Abstract
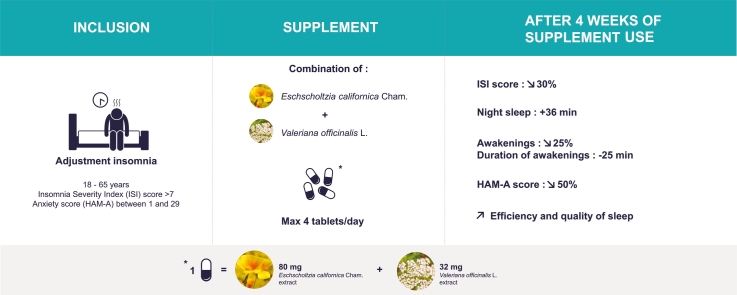
Eschscholtzia californica Cham. and Valeriana officinalis L. have long been used for the management of sleep disorders and anxiety. Use of a fixed combination of these two plant extracts (Phytostandard® d’Eschscholtzia et de Valériane, PiLeJe Laboratoire, France) was investigated in an observational study. Adults with adjustment insomnia according to the criteria of the International Classification of Sleep Disorders and with an insomnia severity index (ISI) score >7 enrolled by GPs took a maximum of four tablets of the eschscholtzia and valerian combination every night for four weeks. Within one month, ISI score decreased by approximately 30% (from 16.09 ± 3.67 at inclusion (V1) to 11.32 ± 4.78 at 4 weeks (V2); p < 0.0001). Night sleep duration significantly increased between the first and the fourth week of supplement intake, sleep efficiency increasing from 78.4% ± 12.5 to 84.6% ± 10.2 (p = 0.002). There was no improvement in sleep latency. The number of awakenings decreased by approximately 25% and their total duration by approximately 25 min. Anxiety score significantly decreased by 50% from 13.9 ± 7.3 at V1 to 6.7 ± 6.3 at V2 (p < 0.0001). The supplement was well tolerated. These results suggest that the tested combination of eschscholtzia and valerian extracts could be beneficial for the management of insomnia in adults and deserves further investigation.
Keywords: Valerian, Eschscholtzia, Insomnia disorders, Adjustment insomnia
Graphical abstract
1. Introduction
Insomnia is defined as difficulty in initiating or maintaining sleep that is associated with daytime consequences and is not attributable to environmental circumstances or inadequate opportunity for sleep.1,2 In the International Classification of Sleep Disorders, insomnia is considered as chronic when it has persisted for at least three months at a frequency of at least three times per week; it is defined as short-term insomnia, also called adjustment insomnia, acute insomnia or anxiety-related insomnia, when it meets the symptom criteria but has persisted for less than three months.1 The definition of chronicity is variable, but a minimum duration of one month is stipulated by the International Classification of Diseases.3
Adjustment insomnia is a remarkably common problem, most adults experiencing insomnia or sleeplessness at one time or another in their lives. According to epidemiological studies, about one-third of the general population suffers from at least one insomnia symptom (difficulty in initiating or in maintaining sleep, or non-restorative sleep).4,5 One study specifically focusing on acute insomnia reported a prevalence of 7.9% in a UK sample, with an annual incidence of 36.6%.6 Adjustment insomnia is triggered by a stressor — such as interpersonal conflicts, stress at work, a temporary change of schedule or location, a new situation requiring adjustment, or temporary abuse of a licit or illicit stimulating substance — and is generally relieved once that stressor is no longer present.2,7 In view of the transient nature of adjustment insomnia, individuals generally do not seek medical treatment and often turn to nonpharmacological or over-the-counter remedies,4,8 herbal medicines representing one of the most frequently used types of complementary or alternative treatment for insomnia.8,9
Among herbal medicines, valerian has long been used to aid sleep and relieve anxiety and is also among those most extensively studied. The effects of valerian have been attributed to interactions of valepotriates, valerenic acid, and other components with the GABAergic system, GABA being a key sleep-promoting neurotransmitter.10,11 It has also been suggested that its effects could be induced by the GABA contained in the extracts tested. However, whether exogenous GABA can cross the blood-brain barrier is far from clear.10, 12, 13, 14 Overall, the results of in vitro and in vivo studies suggest both a sedative and an anxiolytic effect of valerian, mediated by various interacting components which might promote sleep and improve nervous state.
The effects on sleep of valerian alone or in combination with hops have been studied in many clinical trials that vary in their conclusions. Reviews and meta-analyses either found no difference in sleep outcomes between valerian and placebo,4,9 or concluded that valerian may improve sleep quality, but that methodological problems limit the ability to draw firm conclusions.15,16 Most of the clinical studies included in these analyses were considered to be of poor quality with regard to their design, the diagnosis of the patients included and/or sample size.4,9 In addition, a wide range of preparations, doses and treatment durations were tested in these clinical studies, and it is well known that the method of extraction can affect the active components, and thus the efficacy of preparations. On the other hand, in its assessment of Valeriana officinalis L., radix, the Herbal Medicinal Products Committee of the European Medicines Agency (HPMC/EMA) concluded that the herbal substance and derived preparations of this plant have a positive risk/benefit ratio in view of their minimal adverse events in relation to their efficacy. The committee consequently considered as well established the use of one specific extract of Valeriana officinalis L., radix for the relief of mild nervous tension and sleep disorders and as traditional the use of a dozen other extracts for the relief of mild symptoms of mental stress and to aid sleep.17 This assessment was based on the analysis of a large body of evidence that may serve as a proof of efficacy and safety (derived not only from clinical trials but also from experimental, post-marketing, epidemiological studies, etc.) and notably took into account the type of preparation used (in contrast to published meta-analyses).
The HMPC/EMA also recognizes the traditional use of extracts of the aerial parts of Eschscholtzia californica Cham., herba for the relief of mild symptoms of mental stress and sleep aid on the basis of their long-standing use.18 The sedative and anxiolytic properties of E. californica (California poppy) extracts have been demonstrated in several preclinical studies.18, 19, 20, 21 In particular, prolongation of sleeping time and reduced locomotor activity were observed in mice and rats receiving various doses of E. californica extracts.19,20 In contrast, clinical data on eschscholtzia are very scarce.22,23 The effects of E. californica result from its chemical composition and in particular the presence of specific alkaloids, such as californidine and eschscholtzine.18 Eschscholtzia would act by binding to benzodiazepine receptors.24,25
We hypothesized that use of a supplement combining Valeriana officinalis L. and Eschscholtzia californica Cham. might be of benefit for patients with adjustment insomnia. We therefore conducted an observational study to evaluate one-month use of a supplement comprising a fixed combination of eschscholtzia and valerian extracts in adults diagnosed with adjustment insomnia. The study was conducted in the primary care setting as general practitioners (GPs) are consulted for insomnia in the first instance, this disorder constituting a frequent reason for consultation in primary care.2
2. Materials and methods
2.1. Study design and ethics statement
This was an open-label, observational, longitudinal study performed in France between September 2016 and January 2017. The study was conducted in a routine practice context without any additional or unusual procedure as regards diagnosis, monitoring or supplement use. The study was conducted by GPs who habitually recommended the dietary supplement to patients suffering from insomnia, so patients would have received the combination regardless of whether they were enrolled in the study. The study was approved by the Advisory Committee on Information Processing in Material Research in the Field of Health (agreement no. 15.859) and the National Commission on Computerization and Freedom. The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki and the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines. It was registered on the ClinicalTrials.gov site in November 2016 (identifier: NCT02981238).
2.2. Participants and recruitment
Patients aged 18–65 years of either sex were recruited by GPs.
Patients had to meet both of the following inclusion criteria: (1) current presence of adjustment insomnia and/or history of this disorder within the last 3 months. The diagnosis of insomnia was based on the patient’s complaint: sleep perceived by the patient as difficult to achieve, insufficient or non-restorative1; (2) deterioration of sleep within the previous month resulting in a score strictly greater than 7 on the insomnia severity index (ISI).26
Exclusion criteria were: (1) an anxiety score on the Hamilton anxiety rating scale (HAM-A) ≥ 30; (2) a score on the Epworth sleepiness scale > 10; (3) symptoms suggestive of sleep apnoea or restless leg syndrome; (4) chronic pain syndrome requiring daily intake of analgesics; and (5) ongoing psychotropic (neuroleptic, anxiolytic, antidepressant or hypnotic) treatment or under treatment that might adversely affect the waking state (antihistamines, beta-blockers, cough syrups, etc.). Patients with illnesses and/or undergoing treatments liable to interfere with sleep disorders were not eligible for this study. All patients received written information about the study and gave their written consent before participating in it.
2.3. Supplement
The dietary supplement was a combination of eschscholtzia and valerian extracts (Phytostandard® d’Eschscholtzia et de Valériane, PiLeJe Laboratoire, France) marketed in France since 2010. One tablet contains 80 mg of eschscholtzia extract (Eschscholtzia californica Cham., flowering aerial parts) and 32 mg of valerian extract (Valeriana officinalis L., roots). Patients were instructed to take a maximum of four tablets of the supplement every night for one month.
2.4. Procedure
The procedure complied with routine practice and official recommendations for the management of sleep disorders.2
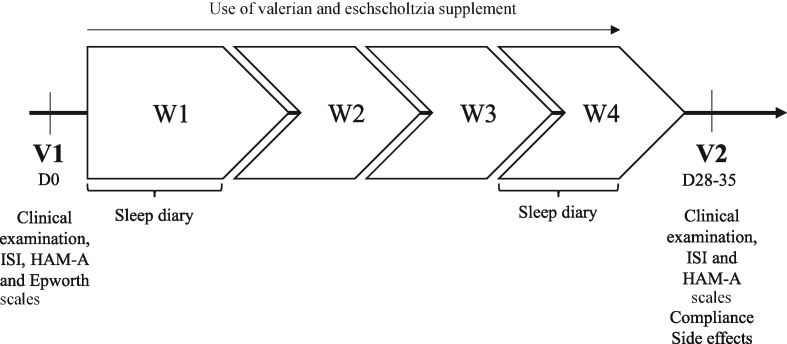
On the first visit (V1), patient eligibility was verified. GPs had to complete an electronic inclusion form including the patient’s socio-demographic (age and sex) and anthropometric (height and weight) characteristics, their medical and sleep disorder history, and information on sleep, associated symptoms, concomitant illnesses and treatments (Fig. 1). Anxiety status (HAM-A), daytime sleepiness (Epworth sleepiness scale) and severity of insomnia (ISI) were assessed. Patients also evaluated the intensity of insomnia (from no impact to high impact on their life), quality of sleep (from excellent to bad) and quality of waking state (from daytime somnolence to good waking state) on a 10 cm visual analogue scale (VAS).
Fig. 1.
Study design.
During the first week (W1) and the last week (W4) of supplement intake, patients had to report sleep characteristics in an electronic sleep diary to estimate sleep latency (interval between going to bed and sleep onset); total sleep duration ([time of falling asleep to time of waking] - total duration of awakenings in the night); number and duration of awakenings; and index of sleep efficiency ([total sleep time/time spent in bed] x 100).
On the second visit (V2; Day 28–35), after one month of supplement intake, GPs had to collect information on adverse events and concomitant treatments. Anxiety (HAM-A scale), insomnia severity (ISI), and insomnia intensity and quality of sleep and waking state (VAS) were re-assessed. Information provided in the electronic sleep diary and compliance with supplement intake were also checked.
2.5. Main evaluation scales
The Epworth sleepiness scale rates on a 4-point scale (from 0 to 3) the patient’s usual likelihood of dozing off or falling asleep while engaged in eight different activities differing widely in their somnificity.27 The sum of the eight individual scores gives an estimate of the person’s ‘average sleep propensity’ across a wide range of activities in their everyday life.
The HAM-A anxiety scale grades the severity of 14 symptoms from 0 = absent to 4 = maximum/disabling giving a total score ranging from 0 to 56. The severity of anxiety was classified as mild (scores 0–17), mild to moderate (scores 18–24) or moderate to severe (scores 25–30). Patients with a score ≥30 were not included.28
The ISI is a self-assessment questionnaire evaluating the nature, severity, and impact of insomnia.26,29 The usual recall period comprises the past month. The ISI components are: 1) sleep disorder severity (subcomponents: difficulty falling asleep, difficulty staying asleep, early morning awakening), 2) sleep satisfaction, 3) interference of sleep difficulties with daytime functioning, 4) extent to which the patient’s sleep problems are noticed by others, and 5) the patient’s concern about his/her sleep disorder. A 5-point Likert scale is used to rate each item (from 0 = no problem to 4 = very severe problem) yielding a total score ranging from 0 to 28. The total score is interpreted as follows: absence of insomnia (scores 0–7); sub-threshold insomnia (scores 8–14); moderate insomnia (scores 15–21); and severe insomnia (scores 22–28).
2.6. Evaluation endpoints
The primary endpoint was change in total ISI score (components 1 to 5) between V1 and V2.26,30 Secondary endpoints comprised changes in each of the seven ISI components and subcomponents, anxiety score (HAM-A scale), intensity of insomnia, quality of sleep and waking state (VAS) between V1 and V2; evolution of sleep latency, sleep duration (nap and night sleep separately and cumulated), number and duration of awakenings and index of sleep efficiency between W1 and W4 (means for the entire week, for working days and for the weekend). Patient satisfaction, safety and compliance were also assessed.
2.7. Statistical analysis
For the calculation of sample size, we postulated a 4-point difference in total ISI score between V1 and V2, with a standard deviation (SD) of 6 points.30, 31, 32 Based on Student’s t-test for paired data, a bilateral test, a power of 80% and an error probability of 0.05, the number of participants required to observe a significant difference was estimated to be 40.
Continuous variables are presented as means ± SD, categorical variables being presented as percentages. The chi-squared test was used to assess differences between categorical variables. The Shapiro-Wilk test was used to test each variable for normality. Student’s t-test or the Mann-Whitney U test was used depending on the normality or non-normality of the data distribution. In all tests, p values < 0.05 were considered statistically significant. The principal analysis was performed on the intention to treat (ITT) population (i.e. all included patients).33 A second confirmatory analysis was performed in the per protocol (PP) population (i.e. patients with no major protocol deviation).33 The safety analysis was performed on all subjects who had received at least one dose of treatment.
Before the multivariate analysis, several univariate analyses were conducted by the addition of a single covariate (age, gender, menopausal status, physical activity, shift work, lifestyle recommendations [diet and behaviour], allopathic treatment in addition to supplement intake, concomitant pathology, and tea, coffee and alcohol consumption) in the statistical model of the primary endpoint. A covariate was considered as a candidate for the multivariate analysis if the p value of this covariate was below the 20% significance threshold (p < 0.20). The ISI score was then analysed using a mixed linear model (to account for repeated measurements in the same subject) using the backward method and forcing the “Visit” variable in the model. Significant covariates at the 5% threshold (p < 0.05) were retained in the final statistical model of the primary endpoint. The difference in ISI score between V2 and V1 was calculated. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC, US).
3. Results
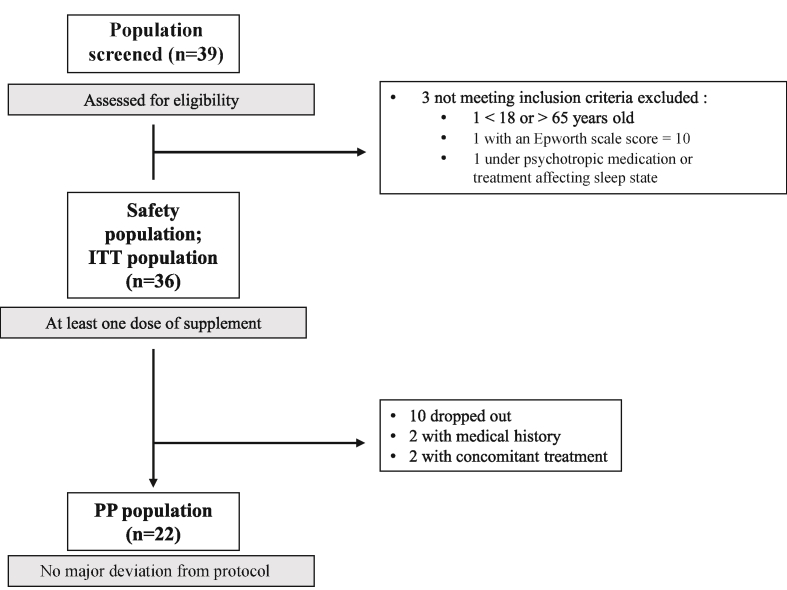
A total of 39 patients were screened by 18 GPs between September 2016 and January 2017 (Fig. 2). Three patients were excluded because they did not meet the inclusion criteria, so finally 36 patients (Safety Population and ITT Population) started to take the supplement at V1. Ten (27.8%) of these patients terminated the study prematurely (1 included by error, 3 lost to follow up and 6 who failed to attend V2), 26 patients completing the study. Four of these latter patients were excluded from the analysis, two on the grounds of a medical history (menopause and migraine, respectively) and two because they were receiving a concomitant treatment (a β-blocker for chronic hypertension in both cases) that might have had an impact on efficacy criteria. Twenty-two patients were included in the PP population.
Fig. 2.
Flow diagram.
3.1. Baseline characteristics (ITT population)
The patients enrolled comprised mainly women (77.8%, 28/36 patients; ITT population). Mean age at inclusion was 49.6 ± 11.3 years.
Mean ISI score was 16.1 ± 3.7 (n = 35). Among the 21 patients who provided information on their sleep disorder in their sleep diary, 7 patients (43.8%, 7/16 with available data) reported sleep-onset insomnia, 16 patients (84.2%, 16/19) sleep-maintenance insomnia, 6 patients (31.6%, 6/19) early morning awakening and 10 patients (55.6%, 10/18) sleep dissatisfaction (patients might have declared several insomnia characteristics).
At V1, 4 patients in the ITT population (18.2%, 4/22 who provided information) declared having hypertension, 2 patients (9.1%, 2/22) a respiratory disorder, 1 patient (4.5%, 1/22) a neuropsychological disorder and 3 patients (14.3%, 3/22) thyroid problems, a further 7 patients (31.8%, 7/22) being treated for other (unspecified) diseases.
3.2. Primary endpoint: ISI score
A significant decrease in the mean ISI score was observed after one month of use of the supplement combining eschscholtzia and valerian extracts. The mean ISI score decreased by approximately 30% from 16.09 ± 3.67 (n = 35) at V1 to 11.32 ± 4.78 (n = 28) at V2 (−4.93 ± 4.83 [n = 28]; p < 0.0001, difference V2-V1 [CI95%] = −4.82 [−6.58; −3.07]). The median score was 16.0 (range: 10.0–24.0) at V1 and 12.0 (range: 3.0–21.0) at V2.
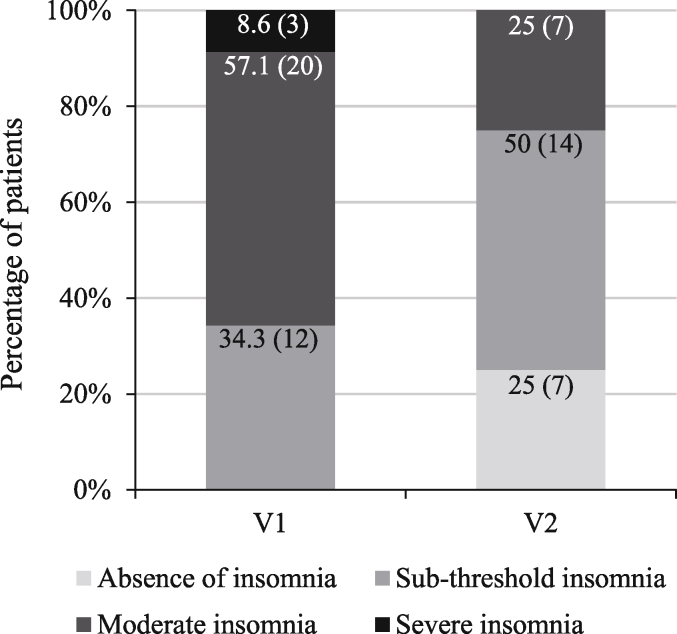
Patient distribution between the four ISI classes at V2 was significantly different from that observed at V1 (p < 0.0001, OR [CI95%] = 0.13 [0.06; 0.29]; Fig. 3). At V2, 7 patients (25.0%, 7/28) no longer suffered from insomnia. None of the patients had severe insomnia at V2 compared to 3 (8.6%, 3/35) at V1.
Fig. 3.
Patient distribution into ISI classes at V1 and V2.
The multivariate analysis confirmed the significant decrease in the ISI score between V1 and V2.
3.3. Secondary endpoints
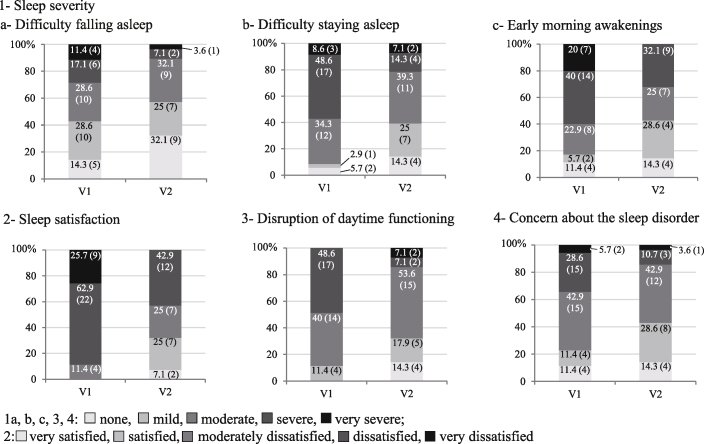
3.3.1. ISI component scores
At V2, patient distribution into the different classes of four of the five ISI components was significantly different from that reported at V1 (Fig. 4). After one month of supplement intake (V2), patients were less likely to have difficulties in falling asleep (p < 0.01, OR [CI95%] = 0.43 [0.23; 0.80]), staying asleep (p < 0.001, OR [CI95%] = 0.21 [0.09; 0.52]), and were less likely to experience early morning awakenings (p < 0.005, OR [CI95%] = 0.29 [0.13; 0.61]). Overall, their sleep disorders interfered less with daytime functioning (p < 0.005, OR [CI95%] = 0.23 [0.09; 0.60]). In addition, patients were more satisfied with their sleep (p < 0.0001, OR [CI95%] = 14.78 [5.14; 42.47]) and less concerned about their sleep disorder (p < 0.05, OR [CI95%] = 0.40 [0.19; 0.84]). The proportions of patients in the “none” and “mild” classes were higher for these ISI components and subcomponents.
Fig. 4.
Patient distribution into the different classes of four ISI components (3 subcomponents 1a, b, c) at V1 and V2 (ITT population; n = 35 at V1, n = 28 at V2; % [n]).
There was no significant change in the fifth component “noticeability of sleep problems to others in terms of impairing patient quality of life” (data not shown).
3.3.2. Sleep diary
Analysis of the data recorded in patients’ sleep diaries revealed that total sleep duration (during an entire week) significantly increased between W1 and W4 by about half an hour (p = 0.016, difference W1-W4 [CI95%] = 0.56 [0.12; 1.00]; Table 1). There was similarly a significant increase in night sleep duration of about half an hour (p = 0.009, difference W1-W4 [CI95%] = 0.57 [0.16; 0.98]), but no change in the mean duration of naps. Total sleep duration was significantly improved during working days (p = 0.01, difference W1-W4 [CI95%] = 0.61 [0.16; 1.07]) whereas there was no significant difference on weekends.
Table 1.
Changes in sleep latency, duration and number of awakenings, and sleep efficiency between W1 and W4 in the ITT population.
| Working days |
Weekends |
Entire week |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| W1 (n = 20* or 21) |
W4 (n = 21) |
p value | W1 (n = 20* or 21) |
W4 (n = 21) |
p value | W1 (n = 20* or 21) |
W4 (n = 21) |
p value | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Total sleep duration (h) | 6.8 ± 1.1* | 7.4 ± 1.3 | 0.01 | 7.4 ± 1.7* | 7.8 ± 1.5 | 0.29 | 6.9 ± 1.22* | 7.5 ± 1.20 | 0.016 |
| Night sleep duration (h) | 6.7 ± 1.1 | 7.3 ± 1.2 | 0.008 | 7.2 ± 1.6 | 7.7 ± 1.2 | 0.19 | 6.8 ± 1.15 | 7.4 ± 1.08 | 0.009 |
| Nap sleep duration (h) | 0.1 ± 0.24* | 0.1 ± 0.20 | 0.48 | 0.1 ± 0.34* | 0.1 ± 0.32 | 0.71 | 0.1 ± 0.24* | 0.1 ± 0.22 | 0.33 |
| Sleep efficiency (%) | 77.5 ± 11.6 | 83.9 ± 12.0 | 0.003 | 80.6 ± 16.0 | 86.8 ± 7.0 | 0.04 | 78.4 ± 12.5 | 84.6 ± 10.2 | 0.002 |
| Sleep latency (min) | 36.5 ± 21.9 | 31.2 ± 22.7 | 0.3 | 32.5 ± 23.7 | 30.1 ± 26.7 | 0.7 | 35.8 ± 20 | 31.6 ± 21.2 | 0.4 |
| Duration of awakenings (min) | 58.5 ± 46.2 | 34.1 ± 34.2 | 0.001 | 43.6 ± 50.8 | 15.8 ± 20 | 0.02 | 53.8 ± 44.8 | 28.8 ± 27.0 | 0.001 |
| Number of awakenings | 1.1 ± 0.69 | 0.8 ± 0.64 | 0.0004 | 0.9 ± 0.71 | 0.7 ± 0.73 | 0.1 | 1.0 ± 0.66 | 0.8 ± 0.64 | 0.001 |
A significant improvement in sleep efficiency was observed between the first and last study week as a whole, including both working days and weekends (Table 1). With respect to each of these weeks as a whole, sleep efficiency increased from 78.4% ± 12.5 for W1 to 84.6% ± 10.2 for W4 (p = 0.002, difference W1-W4 [CI95%] = 6.16 [2.68; 9.65]).
There was no improvement of sleep latency: patients needed 35.8 ± 20 min in W1 and 31.6 ± 21.2 min in W4 to fall asleep, the difference not being statistically significant (Table 1).
A significant decrease was observed in both the number and duration of nocturnal awakenings during the entire week, including both working days and the weekend (but not in the number of awakenings during weekends; Table 1). Comparison of the entire first and last study weeks showed a decrease of approximately 25% in the number of awakenings (p = 0.001, difference W1-W4 [CI95%] = −0.25 [-0.39; −0.11]) and a decrease of approximately 25 min in their total duration (p = 0.001, difference W1-W4 [CI95%] = −25.03 [-38.82; −11.25]).
3.3.3. Intensity of insomnia, quality of sleep and quality of daytime waking state
Both a decrease in insomnia intensity (impact of insomnia on the patient’s life) and an improvement in quality of life were observed. The VAS score of insomnia intensity significantly decreased by 44.8% from 70.8 ± 18.5 (n = 24) at V1 to 39.1 ± 21.5 (n = 18) at V2 (p < 0.0001), the quality of sleep score decreasing by 42.7% from 76.5 ± 16.0 (n = 24) at V1 to 43.8 ± 26.7 (n = 18) at V2 (p < 0.0001). No significant improvement in the quality of daytime waking state was observed between V1 and V2.
3.3.4. Anxiety score
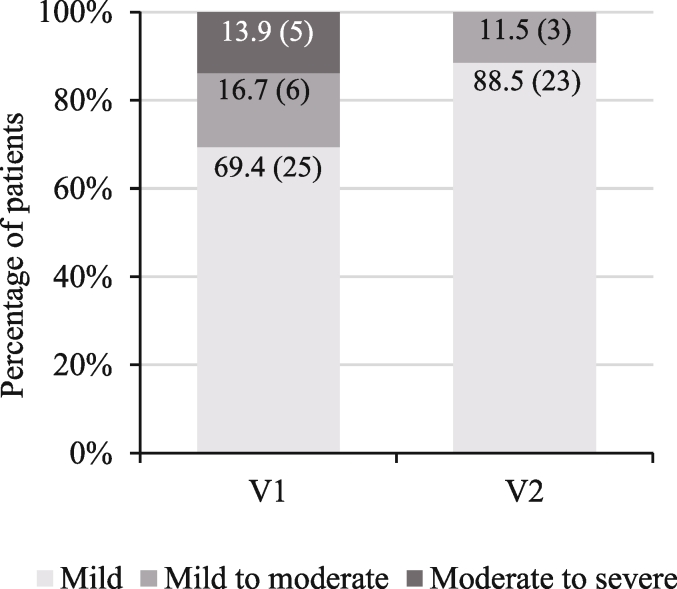
The HAM-A score significantly decreased after one month of supplement use, from 13.9 ± 7.3 (n = 36) at V1 to 6.7 ± 6.3 (n = 26) at V2 (−6.7 ± 6.2 [n = 26]; p < 0.0001; difference V2-V1 [CI95%] = −6.87 [-9.33;-4.41]). The median score was 12.5 (range: 4.0–29.0) at V1 and 5.0 (range: 0.0–23.0) at V2.
There was a significant difference in patient distribution into the different HAM-A classes between V1 and V2 (p = 0.016, OR [CI95%] = 0.27 [0.10; 0.79]; Fig. 5). At V2, most patients (88.5%, 23/26) presented mild anxiety (score <17). Three patients (11.5%, 3/26) showed mild to moderate anxiety at V2. No patient manifested moderate to severe anxiety at V2 compared to 5 patients (13.9%, 5/36) at V1.
Fig. 5.
Patient distribution into HAM-A classes at V1 and V2.
3.3.5. Compliance
According to data collected by GPs for 27 patients, the mean total number of supplement tablets taken during the study period was 49.2 ± 32.7, the median number being 54 (range: 0–96).
3.3.6. Safety
The supplement was well tolerated. One patient out of 36 (safety population) experienced an adverse event comprising nocturnal pollakiuria. This adverse event was mild and did not lead to discontinuation of supplement use. No serious adverse events were reported.
All statistically significant results reported for the ITT population were also observed in the PP population except for sleep efficiency at night during weekends (p = 0.06).
4. Discussion
In this study performed with recognised and validated criteria and measurement scales for the diagnosis and assessment of insomnia and anxiety, use of a supplement combining eschscholtzia and valerian extracts was associated with a significant decrease in the severity of adjustment insomnia as assessed by the ISI score. Mean ISI score decreased by approximately 30% (approximately 5 points) between the first visit and the last visit four weeks later. Similar decreases in the overall ISI score have been previously reported with other herbal medicines and also psychological/behavioural interventions. For instance, in a double-blind, randomized, controlled trial, the efficacy of a polyherbal medicine containing valerian (valerian, passion flower and hop combination) was compared to that of zolpidem in patients with primary insomnia (fewer than 6 h of sleep per night, ISI score > 7).34 A two-week treatment induced an ISI decrease of approximately 5.5 points, an effect that did not differ from that observed with zolpidem. A similar decrease was reported in another study performed in patients with cancer-related sleep disturbance who received Gamiguibi-tang, a traditional herbal formula, for two weeks.35 In a single-blind, randomized, controlled study performed in adults with chronic insomnia, mindfulness-based cognitive therapy induced a 4-point decrease in ISI score at 2 months.36 Similarly, a 6–8 point decrease in ISI score was reported after 8–9 weeks of cognitive behavioural therapy in two controlled studies enrolling adults with insomnia.37,38
In our study, patients were also less likely to have difficulties in falling asleep (one of the ISI components) one month after starting to use a supplement combining eschscholtzia and valerian extracts. In contrast, according to data from sleep diaries there was no improvement in sleep latency. However, the time of falling asleep was certainly not appropriately recorded in sleep diaries. Sleep latency should therefore also be measured objectively by actigraphy and its evaluation should not be based solely on the patient’s subjective assessment. Similar results on sleep maintenance and duration of sleep were nevertheless observed with regard to ISI scores and sleep diary data. ISI scores revealed that patients were less likely to have difficulties staying asleep and were also less likely to wake up too early. The analysis of information collected in sleep diaries showed a significant increase in night sleep duration (of approximately 36 min) between the first and the fourth week of supplement use. This improvement in the duration of sleep had a significant impact on sleep efficiency. Sleep efficiency was nearly 85% at the end of the one-month study period, which is the generally accepted cut-off for “normal” sleep efficiency.39 In addition, a 25% decrease in the number of awakenings was observed, associated with a 25-min reduction in their duration. According to ISI scores, there was also a lesser impact of sleep disorders on daytime functioning at the end of the study period. Patients were more satisfied with their sleep and less concerned about their sleep disorder. VAS scores confirmed the improvement in quality of life.
In addition to the improvement in sleep characteristics, a reduction in the level of anxiety was observed. Mean HAM-A score was significantly decreased by more than 50% (approximately 7 points) after one month of supplement use. This suggests that the combination tested could benefit sleep by inducing a general state of relaxation. Valerian has well-known beneficial effects on anxiety.17 We previously showed that a valerian extract had a relaxant effect on skeletal muscle40 and it is well known that muscle relaxation facilitates sleep.41,42
The main limitation of our observational study is obviously the absence of a control group. Due to the placebo effect, sometimes high in the context of insomnia treatment,2,7 the effect of the combination tested might be overestimated. It is also worth noting that the majority of the patients included were women of menopause age, but the multivariate analysis showed that this covariate had no impact on the results.
The management of insomnia disorders includes both pharmacological and non-pharmacological approaches. Cognitive behavioural therapy (CBT) is a first-line therapeutic approach for insomnia based on sleep hygiene measures and changes in maladaptive behaviour patterns.11 The goals of this approach are to control the patient’s environment, restrict the amount of time spent in bed, reduce outside stimuli, promote relaxation and mindfulness, limit caffeine and alcohol, and avoid daytime napping and exercise close to bedtime. The benefits of CBT are generally not evident straight away and their impact is not immediate.11 In addition, treatment access is poor since qualified CBT-therapists are rare and expensive. Hypnotic drugs are regularly used for insomnia and other sleep disorders, over 95% of insomniac patients being prescribed hypnotics in some countries.43 When prescribed, hypnotic medication should be continued for the shortest period of time as hypnotics and sedatives, such as benzodiazepines and barbiturates, are associated with undesirable effects, including adaptation, dependency, hang-over effects, increased sleep, apnoea and anterograde amnesia.17 There is an obvious need for treatments that are better tolerated and easier to implement.17 Adjustment insomnia is transient by definition and generally resolves once the triggering stressor disappears.2,7 It is therefore acknowledged that, in contrary to chronic insomnia, adjustment insomnia does not always require treatment. The recent European guideline for the diagnosis and treatment of insomnia states that “acute insomnia does not need a specific treatment in all cases”.7 The French guidelines recommend in the case of adjustment insomnia “first to take the drama out of the situation and provide psychological support; if needed, pharmacological treatment, whether sedative, anxiolytic or hypnotic, should be as light (phytotherapy) and brief as possible”.2 It is nevertheless important to keep in mind that adjustment insomnia can also be recurrent and may become chronic.2 Certain mechanisms inducing chronicity can be at work from the first month of insomnia. Therapeutic options capable of preventing the development of chronic insomnia17 and limiting its repercussions on health should therefore be available to patients.
Herbal medicines with relaxing and soothing properties could help the practitioner to manage insomnia, especially when the risk/benefit ratio of hypnotic drug prescription is likely to be unfavourable. For instance, elderly people are more sensitive to the potential adverse effects of drugs, and the risks generally outweigh any marginal benefits of hypnotics in this population.43 The usual adverse effects of benzodiazepines occur more frequently in the elderly, increasing in particular the risk of falls or driving accidents.2 Herbal medicines may offer an alternative treatment option that is both effective and devoid of the typical undesirable effects observed with conventional treatments. In our study, the combination tested was well tolerated with only one undesirable effect reported. Valerian is generally well tolerated.4,15,44 Minor adverse effects have been reported with chronic use of valerian extracts, including headaches, excitability, uneasiness, and insomnia.45 Very large doses may cause bradycardia and arrhythmias, and decrease intestinal motility. Data on the safety of eschscholtzia extracts are scarce; nonetheless, due to their opioid-like effects, they should not be used in patients manifesting sleep apnoea.
5. Conclusion
The results of this observational study performed in the primary care context suggest that the tested proprietary supplement containing eschscholtzia and valerian extracts could be of interest for the management of insomnia. An improvement in sleep quality and quantity, with a decrease in insomnia-induced daytime impairments were seen, all these factors constituting insomnia treatment goals.7 Whether this combination could be an effective alternative to conventional treatments for insomnia would need to be further investigated in a double-blind placebo-controlled study including actigraphy and polysomnography as objective measurements.
Conflicts of interest
AG was the Scientific Department Manager at Pileje Laboratoire at the time of the study; IG is Research Project Manager, AB and CB are Scientific and Medical Writers and SAA is Clinical Project Manager at PiLeJe Laboratoire. FD and MB are consultants for PiLeJe Laboratoire and gave advice on the study design.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Samira Ait Abdellah, Email: s.aitabdellah@pileje.com.
Aurélie Berlin, Email: a.berlin@pileje.com.
Claude Blondeau, Email: c.blondeau@pileje.com.
Isabelle Guinobert, Email: i.guinobert@pileje.com.
Angèle Guilbot, Email: angele.guilbot@gmail.com.
Marc Beck, Email: marc@beck31.net.
François Duforez, Email: fduforez@europeansleepcenter.fr.
References
- 1.American Academy of Sleep Medicine . third ed. 2014. International Classification of Sleep Disorders. [Google Scholar]
- 2.Haute Autorité de Santé Recommandations pour la pratique clinique: Prise en charge du patient adulte se plaignant d’insomnie en médecine générale. Revue de Pneumologie Clinique. [Recommandations] 2006;62:283–284. [Google Scholar]
- 3.World Health Organization The ICD10 classification of mental and behavioural disorders: diagnostic criteria for research. http://www.who.int/classifications/icd/en/GRNBOOK.pdf
- 4.Culpepper L., Wingertzahn M.A. Over-the-Counter agents for the treatment of occasional disturbed sleep or transient insomnia: a systematic review of efficacy and safety. Prim Care Companion CNS Disord. 2015;17 doi: 10.4088/PCC.15r01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohayon M.M. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 6.Ellis J.G., Gehrman P., Espie C.A., Riemann D., Perlis M.L. Acute insomnia: current conceptualizations and future directions. Sleep Med Rev. 2012;16:5–14. doi: 10.1016/j.smrv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Riemann D., Baglioni C., Bassetti C. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017 doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 8.Bertisch S.M., Wells R.E., Smith M.T., McCarthy E.P. Use of relaxation techniques and complementary and alternative medicine by American adults with insomnia symptoms: results from a national survey. J Clin Sleep Med. 2012;8:681–691. doi: 10.5664/jcsm.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leach M.J., Page A.T. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1–12. doi: 10.1016/j.smrv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhou E.S., Gardiner P., Bertisch S.M. Integrative medicine for insomnia. Med Clin. 2017;101:865–879. doi: 10.1016/j.mcna.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Schroeck J.L., Ford J., Conway E.L. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340–2372. doi: 10.1016/j.clinthera.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Cavadas C., Araujo I., Cotrim M.D. In vitro study on the interaction of Valeriana officinalis L. extracts and their amino acids on GABAA receptor in rat brain. Arzneim Forsch. 1995;45:753–755. [PubMed] [Google Scholar]
- 13.Yuan C.-S., Mehendale S., Xiao Y., Aung H.H., Xie J.-T., Ang-Lee M.K. The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anesth Analg. 2004;98:353–358. doi: 10.1213/01.ANE.0000096189.70405.A5. [DOI] [PubMed] [Google Scholar]
- 14.Boonstra E., Kleijn R de, Colzato L.S., Alkemade A., Forstmann B.U., Nieuwenhuis S. Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front Psychol. 2015;6:1520. doi: 10.3389/fpsyg.2015.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bent S., Padula A., Moore D., Patterson M., Mehling W. Valerian for sleep: a systematic review and meta-analysis. Am J Med. 2006;119:1005–1012. doi: 10.1016/j.amjmed.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández S., Wasowski C., Paladini A.C., Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol Biochem Behav. 2004;77:399–404. doi: 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency European Union herbal monograph on Valeriana officinalis L., radix. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2016/04/WC500205373.pdf Published.
- 18.European Medicines Agency European Union herbal monograph on Eschscholzia californica Cham., herba. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2015/05/WC500186550.pdf Published.
- 19.Vincieri F.F., Celli S., Mulinacci N., Speroni E. An approach to the study of the biological activity of Eschscholtzia californica Cham. Pharmacol Res Commun. 1988;20:41–44. doi: 10.1016/s0031-6989(88)80837-3. [DOI] [PubMed] [Google Scholar]
- 20.Rolland A., Fleurentin J., Lanhers M.C. Behavioural effects of the American traditional plant Eschscholzia californica: sedative and anxiolytic properties. Planta Med. 1991;57:212–216. doi: 10.1055/s-2006-960076. [DOI] [PubMed] [Google Scholar]
- 21.PDR for Herbal Medicines. third ed. Thomson PDR; Montvale: 2004. [Google Scholar]
- 22.Baldacci R. Contribution à l’utilisation en médecine de ville de l’Eschscholtzia californica. Phytotherapy. 1984;9:31–32. [Google Scholar]
- 23.Hanus M., Lafon J., Mathieu M. Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium in mild-to-moderate anxiety disorders. Curr Med Res Opin. 2004;20:63–71. doi: 10.1185/030079903125002603. [DOI] [PubMed] [Google Scholar]
- 24.Schäfer H.L., Schäfer H., Schneider W., Elstner E.F. Sedative action of extract combinations of Eschscholtzia californica and Corydalis cava. Arzneim Forsch. 1995;45:124–126. [PubMed] [Google Scholar]
- 25.Rolland A., Fleurentin J., Lanhers M.C., Misslin R., Mortier F. Neurophysiological effects of an extract of Eschscholzia californica Cham. (Papaveraceae) Phytother Res. 2001;15:377–381. doi: 10.1002/ptr.884. [DOI] [PubMed] [Google Scholar]
- 26.Morin C.M. Guilford Press; New York, London: 1993. Insomnia: Psychological Assessment and Management. (Treatment manuals for practitioners) [Google Scholar]
- 27.The Epworth Sleepiness Scale http://epworthsleepinessscale.com/about-the-ess/
- 28.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Bastien C. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 30.Morin C.M., Belleville G., Bélanger L., Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho Y.W., Song M.L., Morin C.M. Validation of a Korean version of the insomnia severity index. J Clin Neurol. 2014;10(3):210–215. doi: 10.3988/jcn.2014.10.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagnon C., Bélanger L., Ivers H., Morin C.M. Validation of the insomnia severity index in primary care. J Am Board Fam Med. 2013;26:701–710. doi: 10.3122/jabfm.2013.06.130064. [DOI] [PubMed] [Google Scholar]
- 33.Kay R. Statistical principles for clinical trials. J Int Med Res. 1998;26:57–65. doi: 10.1177/030006059802600201. [DOI] [PubMed] [Google Scholar]
- 34.Maroo N., Hazra A., Das T. Efficacy and safety of a polyherbal sedative-hypnotic formulation NSF-3 in primary insomnia in comparison to zolpidem: a randomized controlled trial. Indian J Pharmacol. 2013;45:34–39. doi: 10.4103/0253-7613.106432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.Y., Oh H.K., Ryu H.S., Yoon S.S., Eo W., Yoon S.W. Efficacy and safety of the traditional herbal medicine, gamiguibi-tang, in patients with cancer-related sleep disturbance: a prospective, randomized, wait-list-controlled, pilot study. Integr Canc Ther. 2017:524–530. doi: 10.1177/1534735417734914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong S.Y.-S., Zhang D.-X., Li C.C.-K. Comparing the effects of mindfulness-based cognitive therapy and sleep psycho-education with exercise on chronic insomnia: a randomised controlled trial. Psychother Psychosom. 2017;86:241–253. doi: 10.1159/000470847. [DOI] [PubMed] [Google Scholar]
- 37.Kaldo V., Jernelöv S., Blom K. Guided internet cognitive behavioral therapy for insomnia compared to a control treatment - a randomized trial. Behav Res Ther. 2015;71:90–100. doi: 10.1016/j.brat.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Bothelius K., Kyhle K., Espie C.A., Broman J.-E. Manual-guided cognitive-behavioural therapy for insomnia delivered by ordinary primary care personnel in general medical practice: a randomized controlled effectiveness trial. J Sleep Res. 2013;22:688–696. doi: 10.1111/jsr.12067. [DOI] [PubMed] [Google Scholar]
- 39.Reed D.L., Sacco W.P. Measuring sleep efficiency: what should the denominator Be? J Clin Sleep Med. 2016;12:263–266. doi: 10.5664/jcsm.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caudal D., Guinobert I., Lafoux A. Skeletal muscle relaxant effect of a standardized extract of Valeriana officinalis L. after acute administration in mice. Journal of Traditional and Complementary Medicine. 2017;8(2):335–340. doi: 10.1016/j.jtcme.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yilmaz C.K., Kapucu S. The effect of progressive relaxation exercises on fatigue and sleep quality in individuals with COPD. Holist Nurs Pract. 2017;31:369–377. doi: 10.1097/HNP.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 42.Machado FdS., Souza RCdS., Poveda V.B., Costa A.L.S. Non-pharmacological interventions to promote the sleep of patients after cardiac surgery: a systematic review. Rev Lat Am Enfermagem. 2017;25 doi: 10.1590/1518-8345.1917.2926. e2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmieri G., Contaldi P., Fogliame G. Evaluation of effectiveness and safety of a herbal compound in primary insomnia symptoms and sleep disturbances not related to medical or psychiatric causes. Nat Sci Sleep. 2017;9:163–169. doi: 10.2147/NSS.S117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-San-Martín M.I., Masa-Font R., Palacios-Soler L., Sancho-Gómez P., Calbó-Caldentey C., Flores-Mateo G. Effectiveness of Valerian on insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2010;11:505–511. doi: 10.1016/j.sleep.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization . 1999. WHO Monographs on Selected Medicinal Plants - Volume 1: Radix Valerianae.http://apps.who.int/medicinedocs/fr/d/Js2200e/29.html#Js2200e.29 [Google Scholar]