way as Cl− [16]. After being excreted into the stomach, 131I is reported to flow into the small intestine [17]. As shown in Figure 1, the %ID, %ID/g and residence time of the stomach contents were higher than those of the stomach wall, suggesting a similar absorption-excretion mechanism of 211At in the stomach. Although a previous study reported pathological changes found in the gastrointestinal tract on day 3, treated with 28 kBq/g of 211At-NaAt, we discovered no changes at the same dose [15].
Although the salivary glands, spleen and kidneys were thought to be the next high-risk organs, because of the moderate absorbed doses, we did not discover any significant histological changes. Despite salivary gland dysfunction being occasionally observed in the 131I therapy, we discovered no significant histological abnormality in the salivary gland treated with 0.1 MBq and 1 MBq of 211At-NaAt [18]. Although 211At is excreted mainly from the kidneys, no impairments in kidney function were evident. In addition, no renal fibrosis or tubular cell necrosis was observed. The lungs also had moderate absorbed doses in mice but not in humans, without any histological changes.
In the testis, the total number of germ cells decreased, and seminiferous tubules atrophied in one mouse on day 29, but no abnormality was observed on day 60. 131I therapy has been reported to be related to the reduction of testis function, and to cause potential infertility [19,20]. Cobb et al. also reported that when treated with 28 kBq/g 211At-NaAt, the sperm count reduces by 80% on day 3 [15]. Our results reflect these previous findings, although the degree of severity was relatively mild.
The history of 211At goes back to as early as the 1950s. In 1954, Hamilton et al. reported the acute and chronic changes in rats and monkeys [21,22]. Two weeks after being injected with 44 kBq/g of 211At-NaAt, some of the rats showed hemorrhage in the spleen, lymph nodes, lungs, adrenal glands and died [22]. However, we did not observe such results when we injected the mice with 26 kBq/g of 211At-NaAt. In addition, Cobb et al. also did not mention hemorrhage even when they injected mice with 61 kBq/g of 211At-NaAt [23]. Overall, the hemorrhagic reaction is not an inevitable phenomenon, but it could be a part of the problem due to a non-specific-reaction or type of species. Hamilton et al. also reported the atrophy of the spleen, thymus, and thyroid after administration of 44 kBq/g 211At-NaAt in rats. We also observed atrophy in the thyroid when we injected mice with 26 kBq/g 211At-NaAt, compatible with their results. As described above, the evaluation of radiation induced toxicity of 211At had already begun in 1950s; however, there was a long era when it was not utilized clinically. Since the safety of alpha emitters was still unknown and because of the use of beta emitters (131I) for therapy, it was considered not necessary to use alpha particles against the unknown risk. After a long time, finding out the benefit of alpha particles and considering the balance of advantages and risks, it is on our radar again due to the promising clinical results in the targeted alpha therapy.
For targeted alpha therapy, bone marrow suppression could be a limitation, and this requires attention. However, due to the relatively short circulation half-life of 211At, bone marrow toxicity is not a major problem compared with other radiolabeled antibodies. Hematotoxicity is thought to be related to the residence time of alpha-particle emitting nuclides in the blood, as the radiation exposure to the bone marrow is delivered from the bloodstream. Since the residence time in blood was only 0.3 h with 211At-NaAt in this study, and the hematological analysis indicated that the abnormality of WBC and lymphocytes is transient in the 1 MBq group, and there is limited hematotoxicity. The results on the hematological toxicity in the previous studies were also different from the present study. Cobb et al. reported a significant fall in the lymphocyte and PLT counts on day 3, which recovers from week 8 [15]. Since we used different kinds of mice and added ascorbic acid in the solution, the toxicity may have been affected. Meanwhile, we should be careful about the interpretation of statistical significance in PLT counts because it showed considerable variability.
TC, ALB, and TP levels also increased in the 1 MBq group with no ALT or AST changes. We thought the increase in TC levels was not caused by an impairment of liver function but by hypothyroidism since thyroid hormone has effects on the lipid metabolism and thyroid functions are associated with it [24,25]. Hypothyroidism leads to a high serum level of TC [26,27]. ALB and TP levels are also enhanced in hypothyroidism, so we thought the elevation in the levels of ALB and TP was caused by hypothyroidism after 211At therapy [28,29]. Regarding the effect caused by hypothyroidism, thyroid hormone supplementation therapy is usually given after thyroid resection surgery, the toxicity we showed can be compensated. No significant difference in ALT, AST, T-BIL, LD levels and no histological damage was observed in the liver, suggesting normal hepatic function and no severe liver injury. Normal serum levels of CK, AMY, and GLU revealed the normal function of myocardium and pancreas.
In the first week after injection, the body weight change showed decreased trend both in 0.1 MBq group and 1 MBq group compared to control mice. It was suggested that injection of the 211At-NaAt solution might affect the general condition of mice. While after 1 week, the bodyweight in the 0.1 MBq group showed recovery, but the weight increase rate of 1 MBq group still didn't reach a normal level. We thought it might be because of hypothyroidism and the loss of appetite as shown in the decrease of food intake.
We found that mice had higher absorbed doses in lungs and bladder compared with humans. Since the size of alveoli and bladder are smaller in mice than humans, alpha particles are more likely to affect the lungs and the bladder wall in mice, due to short distance in the tissue (e.g. self-absorption of alpha-ray in the urine is small in mice compared to human due to its small volume). In contrast, we also found that the absorbed dose in the testis of mice was lower than the estimated dose in humans, but the underlying calculation algorithm is currently unknown.
Nevertheless, regarding the translation of murine data to the expected human data, similar distribution is expected as it is mainly regulated by the NIS expression as we can see in the radioiodine distribution in murine and humans. We translated absorbed doses of mice to humans. Previous studies also reported that there was no significant difference in the toxicity after the administration of 211At between rodents and non-rodents [21,22]. In comparison with biodistribution study in rats, thyroid gland showed similar uptakes, while stomach and salivary gland showed less uptake than those in our study using mice [30]. Although we still need to be careful about the translation of murine data to the expected human data, especially for the therapeutic setting, the biodistribution of 211At in humans can be estimated based on the knowledge of 131I.
A very narrow therapeutic window has been reported in a previous study using 211At [31]. In our previous study, even 0.1 MBq (2.6 kBq/g) showed tumor growth suppression effect, so the therapeutic window of 211At-NaAt is not that narrow. However, we still should pay attention to the individual variability of distribution in each patient. In the clinical application, we should be careful about over-dose, and repeated therapy can be considered with a lower dose for one injection.
Although we found no abnormalities in the cell count, serum level biomarkers and histologic analysis in the 0.1 MBq group, RNA levels could change. In the previous studies, dose -dependent changes in transcript regulations were found in the thyroid gland and non-thyroid tissues without protein or tissue function biomarker changing with low dose 211At-NaAt solution [32,33]. So even if we revealed no damages, the hidden side effects such as the risk of secondary cancers in the clinical application.
This study has several limitations. First, we only used two different doses. Since almost no change was observed in the 0.1 MBq group, we cannot confirm the linear-relationship between dose and radiation-induced toxicity. Second, we only studied the side effects of the treatment in male mice. Some reports show that some side effects only appear in females and we could not rule out this possibility for 211At even though no sex-dependent side effects of 131I was detected. Third, we only evaluated short and intermediate-term side effects. However, renal toxicity can develop in the late-stage or even if it is not apparent at the intermediate-stage [34,35]. It needs to be evaluated in future studies since more prolonged survival is expected with the use of alpha-emitters. In addition, we should be careful, as creatine is not a sensitive marker to predict renal dysfunction. Fourth, we only evaluated the toxicity in mice with a limited number to demonstrate the feasible tolerability as an initial evaluation. There can still be possible unexpected side effects on humans. And hence, we need to pay attention while using 211At-NaAt clinically.
Conclusion
This preliminary study shows that high dose administration of 211At-NaAt causes a transient decrease in the WBC and lymphocytes, atrophy in the thyroid gland and transient hypospermatogenesis. No severe toxicity, such as renal dysfunction, or significant bone marrow depletion were observed, which demonstrates the tolerable feasibility of 211At-NaAt within the administered dose and follow-up period in this study.
The following are the supplementary data related to this article.
Financial Disclosure
This study was funded by the QiSS program of the OPERA (Grant Number: JPMJOP1721) from the Japan Science and Technology Agency (JST), Japan. There is no other potential conflict of interest relevant to this article to disclose.
Acknowledgments
We thank Takanori Kobayashi for his excellent technical assistance. The 211At was partly supplied through the Supply Platform of Short-lived Radioisotopes, supported by the funding of JSPS Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number 16H06278.
Footnotes
This study was funded by the QiSS program of the OPERA (Grant Number: JPMJOP1721) from the Japan Science and Technology Agency (JST).
Contributor Information
Yuwei Liu, Email: liu@tracer.med.osaka-u.ac.jp.
Tadashi Watabe, Email: watabe@tracer.med.osaka-u.ac.jp.
References
- 1.Maxon H.R., 3rd, Englaro E.E., Thomas S.R. Radioiodine-131 therapy for well-differentiated thyroid cancer--a quantitative radiation dosimetric approach: outcome and validation in 85 patients. J. Nucl. Med. 1992;33:1132–1136. [PubMed] [Google Scholar]
- 2.Maheshwari Y.K., Hill C.S., Jr., Haynie T.P., 3rd, Hickey R.C., Samaan N.A. 131I therapy in differentiated thyroid carcinoma: M. D. Anderson Hospital experience. Cancer. 1981;47:664–671. doi: 10.1002/1097-0142(19810215)47:4<664::aid-cncr2820470408>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Cobb L.M., Harrison A., Dudley N.E., Carr T.E., Humphreys J.A. Relative concentration of astatine-211 and iodine-125 by human fetal thyroid and carcinoma of the thyroid in nude mice. Radiother. Oncol. 1988;13:203–209. doi: 10.1016/0167-8140(88)90057-6. [DOI] [PubMed] [Google Scholar]
- 4.Jin Y., Van Nostrand D., Cheng L., Liu M., Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol. 2018;125:111–120. doi: 10.1016/j.critrevonc.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M., Brose M., Elisei R. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356–358. doi: 10.1016/S2213-8587(13)70215-8. [DOI] [PubMed] [Google Scholar]
- 6.Zalutsky M.R., Vaidyanathan G. Astatine-211-labeled radiotherapeutics: an emerging approach to targeted alpha-particle radiotherapy. Curr. Pharm. Des. 2000;6:1433–1455. doi: 10.2174/1381612003399275. [DOI] [PubMed] [Google Scholar]
- 7.Dahle J., Abbas N., Bruland O.S., Larsen R.H. Toxicity and relative biological effectiveness of alpha emitting radioimmunoconjugates. Curr. Radiopharm. 2011;4:321–328. doi: 10.2174/1874471011104040321. [DOI] [PubMed] [Google Scholar]
- 8.Andersson H., Palm S., Lindegren S. Comparison of the therapeutic efficacy of 211At- and 131I-labelled monoclonal antibody MOv18 in nude mice with intraperitoneal growth of human ovarian cancer. Anticancer Res. 2001;21:409–412. [PubMed] [Google Scholar]
- 9.Kratochwil C., Bruchertseifer F., Giesel F.L. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 10.DeNardo G.L., DeNardo S.J., Macey D.J., Shen S., Kroger L.A. Overview of radiation myelotoxicity secondary to radioimmunotherapy using 131I-Lym-1 as a model. Cancer. 1994;73:1038–1048. doi: 10.1002/1097-0142(19940201)73:3+<1038::aid-cncr2820731343>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Kratochwil C., Bruchertseifer F., Rathke H. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J. Nucl. Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 12.Rahbar K., Ahmadzadehfar H., Kratochwil C. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 13.Watabe T., Kaneda-Nakashima K., Liu Y. Enhancement of (211)At uptake via the sodium iodide symporter by the addition of ascorbic acid in targeted alpha-therapy of thyroid cancer. J. Nucl. Med. 2019;60:1301–1307. doi: 10.2967/jnumed.118.222638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson M, Johansson L, Eckerman K, Mattsson S. IDAC-Dose 2.1, an internal dosimetry program for diagnostic nuclear medicine based on the ICRP adult reference voxel phantoms. EJNMMI Res. 2017;7:88. [DOI] [PMC free article] [PubMed]
- 15.Cobb L.M., Butler S.A., Harrison A. The effect of the alpha-particle emitter astatine-211 in the mouse at the minimum toxic dose. Hum Exp Toxicol. 1990;9:289–293. doi: 10.1177/096032719000900505. [DOI] [PubMed] [Google Scholar]
- 16.Howell G., Van Middlesworth L. Gastric iodide and chloride clearances in dogs. Proc. Soc. Exp. Biol. Med. 1956;93:602–605. doi: 10.3181/00379727-93-22835. [DOI] [PubMed] [Google Scholar]
- 17.Sfakianakis G., Sfakianaki E. The sodium-iodine symporter and the proton-pump inhibitors in - related to the side effects of- the treatment of thyroid cancer with iodine-131. Hell J Nucl Med. 2007;10:2–5. [PubMed] [Google Scholar]
- 18.Upadhyaya A, Meng Z, Wang P, et al. Effects of first radioiodine ablation on functions of salivary glands in patients with differentiated thyroid cancer. Medicine (Baltimore). 2017;96:e7164. [DOI] [PMC free article] [PubMed]
- 19.Andresen N.S., Buatti J.M., Tewfik H.H., Pagedar N.A., Anderson C.M., Watkins J.M. Radioiodine ablation following thyroidectomy for differentiated thyroid cancer: literature review of utility, dose, and toxicity. Eur Thyroid J. 2017;6:187–196. doi: 10.1159/000468927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fard-Esfahani A., Emami-Ardekani A., Fallahi B. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl. Med. Commun. 2014;35:808–817. doi: 10.1097/MNM.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton J.G., Asling C.W., Garrison W.M., Scott K.G. The accumulation, metabolism and biological effects of astatine in rats and monkeys. Univ Calif Publ Pharmacol. 1953;2:283–312. [PubMed] [Google Scholar]
- 22.Hamilton JG, Durbin, P.W., and Parrott, M.W. Comparison of acute and chronic changes produced in rats by iodine-131 and astatine-211 at lethal levels. Preliminary clinical data on the uptake of astatine in patients with thyroid disease. Animal physiology and pathology 2 1954:12.
- 23.Cobb L.M., Harrison A., Butler S.A. Toxicity of astatine-211 in the mouse. Hum. Toxicol. 1988;7:529–534. doi: 10.1177/096032718800700602. [DOI] [PubMed] [Google Scholar]
- 24.Pearce E.N. Update in lipid alterations in subclinical hypothyroidism. The Journal of Clinical Endocrinology & Metabolism. 2012;97:326–333. doi: 10.1210/jc.2011-2532. [DOI] [PubMed] [Google Scholar]
- 25.Gutch M., Rungta S., Kumar S., Agarwal A., Bhattacharya A., Razi S.M. Thyroid functions and serum lipid profile in metabolic syndrome. Biom. J. 2017;40:147–153. doi: 10.1016/j.bj.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampaolo G., Campanella N., Catozzo V., Ferretti M., Vichi G., Morosini P. Relationship between hypothyroidism and cholesterol out of the records of 1756 patients. Recenti Prog. Med. 2014;105:79–82. doi: 10.1701/1417.15701. [DOI] [PubMed] [Google Scholar]
- 27.Morris M.S., Bostom A.G., Jacques P.F., Selhub J., Rosenberg I.H. Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis. 2001;155:195–200. doi: 10.1016/s0021-9150(00)00537-2. [DOI] [PubMed] [Google Scholar]
- 28.Chertow B.S., Motto G.S., Shah J.H. A biochemical profile of abnormalities in hypothyroidism. Am. J. Clin. Pathol. 1974;61:785–788. doi: 10.1093/ajcp/61.6.785. [DOI] [PubMed] [Google Scholar]
- 29.Gildea E.F., Man E.B., Peters J.P. Serum lipoids and proteins in hypothyroidism. J. Clin. Invest. 1939;18:739–755. doi: 10.1172/JCI101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spetz J., Rudqvist N., Forssell-Aronsson E. Biodistribution and dosimetry of free 211At, 125I- and 131I- in rats. Cancer Biother. Radiopharm. 2013;28:657–664. doi: 10.1089/cbr.2013.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohshima Y., Sudo H., Watanabe S. Antitumor effects of radionuclide treatment using alpha-emitting meta-(211)At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:999–1010. doi: 10.1007/s00259-017-3919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudqvist N., Spetz J., Schuler E. Transcriptional response in mouse thyroid tissue after 211At administration: effects of absorbed dose. Initial Dose-Rate and Time after Administration. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langen B, Rudqvist N, Parris TZ, et al. Transcriptional response in normal mouse tissues after i.v. (211)At administration - response related to absorbed dose, dose rate, and time. EJNMMI Res. 2015;5:1. [DOI] [PMC free article] [PubMed]
- 34.Dorso L., Bigot-Corbel E., Abadie J. Long-term toxicity of 213Bi-labelled BSA in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson M.K., Shaller C., Garmestani K. Effective treatment of established human breast tumor xenografts in immunodeficient mice with a single dose of the alpha-emitting radioisotope astatine-211 conjugated to anti-HER2/neu diabodies. Clin. Cancer Res. 2008;14:875–882. doi: 10.1158/1078-0432.CCR-07-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]