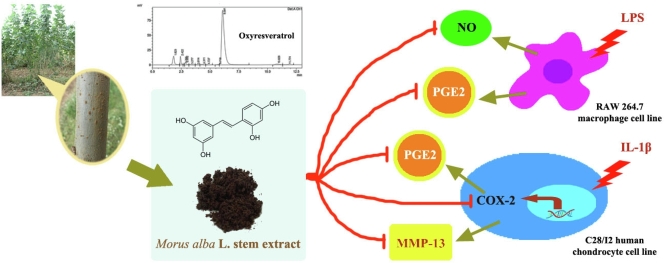
Abstract
This study aimed to investigate the effects of Morus alba stem extract (MSE) and oxyresveratrol on the suppression of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages and IL-1β-stimulated C28/I2 human chondrocyte cell line. The chondroprotective effect was also investigated using the chondrocyte cell line. First, MSE was prepared and analyzed for the amount of oxyresveratrol. The anti-inflammatory effects of MSE at various concentrations were evaluated through the inhibition of nitric oxide (NO), prostaglandin (PG)-E2 and cyclooxygenase (COX)-2 production. Oxyresveratrol at the equivalent amount found in the extract was investigated in the same manner. The chondroprotective effect was investigated through the suppression of MMP-13 production. The results showed that oxyresveratrol content in MSE was 15%. In RAW 264.7 cells, MSE (5–50 μg/mL) could inhibit the NO (24–30%) and PGE2 (11–82%) production. Oxyresveratrol at 0.75 and 7.5 μg/mL could suppress NO and also inhibited PGE2 but at only at high concentration. In the chondrocyte cell line, MSE at 5–100 μg/mL significantly decreased the PGE2 and COX-2 production by 44–93% and 17–65%, respectively. Again, oxyresveratrol at both concentrations could significantly inhibit PGE2 production by 50–92% but it inhibited COX-2 only at high concentration. In addition, MSE and oxyresveratrol was shown to significantly inhibit MMP-13 production by 14–57% and 16–56%, depending on their concentrations. The MSE demonstrates the potential to be used as an alternative treatment for reducing inflammation and preventing cartilage degradation. Its component, oxyresveratrol, may exert these effects to some extent.
Keywords: Mulberry stem, Oxyresveratrol, Chondrocytes, Chondroprotective, Inflammation
Graphical abstract
1. Introduction
Morus alba, known as mulberry, has been widely used in traditional medicine in Asia. In Korea and Japan, mulberry leaves are used as an antihyperglycemic food supplement for diabetic patients.1 In Chinese medicine, M. alba has long been used to treat fever, protect the liver, improve eyesight, facilitate discharge of urine, lower blood pressure and prevent cardiovascular disease.2,3 For arthritis used, decoction of M. alba twig (called Sang zhi) has been for the treatment of rheumatic and arthritic edema and pain.4 In Guangdong, M. alba twig and older stem have been used to benefit the joints.5
Osteoarthritis (OA) is a slowly progressive degenerative joint disease occurring worldwide, most frequently in the elderly. Inflammation plays an important role in the progression of OA.6 Chondrocytes, the cells found in articular cartilage, are known to proliferate and secrete the extracellular matrix components which consist mainly of collagens and proteoglycans in order to maintain articular cartilage. The balance between the synthesis and degradation of matrix components changes when the chondrocytes are activated by many factors including aging, gender, obesity and joint injury.7 Chondrocytes respond to mechanical injury and biochemical stresses by overexpressing inflammatory mediators such as IL-1β, tumor necrosis factor (TNF)-α, and matrix degrading enzymes including the matrix metalloproteinases (MMPs).8 IL-1β and TNF-α play important roles in the initiation and the development of OA by modifying extracellular matrix turnover, accelerating the degradation of cartilage and inducing chondrocyte apoptosis.9 In addition, both cytokines can induce chondrocytes to produce the inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) which continue to produce nitric oxide (NO) and prostaglandin (PG), especially PGE2.10 Moreover, IL-1β and TNF-α induce chondrocytes to express the MMPs which can degrade all components of the extracellular matrix causing the loss of tensile properties in cartilage. MMP-13 is a major enzyme responsible for the cartilage degradation. It is the predominant collagenase for cleaving type II collagen which is the major component in human articular cartilage and also degrading aggrecan.11
As state above, M. alba has been widely used in traditional medicine for the treatment of rheumatic and arthritic edema and pain. However, neither MSE nor its component (i.e. oxyresveratrol) has been investigated for their effects in human articular chondrocytes. Thus, this study was aimed to investigate the effects of MSE on anti-inflammatory responses through the suppression of NO and PGE2 in LPS-stimulated RAW 264.7 cells and the suppression of PGE2 and COX-2 in IL-1β-stimulated C28/I2 human chondrocyte cell line, a model for osteoarthritis. Oxyresveratrol which was found at the highest amount in the stem compared to twig and leaf12 was also investigated in the same manner. Chondroprotective effects of both compounds were also determined through the inhibition of MMP-13.
2. Materials and methods
2.1. Plant material
The stems of M. alba variety namely Burirum 60 were obtained from Queen Sirikit Sericulture Center (Tak, Thailand) and identified by Dr. Pranee Nangngam. A voucher specimen of the plant has been deposited at the Faculty of Science, Naresuan University (Voucher Specimen No. 004067).
2.2. Preparation of the MSE
The MSE extraction method was modified from Soonthornsit et al.13 Briefly, the bark of M. alba stems was removed. The inner wood was chopped and dried. Then it was macerated in 80% v/v ethanolic solution for 24 h at room temperature. The maceration process was repeated once more for a total of two cycles. After filtration, the filtrates were pooled, evaporated by a rotary evaporator (R-210, Buchi, Flawil, Switzerland) and continued drying on a water bath (M25, Lauda-Brinkmann, Lauda-Königshofen, Germany). The dried crude extract was stored in an airtight and light-protected container until used. The percentage yield was calculated using the following equation.
| % yield = (Weight of dried extract / Weight of chopped and dried stem wood) × 100 |
2.3. High performance liquid chromatography (HPLC) analysis of oxyresveratrol in MSE
Standard oxyresveratrol was purchased from Sigma-Aldrich (Saint Louis, Missouri, USA). The content of oxyresveratrol was analyzed by HPLC and the method modified from Yhirayha and Pitaksuteepong.14 The HPLC system consisted of a UV–Vis detector (SPD-10A, Shimadzu, Kyoto, Japan), an auto sampler (UFLC, Shimadzu, Kyoto, Japan), and a column oven (CTO-10 AS VP, Shimadzu, Kyoto, Japan). The HPLC analysis was performed using a C18 boned-silica gel reverse phase column (Gemini, 5 μm, 150 × 4.6 mm, Phenomenax, Torrance, USA). Mobile phase was composed of acetonitrile and 0.0125 M phosphate buffer (1:3 v/v, pH3). The flow rate of the mobile phase was 1 mL/min. The UV detector wavelength and the column temperature were set at 320 nm and 30 °C, respectively. The running time was set at 13 min.
2.4. Cell line and culture condition
The mouse macrophage (RAW 264.7) and immortalized C28/I2 human chondrocyte cell lines were obtained from the American Type Culture Collection (Manassas, Virginia, USA) and Merck Millipore (Temecula, California, USA) and respectively. The cells were cultured in Dulbecco's modified Eagles' medium (DMEM high glucose) supplemented with 10%v/v fetal bovine serum (FBS), 1% l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin and maintained at 37๐C in a humidified atmosphere of 5% CO2. At 80–90% confluence, they were passaged with 0.25% w/v trypsin-EDTA. All cell culture media and supplements listed above were obtained from Invitrogen (Eggenstein, Germany). The human chondrocyte culture protocol was approved by the Naresuan University Institutional Review Board (IRB No. 0669/60).
2.5. Sample preparation
M. alba stem crude extract was dissolved in pure DMSO (Sigma-Aldrich, Saint Louis, Missouri, USA) to make a stock solution at concentration of 100 mg/mL. The stock solution of oxyresveratrol (Sigma-Aldrich, Saint Louis, Missouri, USA) at concentration of 1 mg/mL was prepared by dissolving oxyresveratrol in 2% v/v DMSO solution. Glucosamine sulfate (Taizhou City Fengrun Biochemical Co., Ltd., Taizhou, China) or diclofenac sodium (Pharmaceutical grade, Suzhou Ausun Chemical Co., Ltd., Zhejiang, China) was used as the positive controls. They were dissolved in water to make stock solutions at concentration of 5 mg/mL. The stock solutions were further diluted with cell culture medium to the required concentrations and filtered through 0.2 μm sterile membrane (GE Healthcare UK Limited, Buckinghamshire, UK) before using in subsequent tests.
2.6. Cell viability assay
The effect of MSE and oxyresveratrol on the viability of RAW 264.7 cells and C28/I2 cells were determined by MTT assay. RAW 264.7 cells were seeded into a 96-well microplate at a density of 7 × 103 cells/well. After overnight incubation, they were treated with various concentrations of MSE or oxyresveratrol under serum-free condition for 24 h in a presence of LPS at 5 μg/mL. For the C28/I2 human chondrocyte cell line, the cells were seeded into a 96-well microplate at a density of 4 × 103 cells/well. They were cultured and treated using the same procedure as that performed in RAW 264.7 cells but LPS was replaced by IL-1β at 10 ng/mL. Then, the supernatant was discarded and 100 μL of 0.5 mg/mL MTT reagent (Thermo Scientific™, Eugene, Oregon, USA) was added into each well. The plate was further incubated for 4 h. After that, the supernatant was again discarded and then 100 μL of DMSO was added into each well to dissolve formazan crystals. After 10 min incubation, the absorbance was measured at 570 nm by using a microplate reader (Bio Tek Instruments Inc., Winooski, USA). The viability of untreated cells was defined as 100%. The percentage viability was calculated using the equation below. All experiments were performed in triplicate.
| % viability = ((Atreat – Abackground) / (Auntreat – Abackground)) x 100 |
Where Atreat is the absorbance of the treated wells which consist of the cells treated with a sample, culture medium and MTT reagent, Abackground is the absorbance of the background control wells which consist of culture medium and MTT reagent, and Auntreat is the absorbance of untreated wells which consist of the cells in culture medium and MTT reagent.
In RAW 264.7 cell line, diclofenac, a nonsteroidal anti-inflammatory drug which also widely used to treat pain, swelling and joint stiffness in OA patient, was used as a positive control. For human chondrocyte cell line, diclofenac and glucosamine sulfate, worldwide used dietary supplement for preventing the cartilage degradation, were used as positive controls.
2.7. Anti-inflammatory activity
2.7.1. Determination of NO and PGE2 production in raw 264.7 cells
RAW 264.7 cells were seeded into a 6-well microplate at a concentration of 5 × 105 cells/well. The cells were pretreated with MSE, oxyresveratrol, and diclofenac sodium and then they were stimulated with LPS 5 μg/mL. The cell culture supernatant was collected. The PGE2 production was measured by using Prostaglandin E2 Assay Kit (R&D Systems, Inc., Minneapolis, USA) according to the manufacturer's instructions. The PGE2 concentrations were calculated from a PGE2 standard curve (0–2500 pg/mL) that was created by using a four-parameter logistic (4-PL) curve fitting program in ELISA software version 3.2. The NO production was performed by determining the nitrite concentration using Griess assay. The nitrite concentration was calculated from a sodium nitrite standard curve (0–100 μM). The nitrite or PEG2 production of LPS-only-treated cells was regarded as 100%. The results were shown as the percentage of activated control which was calculated using the following equation.
| % of activated control = (Nitrite or PGE2 concentration when treated with a sample / Nitrite or PGE2 concentration in LPS-stimulated RAW 264.7 cells) × 100 |
2.7.2. Determination of PGE2 production in C28/I2 chondrocytes
The C28/I2 cells were seeded into a 6-well microplate at a density of 1 × 106 cells/well. After overnight incubation, the cells were pretreated with the MSE, oxyresveratrol, glucosamine sulfate, and diclofenac sodium for 2 h. After that, the cells were stimulated with 10 ng/mL IL-1β for 22 h. Then, the cell supernatants were collected and the PGE2 concentration was measured by using a PGE2 Assay Kit. The PEG2 production of IL-1β-only-treated cells was regarded as 100% and the results were shown as the percentage of activated control as described above.
2.7.3. Determination of COX-2 protein expression
The C28/I2 cells were seeded into a 6-well microplate at a density of 2 × 106 cells/well. After overnight incubation, the medium in each well was discarded and then the cells were pretreated with MSE, oxyresveratrol, glucosamine sulfate, and diclofenac sodium in DMEM without serum. After 2 h incubation period, the cells were stimulated by 10 ng/mL IL-1β for 22 h. The cells were then rinsed twice with 1 mL cold phosphate buffered saline (PBS). Next, the cells were lysed in a lysis buffer consisting of 10 mM Tris-HCl, pH 7.4 (EMD Chemicals Inc., Darmstadt, Germany), 100 mM NaCl (Ajax Finechem Pty Ltd, Auckland, New Zealand), 1 mM EDTA (Sigma-Aldrich, Saint Louis, Missouri, USA), 1% Triton X-100 (Amresco®, Massachusetts, USA), 0.1% Sodium dodecyl sulfate (SDS; Ajax Finechem Pty Ltd, Auckland, New Zealand) and 1% protease inhibitor cocktail (Sigma-Aldrich, Saint Louis, Missouri, USA). After 20 min incubation period on ice, the mixtures were centrifuged at 10,000 rpm, 4 ๐C for 10 min and then the supernatant was collected. Total protein concentrations were determined by using a BCA protein assay kit (Thermo Scientific™, Rockford, Illinois, USA) according to manufacturer's instructions. The cell lysates were mixed with tracking blue dye and then boiled at 90 ๐C for 5 min. After that, the 20 μg of total protein was loaded into each lane of 12% SDS-polyacrylamide gel (PAGE) inside electrophoresis chamber. Next, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% skimmed milk solution (HiMedia Laboratories Pvt. Ltd., Mumbai, India) at 4 ๐C overnight and then incubated with COX-2 mouse monoclonal antibody (Santa Cruz Biotechnology, Inc., CA, USA) at a dilution of 1:500 in 5% skimmed milk solution for 1 h at room temperature. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) mouse monoclonal antibody (Merck Millipore, Darmstadt, Germany) at a dilution of 1:1000 in 5% skimmed milk solution was used as a loading control for western blot. The membranes were washed 3 times with 0.05% Tween 20 in PBS and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Merck Millipore, Temecula, California) diluted 1:10,000 in 5% skimmed milk solution for 1 h at room temperature. After that, membranes were again washed 3 times and incubated with Luminata Forte Western HRP substrate (Merck Millipore, Darmstadt, Germany) for 30 s. Protein bands were performed on scanned immunoblot images with ChemiDoc™ XRS + using Image Lab™ Software version 6.0 (Bio-Rad Laboratories, Inc., California, USA).
2.8. Chondroprotective activity through the determination of MMP-13 production
The C28/I2 cells were seeded into a 6-well microplate at a density of 2 × 106 cells/well. After overnight incubation, the cells were pretreated with MSE, oxyresveratrol, glucosamine sulfate, and diclofenac sodium for 2 h and then stimulated with 10 ng/mL IL-1β for 22 h. Then, the cell supernatants were collected and the MMP-13 concentration was measured by using a Human MMP-13 ELISA Kit (Thermo Scientific™, Rockford, Illinois, USA) according to the manufacturer's instructions. Again, the MMP-13 concentrations were calculated from a MMP-13 standard curve (0–6000 pg/mL) that was created by using a four-parameter logistic (4-PL) curve fitting program in ELISA software version 3.2. The MMP-13 production of IL-1β-only-treated cells was regarded as 100% and the results were shown as the percentage of activated control as described above.
2.9. Statistical analysis
All data are presented as mean ± standard deviation (S.D.) of three experiments. Statistical comparisons were performed by one-way analysis of variance (ANOVA), followed by Tukey's test (SPSS Statistics version 16.0). The p value less than 0.05 was considered a significant difference between groups.
3. Results
3.1. Appearance, percentage yield and amount of oxyresveratrol
The MSE extract was dark brown color and the percentage yield was 4.08%. Oxyresveratrol, 2,3′,4,5′-tetrahydroxy-trans-stilbene is a bioactive compound found in nearly all parts of M. alba tree, especially in stem.12 In this study, the analysis of oxyresveratrol in MSE was carried out by HPLC and the result was shown to be 15.06 ± 0.36%.
3.2. Effect of MSE and oxyresveratrol on cell viability
The effect of MSE and oxyresveratrol on the viability of RAW 264.7 macrophage cells and C28/I2 human chondrocyte cell line were determined by using MTT assay. In this study, the concentrations of samples that presented the cell viability higher than 80% were considered non-cytotoxic and subjected to further studies.
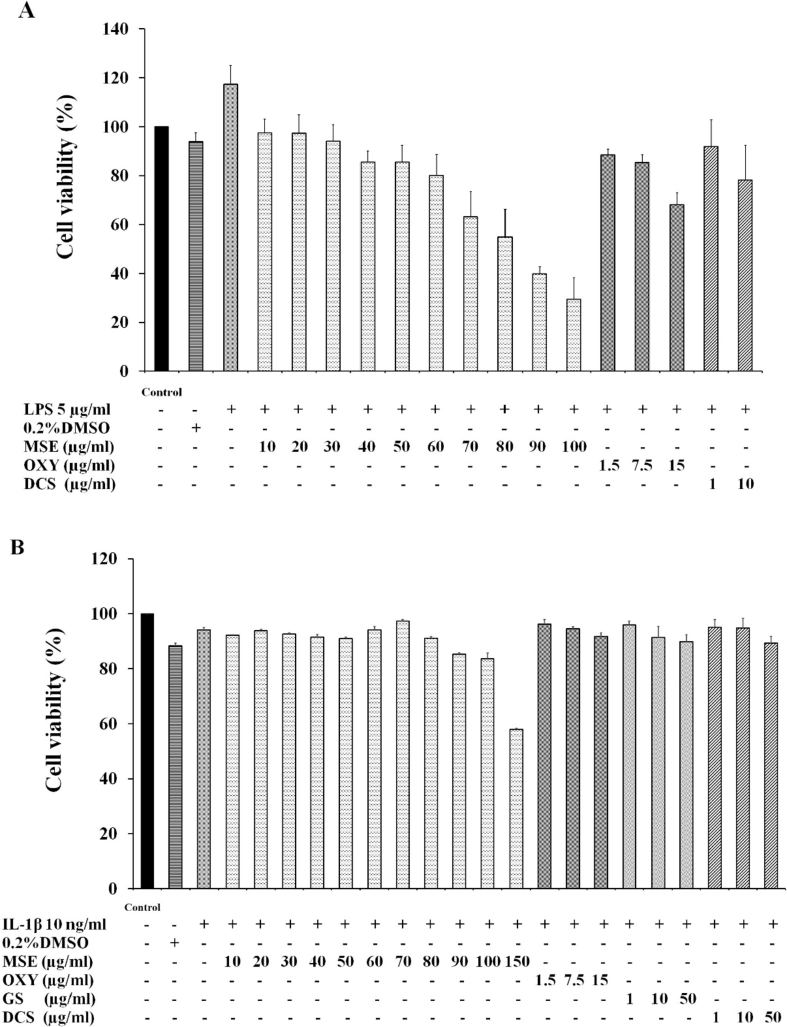
For the RAW 264.7 cells, it was observed that cell viability decreased with increasing concentration of MSE (Fig. 1A). MSE at concentrations ranging of 10–60 μg/mL showed the cell viability higher than 80%. Oxyresveratrol at the equivalent amount found in MSE 10, 50 and 100 μg/mL (i.e., 1.5, 7.5 and 15 μg/mL, respectively) was determined for cell viability. Its concentrations up to 7.5 μg/mL was considered non-cytotoxic. Diclofenac sodium, a positive control, at a concentration of 1 μg/mL showed the cell viability higher than 80%. As a result, MSE at concentration 5, 25 and 50 μg/mL as well as oxyresveratrol 0.75 and 7.5 μg/mL (the equivalent amount found in MSE 5 and 50 μg/mL, respectively) were chosen for further studies in the RAW 264.7 cells.
Fig. 1.
Effects of M. alba stem extract (MSE), oxyresveratrol (OXY), glucosamine sulfate (GS) and diclofenac sodium (DCS) on the viability of RAW 264.7 cell line (A) C28/I2 human chondrocyte cell line (B). The cells were incubated for 24 h and the cell viability was determined using MTT assay. The data are expressed as mean ± S.D. of three independent experiments.
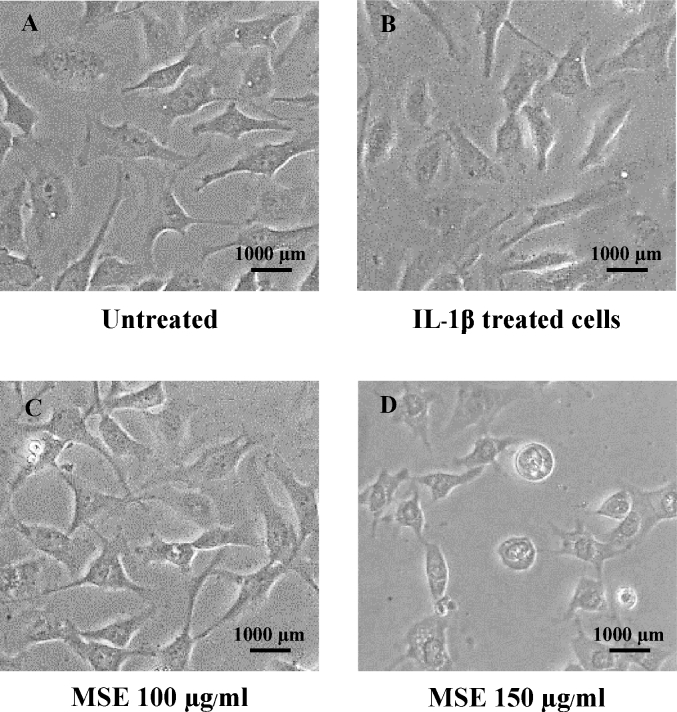
The effects of MSE and oxyresveratrol on the viability of C28/I2 chondrocyte cell line were shown in Fig. 1B. MSE at concentrations between 10 and 100 μg/mL yielded the cell viability higher than 80%. The results were in agreement with the cell morphology (Fig. 2). The morphology of cells which were treated with MSE at concentrations of 10–100 μg/mL were similar to that of the untreated cells and the cells treated with IL-1β (Fig. 2A–C). However, when the cells were incubated with MSE at 150 μg/mL, their morphology changed to round shape (Fig. 2, D). Oxyresveratrol at concentrations of 1.5, 7.5 and 15 μg/mL (equivalent amount found in MSE 10, 50 and 100 μg/mL, respectively) yielded percentage cell viability higher than 80%. In addition, the cell morphology did not change compared to untreated cells and the cells treated with IL-1β. Therefore, MSE at concentrations of 5, 25, 50 and 100 μg/mL as well as oxyresveratrol at concentrations of 0.75 and 15 μg/mL (the equivalent amount found in MSE 5 and 100 μg/mL) were chosen for further investigations.
Fig. 2.
Example photographs of the C28/I2 human chondrocyte cell line in the cell viability test when the cells were untreated (A) and treated with IL-1β 10 ng/mL (B), IL-1β 10 ng/mL + MSE 100 μg/mL (C), and IL-1β 10 ng/mL + MSE 150 μg/mL (D) for 24 h (magnification × 100).
Glucosamine was included as one of the positive controls in this study because it was widely consumed as a dietary supplement to prevent or slow down the degeneration of joint cartilage which caused OA pain. Diclofenac, a NSAIDs which most often used in OA, was also included as a positive control. Both glucosamine and diclofenac at concentrations between 1 and 50 μg/mL were considered non-cytotoxic (Fig. 1B) and their concentration of 10 μg/mL was selected for further studies.
3.3. Anti-inflammatory activity of MSE and oxyresveratrol
3.3.1. Suppression of NO production in raw 264.7 cells
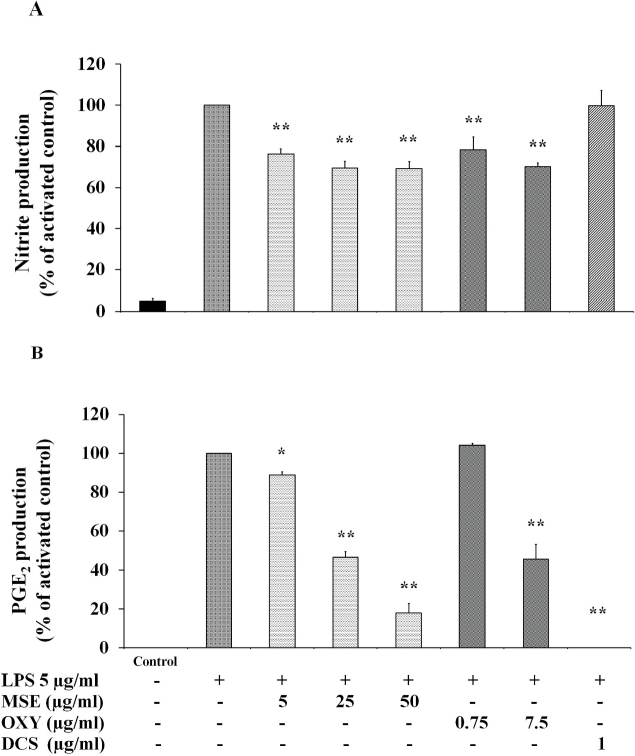
Fig. 3A shows the effect of MSE and oxyresveratrol on NO production in RAW 264.7 macrophages. The MSE at 5 μg/mL could significantly inhibited NO production compared to that produced in LPS-induced RAW 264.7 cells. However, an increased concentration of MSE to 25 and 50 μg/mL has a slight effect on the percentage inhibition of NO. The same trends were observed in oxyresveratrol at concentrations of 0.75 and 7.5 μg/mL.
Fig. 3.
Effects of M. alba stem extract (MSE) and oxyresveratrol (OXY) on NO (A) and PGE2 (B) production in LPS-induced RAW 264.7 cells. Diclofenac sodium (DCS) 1 μg/mL was used as a positive control. The cells were untreated (Bar 1), only stimulated with LPS 5 μg/mL (Bar 2) and pretreated with various concentrations of MSE and OXY for 2 h and then stimulated by 5 μg/mL LPS for 22 h. Levels of NO and PGE2 in cell culture supernatant were quantified by Griess assay and ELISA, respectively. The data are expressed as mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01 compared to the LPS-stimulated cells.
3.3.2. Suppression of PGE2 production in raw 264.7 cells
For the suppression of PGE2 production in LPS-induced RAW 264.7 cells, MSE in the concentration range of 5–50 μg/mL significantly inhibited the PGE2 production from 11 to 82% in a concentration-dependent manner (Fig. 3B). Oxyresveratrol could suppress PGE2 production but only at concentration of 7.5 μg/mL and percentage inhibition was about 55%. Diclofenac, a positive control, could completely inhibit PGE2 production (Fig. 3B).
3.3.3. Suppression of PGE2and COX-2 production in C28/I2 human chondrocyte cell line
The investigations on anti-inflammatory properties of MSE and oxyresveratrol were further performed through the inhibition of PGE2 production and COX-2 enzyme activity in a human chondrocyte cell line. COX-2 is an inducible enzyme which is overexpressed locally at the site of inflammation. COX-2 converts arachidonic acid into PGH2 which is a precursor for the biosynthesis of PGs, especially PGE2.15 PGE2 is the inflammatory mediator that induces pain and joint swelling.16
In this study, glucosamine sulfate and diclofenac were selected as positive controls. As state above, glucosamine sulfate is widely consumed as a dietary supplement in many countries including Thailand to prevent or slow down the degeneration of joint cartilage. It was reported to inhibit PGE2 production via the inhibition of COX-2, which was responsible for the synthesis of PGH2 from arachidonic and microsomal PGE synthase (mPGES)-1, which was responsible for the conversion of PGH2 to PGE2.17 Diclofenac is an intermediate COX-2 selectivity and is most often used in OA. It was reported to inhibit PGE2 production via the suppression of IL-1α and IL-1β.18,19
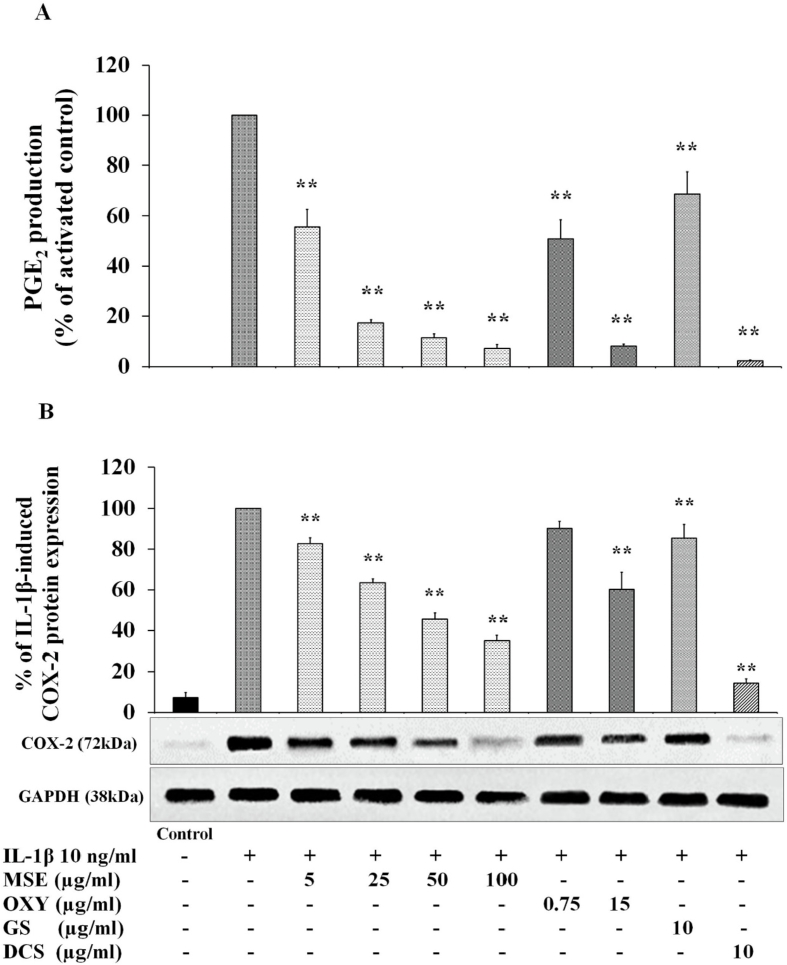
The PGE2 production in the C28/I2 chondrocyte cell line was determined by ELISA assay. MSE at 5 μg/mL significantly inhibited the PGE2 production by 44% compared to activated cells (i.e. IL-1β stimulated cells) (p < 0.01) (Fig. 4A). An increased concentration of MSE to 25 μg/mL, the PGE2 production was further suppressed by 83%. Interestingly, beyond this concentration, MSE could bring the PGE2 production down to the similar level as the untreated cells. However, the percentage inhibition of PGE2 by MSE at the concentrations beyond 25 μg/mL was not different.
Fig. 4.
Effects of M. alba stem extract (MSE), oxyresveratrol (OXY), glucosamine sulfate (GS) and diclofenac sodium (DCS) on IL-1β-induced PGE2 production (A) and COX-2 protein expression (B) in the C28/I2 human chondrocyte cell line. The cells were untreated (Bar 1), only stimulated with IL-1β 10 ng/mL (Bar 2) and pretreated with various concentrations of MSE and OXY as well as GS 10 μg/mL and DCS 10 μg/mL for 2 h and then stimulated by 10 ng/mL IL-1β for 22 h. The cell culture supernatant was collected and determined the PGE2 production by using ELISA kit. The concentration of PGE2 was normalized to the activated control. The expression of COX-2 (72 kDa) was determined by western blotting analysis and GAPDH protein (38 kDa) was used as a loading control. The data are expressed as mean ± S.D. of three independent experiments. **p < 0.01 compared to the IL-1β-stimulated cells.
Similar trend was observed in oxyresveratrol. Oxyresveratrol at 0.75 μg/mL could reduce PGE2 production by 50% while PGE2 production was almost completely inhibited at a concentration of 15 μg/mL. Both glucosamine sulfate and diclofenac (the positive controls), were shown to inhibit PGE2 production confirming the validity of this assay.
The effects of MSE on IL-1β-induced the expression levels of COX-2 protein were investigated by using western blotting analysis. It was found that MSE within the concentration range of 5–100 μg/mL significantly decreased the protein expression level of COX-2 enzyme in a concentration-dependent manner (Fig. 4B). MSE at 5 μg/mL significantly inhibited the COX-2 production by 17% compared to IL-1β activated cells (p < 0.01) (Fig. 4B). An increased concentration of MSE to 25 and 50 μg/mL, the COX-2 production was further suppressed by 37% and 54%. The inhibitory effect of MSE on COX-2 was seemed to be saturated at concentration of 50 μg/mL.
Oxyresveratrol also showed the similar but milder trend in the inhibition of COX-2 as MSE. Oxyresveratrol at 0.75 and 15 μg/mL significantly decreased the protein expression level of COX-2 enzyme by 10 and 40%, respectively. Again, both glucosamine and diclofenac (the positive controls), were shown to inhibit the COX-2 protein expression confirming the validity of this assay.
3.4. Chondroprotective activity of MSE and oxyresveratrol
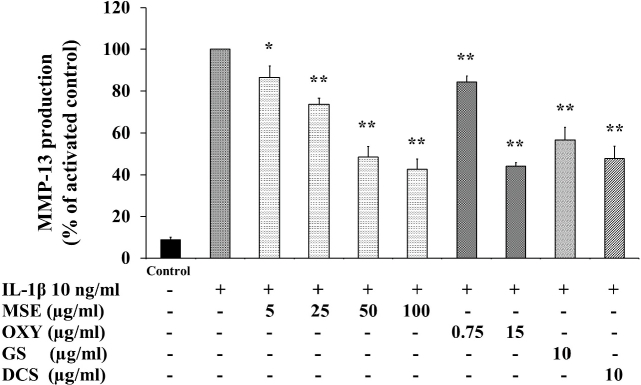
Chondroprotective activity of MSE and oxyresveratrol was investigated through the suppression of MMP-13 production. The inhibition of MMP-13 production by MSE was similar to that of COX-2. MSE at concentrations of 5–100 μg/mL significantly inhibited the production of MMP-13 in IL-1β-activated human chondrocyte cell line in a concentration-dependent manner (Fig. 5). MSE at 5 μg/mL significantly inhibited the MMP-13 production by 14% compared to IL-1β activated cells (p < 0.05) (Fig. 5). An increased concentration of MSE to 25 and 50 μg/mL, the MMP-13 production was further suppressed by 26% and 52%. Again, the inhibitory effect of MSE on MMP-13 was seemed to be saturated at concentration of 50 μg/mL.
Fig. 5.
Effects of M. alba stem extract (MSE), oxyresveratrol (OXY), glucosamine sulfate (GS) and diclofenac sodium (DCS) on IL-1β-induced MMP-13 production in the C28/I2 human chondrocyte cell line. The cells were untreated (Bar 1), only stimulated with IL-1β 10 ng/mL (Bar 2) and pretreated with MSE, OXY, GS and DCS for 2 h and then stimulated by IL-1β 10 ng/mL for 22 h. The cell culture supernatant was collected and determined by ELISA. The concentration of MMP-13 was normalized to the activated control. The data are expressed as mean ± S.D. of three independent experiments. *p < 0.05 and **p < 0.01 compared to the IL-1β-stimulated cells.
Oxyresveratrol at concentrations of 0.75 and 15 μg/mL (i.e. equivalent to the amount found in MSE 5 and 100 μg/mL) significantly inhibited the production of MMP-13 by 16% (p < 0.01) and 56% (p < 0.01) compared to IL-1β activated cells.
Glucosamine sulfate and diclofenac sodium were included as positive controls. Both compounds at 10 μg/mL significantly inhibited the MMP-13 production.
4. Discussion
OA is a non-inflammatory disease, however inflammation plays an important role in the progression of OA.6 Pro-inflammatory cytokines such as IL-1β and TNF-α activate chondrocytes to express iNOS and COX-2 which continue to produce NO and PGE2 leading to suppress matrix synthesis, activate chondrocyte apoptosis and induce joint swelling and pain.16,20
MSE and oxyresveratrol have been previously reported to have the anti-inflammatory activity through the inhibition of pro-inflammatory mediators and cytokines production and their transcription majorly involved in NF-kB pathway.21, 22, 23, 24, 25 NF-kB is a dimeric protein complex which is an inactive state by combining with the inhibitory kappa B (IkB) protein in cytoplasm of resting cells. When a variety of extracellular stimuli including IL-1β, TNF-α and LPS lead to activate IkB degradation, NF-kB dimers can translocate to the nucleus and then further induces gene expression such as iNOS and COX-2 resulting in NO and PGs production, especially PGE2.26 Therefore, in this study the anti-inflammatory activities of MSE and oxyresveratrol were evaluated through the inhibition of NO, PGE2 and COX-2.
In response to the activation of LPS and proinflammatory cytokines such as IL-1β and TNF-α, both macrophages and chondrocytes overproduce inflammatory mediators such as NO and PGE2 through the expression of iNOS and COX-2. These chemical mediators play a major role in progression of OA.10,27,28
In this study, NO and PGE2 production was investigated because they play a crucial role in inflammation. In addition, both mediators have been used in many studies to determine anti-inflammatory effects. In RAW 264.7 macrophage cell model, LPS, a component of the outer membrane of gram-negative bacteria, was used to stimulate macrophage to produce these inflammatory mediators. The results found that both MSE and oxyresveratrol could inhibit NO production in LPS-stimulated RAW 264.7 macrophages. This results correlate with our previous study showing that MSE and oxyresveratrol were able suppress iNOS mRNA and protein expression in RAW 264.7 macrophages.13 In addition, this study showed that both MSE (5–50 μg/mL) and oxyresveratrol (7.5 μg/mL) could inhibit PGE2 production in LPS-induced RAW 264.7 macrophages.
Further investigations on the anti-inflammatory activities of MSE and oxyresveratrol were performed in C28/I2 chondrocyte cell line through the suppression of PGE2 and COX-2. For chondrocyte cell line, IL-1β, one of major pro-inflammatory cytokines involved in the pathogenesis of OA, was used to stimulate inflammation process. In the present study, MSE and oxyresveratrol were clearly shown to inhibit the production of PGE2 via the inhibition of COX-2 protein expression, indicating the ability to relieve pain. This observations may explain the mechanism of anti-nociceptive effect of MSE in our previous study using the anterior cruciate ligament transection (ACLT)-induced rat model of OA.29
MSE seemed to be more potent to suppress PGE2 production and COX-2 protein expression better than its bioactive compound, oxyresveratrol in both RAW 264.7 cells and human chondrocytes. These results suggested that the other phenolic compounds found in MSE including stilbenoids (mulberroside A and resveratrol) and flavonoids (moracin M, dihydromorin and steppogenin)30 may contribute to the anti-inflammatory effects. Mulberroside A and resveratrol, belonging to stilbenoid have been reported to inhibit NO production through suppressing iNOS protein expression31 and to suppress COX-2 expression via inhibition of NF-kB pathway.32 Moreover, Moracin M and steppogenin, flavonoids, were reported to decrease the production of proinflammatory cytokines (i.e. IL-1β, TNF-α, and IL-6), to suppress the production of NO, PGE2 and COX-2.33,34 In addition, Shen et al.9 reported that dietary polyphenols including stilbenoids and flavonoids have the potential effects on progression of OA. Anti-inflammatory activity of dietary polyphenols is reported that it is one of possible mechanisms to manage OA.9
In our parallel study, the contents of total phenolic and total flavonoid compounds in MSE were determined by Folin-Ciocalteu reagent and aluminium chloride colorimetric method. It was found that the total phenolic and total flavonoid contents in the extract were 203.35 ± 7.10 μg GA/mg extract and 11.63 ± 1.64 μg QUE/mg extract, respectively.35
Apart from anti-inflammatory activities, the present study also investigated chondroprotective activity of MSE and oxyresveratrol through the inhibition of MMP-13 production. MMP-13 (also known as collagenase-3) is the distinctive collagenase expressed in chondrocytes and it is upregulated in inflammation condition.36 MMP-13 plays an important role in the early stages of OA. It specially cleaves type II collagen, a major component of extracellular matrix in human articular cartilage, than other collagenases.11 Therefore, MMP-13 was a degenerative enzyme used as an indicator to evaluate cartilage degradation in this study.
The present study is the first to demonstrate the inhibitory effect of MSE and oxyresveratrol on the production of MMP-13 in human chondrocytes. Interestingly, the inhibitory activity of MSE and oxyresveratrol (at the equivalent amount found in MSE) on MMP-13 production did not significantly different. These results suggest that oxyresveratrol is the major compound giving contribution to this effect. The mechanism of oxyresveratrol to inhibit MMP-13 does not known. However, resveratrol, a hydroxyl-substituted stilbene compound similar chemical structure to oxyresveratrol has been reported to have cartilage protective effect in chondrocytes through the suppress MMP-13 expression via NF-kB, JNK and AP-1 signaling pathways.37 Oxyresveratrol was previously reported to suppress NF-kB and MAPK signaling pathways by inhibiting phosphorylation of IkB-α, NF-kB translocation to nucleus and phosphorylation of JNK and p38.25 These pathways may be possible mechanisms link to the MMP-13 inhibition of MSE and oxyresveratrol.
5. Conclusions
This study was clearly demonstrated the anti-inflammatory activities of MSE and a component of MSE, oxyresveratrol, by suppressing NO and PGE2 production in LPS-induced RAW 264.7 macrophage cells. In addition, their anti-inflammatory activities were confirmed by the inhibition of PGE2 production and COX-2 protein expression in IL-1β induced C28/I2 human chondrocyte cell line. Moreover, it was clearly shown that MSE and oxyresveratrol possessed the chondroprotective effect by inhibiting the MMP-13 production. Therefore, we suggest that MSE and oxyresveratrol show significant potential as an alternative treatment for reducing inflammation and cartilage degradation in OA patients.
Author contributions
Assoc. Prof. Tasana Pitaksuteepong was the Principal Investigator of the project, supervised Miss. Thidarat Wongwat and Miss Kanyarat Srihaphon, critically reviewed and edited the manuscript. Miss Wongwat was the graduate student who conducted the experiments, analyzed data and prepared the manuscript in consultation with Assoc. Prof. Pitaksuteepong. Miss Srihaphon was the graduate student who worked together with Miss Wongwat for the preparation of the plant extract. Dr. Chetsadaporn Pitaksutheepong helped in the Western blot analysis. Dr. Worawan Boonyo helped Miss Wongwat to carried some part of experiments. All authors discussed the results and commented on the manuscript.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgements
We would like to acknowledge Naresuan University, Thailand for the financial support of this study (the annual revenue, grant number R2561B003) and for the Graduate Student Scholarship for Miss Thidarat Wongwat and Miss Kanyarat Srihaphon. Many thanks are due to the Queen Sirikit Sericulture Center (Tak, Thailand) for supplying the plant materials, to the Center of Excellence for Innovation in Chemistry (PERCH-CIC) for facility supports and to Mr. Paul Freund of the Naresuan University Language Center for language editing.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Thidarat Wongwat, Email: thidaratw58@nu.ac.th.
Kanyarat Srihaphon, Email: i.cher6058@hotmail.com.
Chetsadaporn Pitaksutheepong, Email: chetsadaporn@biotec.or.th.
Worawan Boonyo, Email: worawan@scphpl.ac.th.
Tasana Pitaksuteepong, Email: tasanap@nu.ac.th.
References
- 1.Katsube T., Imawaka N., Kawano Y., Yamazaki Y., Shiwaku K., Yamane Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006;97(1):25–31. [Google Scholar]
- 2.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 3.Huang H.P., Ou T.T., Wang C.J. Mulberry (桑葚子 Sang Shèn Zǐ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J Tradit Complement Med. 2013;3(1):7–15. doi: 10.4103/2225-4110.106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P., Zheng F., Zhang Y., Gao F., Chen Y., Shi G. Ethnobotanical study of medicinal plants on arthritis used by Chaoshan in Guangdong, China. Med Chem. 2016;6(12):715–723. [Google Scholar]
- 5.Bensky D., Gamble A. revised ed. Eastland Press; Washington: 1993. Chinese Herbal Medicine: Materia Medica. [Google Scholar]
- 6.Goldring M.B., Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldring M.B., Goldring S.R. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 8.Glyn-Jones S., Palmer A.J., Agricola R. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 9.Shen C.L., Smith B.J., Lo D.F. Dietary polyphenols and mechanisms of osteoarthritis. J Nutr Biochem. 2012;23(11):1367–1377. doi: 10.1016/j.jnutbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes J.C., Pelletier J.M., Pelletier J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1-2):237–246. [PubMed] [Google Scholar]
- 11.Burrage P.S., Mix K.S., Brinckerhoff C.E. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 12.Thongsuk P. Naresuan University; Phitsanulok, Thailand: 2007. In-vitro and Clinical Study of M. Alba Extract for Skin Whitening Product [master's Thesis] [Google Scholar]
- 13.Soonthornsit N., Pitaksutheepong C., Hemstapat W., Utaisincharoen P., Pitaksuteepong T. vol. 2017. 2017. In vitro anti-inflammatory activity of Morus alba L. stem extract in LPS-stimulated RAW 264.7 cells. (Evid Based Complement Alternat Med). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yhiraya C., Pitaksuteepong T. Mulberry stem (Morus alba L.) extract in lyotropic liquid crystal of tridecyl salicylate/PGE-7 glyceryl cocoate/water system as a skin whitening product. PACCON. 2012:301–305. 2012. [Google Scholar]
- 15.Kargman S., Charleson S., Cartwright M. Characterization of prostaglandin G/H synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996;111(2):445–454. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- 16.Abramson S.B. The role of COX-2 produced by cartilage in arthritis. Osteoarthritis Cartilage. 1999;7(4):380–381. doi: 10.1053/joca.1998.0217. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor M., Mineau F., Fahmi H., Pelletier J.P., Martel-Pelletier J. Glucosamine sulfate reduces prostaglandin E2 production in osteoarthritic chondrocytes through inhibition of microsomal PGE synthase-1. J Rheumatol. 2012;39(3):635–644. doi: 10.3899/jrheum.110621. [DOI] [PubMed] [Google Scholar]
- 18.O´Neill L.A., Lewis G.P. Inhibitory effects of diclofenac and indomethacin on interleukin-1-induced changes in PGE2 release. A novel effect on free arachidonic acid levels in human synovial cells. Biochem Pharmacol. 1989;38(21):3707–3711. doi: 10.1016/0006-2952(89)90576-5. [DOI] [PubMed] [Google Scholar]
- 19.Al-Kofahi M., Becker F., Gavins F.N. IL-1β reduces tonic contraction of mesenteric lymphatic muscle cells, with the involvement of cyclooxygenase-2 and prostaglandin E2. Br J Pharmacol. 2015;172(16):4038–4051. doi: 10.1111/bph.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koopman W.J., Moreland L.W. fifteenth ed. Lippincott Williams & Wilkins; Philadelphia: 2005. Arthritis and Allied Conditions: A Textbook of Rheumatology. [Google Scholar]
- 21.Chung K.O., Kim B.Y., Lee M.H. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003;55(12):1695–1700. doi: 10.1211/0022357022313. [DOI] [PubMed] [Google Scholar]
- 22.Shibata Y., Kume N., Arai H. Mulberry leaf aqueous fractions inhibit TNF- α-induced nuclear factor kB (NF-kB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193(1):20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Park E., Lee S., Lee J., Kim J. Anti-inflammatory activity of mulberry leaf extract through inhibition of NF-kB. J Funct Foods. 2013;5(1):178–186. [Google Scholar]
- 24.Eo H.J., Park J.H., Park G.H. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement Altern Med. 2014;14:200. doi: 10.1186/1472-6882-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H.S., Kim D.H., Hong J.E., Lee J.Y., Kim E.J. Oxyresveratrol suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages. Hum Exp Toxicol. 2015;34(8):808–818. doi: 10.1177/0960327114559989. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio F., Manning A.M. Multiple signals converging on NF-kB. Curr Opin Cell Biol. 1999;11(2):226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 27.Lin W.W., Chen B.C., Hsu Y.W., Lee C.M., Shyue S.K. Modulation of inducible nitric oxide synthase induction by prostaglandin E2 in macrophages: distinct susceptibility in murine J774 and RAW 264.7 macrophages. Prostag Other Lipid Mediat. 1999;58(2-4):87–101. doi: 10.1016/s0090-6980(99)00023-4. [DOI] [PubMed] [Google Scholar]
- 28.Pan M.H., Chiou Y.S., Tsai M.L., Ho C.T. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011;1(1):8–24. doi: 10.1016/s2225-4110(16)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khunakornvichaya A., Lekmeechai S., Pham P.P. Morus alba L. stem extract attenuates pain and articular cartilage damage in the anterior cruciate ligament transection-Induced rat model of osteoarthritis. Pharmacology. 2016;98(5-6):209–216. doi: 10.1159/000447973. [DOI] [PubMed] [Google Scholar]
- 30.Rivière C., Krisa S., Péchamat L. Polyphenols from the stems of Morus alba and their inhibitory activity against nitric oxide production by lipopolysaccharide-activated microglia. Fitoterapia. 2014;97:253–260. doi: 10.1016/j.fitote.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Shi L. Anti-inflammatory and analgesic properties of cis-mulberroside A from Ramulus mori. Fitoterapia. 2010;81(3):214–218. doi: 10.1016/j.fitote.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Shakibaei M., Csaki C., Nebrich S., Mobasheri A. Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008;76(11):1426–1439. doi: 10.1016/j.bcp.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.H., Ko H.J., Woo E.R. Moracin M inhibits airway inflammation by interrupting the JNK/C-Jun and NF-kB pathways in vitro and vivo. Eur J Pharmacol. 2016;783:64–72. doi: 10.1016/j.ejphar.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 34.Kim D.C., Quang T.H., Oh H., Kim Y.C. Steppogenin isolated from Cudrania tricuspidata shows antineuroinflammatory effects via NF-kB and MAPK pathways in LPS-stimulated BV2 and primary rat microglial cells. Molecules. 2017;22(12) doi: 10.3390/molecules22122130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srihapon K. Naresuan University; Phitsanulok, Thailand: 2018. The Development of Nanoparticulate Delivery Systems of Mulberry Stem Extract for Inflammatory Acne [master's Thesis] [Google Scholar]
- 36.Reboul P., Pelletier J.P., Tardif G., Cloutier J.M., Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. J Clin Investig. 1996;97(9):2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F.C., Hung L.F., Wu W.L. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res Ther. 2010;12(5):R167. doi: 10.1186/ar3127. [DOI] [PMC free article] [PubMed] [Google Scholar]