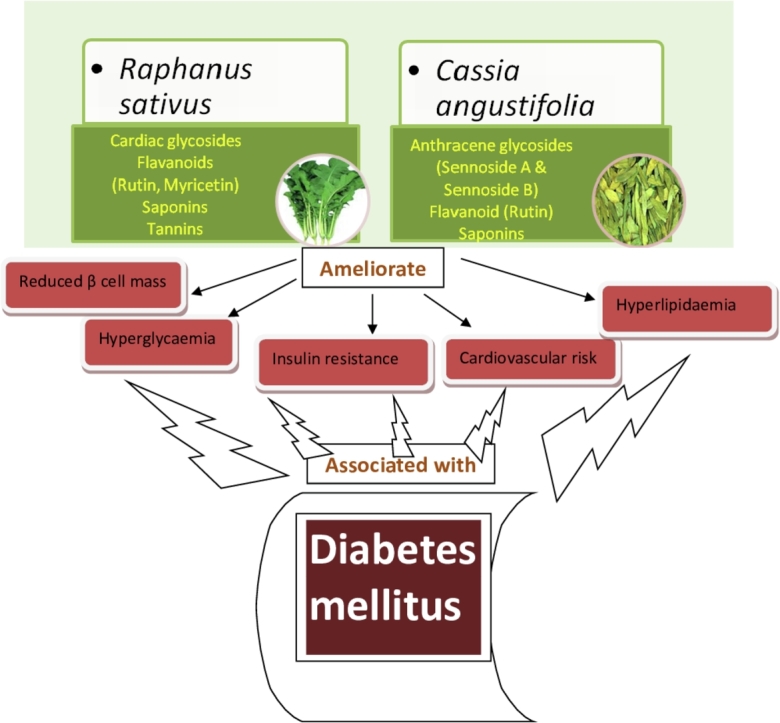
Graphical abstract
Keywords: Diabetes, High fat diet, Streptozotocin, Metformin, Herbal medicine
Abbreviations: AC, Atherogenic coefficient; AIP, Atherogenic index of plasma; ANOVA, analysis of variance; CA, Cassia angustifolia Vahl.; CRI-I, Castelli risk index I; CRI-II, Castelli risk index II; CMC, carboxymethylcellulose; GTT, Glucose tolerance test; HDL, High density lipoprotein; HFD, High fat diet; HOMA-IR, Homeostasis model assessment-estimated insulin resistance; HPLC, high performance liquid chromatography; ITT, Insulin tolerance test; LDL, Low density lipoprotein; MET, metformin; NPD, Normal pellet diet; RS, Raphanus sativus Linn.; SEM, standard error of mean; STZ, streptozotocin; TC, Total cholesterol; TG, Triglyceride; VLDL, Very low density lipoprotein
Highlights
-
•
Senna and radish leaf extracts reduced hyperglycemia in diabetic animals.
-
•
Treatment with study extracts improved lipid profile and decreased atherogenicity.
-
•
Treatment reduced insulin resistance and ameliorated pancreatic histology.
-
•
Antidiabetic action attributed to synergistic effect of phytoconstituents.
1. Introduction
Metabolic disorders are a worldwide problem as declared by WHO. Metabolic disorders especially diabetes and obesity are of growing concern due to associated adverse impact on quality of life.1,2 All over the world, one of the leading causes of morbidity as well as mortality is diabetes mellitus. The number of people affected with diabetes has risen worldwide from 108 million in 1980 to 422 million in 2014. In 2015, diabetes caused an estimated 1.6 million deaths globally. WHO has declared that diabetes will be the seventh leading cause of death in 2030.3 Rising prevalence of type-2 diabetes and adverse effects associated with currently available synthetic anti-diabetic drugs are also an important point of concern.4
Traditionally used herbal medicines are getting significantly increased attention globally. There is also increase in public funding for international traditional herbal medicine research. WHO also promotes safe and effective use of herbal medicines. Since many years herbal medicines are reliable, satisfactory and preferable treatment option among people world-wide. Market value of herbal medicines is increasing day by day and due to same reason there is increase in number of investors in herbal medicine production and research.5,6
Many studies have done in past in search of new herbal drugs to treat diabetes, still there is quest for a better and effective anti-diabetic herbal medicine. Leaves of senna (番泻叶 fān xiè yè, Cassia angustifolia Vahl.) and leaves of radish (菜头 cài tóu, Raphanus sativus Linn.) are reported to possess antioxidant,7,8 antihyperlipidemic9, antihyperglycemic10,11 and α-glucosidase inhibitory activity,12 thus both plants possess ability to ameliorate diabetes and associated consequences. The lack of sufficient research on the effectiveness of Raphanus sativus (RS) and Cassia angustifolia (CA) leaf extracts as treatment option in diabetes indicates need for evaluation of anti-diabetic potential of these herbs, so the current study is planned to evaluate anti-diabetic activity of RS and CA leaf extracts in high fat diet and low dose streptozotocin-induced diabetes mellitus.
2. Materials and methods
2.1. Experimental animals and ethical approval
Female Sprague-dawley rats were used for the study. Standard housing conditions (well ventilated, temperature 22 ± 2 °C, relative humidity 50–60% and 12 h day and night cycle) were maintained. Food and water was provided ad libitum. Study was conducted in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, Govt. of India. Animal study protocol was approved by IAEC committee, CPCSEA, Govt. of India (PhD/13-15/01, BIP/IAEC/2014/16).
2.2. Chemicals and kits
Glucophage@® tablets (Sun Pharma Advanced Research Centre, Vadodara, India) and Streptozotocin (Sisco Research Laboratories Pvt. Ltd., Mumbai, India) were purchased for the study. Kits for SGPT, SGOT, total cholesterol, triglyceride, HDL and creatinine were purchased from Labcare Diagnostics Pvt. Ltd. Valsad, India. Insulin kit was purchased from GenXbio, Delhi, India.
2.3. Plant materials and extraction
RS leaves were procured by field collection and authenticated at K.N.K. College of horticulture, Mandsaur, M.P., India. RS leaves were dried, powdered and hydroalcoholic (70%) extract was prepared by cold maceration method. CA leaves aqueous extract was obtained as a gift sample from Vasu Research Centre, Vadodara, Gujarat, India.
2.4. Phytochemical analysis
Qualitative phytochemical evaluation was done as per the standard phytochemical tests.13, 14, 15 Quantitative analysis was done, by Gravimetric analysis16,17 and HPLC method. In HPLC method, quantification of sennosides was done by using a modular HPLC (Shimadzu Corporation, Kyoto, Japan), LC system having RP-18 column (100 mm × 4.6 mm, 3 μm) using the gradient solvent system consisting of methanol and 1.25% acetic acid in water at a flow rate of 1.0 ml/min at 40 °C, at λ max 270 nm for sennosides. Mobile phase used for the separation of flavonoids was acetonitrile (45%) and 0.1% formic acid in water (55%) at a flow rate of 1.0 ml/min at 40 °C, at λ max 370 nm.
2.5. Dose optimization study
In this study, the animals were divided into XI groups (n = 6) i.e. Group I-vehicle control [1% carboxymethylcellulose (CMC), p.o.], Group II-glucose control (glucose, 3 g/kg, p.o.), Group III (RS2), IV (RS4), V (RS8), VI (RS16) treated with 200, 400, 800 and 1600 mg/kg, p.o. of RS leaf extract respectively, VII (CA2), VIII (CA4), IX (CA8), X (CA16) treated with 200, 400, 800 and 1600 mg/kg, p.o. of CA leaf extract respectively and Group XI was treated with MET (metformin, 100 mg/kg, p.o.).
Dose optimization study was done by using glucose tolerance test (GTT) model. After 18 h of food deprivation, basal blood glucose was measured for randomization. After randomization, respective treatment was given to all the animals and glucose levels were measured 1 h after treatment. Thereafter, oral glucose load was given to all the groups except group I. Blood glucose levels were measured by using Accu-Chek active glucometer strips at 0, 30, 60, 90 and 120 min after the oral glucose load.18
2.6. Streptozotocin and high fat diet-induced diabetes
Modification was done in the method reported previously by Srinivasan et al.19 for the induction of type II diabetes, i.e. high fat diet (Table 1) was given for 16 weeks before the induction of diabetes in place of two weeks high fat diet. After 16 weeks of dietary manipulation, rats were injected with streptozotocin (STZ) at low dose of 35 mg/kg, i.p.. Seven days after STZ injection, rats with the non-fasting glucose level of ≥200 mg/dL were considered diabetic. Animals were divided into VIII groups:
Table 1.
Composition of high fat diet.
| Normal Pellet Diet (NPD), 9.67% | High Fat Diet (HFD), 58.4% |
|---|---|
| Carbohydrate: 63.22% | Hydrogenated vegetable oil: 34% |
| Crude protein: 22.12% | Casein: 5% |
| Crude oil: 4.06% | Powder of normal pellets: 44.4% |
| Crude fiber: 3.76% | Sucrose: 14% |
| Ash: 5.64% | Choline bitartrate: 0.6% |
| Sand silica: 1.2%. | Vitamin mix: 1% |
| Mineral mix: 1% |
Group I: NPD (normal pellet diet, 1% CMC, p.o.) having NPD fed animals without STZ treatment.
Group II: NPD + STZ (normal pellet diet, 1% CMC, p.o.) having NPD fed animals with STZ treatment.
HFD fed STZ treated animals were included in following groups:
Group III: HFD + STZ (high fat diet, 1% CMC, p.o.)
Group IV: RS4 (high-fat diet and RS extract, 400 mg/kg, p.o.)
Group V: RS8 (high-fat diet and RS extract, 800 mg/kg, p.o.)
Group VI: CA4 (high-fat diet and CA extract, 400 mg/kg, p.o.)
Group VII: CA8 (high-fat diet and CA extract, 800 mg/kg, p.o.)
Group VIII: MET (high fat diet and metformin, 100 mg/kg, p.o.)
All the animals, normal as well as diabetic, were given respective diet and treatment for three weeks. Blood glucose level was checked before and after the study. Food intake was measured daily. Body weight was measured weekly. Other parameters studied at the end of the study were nutritional parameters (energy intake, feed efficiency ratio), lipid profile [total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), very low density lipoprotein (VLDL)], creatinine, glucose, insulin, atherogenic indices [atherogenic coefficient (AC), Castelli's risk index-I (CRI-I), Castelli's risk index-II (CRI-II)], HOMA-IR, organ weight (kidney, spleen, heart) and histopathological study (pancreas, kidney).20, 21, 22, 23, 24, 25, 26, 27
2.7. Statistical analysis
Data were analyzed using analysis of variance (ANOVA) followed by Bonferroni multiple comparison test and p value < 0.05 was considered statistically significant. Data presented as mean ± standard error of mean (SEM). Graph Pad Prism 5 software was used for statistical analysis.
3. Results
3.1. Phytochemical analysis
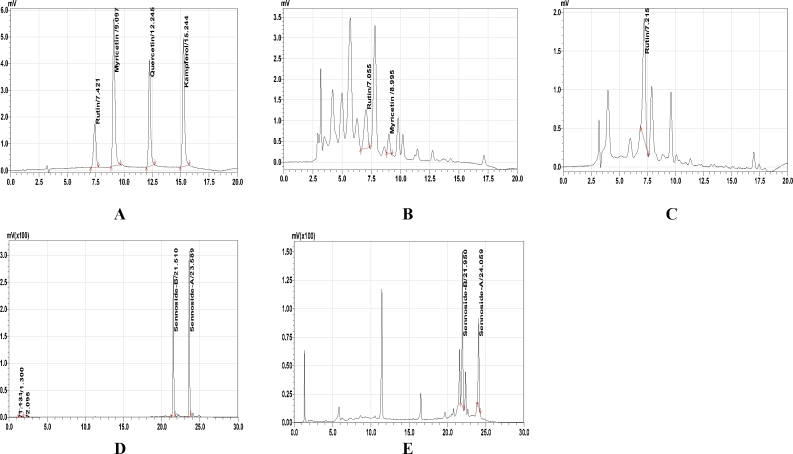
Phytochemical study showed presence of flavonoids, saponins and glycosides in both the extracts. Tannins were also present in RS extract. Gravimetric analysis for both the extracts indicated presence of flavonoids: 5.2%, cardiac glycosides: <2%, saponins: 24.52% & tannins: 2.91% in RS extract and Glycoside: 2.62%, flavonoids: 4.62%, saponins: <2% in CA extract. Further, HPLC method, indicated presence of rutin: 0.12%, myricetin: 0.03% in RS extract (Fig. 1B) and Sennoside A: 0.73%, Sennoside B: 1.55%, and rutin: 0.15% in CA extract (Fig. 1C and E).
Fig. 1.
High-performance liquid chromatography (HPLC) spectrum of RS and CA extracts
A: Standard HPLC chromatogram of flavonoids B. HPLC chromatogram of hydroalcoholic extract of RS leaves, C: HPLC chromatogram of aqueous extract of CA leaves, D: Standard HPLC chromatogram of sennosides, E: HPLC chromatogram of aqueous extract of CA leaves
Mobile system was consisting of methanol and 1.25% acetic acid in water at a flow rate of 1.0 ml/min at 40 °C, at λ max 270 nm for sennosides; Mobile system was consisting of acetonitril (45%) and 0.1% formic acid in water (55%) at a flow rate of 1.0 ml/min at 40 °C, at λ max 370 nm for the separation of flavonoids.
3.2. Dose optimization study
Decrease in glucose excursion (%) of each plant extract at each dose was calculated. Results indicated that RS produced decrease in glucose excursion at the dose of 400 mg/kg, 800 mg/kg and 1600 mg/kg as compared to glucose control. Dose of 1600 mg/kg showed similar outcomes as with dose of 800 mg/kg. No significant benefit observed by increase in dose more than 800 mg/kg, therefore two doses of RS i.e. 400 mg/kg and 800 mg/kg were selected for further study. Results also indicated that CA produced decrease in glucose excursion with the dose of 400 mg/kg and 800 mg/kg as compared to glucose control, while undesirable decrease in glucose excursion was observed at the dose of 1600 mg/kg. Therefore, two doses of both the extracts i.e. 400 and 800 mg/kg were selected for further study in chronic model of diabetes (Table 2).
Table 2.
Decrease in glucose excursion (%) of each plant extract at each dose.
| Group | % Decrease in glucose excursion |
|||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |
| Glucose + RS2 (200 mg/kg, p.o.) | 18.79 | −45.01 | −46.88 | −69.23 |
| Glucose + RS4 (400 mg/kg, p.o.) | 23.66 | 20.34 | 18.75 | 17.08 |
| Glucose + RS8 (800 mg/kg, p.o.) | 37.20 | 38.08 | 51.03 | 49.54 |
| Glucose + RS16 (1600 mg/kg, p.o.) | 38.35 | 35.51 | 57.28 | 52.97 |
| Glucose + CA2 (200 mg/kg, p.o.) | −15.05 | −39.38 | −49.47 | −76.92 |
| Glucose + CA4 (400 mg/kg, p.o.) | 23.30 | 11.69 | 16.13 | 6.82 |
| Glucose + CA8 (800 mg/kg, p.o.) | 25.20 | 22.52 | 40.63 | 70.97 |
| Glucose + CA16 (1600 mg/kg, p.o.) | 30.81 | 38.52 | 82.28 | 132.46 |
| MET (100 mg/kg, p.o.) | 49.63 | 50.21 | 65.09 | 90.62 |
3.3. Streptozotocin and high fat diet-induced diabetes
3.3.1. Effect of RS and CA extracts on body weight and food intake
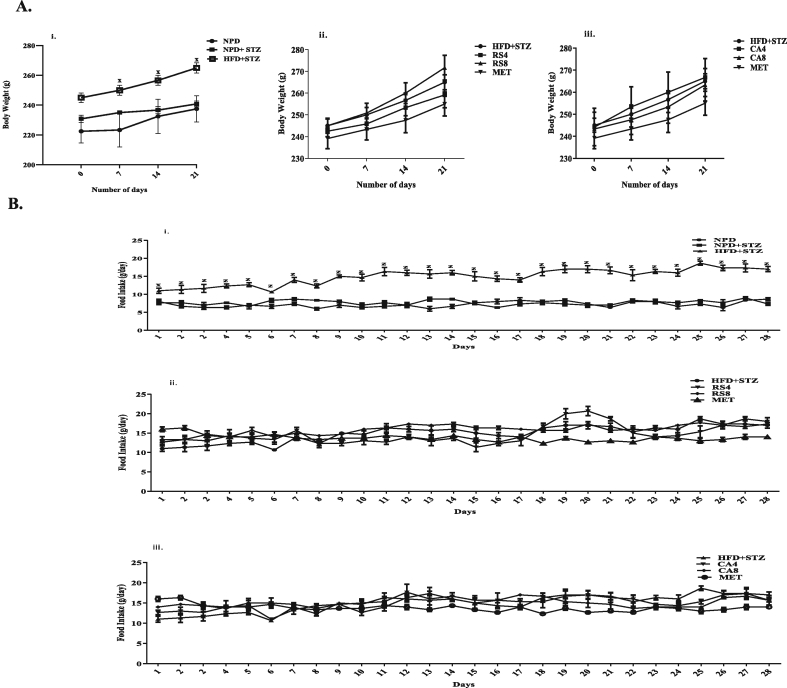
Significant increase in body weight and food intake was observed in the HFD + STZ group as compared to the NPD group (Fig. 2Ai and Bi). Significant decrease in food intake was observed in MET group as compared to the HFD + STZ group. No change was observed in the NPD + STZ group and extract treated groups (Fig. 2Aii, Aiii, Bii and Biii).
Fig. 2.
Effect of RS and CA extracts on the weekly body weight (g) and daily food intake (g).
Values are expressed as Mean ± SEM (n = 6), Data analyzed by two way ANOVA followed by Bonferroni Test.
A. i: Body weight of NPD, NPD + STZ and HFD + STZ groups, ii: Body weight of HFD + STZ, RS4, RS8 and MET groups, iii: Body weight of HFD + STZ, CA4, CA8 and MET groups.
B. i: Food intake of NPD, NPD + STZ and HFD + STZ groups, ii: Food intake of HFD + STZ, RS4, RS8 and MET groups, iii: Food intake of HFD + STZ, CA4, CA8 and MET groups.
x denotes P < 0.05 & z denotes P < 0.001 for HFD + STZ as compared with NPD.
3.3.2. Effect of RS and CA extracts on nutritional parameters
Significant increase in energy intake (p < 0.001) and feed efficiency ratio (p < 0.05) was observed in the HFD + STZ group as compared to the NPD group. Significant decrease in energy intake was observed in the MET group as compared to the HFD + STZ group (Table 3).
Table 3.
Effect of RS and CA extracts on study parameters.
| Groups | Body weight gain (g) | Food intake (g/rat/day) | Energy intake (Kcal/rat/day) | Feed efficiency ratio (%) | Glucose (mg/dL), Day 0 |
Glucose (mg/dL), Day 21 |
|---|---|---|---|---|---|---|
| NPD | 11.67 ± 1.67 | 9.59 ± 0.04 | 36.24 ± 0.14 | 15.36 ± 2.23 | 92.50 ± 3.94 | 90.00 ± 3.15 |
| NPD + STZ | 10.00 ± 2.89 | 10.25 ± 0.04 | 38.76 ± 0.14 | 12.29 ± 3.58 | 156.33 ± 4.78 | 97.17 ± 3.46 |
| HFD + STZ | 20.00 ± 1.83 | 19.90 ± 0.13z | 109.87 ± 0.72z | 8.67 ± 0.79x | 349.67 ± 19.12z | 405.83 ± 20.79z |
| RS4 | 16.67 ± 4.22 | 19.62 ± 0.18 | 108.29 ± 1.02 | 7.35 ± 1.87 | 341.00 ± 38.16 | 200.33 ± 41.89c |
| RS8 | 26.67 ± 3.07 | 21.11 ± 0.25 | 116.53 ± 1.39 | 10.89 ± 1.25 | 335.83 ± 39.00 | 144.33 ± 25.12c |
| CA4 | 22.50 ± 2.50 | 20.14 ± 0.69 | 111.19 ± 3.80 | 9.70 ± 1.19 | 353.50 ± 25.42 | 258.33 ± 36.56b |
| CA8 | 20.00 ± 1.83 | 19.98 ± 0.18 | 110.31 ± 0.99 | 8.62 ± 0.76 | 322.17 ± 33.81 | 136.33 ± 14.76c |
| MET | 15.83 ± 2.01 | 18.38 ± 0.09b | 101.46 ± 0.48b | 7.43 ± 0.94 | 323.17 ± 38.29 | 129.00 ± 18.99c |
Values are expressed as Mean ± SEM (n = 6), analyzed by one way ANOVA followed by Bonferroni test. x denotes p < 0.05, z denotes p < 0.001 as compared to NPD, b denotes p < 0.01 & c denotes p < 0.001 as compared to HFD + STZ.
3.3.3. Effect of RS and CA extracts on serum biochemical parameters
3.3.3.1. Glucose and insulin
Significant increase in glucose day 0 (p < 0.001), glucose day 21 (p < 0.001) and significant decrease in insulin (p < 0.01) was observed in the HFD + STZ group as compared to the NPD group. Significant decrease was observed in glucose level of treatment groups on day 21 as compared to the HFD + STZ group (Table 3).
Significant increase in insulin level was observed in RS4 and RS8 groups as compared to the HFD + STZ group. No significant change was observed in the NPD + STZ group as compared to the NPD group (Table 4).
Table 4.
Effect of RS and CA extracts on study parameters.
| Groups | Insulin (μg/L) | HOMA-IR | Creatinine (mg/dL) | Relative Organ Weight, % |
||
|---|---|---|---|---|---|---|
| Heart | Spleen | Kidney | ||||
| NPD | 2.26 ± 0.10 | 9.10 ± 0.52 | 0.72 ± 0.02 | 0.29 ± 0.04 | 0.19 ± 0.01 | 0.32 ± 0.04 |
| NPD + STZ | 2.12 ± 0.17 | 9.10 ± 0.79 | 0.78 ± 0.07 | 0.29 ± 0.01 | 0.14 ± 0.01 | 0.34 ± 0.01 |
| HFD + STZ | 1.57 ± 0.09y | 28.00 ± 0.89z | 0.87 ± 0.05 | 0.28 ± 0.03 | 0.12 ± 0.03 | 0.44 ± 0.03 |
| RS4 | 2.26 ± 0.12a | 19.70 ± 3.81 | 0.81 ± 0.04 | 0.31 ± 0.01 | 0.15 ± 0.01 | 0.34 ± 0.01 |
| RS8 | 2.40 ± 0.13b | 15.50 ± 3.07b | 0.94 ± 0.03 | 0.33 ± 0.01 | 0.16 ± 0.01 | 0.35 ± 0.01 |
| CA4 | 1.69 ± 0.14 | 18.60 ± 2.32 | 0.82 ± 0.04 | 0.37 ± 0.01 | 0.18 ± 0.01 | 0.36 ± 0.01 |
| CA8 | 1.85 ± 0.08 | 10.40 ± 1.22c | 0.92 ± 0.07 | 0.31 ± 0.01 | 0.16 ± 0.01 | 0.41 ± 0.04 |
| MET | 1.73 ± 0.16 | 10.50 ± 1.57c | 0.81 ± 0.04 | 0.30 ± 0.04 | 0.13 ± 0.02 | 0.35 ± 0.04 |
Values are expressed as Mean ± SEM (n = 6), analyzed by one way ANOVA followed by Bonferroni test. y denotes p < 0.01 & z denotes p < 0.001 as compared to NPD, a denotes p < 0.05, b denotes p < 0.01 & c denotes p < 0.001 as compared to HFD + STZ.
3.3.3.2. Creatinine
No significant change was observed in creatinine level (Table 4).
3.3.3.3. Lipid profile
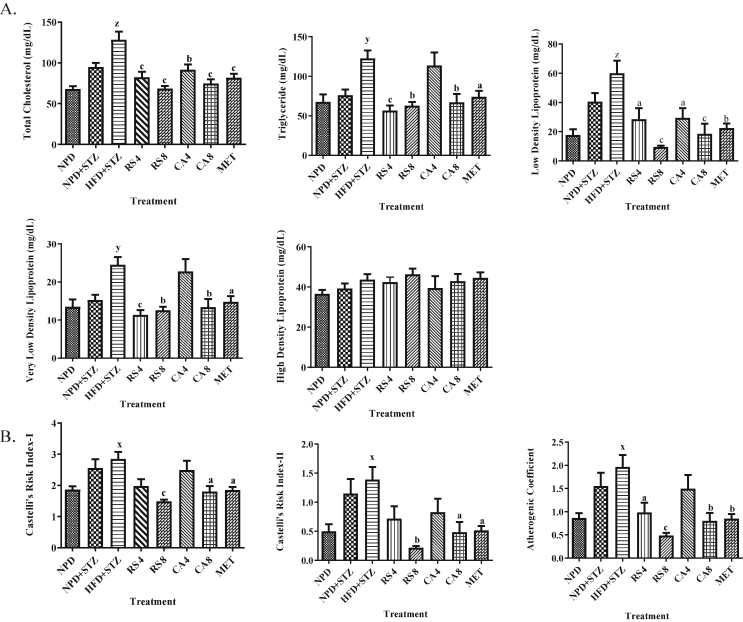
The levels of TC (p < 0.001), TG (p < 0.01), LDL (p < 0.001) and VLDL (p < 0.01) were significantly increased in the HFD + STZ group as compared to the NPD group. Significant decrease in the level of levels of TC, TG, LDL and VLDL was observed in RS4, RS8, CA8 and MET groups, while CA4 group showed significant decrease in TC and LDL, as compared with the HFD + STZ group. No significant change was observed in the NPD + STZ group as compared to the NPD group (Fig. 3).
Fig. 3.
Effect of RS and CA extracts on lipid profile and atherogenic indices.
Values are expressed as Mean ± SEM (n = 6), Data analyzed by two way ANOVA followed by Bonferroni Test.
A. Lipid profile includes level of total cholesterol, triglyceride, low density lipoprotein, very low density lipoprotein, high density lipoprotein in control and treatment groups.
B. Atherogenic indices includes estimation of Castelli's risk index-I, Castelli's risk index-II and atherogenic coefficient for control and treatment groups.
x denotes P < 0.05, y denotes P < 0.01 & z denotes P < 0.001 for HFD + STZ as compared with NPD,
a denotes P < 0.05, b denotes P < 0.01 & c denotes P < 0.001 for treatment groups as compared with HFD + STZ.
3.3.4. Effect of RS and CA extracts on HOMA-IR and atherogenic indices
Significant increase in HOMA-IR (p < 0.001) was observed in the HFD + STZ group as compared to the NPD group. Significant decrease in HOMA-IR was observed in RS8, CA8 and MET groups as compared to the HFD + STZ group (Table 4).
Results indicated significant increase (p < 0.05) in CRI-I, CRI-II and AC in the HFD + STZ group as compared to the NPD group, while significant decrease was observed in RS8, CA8 and MET groups as compared to HFD + STZ group. Significant decrease in AC was observed in RS4 group as compared to the HFD + STZ group. No significant change was observed in the CA4 group and NPD + STZ group (Fig. 3).
3.3.5. Effect of RS and CA extracts on relative organ weight, %
No significant change was observed in relative organ weight in all the treatment groups (Table 4).
3.3.6. Effect of RS and CA extracts on histopathology of pancreas and kidney
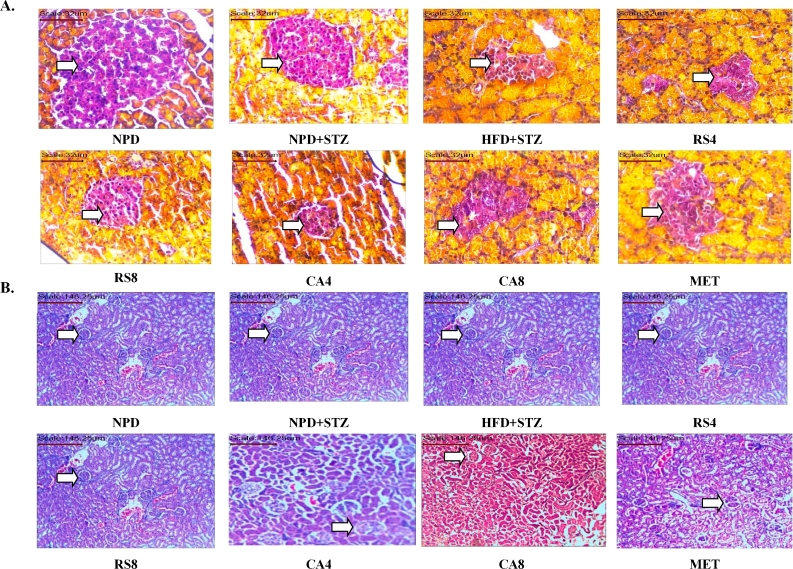
Histopathology of pancreas revealed that a decrease in β cells was seen in the HFD + STZ group, whereas no histological abnormalities were seen in NPD and NPD + STZ groups. Significant increase in β cells was observed in RS4, RS8, CA4 and CA8 groups as compared to the HFD + STZ group (Fig. 4A). No histopathological abnormalities were seen in kidney of rats in NPD and NPD + STZ groups. In HFD + STZ group mild multifocal tubular vacuolation was observed. Significant reduction was observed in the tubular vacuolation in treatment groups as compared to the HFD + STZ group (Fig. 4B).
Fig. 4.
Effect of RS and CA extracts on the histopathology of pancreas and kidney. A. Histopathology of pancreas using Aldehyde-fuchsin staining at 40× magnification, arrow indicates Islet of Langerhans, B. histopathology of kidney using hematoxylin and eosin staining at 10× magnification, arrow indicates glomerulus.
4. Discussion
Phytochemical analysis revealed the presence of various bioactive phytoconstituents in RS and CA extracts. Both the extracts contain flavonoids i.e. rutin and myricetin in RS extract and rutin in CA extract. It is well reported that flavonoids possess antihyperglycemic, antiobesity and antioxidant activity.28,29 Rutin and myricetin are reported to possess anti-diabetic activity.30 Another constituents of chosen extracts i.e. saponin is indicated to use as renoprotective, antihyerglycemic, antihyperlipidemic and antioxidant, while tannin possesses antioxidant and renoprotective activity.28,29 HPLC study revealed presence of glycosides i.e. Sennoside A and Sennoside B in CA extract. In the literature, glycosides are indicated to use for antihyerglycemic and hepatoprotective activity.28,29 Presence of all these constituents in the extracts of study plants strongly supports their synergistic effect for the treatment of diabetes.
Glucose lowering medications act by modifying the actions of glucagon and insulin directly. As glucose tolerance test determines the blood glucose clearance rate, thus comparisons of glucose levels using this test at various time points, with and without medications, lead to a better understanding of utility of study medications in the treatment of diabetes.31 Therefore, with dose optimization study using glucose tolerance test, two doses of both the extracts were selected for further screening.
Ishak et al. showed that diabetes induced in rats by combination of low dose STZ and high fat diet closely resembles with the natural process of the diabetic occurrence and metabolic disturbance in type-2 diabetic human.32 In the present study, diabetic model was used with modification of method reported by Srinivasan et al.19 Duration of high fat diet feeding was increased up to 16 weeks in place of two weeks for better induction of diabetes and associated consequences.
Food intake and energy intake were better controlled with metformin treatment only, while comparable results were obtained between extracts treated groups and metformin treated group in body weight, body weight gain and feed efficiency ratio of diabetic rats.
The glucose level of current study was similar with the findings of Poitout and Robertson,33 where they showed that STZ produces destruction of pancreatic β-cells and decrease the sensitivity of the cells for insulin mediated glucose uptake and this resulted into high glucose concentration in blood. Extract treated groups significantly reduced blood glucose level. Choi et al. reported that glycosides i.e. sennoside A and B decrease carbohydrate digestion.34 Therefore, glucose lowering activity of CA extracts is partially attributed to the glycoside content of the extract. RS leaves possess intestinal α-glucosidase inhibitory activity as reported by Kim et al.,35 while Kang et al. reported that the myricetin alleviates postprandial hyperglycaemia conditions via inhibition of the α-glucosidase activity.36 Therefore, glucose lowering activity of RS extract is due to the α-glucosidase inhibitory activity.37 Antihyperglycemic activity of RS and CA extracts was found to be as good as the metformin treatment in diabetic rats suggesting that the natural constituents could have act separately or synergistically to induce the hypoglycemic effect.
Flavonoids possess the ability to regenerate β cells, whereas saponins improve insulin signalling, inhibit gluconeogenesis and α-glucosidase activity28,29. Hossain et al. reported that diabetic animals fed with rutin showed significant reductions in glucose levels and increased insulin levels.30 Ong and Khoo reported that myricetin stimulates glucose uptake without the presence of fully functional insulin receptor.38 Li et al. reported that the myricetin stimulates the secretion of insulin.39 Thus, increase in insulin level with RS extract treatment clearly indicated insulin secretagogue action of RS in diabetic rats, attributed to the presence of phytoconstituents. Along with this, comparable reduction in HOMA-IR was observed between high dose of extracts and metformin treatment, which also supports antidiabetic activity of RS and CA extracts.
Type-2 diabetes is often associated with dyslipidemia.40 Chronic dyslipidemia leads to lipid deposition and pancreatic lipotoxicity, thus impairing insulin secretion and promoting the progression of type-2 diabetes.41 In the present study, supplementation with RS and CA extracts was observed to attenuate dyslipidemia significantly. The treatment also showed trend of increase in HDL level in a dose dependent manner. Both extracts contain flavonoids and saponins and it is reported that flavonoids and saponins both are pancreatic lipase inhibitors, thus they inhibit fat absorption.42 CA extract also contain sennosides and Choi et al. reported that sennoside B decreases triglyceride accumulation while both sennoside A and B inhibit α-glucoamylase activity.34 RS extract contains myricetin and Choi et al. reported that myricetin improves hypertriglyceridemia and hypercholesterolemia without a significant influence on food intake in animals fed with high fat diet.43 Thus, treatment with RS and CA extracts significantly ameliorated dyslipidemia, which was due to the presence of various phytoconstituents as well as enzyme inhibitory actions. Interestingly, alleviation of dyslipidemia with RS and CA extracts treatment was comparable to the metformin treatment.
CRI and AC are the ratios for predicting the risk of cardiovascular disease.44,45 Significant reduction in these indices was observed with the treatment of RS and CA extracts, which was similar to the metformin treatment. This clearly indicates reduction in the risk of cardiovascular diseases by the treatments given.
In the extract treated groups, renal function marker creatinine was found to be near to normal and histopathological study of the kidney revealed no sign of diabetic nephropathy. Histopathological findings of pancreas indicated that metformin could repair the damage incurred by the pancreatic islets due to exposure to STZ, whereas the groups fed with RS and CA showed effects which were comparable to metformin group in terms of the recovery of STZ-induced impairment of pancreatic islets. It has been reported previously that flavanoids acts as an antioxidant and prevent the progressive impairment of pancreatic beta-cell function due to oxidative stress and may thus reduce the occurrence of type 2 diabetes,37 thus, significant amelioration of diabetes induced changes in kidney and pancreas with extract treatment groups is attributed to bioactive phytoconstituents of both the extracts.
Free radicals enhance the metabolic dysfunctions in diabetes; therefore antioxidant property of herbal medicines reduces metabolic consequences associated with diabetes.42,43 Both extracts are reported to possess antioxidant activity,6,7 that additionally favours ability of extracts to reduce metabolic dysfunctions in diabetes.
5. Conclusion
This study provides evidence that extracts of RS and CA, ameliorates metabolic abnormalities associated with diabetes and can retard the risk of complications due to chronic hyperglycemia. The beneficial effects of both the extracts in diabetes seem to be attributed to the synergistic effects of its bioactive compounds such as flavonoids, saponins, and glycosides.
Declarations
Conflict of interest
The Authors declare that there is no conflict of interest.
Acknowledgement
We express our sincere gratitude to Dr. Madhav Marathe, Ex-Vice president (Toxicology and Laboratory Animal House), Sun Pharma Advanced Research Co. Ltd, Vadodara, India for providing animals for research purpose and to Dr. Narendra Gajbhiye, Senior Scientist, DMAPR, Anand, India for help and guidance in phytochemical investigation. We are also grateful to Dr. Hardik Soni, Manager (R&D), Vasu Research Centre, Vadodara, India for their invaluable support and help in the successful conduction of this study. We express sincere thanks to Dr. S. N. Mishra, Principal Scientist, AICRP on M & A P, KNK College of Horticulture, Mandsaur (M.P.), India for timely authentication of herbs under study.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.WHO . WHO; 2016. Obesity and Overweight.http://www.who.int/mediacentre/factsheets/fs311/en/ Published. [Google Scholar]
- 2.WHO . Who; 2016. World Health Day 2016: WHO Calls for Global Action to Halt Rise in and Improve Care for People with Diabetes; pp. 2014–2016.http://www.who.int/diabetes/global-report/WHD16-press-release-EN_3.pdf%0Ahttp://www.who.int/mediacentre/news/releases/2016/world-health-day/en/ [Google Scholar]
- 3.WHO Diabetes fact sheet. Sci Total Environ. 2017;20:0–1. [Google Scholar]
- 4.Chaudhury A., Duvoor C., Reddy Dendi V.S. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol. 2017;8(JAN) doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Risco M.R., Mouhid L., Salas-Pérez L. Biological activities of Asteraceae (Achillea millefolium and Calendula officinalis) and Lamiaceae (Melissa officinalis and Origanum majorana) plant extracts. Plant Foods Hum Nutr. 2017;72(1):96–102. doi: 10.1007/s11130-016-0596-8. [DOI] [PubMed] [Google Scholar]
- 6.Gupta B.P., Sharma I., Kohli N., Sharma S., Rathi A., Sharma A.K. Preliminary clinical assessment and non- toxicity evaluation of an ayurvedic formulation BGR-34 in NIDDM. J Tradit Complement Med. 2018 doi: 10.1016/j.jtcme.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S.I., Hayat M.Q., Tahir M. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement Altern Med. 2016;16(1):460. doi: 10.1186/s12906-016-1443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beevi S.S., Narasu M.L., Gowda B.B. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum Nutr. 2010;65(1):8–17. doi: 10.1007/s11130-009-0148-6. [DOI] [PubMed] [Google Scholar]
- 9.Jani Deepti K.G.S. Ameliorative effect of Raphanus sativus and Cassia angustifolia in experimentally induced hyperlipidemia and cardiovascular risk reduction. Int J PharmTech Res. 2017;10(4):273–279. [Google Scholar]
- 10.Banihani S.A. Radish (Raphanus sativus) and diabetes. Nutrients. 2017;9(9) doi: 10.3390/nu9091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Adhal A. The effect of Cassia angustifolia (senna) leaves on the fasting blood sugar in a sample of mild diabetic Yemeni patients. Yemeni J Med Sci. 2009;3:9. [Google Scholar]
- 12.Banihani S.A. Radish (Raphanus sativus) and diabetes. Nutrients. 2017 doi: 10.3390/nu9091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KR K.P. Practical Pharmacognosy. twenty-first ed. Vol. 25.1. Nirali Prakashan; 2011. Preliminary phytochemical screening. [Google Scholar]
- 14.Trease G.E., EWC . thirteenth ed. Bailliere Tindall; London: 1989. Pharmacognosy. [Google Scholar]
- 15.Harborne J.B. Chapman and Hall Ltd.; London: 1973. Phytochemical Methods. [Google Scholar]
- 16.Senguttuvan J., Paulsamy S., Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac J Trop Biomed. 2014;4:S359–S367. doi: 10.12980/APJTB.4.2014C1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakulpanich A., Gritsanapan W. Determination of anthraquinone glycoside content in Cassia fistula leaf extracts for alternative source of laxative drug. Int J Biomed Pharm Sci. 2009;3(1):42–45. [Google Scholar]
- 18.Yamazaki K., Yasuda N., Inoue T. Effects of the combination of a dipeptidyl peptidase IV inhibitor and an insulin secretagogue on glucose and insulin levels in mice and rats. J Pharmacol Exp Ther. 2007;320(2):738–746. doi: 10.1124/jpet.106.112011. jpet.106.112011 [pii]∖r10.1124/jpet.106.112011 [doi] [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan K., Viswanad B., Asrat L., Kaul C.L., Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Bas A.L., Demirci S., Yazihan N., Uney K., Ermis Kaya E. Nerium oleander distillate improves fat and glucose metabolism in high-fat diet-fed streptozotocin-induced diabetic rats. Internet J Endocrinol. 2012 doi: 10.1155/2012/947187. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalelioglu T., Genc A., Karamustafalioglu N., Emul M. Assessment of cardiovascular risk via atherogenic indices in patients with bipolar disorder manic episode and alterations with treatment. Diabetes and Metabolic Syndrome. 2017;11(1):S473–S475. doi: 10.1016/j.dsx.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Castelli W.P., Abbott R.D.M.P.M. Summary estimates of cholesterol used to predict coronary heart disease. Circulation. 1983;67(4):730–734. doi: 10.1161/01.cir.67.4.730. [DOI] [PubMed] [Google Scholar]
- 23.Novelli E.L., Diniz Y.S., Galhardi C.M. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41(1):111–119. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 24.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 25.Chaudhari H.S., Bhandari U., Khanna G. Embelia ribes extract reduces high fat diet and low dose streptozotocin-induced diabetic nephrotoxicity in rats. EXCLI J. 2013;12:858–871. [PMC free article] [PubMed] [Google Scholar]
- 26.Gajdosík a, Gajdosíková a, Stefek M., Navarová J., Hozová R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999;18 Spec:54–62. [PubMed] [Google Scholar]
- 27.Hassanzadeh-Taheri M., Hassanpour-Fard M., Doostabadi M., Moodi H., Vazifeshenas-Darmiyan K., Hosseini M. Co-administration effects of aqueous extract of turnip leaf and metformin in diabetic rats. J Tradit Complement Med. 2018 doi: 10.1016/j.jtcme.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh R., Kaur N., Kishore L., Kumar G.G. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol. 2013;150(1):51–70. doi: 10.1016/j.jep.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 29.Gaikwad S.B., Mohan G.K.R.M. Phytochemicals for diabetes management. Pharmaceut Crop. 2014;5(1):11–28. [Google Scholar]
- 30.Hossain M.K., Dayem A.A., Han J. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci. 2016;17(4) doi: 10.3390/ijms17040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul Ernsberger, Koletsky Richard J. In: Glucose Tolerance. Chackrewarthy S., editor. In Tech; 2012. [Google Scholar]
- 32.Ishak N.A., Ismail M., Hamid M., Ahmad Z., Abd Ghafar S.A. Antidiabetic and hypolipidemic activities of Curculigo latifolia fruit:Root extract in high fat fed diet and low dose STZ induced diabetic rats. Evid Based Complement Altern Med. 2013 doi: 10.1155/2013/601838. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poitout V., Robertson R.P. Minireview: secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143(2):339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 34.Choi S.B., Ko B.S., Park S.K., Jang J.S., Park S. Insulin sensitizing and α-glucoamylase inhibitory action of sennosides, rheins and rhaponticin in Rhei Rhizoma. Life Sci. 2006;78(9):934–942. doi: 10.1016/j.lfs.2005.05.101. [DOI] [PubMed] [Google Scholar]
- 35.Kim M., Kim E., Kwak H.S., Jeong Y. The ingredients in Saengshik, A formulated health food, Inhibited the activity of α-amylase and α-glucosidase as anti-diabetic function. Nutr Res Pract. 2014;8(5):602–606. doi: 10.4162/nrp.2014.8.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang S.-J., Park J.-H.Y., Choi H.-N., Kim J.-I. Α-glucosidase inhibitory activities of myricetin in animal models of diabetes mellitus. Food Sci Biotechnol. 2015;24(5):1897–1900. [Google Scholar]
- 37.Vadivelan R., Gopala Krishnan R., Kannan R. Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of α-amylase and α-glucosidase. J Tradit Complement Med. 2019 doi: 10.1016/j.jtcme.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong K.C., Khoo H.E. Insulinomimetic effects of myricetin on lipogenesis and glucose transport in rat adipocytes but not glucose transporter translocation. Biochem Pharmacol. 1996;51(4):423–429. doi: 10.1016/0006-2952(95)02195-7. [DOI] [PubMed] [Google Scholar]
- 39.Li Y., Zheng X., Yi X. Myricetin: a potent approach for the treatment of type 2 diabetes as a natural class b gpcr agonist. FASEB J. 2017;31(6):2603–2611. doi: 10.1096/fj.201601339R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian J., Chen H., Jia F. Trends in the levels of serum lipids and lipoproteins and the prevalence of dyslipidemia in adults with newly diagnosed type 2 diabetes in the Southwest Chinese Han population during 2003-2012. Internet J Endocrinol. 2015;2015 doi: 10.1155/2015/818075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Litwak S.A., Wali J.A., Pappas E.G. Lipotoxic stress induces pancreatic β-cell apoptosis through modulation of Bcl-2 proteins by the ubiquitin-proteasome system. J Diabetes Res. 2015;2015 doi: 10.1155/2015/280615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seyedan A., Alshawsh M.A., Alshagga M.A., Koosha S., Mohamed Z. Medicinal plants and their inhibitory activities against pancreatic lipase: a review. Evid Based Complement Altern Med. 2015;2015 doi: 10.1155/2015/973143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi H.-N., Kang M.-J., Lee S.-J., Kim J.-I. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr Res Pract. 2014;8(5):544–549. doi: 10.4162/nrp.2014.8.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhardwaj S., Bhattacharjee J., Bhatnagar M.K., Tyagi S., Delhi N. Atherogenic index of plasma, castelli risk index and atherogenic coefficient - new parameters in assessing cardiovascular risk. Int J Pharm Biol Sci. 2013;3(3):359–364. [Google Scholar]
- 45.Singh M. A study on atherogenic indices of pregnancy induced hypertension patients as compared to normal pregnant women. J Clin Diagnostic Res. 2015 doi: 10.7860/JCDR/2015/13505.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]