Abstract
Bidens pilosa (BP) is an edible Asteraceae plant found worldwide that has traditionally been used as food without noticeable side effects. BP has also been used as an herbal medicine to treat over 41 categories of disease in humans and animals. However, to date no long-term toxicity study of BP has been conducted in animals. In this study, 24-week oral toxicity of BP at doses of 0%, 0.5%, 2.5%, 5% and 10% of food was investigated in mice. Mortality, body weight, organ weight, food intake, water consumption, hematology, serum biochemistry, urinalysis, genotoxicity and organ histopathology of animals of both sexes were analyzed. No significant difference in the above parameters was observed between control and BP-fed mice except that body weight and food intake in those fed with 10% BP were significantly less than controls. In addition, similar results were seen in chickens fed with BP for 28 days. Collectively, the data demonstrate that BP has no adverse effects in mice and chickens at dose of 5% or less of food.
Keywords: Asteraceae, Mortality, Chronic toxicity, Adverse effect, Mice
Graphical abstract
1. Introduction
B. pilosa (BP) is widely distributed across different regions of the world. The Food and Agriculture Organization of the United Nations has reported BP as a staple food and promoted its cultivation in Africa since 1975.1 The Taiwan Ministry of Health and Welfare has legalized the use of BP as an ingredient in food for human and animal consumption.2 Whole plant or parts of BP, such as leaves, flowers, seeds, stems, and/or the roots, are used as the components of potherbs or medicinal herbs. In addition, dry powder, decoction, maceration and tincture are usual formulations for its internal as well as external use.3 BP has been reported to be used as a herbal medicine to treat over 41 categories of disease in humans and animals.1 Most of the functionality studies for BP have been conducted in cells and rodent models.4, 5, 6 More recently, one clinical pilot test showed that BP was therapeutically effective against diabetes in human patients.7
Systematic toxicology is crucial for the further development of BP or its parts as nutraceuticals and botanicals for human or animal use. So far, oral acute and 4-week toxicity of BP in rats and/or mice have been partially assessed. For instance, the median lethal dose (LD50) of the water and ethanol extracts of BP in oral acute toxicity study in mice was 12.3 g/kg body weight (BW) and 6.2 g/kg BW, respectively.8 Besides, oral delivery of the BP water extract at up to 1 g/kg BW/day, once a day, showed no obvious fatality or changes in rats over 4 weeks, as evidenced by survival rate, body weight and gross examination of organs.8,9 The overall data suggest that consumption of the aqueous extract of BP at a daily dose of 1 g/kg BW is generally recognized as safe in rats. However, current information about the toxicology studies of BP is fragmented and insufficient for a complete safety assessment. Therefore, a comprehensive investigation of long-term toxicology of BP is absolutely required for its clinical application for human or veterinary purposes.
In this study, the toxicity of the whole plant of BP was evaluated in both sexes of mice and chickens. Survival, body weight, organ weight, food intake, water consumption, hematology, serum biochemistry, urinalysis, organ pathology, and genotoxicity were investigated in all the animals.
2. Methods
2.1. Animals, diet and CO2 euthanasia
A total of 80 ICR mice, 40 mice of each sex, aged 5 weeks, were purchased from BioLASCO (Taipei, Taiwan). And one-day-old disease free 15 male Lohmann chicks were purchased from a local hatchery in Taichung, Taiwan. All the animals were health monitored followed by previous method10 and maintained on a 12-h light-dark cycle with controlled temperature (22 ± 2 °C) and humidity (55 ± 10%) in a specific pathogen-free animal facility. They were acclimatized on a free access to rodent standard diet (LabDiet 5010, St. Louis, MO) or and water for one week before study. Whole-plant powder of B. pilosa (BP) was produced in compliance with the good manufacturing practices guidelines and purchased from Ta-Fong Pharmaceutical Company (Taichung, Taiwan) as previously described.11 The powder of B. pilosa was ground into the particles and the size of these particles, ranging from 0.149 to 0.177 mm (i.e., 100 - 80 mesh), were collected for diet preparations. Rodent or chicken standard diet (0% BP) or the diet containing BP at 0.5%, 2.5%, 5%, and/or 10% were formulated as a modification of a previous publication4,12 and the gross energy in the diet supplemented with B. pilosa is shown in Sup. Table S3. Mortality, appearance, body weight, food and water intake were recorded weekly. In order to avoid effects of handling-associated stress, all mice were euthanized in their home cage and followed as previously report.13 The mouse home cage (Type 22, Biozone, Ramsgate, UK) was placed in a euthanasia chamber (44 cm × 24 cm × 21 cm) and covered with an acrylic lid. Compressed CO2 gas was provided from CO2 cylinder and controlled by a CO2-specific regulator (C.C. gaseous corporation, New Taipei City, Taiwan). Chamber air was replaced with CO2 at chamber replacement rates (CRR) of 50%–100%. Mice were monitored continuously during the procedure and mice were immobile. All procedures were approved by the Institutional Animal Care and Use Committee of Academia Sinica (approved protocols no. 12-12-478 and 103–34) and followed the guidance for the Use of Laboratory Animals (National Academy Press, Washington, DC).
2.2. Toxicity study
Each gender of ICR mice was assigned to 5 groups (8 mice/sex/group). The mice in groups 1 to 4 were given 0.5%, 2.5%, 5% or 10% BP, once a day, for 24 weeks, respectively, and the control mice in group 5 were fed with standard diet (0% BP). Survival rate, water intake, food consumption, body weight and gross examination of the mice were monitored on a daily basis. Similarly, 3 groups of 1-day-old chickens (5 chickens/group) were given standard diet or standard diet containing BP at 0.5% and 5% for 28 days. The same parameters as those measured in mice were monitored. By the end of the experiment, all the animals were sacrificed. Cardiac blood samples were collected for blood smear, hematologic and clinical chemistry analysis. In addition, the organs were removed, weighed and selected for histological examination.
2.3. Urinalysis, hematological and biochemical analysis
In the last week of treatment, mice were placed in metabolic cages (Shinano Manufacturing, Tokyo, Japan) for urine collection. A urinalysis of each group was performed using an AE-4020 urine analyzer (Arkray, Tokyo, Japan) according to the manufacturer's protocols. Cardiac blood in the presence of EDTA was collected for hematological parameters using a Cell-Dyn 3700 hematology counter (Abbott, IL, USA). Similarly, blood samples of the animals were collected in vacutainers without anticoagulant for biochemical estimation. Their sera were analyzed using a Dri-Chem 4000i analyzer (Fuji, Tokyo, Japan).
2.4. Histochemical and blood film staining
Tissues were collected at necropsy for histological examination: liver, kidneys, lungs, spleen, skeletal muscle, heart, aorta, adrenal glands, brain, pancreas, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, mesenteric lymph node, salivary glands, mandibular lymph node, thymus, larynx/pharynx with tongue, thyroid gland, parathyroid glands, trachea, esophagus, skin, mammary glands (females only), urinary bladder, female and male reproductive organs. All tissues were fixed in 10% neutral buffered formalin, processed into paraffin-embedded blocks and then sectioned. After staining with hematoxylin and eosin (H&E), the histological changes where examined via light microscopy. The mounted specimens were observed and were scored under light microscopy. For a semi-quantitative comparison of the structural changes, the abnormalities of tissue damage in the sections were graded from 0 (normal structure) to 3 (severe pathological changes).14 Blood films were made to analyze blood cells by smearing a drop of mouse blood on the glass slide. The drop was spread by using another slide, placing the spreader at a 45° angle and backing into the drop of blood. The spreader catches the drop, and it spreads by capillary action along its edge. The smear is air-dried for 1 min and stained with ASK Quick Blood Cell Stain kit (Tonyar Biotech, Taoyuan, Taiwan), followed by microscopic examination.
2.5. Genotoxicity study
The mouse peripheral blood micronucleus assay was carried out for genotoxicity evaluation. The micronucleus assays using acridine orang (AO) coated technique was described elsewhere.15 Briefly, AO (125 μg/mL in pH 6.8 phosphate buffer) was spread on the slide by moving a glass rod back and forth over the slide and then air-dried. To detect micronucleus, 5–10 μL of peripheral blood was collected from tail vein of the mice. The blood samples were placed on the glass slides and covered with coverslips. All the slides were kept in plastic bags in the dark at 4 °C. Using a fluorescence microscope, PCE were identified by red fluorescing reticulum in the cytoplasm, and MNPCE were identified by greenish yellow (Sup. Fig. S4). For each animal, one thousand erythrocytes and PCE were examined and MNPCE were scored in PCE.
2.6. Statistical analysis
The data are represented as mean ± standard error of mean (SEM), and all data were analyzed using a one-way ANOVA with and Tukey's multiple comparison test. P values of less than 0.05 were considered to be statistically significant.
3. Results
3.1. General conditions
In-feed supplementation with 0%, 0.5%, 2.5%, 5% or 10% BP was evaluated for toxicity to comply with the guidance for the toxicology assessment of new animal drugs in rodents (Center for Veterinary Medicine, GFI#149 and VICH#33). In this study, the mice, given a daily dose of 0%, 0.5%, 2.5%, 5% or 10% BP, over 24 weeks, appeared healthy. No tail lift, color change in skin or hair, abnormal behavior, gait, or posture was observed in any mouse. In addition, no noticeable abnormal behavior and appearance were noticed in any BP-fed chicken.
3.2. BW, organ weight, and food/water consumption
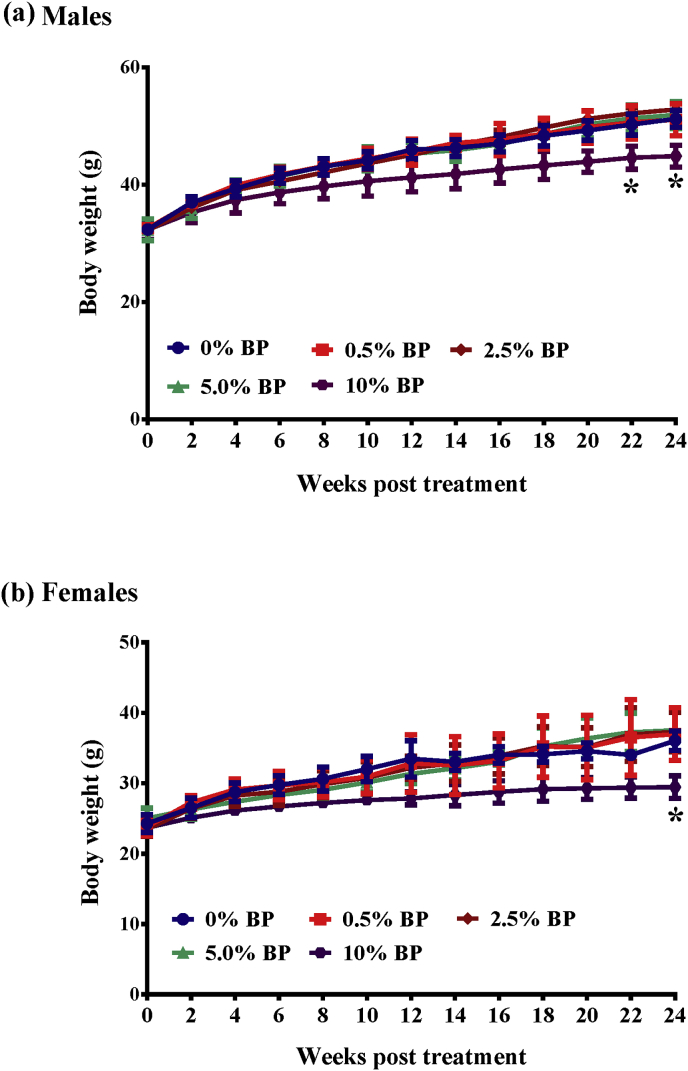
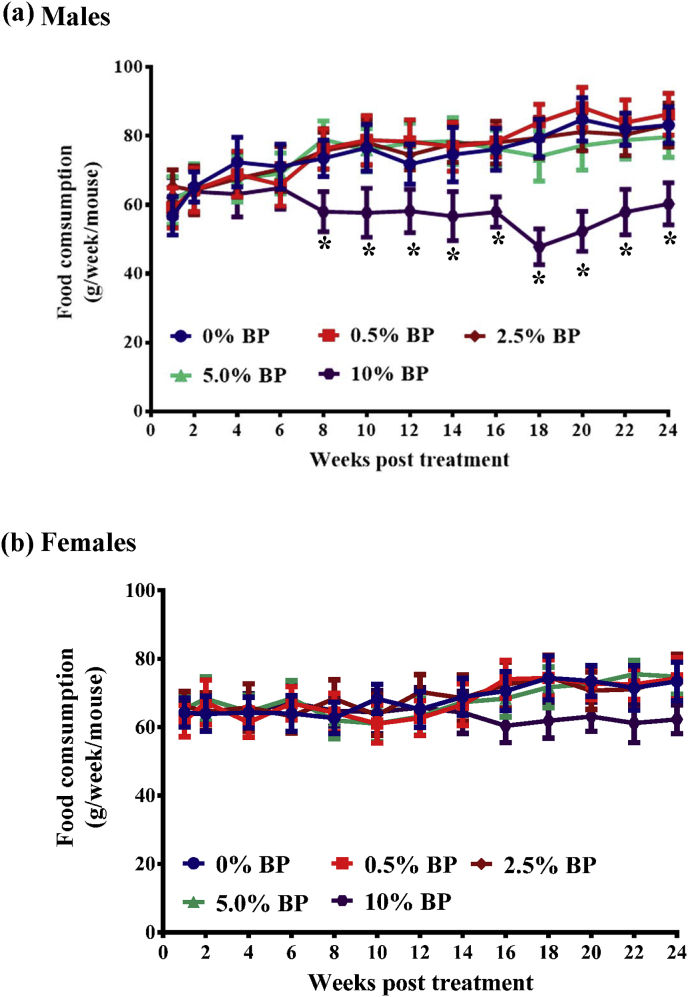
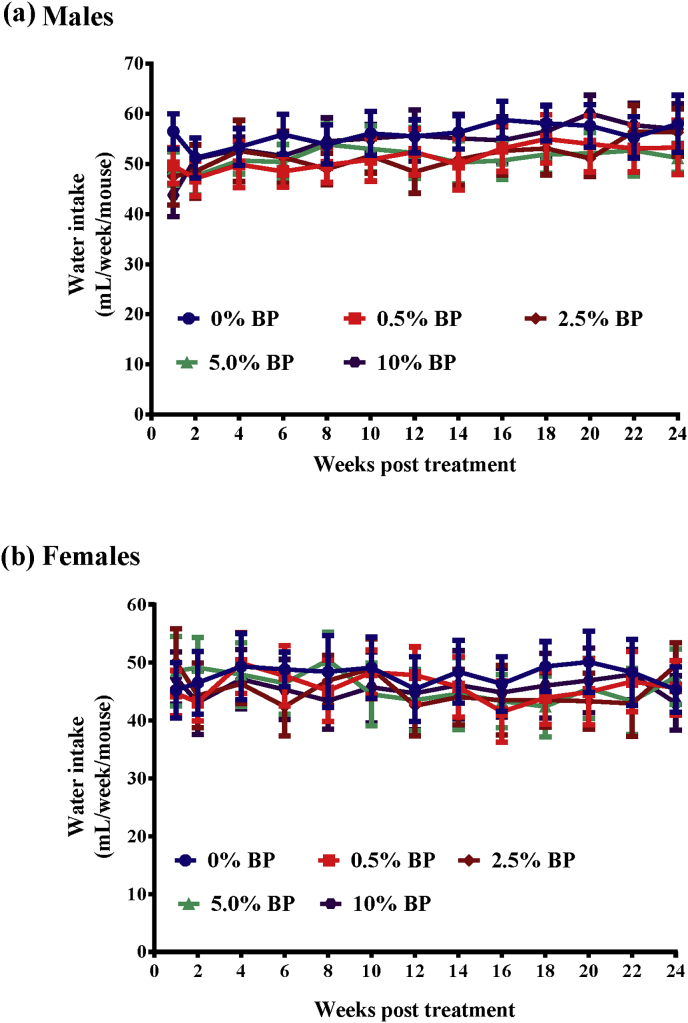
All the animals fed with different doses of BP were monitored daily for their BW and food/water consumption. We first found that all the mice fed with 0%–5% of BP steadily increased body weight with time (Fig. 1). Besides, BW, weight gain, and food intake were comparable in both sexes in the control (0% BP) and treatment groups (0.5%, 2.5% and 5% BP) (Fig. 1, Fig. 2). In contrast, mice of both sexes fed with 10% BP decreased body weight (10% BP, Fig. 1) and food intake (10% BP, Fig. 2). Of note, the body weight and food intake in mice fed with 10% BP were significantly less than both in mice of any the other groups after 24-week treatment (Fig. 1, Fig. 2). However, water consumption (Fig. 3) was similar in all groups. At the end of the 24-week treatment period, the mice were sacrificed and main organ weights such as heart, liver, spleen, lung, kidney, brain and sexual organs showed no significant differences between the control and BP-fed groups in either sex (Table 1). Similarly, chickens fed with 0%–5% of BP steadily increased body weight with time (Sup. Fig. S1a). However, no difference in food intake (Sup. Fig. S1b), water consumption (Sup. Fig. S1c) and main organ weights (Sup. Table S1) between the control and BP-fed chickens was seen.
Fig. 1.
Changes in body weight in ICR male and female mice fed with BP for 24 weeks. All ICR mice, aged 5 weeks, were randomly assigned into 4 groups, 5 males/females a group. Each group was given a standard diet and a standard diet containing 0.5% BP, 1.5% BP, 2.5% BP, 5% BP and 10% BP for 24 weeks. Body weight in each group of males (a) and females (b) was monitored before and after 13-week treatment. The data from each group are expressed as mean ± SEM. ANOVA was used to compare the difference between the control and treatment groups. P values of less than 0.05 (*) are considered statistically significant.
Fig. 2.
Food consumption in ICR males and females fed with BP for 24 weeks. Food intake in males (a) and females (b) from Fig. 1 was monitored before and after 24-week treatment. The data from each group are expressed as mean ± SEM. ANOVA was used to compare the difference between the control and treatment groups. P values of less than 0.05 (*) are considered statistically significant.
Fig. 3.
Water intake in ICR males and females fed with BP for 24 weeks. Water intake in males (a) and females (b) from Fig. 1 was monitored before and after 24-week treatment. The data from each group are expressed as mean ± SEM. ANOVA was used to compare the difference between the control and treatment groups.
Table 1.
Organ weight of ICR mice in 24-week feed study of B. pilosa.
| Organ (g) | Males |
Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% BP | 0.5% BP | 2.5% BP | 5% BP | 10% BP | 0% BP | 0.5% BP | 2.5% BP | 5% BP | 10% BP | |
| Heart | 0.222 ± 0.023 | 0.217 ± 0.015 | 0.209 ± 0.024 | 0.235 ± 0.021 | 0.227 ± 0.033 | 0.155 ± 0.011 | 0.165 ± 0.014 | 0.154 ± 0.011 | 0.162 ± 0.023 | 0.149 ± 0.025 |
| Liver | 2.237 ± 0.254 | 2.114 ± 0.314 | 2.406 ± 0.223 | 2.223 ± 0.155 | 2.233 ± 0.221 | 1.698 ± 0.165 | 1.887 ± 0.215 | 1.797 ± 0.311 | 1.788 ± 0.254 | 1.777 ± 0.183 |
| Spleen | 0.122 ± 0.013 | 0.123 ± 0.016 | 0.118 ± 0.023 | 0.131 ± 0.025 | 0.127 ± 0.028 | 0.128 ± 0.029 | 0.132 ± 0.024 | 0.124 ± 0.042 | 0.124 ± 0.012 | 0.123 ± 0.013 |
| Lung | 0.255 ± 0.034 | 0.258 ± 0.022 | 0.265 ± 0.037 | 0.238 ± 0.045 | 0.247 ± 0.036 | 0.275 ± 0.035 | 0.229 ± 0.034 | 0.242 ± 0.043 | 0.247 ± 0.036 | 0.253 ± 0.037 |
| Kidney | 0.644 ± 0.062 | 0.634 ± 0.059 | 0.665 ± 0.044 | 0.678 ± 0.062 | 0.657 ± 0.038 | 0.427 ± 0.071 | 0.417 ± 0.053 | 0.439 ± 0.066 | 0.426 ± 0.065 | 0.414 ± 0.039 |
| Brain | 0.501 ± 0.036 | 0.511 ± 0.032 | 0.541 ± 0.056 | 0.512 ± 0.029 | 0.527 ± 0.047 | 0.530 ± 0.033 | 0.497 ± 0.054 | 0.511 ± 0.046 | 0.502 ± 0.076 | 0.524 ± 0.023 |
| Testis | 0.295 ± 0.021 | 0.301 ± 0.037 | 0.287 ± 0.054 | 0.303 ± 0.027 | 0.291 ± 0.016 | – | – | – | – | – |
| Ovary | – | – | – | – | – | 0.029 ± 0.006 | 0.025 ± 0.004 | 0.028 ± 0.004 | 0.026 ± 0.007 | 0.028 ± 0.005 |
3.3. Clinical biochemistry
Serum and urine samples of the animals fed with different doses of BP for 24 weeks were collected for clinical biochemical parameters. The data on the effect of BP on mouse serum chemistry are summarized in Table 2. Glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), alkaline phosphatase (ALP), total protein (TP), albumin (Alb), blood urine nitrogen (BUN), creatinine (CRE), lactate dehydrogenase (LDH), and creatine phosphokinase (CPK) showed no statistically significant difference between the control and BP-fed mice. The effect of BP on mouse urine parameters is presented in Table 3. The results indicated that specific gravity (SG), pH, urobilinogen (Uro), protein (Pro), glucose (Glu), bilirubin (Bili), ketone (Ket), blood (Bld), leucocyte (Leu) and nitrite were all within the normal physiological range in the control and BP-fed mice. Likewise, no difference in clinical biochemistry between the control and BP-fed chickens was observed (Sup. Table S2). Taken together, these results suggest that BP at the indicated doses did not compromise the function of the liver, kidney, heart, and muscle in animals.
Table 2.
Biochemical parameters for ICR mice in 24-week feed study of B. pilosa.
| Group | General parameter |
Cardiac function parameter |
Renal function parameter |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | p value | LDH (U/L) | p value | CPK (U/L) | p value | BUN (mg/dL) | p value | CRE (mg/dL) | p value | ||
| Male (N = 5) | |||||||||||
| 0% BP | 179.6 ± 32.8 | – | 286.8 ± 38.7 | – | 54.3 ± 11.3 | – | 27.3 ± 7.6 | – | 0.31 ± 0.05 | – | |
| 0.5% BP | 147.5 ± 42.8 | 0.588 | 303.9 ± 51.6 | 0.501 | 52.6 ± 19.2 | 0.907 | 21.1 ± 2.3 | 0.410 | 0.29 ± 0.03 | 0.511 | |
| 2.5% BP | 181.4 ± 28.6 | 0.887 | 271.4 ± 41.3 | 0.657 | 57.1 ± 15.8 | 0.879 | 28.8 ± 5.6 | 0.702 | 0.33 ± 0.08 | 0.624 | |
| 5% BP | 161.2 ± 29.2 | 0.791 | 294.6 ± 35.7 | 0.589 | 58.8 ± 23.4 | 0.803 | 26.9 ± 9.1 | 0.803 | 0.24 ± 0.07 | 0.352 | |
| 10% BP | 151.2 ± 33.7 | 0.543 | 308.5 ± 43.2 | 0.486 | 48.7 ± 26.1 | 0.713 | 27.5 ± 4.3 | 0.867 | 0.29 ± 0.06 | 0.543 | |
| Female (N = 5) | |||||||||||
| 0% BP | 157.3 ± 41.3 | – | 297.8 ± 54.5 | – | 49.7 ± 10.8 | – | 24.5 ± 7.5 | – | 0.35 ± 0.06 | – | |
| 0.5% BP | 166.5 ± 33.9 | 0.437 | 285.0 ± 44.7 | 0.719 | 58.4 ± 16.3 | 0.643 | 20.6 ± 5.8 | 0.506 | 0.31 ± 0.07 | 0.582 | |
| 2.5% BP | 151.6 ± 38.1 | 0.898 | 281.2 ± 40.5 | 0.701 | 52.5 ± 9.60 | 0.715 | 24.1 ± 8.7 | 0.881 | 0.29 ± 0.04 | 0.407 | |
| 5% BP | 154.8 ± 27.6 | 0.913 | 274.3 ± 65.8 | 0.572 | 50.1 ± 12.1 | 0.812 | 21.3 ± 6.3 | 0.568 | 0.25 ± 0.08 | 0.322 | |
| 10% BP |
148.7 ± 41.5 |
0.477 |
292.7 ± 55.8 |
0.811 |
50.4 ± 17.2 |
0.887 |
25.9 ± 5.8 |
0.783 |
0.24 ± 0.09 |
0.286 |
|
| Group | Hepatic function parameter | ||||||||||
| GOT (U/L) |
p value |
GPT (U/L) |
p value |
ALP (U/L) |
p value |
TP (g/dL) |
p value |
Alb (g/dL) |
p value |
||
| Male (N = 5) | |||||||||||
| 0% BP | 40.8 ± 7.9 | – | 28.5 ± 5.7 | – | 125.8 ± 26.7 | – | 6.32 ± 0.56 | – | 4.14 ± 0.47 | – | |
| 0.5% BP | 48.1 ± 9.3 | 0.469 | 24.3 ± 6.3 | 0.349 | 133.4 ± 51.9 | 0.407 | 5.86 ± 0.73 | 0.269 | 4.61 ± 0.39 | 0.258 | |
| 2.5% BP | 39.8 ± 7.7 | 0.601 | 24.1 ± 9.2 | 0.316 | 115.2 ± 47.8 | 0.243 | 6.13 ± 0.68 | 0.574 | 4.25 ± 0.65 | 0.614 | |
| 5% BP | 42.6 ± 8.2 | 0.553 | 29.7 ± 8.6 | 0.679 | 129.6 ± 32.4 | 0.685 | 6.07 ± 0.82 | 0.423 | 3.98 ± 0.73 | 0.683 | |
| 10% BP | 41.2 ± 5.8 | 0.723 | 23.2 ± 6.1 | 0.277 | 122.5 ± 54.8 | 0.746 | 6.45 ± 0.31 | 0.618 | 4.38 ± 0.51 | 0.437 | |
| Female (N = 5) | |||||||||||
| 0% BP | 43.0 ± 7.8 | – | 26.1 ± 7.4 | – | 139.8 ± 35.6 | – | 6.61 ± 0.44 | – | 3.81 ± 0.72 | – | |
| 0.5% BP | 41.9 ± 4.9 | 0.446 | 28.7 ± 8.7 | 0.458 | 128.5 ± 38.2 | 0.476 | 6.42 ± 0.37 | 0.665 | 4.03 ± 0.45 | 0.415 | |
| 2.5% BP | 40.5 ± 8.7 | 0.312 | 22.3 ± 6.4 | 0.415 | 123.4 ± 41.6 | 0.363 | 6.18 ± 0.85 | 0.392 | 4.32 ± 0.82 | 0.376 | |
| 5% BP | 40.1 ± 6.4 | 0.323 | 20.8 ± 7.5 | 0.347 | 132.7 ± 23.6 | 0.691 | 6.24 ± 0.36 | 0.491 | 3.75 ± 0.61 | 0.676 | |
| 10% BP | 42.6 ± 8.4 | 0.682 | 25.6 ± 8.2 | 0.532 | 126.9 ± 37.2 | 0.403 | 6.47 ± 0.29 | 0.652 | 3.98 ± 0.85 | 0.631 | |
Table 3.
Urinalysis of ICR mice in 24-week feed study of B. pilosa.
| Parameter (units) | Male |
Female |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% BP | 0.5% BP | 2.5% BP | 5% BP | 10% BP | 0% BP | 0.5% BP | 2.5% BP | 5% BP | 10% BP | |
| SGa | 1.010 ± 0.005 | 1.012 ± 0.005 | 1.014 ± 0.003 | 1.011 ± 0.003 | 1.011 ± 0.005 | 1.015 ± 0.004 | 1.010 ± 0.006 | 1.014 ± 0.004 | 1.013 ± 0.004 | 1.011 ± 0.005 |
| pHa | 6.44 ± 0.37 | 6.52 ± 0.23 | 6.39 ± 0.54 | 6.50 ± 0.41 | 6.51 ± 0.36 | 6.51 ± 0.28 | 6.42 ± 0.49 | 6.51 ± 0.47 | 6.41 ± 0.63 | 6.47 ± 0.35 |
| Uro (mg/dL)b | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Pro (mg/dL)c | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Glu (mg/dL)d | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Bili (mg/dL)d | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Ket (mg/dL)d | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Bld (μmol/L)d | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Leu (cells/μL)d | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Nitrited | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
Urine samples of both sexes of mice from Fig. 1 were analyzed after 24-week treatment. Abbreviations: SG, specific gravity; Uro, urobilinogen; Pro, protein; Glu, glucose; Bili, bilirubin; Ket, ketone; Bld, blood; and Leu, leucocyte.
Each value represents mean ± SEM.
Normal means that all values are less than or equal to 0.1 mg/dL.
Normal means that all values are less than 50 mg/dL.
Negative connotes that these analytes are within the physiological level in the urine samples.
3.4. Hematological parameters
Blood samples of the mice fed with different doses of BP for 24 weeks were collected for hematological analysis. The data on the effect of BP on hematological values are summarized in Table 4. The number of white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), platelets, lymphocytes, monocytes, eosinophils and neutrophils in the blood of the control and BP-fed mice showed no statistically significant difference. Those hematological parameters revealed that the BP at the indicated doses did not cause any hematologic toxicity in animals.
Table 4.
Hematological values for ICR mice in 24-week feed study of B. pilosa.
| Parameter | Unit | Males |
Females |
References25 range of 20–24 week ICR mice |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% BP | 0.5% BP | 2.5% BP | 5% BP | 10% BP | 0% BP | 0.5% BP | 2.5% BP | 5% BP | 10% BP | Male | Female | ||
| RBC | 1012/L | 9.52 ± 0.61 | 9.68 ± 0.65 | 9.33 ± 0.61 | 9.52 ± 0.39 | 9.85 ± 0.79 | 9.71 ± 0.39 | 9.88 ± 0.73 | 9.98 ± 0.31 | 9.65 ± 0.44 | 9.98 ± 0.93 | 9.02–10.42 | 9.74–10.32 |
| WBC | 109/L | 9.88 ± 1.18 | 10.15 ± 2.32 | 9.71 ± 0.66 | 9.26 ± 0.83 | 9.53 ± 1.06 | 9.12 ± 1.74 | 9.25 ± 1.16 | 9.22 ± 0.62 | 9.55 ± 0.73 | 9.72 ± 1.14 | 9.13–14.71 | 8.87–14.69 |
| HGB | g/dL | 13.5 ± 1.6 | 14.3 ± 0.8 | 14.9 ± 1.4 | 16.1 ± 1.1 | 14.5 ± 0.6 | 14.8 ± 0.3 | 14.9 ± 0.2 | 14.5 ± 0.7 | 14.2 ± 1.2 | 15.0 ± 0.8 | 13.5–17.2 | 14.4–15.8 |
| HCT | % | 51.2 ± 2.1 | 53.0 ± 1.4 | 49.8 ± 3.0 | 52.9 ± 1.3 | 51.7 ± 2.5 | 52.1 ± 1.8 | 52.2 ± 0.9 | 51.1 ± 1.7 | 50.0 ± 1.7 | 51.8 ± 1.6 | 49.4–55.6 | 49.0–56.9 |
| MCV | fL | 55.5 ± 2.5 | 56.4 ± 3.2 | 55.5 ± 1.5 | 56.0 ± 2.7 | 56.1 ± 2.7 | 54.3 ± 0.8 | 55.8 ± 2.9 | 56.4 ± 2.9 | 54.7 ± 1.6 | 55.2 ± 2.4 | 49.9–56.5 | 49.0–57.9 |
| MCH | pg | 15.6 ± 0.6 | 15.7 ± 0.9 | 15.6 ± 0.4 | 16.2 ± 1.3 | 16.2 ± 1.7 | 16.5 ± 0.7 | 15.5 ± 0.6 | 15.8 ± 0.8 | 16.1 ± 0.8 | 16.3 ± 0.9 | 14.5–16.9 | 14.7–16.5 |
| Plates | 1010/L | 129.9 ± 16.1 | 104.6 ± 4.3 | 102.6 ± 14.8 | 119.8 ± 15.1 | 118.2 ± 16.1 | 109.2 ± 11.9 | 108.1 ± 6.9 | 111.0 ± 11.7 | 115.2 ± 4.3 | 112.1 ± 13.7 | 99.38–144.3 | 109.9–148.7 |
| Lymphocytes | % | 80.0 ± 4.1 | 76.6 ± 9.3 | 75.3 ± 5.7 | 70.2 ± 8.7 | 77.9 ± 5.8 | 78.2 ± 4.9 | 77.9 ± 5.3 | 74.4 ± 4.9 | 73.5 ± 8.9 | 75.3 ± 4.7 | 63.9–90.2 | 74.8–82.8 |
| Monocytes | % | 3.7 ± 0.8 | 4.1 ± 1.1 | 5.2 ± 1.2 | 4.5 ± 1.7 | 4.9 ± 1.3 | 4.1 ± 0.9 | 3.5 ± 0.8 | 6.4 ± 1.8 | 4.3 ± 0.8 | 4.9 ± 1.1 | 3.2–6.4 | 2.1–7.1 |
| Eosinophils | % | 1.2 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.7 | 0.8 ± 1.3 | 1.8 ± 1.3 | 0.7 ± 0.1 | 1.0 ± 0.4 | 1.2 ± 0.6 | 1.9 ± 0.7 | 1.7 ± 1.3 | 0.8–3.1 | 0.1–5.1 |
| Neutrophils | % | 16.3 ± 4.4 | 13.3 ± 3.1 | 16.7 ± 5.2 | 18.1 ± 6.4 | 16.5 ± 5.4 | 13.7 ± 1.1 | 15.7 ± 2.6 | 15.3 ± 5.5 | 19.3 ± 5.8 | 14.7 ± 2.8 | 2.1–27.3 | 9.1–19.3 |
Blood samples of both sexes of mice from Fig. 1 were analyzed after 24-week treatment. The data are expressed as mean ± SEM of hematological values. Statistical comparisons of control mice (0% BP) and BP-fed mice were performed by one-way ANOVA plus the Tukey multiple range test and no statistical difference between treatment and control groups was observed. Abbreviations: WBC, white blood count; RBC, red blood cell; HGB, hemoglobin; MCV, mean corpuscular volume; and MCH, mean corpuscular hemoglobin.
3.5. Histology in organs
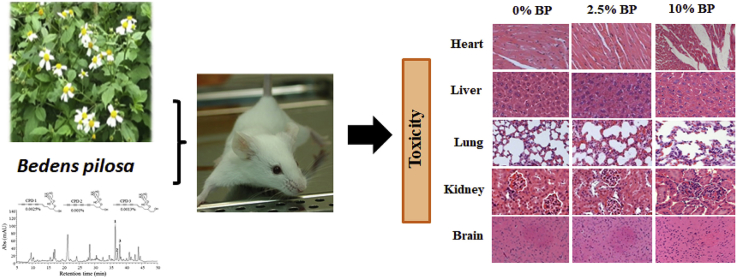
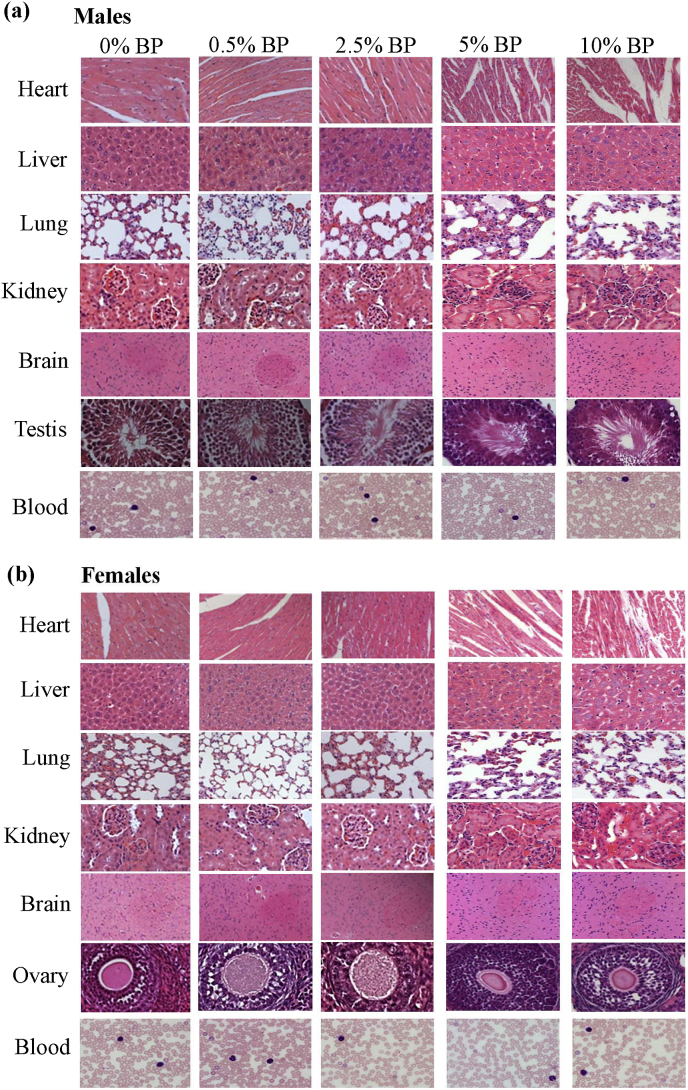
We also examined the histopathology of the organs from the control and BP-fed animals in an attempt to investigate toxicity of BP. After 24-weeks of treatment, the vital organs of the sacrificed mice, including heart, liver, lung, kidney, brain and sexual organs, were fixed with formalin and embedded in paraffin. Sections from these organs were stained with hematoxylin and eosin. Representative images of the organs were photographed under low-power microscopes (Fig. 4). The gross examination data showed no difference in the organs of animals between the control and BP-fed groups as characterized by tissue damage, inflammation, lesions, hemorrhage, hyperplasia and cell death. Further, blood films were used to analyze the peripheral blood samples from both sexes of mice fed with standard diet (0% BP) and BP-supplemented diets. The blood film data indicated that no difference in the number and shape of red blood cells, platelets and white blood cells from control and BP-fed mice was observed (Fig. 4). Similarly, no difference in histology between control and BP-fed chickens was seen (Sup. Fig. S2). The histological examination scores also showed no pathological changes (pathological score = 0) in the organs of animals between all groups (Sup. Fig S2 and S3). Taken together, the histological data showed that BP had no obvious toxicity in the main vital organs of the animals fed with the indicated doses.
Fig. 4.
Histochemical staining of the selected organs of ICR males and females in 24-week feed study of BP. Major organs (heart, liver, lung kidney, brain, sexual organs and blood) of ICR mice from Fig. 1 were taken and analyzed with hematoxylin and eosin staining or blood film technique. Their representative images are shown.
3.6. Survival rate and genotoxicity study
All the mice of each group, given a daily dose of 0%–10% BP, had 100% survival rate by the end of the 24-week treatment period. No tumors were developed in all mice of each group. The genotoxic effect of BP was evaluated using mouse peripheral blood micronucleus assays on erythocytes. Micronucleus assay data indicated that the average number of micronucleated polychromatic erythrocytes (MNPCE) in 1000 polychromatic erythrocytes (PCE) from animals of each group ranged from 0.9 to 1.8 (Table 5). Further, representative florescent photomicrographs of MNPCE and PCE in the animals of every group are shown in Sup. Fig. S4. That data suggests no significant difference in genotoxicity in mice fed with 10% BP or less. Likewise, survival rate of control and BP-fed chickens was 100%.
Table 5.
The average number of MNPCE/1000PCE in peripheral blood of mice fed with different doses of B. pilosa.
| Treatment | MNPCE/1000 PCEa |
|
|---|---|---|
| Males | Females | |
| 0% BP | 1.1 ± 0.6 | 1.3 ± 0.6 |
| 0.5% BP | 1.0 ± 0.9 | 1.6 ± 1.1 |
| 2.5% BP | 0.9 ± 0.7 | 1.1 ± 0.9 |
| 5.0% BP | 1.4 ± 0.8 | 1.8 ± 0.9 |
| 10% BP | 1.4 ± 1.0 | 1.2 ± 0.6 |
MNPCE, micronucleated polychromatic erythrocyte; and PCE, polychromatic erythrocyte.
Overall, this work suggests no toxicity in mice and chickens fed with a daily dose of 5% BP or less for one month or longer.
3.7. HPLC and mass spectrometry analyses of phytochemicals in BP extracts
Apparently, BP toxicity depends on the property of phytochemicals in its different extracts. Lipid-soluble phytochemicals in BP ethanol extract could show higher toxicity than water-soluble ones in its water extract.1 Over 200 compounds, comprising aliphatics, flavonoids, terpenoids, phenylpropanoids, aromatics, porphyrins, polyynes, and other compounds, have been identified from BP as reported previously.1 Since the active phytochemicals of BP against chicken coccidiosis are yet identified, we failed to directly correlate BP toxicity with the content of its active compounds. Instead, we performed high performance liquid chromatography and mass spectrometry to characterize the phytochemical profile of BP (Sup. Fig. S5). Three polyynes, 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7 (CPD 1), 9,11-triyne, 2-β-D-glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne (CPD 2) and 3-β-D-glucopyranosyloxy-1-hydroxy-6(E)-tridecene-8,10,12-triyne (CPD 3), were identified. Using these polyynes as phenolic compounds, we were able to correlate the BP toxicity with the content of these polyynes (Sup. Fig. S5). BP at 10%, containing 2.5 mg/kg CPD 1, 1 mg/kg CPD 2 and 1.3 mg/kg CPD 3, correlated with a reduction of body weight and food consumption in mice.
4. Discussion
BP has traditionally been used as an ingredient of foodstuffs and botanical medicine for human and animal consumption. BP is generally recognized as safe1,2; however, its studies on systemic toxicity (e.g., acute, sub-acute, chronic and sub-chronic toxicities) in humans and animals are still inadequate and insufficient. So far, acute and/or sub-chronic toxicities have been evaluated in rats and mice. Oral acute and 28-day toxicities of water and/or ethanol extracts of BP were evaluated in rodents.8,9 The data suggest the toxicity of different extracts of BP is distinct since the LD50 of the water and ethanol extracts of BP in mice was 12.3 g/kg BW and 6.2 g/kg BW, respectively.8 The BP water extract at a daily dose of 1 g/kg BW/day showed no noticeable sub-chronic toxicity in rats over 28 days.8 The data are consistent with the observations in our publications.4, 5, 6 The objective of this study was to provide oral sub-chronic toxicity of the whole ground mass of BP in experimental animals (mice), which is required by the regulation of the investigational animal drug application in Taiwan and other countries. From scientific and practical aspects, this study provided, for the first time, the 24-week toxicity of the whole-plant powder of BP in mice compared to 4-week toxicity study of BP in chickens. BP at 5% or less, equal to 15 g BP/kg BW/day or less, once a day, over 24 weeks, showed no obvious toxicity in mice, which was related to the presence of its phytochemicals. Compared with the water and ethanol extracts of BP, the whole ground mass of BP seemed to have higher safety. In contrast with the previous toxicity studies in the water or ethanol extract of BP, this work was a long-term evaluation of the whole plant of BP. We also discovered that 10% BP (32.4 g/kg BW/day) slightly decreased body weight and food intake in mice. Such study could validate and benefit the use of BP as herbal medicine for animals and, eventually, humans. These data provided good information for further application of BP. Despite modest novelty, this work has high impact on food safety, agriculture and bio-industry and is not just a prolongation of the experimental time period. Particularly, phytogenics are emerging as an alternative approach to maintain health and/or treat diseases in animals.
More recently, we reported that the whole plant powder of BP at 0.5%–5% significantly suppressed chicken coccidiosis in a dose-dependent manner.7,16 In addition, BP had little or no drug resistance.16 Further, large-scale field trials of BP at 0.025% and 0.05% confirmed the superior action against coccidiosis.11 Toxicology study and safety of residues of veterinary drugs in rodents and target animals need to be assessed prior to filing an investigational new animal drug. In this work, BP at 5% and less had no oral toxicity in mice over a period of 24 weeks as evidenced by survival rate, body weight (Fig. 1), organ weight (Table 1), food intake (Fig. 2), water consumption (Fig. 3), serum biochemistry (Table 2), urinalysis (Table 3), hematology (Table 4), organ histopathology (Fig. 4), and genotoxicity (Table 5). In contrast, mice given a daily dose of 10% BP had the same toxicological parameters as those of any other group except that body weight and food intake in mice were slightly reduced. Since current commercial broilers can achieve a 2-kg market weight in 4–5 weeks,17,18 toxicity study of BP at 5% and 0.5% in chickens was conducted over a similar period of time (28 days). Similar to the data on BP toxicity in mice, body weight, food consumption, water intake and organ weight showed no significant difference between control and BP fed groups in chickens (Sup. Fig. S1-S2 and Sup. Tables S1-S2). Values of serum biochemistry (Sup. Table S2) between the control and BP fed chickens were within physiological range of refernces.19, 20, 21, 22, 23 Therefore, application of BP in food animals such as chickens should be feasible with noticeable toxicity.
Since two polyynes (CPD1 and 3) present in BP (Sup. Fig. S5) showed anoxic effect in diabetic mice,24 the body weight loss in mice fed with 10% BP could partially be attributed to decreased food intake. However, this loss had nothing to do with the type of diets because standard diet and BP-supplemented diets had similar gross energy (Sup. Table S3). The macroscopic and microscopic examination of the vital organs also implies that BP has no carcinogenic property. The dose of BP at 5% used in experimental mice and chickens is 200 times higher than its therapeutic dose (0.025% BP) used in chickens. The overall data on the toxicity study of BP in experimental animals (mice) and target animals (chickens) suggests its high safety for veterinary purpose.
Conflicts of interest
The authors have declared that no competing interests exist.
Funding source
This work was supported by grants from the Ministry of Science and Technology (MOST 106-3114-B-005-001, MOST 107-2321-B-001-033 and MOST 107-2321-B-001-038), Taiwan.
Author contributions
Y.C.L., C.J.L., C.Y.Y., Y.H.C., M.T.Y. and F.S.C. performed the experiments. C.L.T.C and W.C.Y. conceived the project idea, designed the study and supervised the project. Y.C.L. and W.C.Y. wrote the manuscript.
Acknowledgements
The authors thank the animal core facility of ABRC and the Taiwan Mouse Clinic for their excellent technical assistance. They also thank Ms. Miranda Loney for manuscript editing.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.04.002.
Major organs of male and female mice from Fig. 1 were selected and weighed after 24-week treatment. The data are expressed as mean ± SEM. Statistical comparisons of control mice (0% BP) and BP-fed mice were performed by one-way ANOVA with the Tukey multiple range test and no statistical difference between treatment and control groups was observed.
Blood samples of male and female mice from Fig. 1 were analyzed after 24-week treatment. The data are expressed as mean ± SEM. Statistical comparisons of control mice (0% BP) and BP-fed mice were performed by one-way ANOVA by the Tukey multiple range test. P values are calculated in relation to the control. Abbreviations: GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; CRE, creatinine; TP, total protein; and Alb, albumin.
Contributor Information
Wen-Chin Yang, Email: wcyang@sinica.edu.tw.
Cicero Lee-Tian Chang, Email: ltchang@nchu.edu.tw.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Bartolome A.P., Villasenor I.M., Yang W.C. Bidens pilosa L. (asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evid Based Complement Alternat Med. 2013;2013:340215. doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young P.H., Hsu Y.J., Yang C.W. Bidens pilosa L. and its medicinal use. In: Awaad A.S., Singh V.K., Govil J.N., editors. Recent Progress in Medicinal Plants Drug Plant II. vol. 28. Standium Press Pvt. Ltd.; New Delhi, India: 2010. [Google Scholar]
- 3.Redl K., Breu W., Davis B., Bauer R. Anti-inflammatory active polyacetylenes from Bidens campylotheca. Planta Med. 1994;60:58–62. doi: 10.1055/s-2006-959409. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y.C., Yang M.T., Lin C.J., Chang C.L., Yang W.C. Bidens pilosa and its active compound inhibit adipogenesis and lipid accumulation via down-modulation of the C/EBP and PPARgamma pathways. Sci Rep. 2016;6:24285. doi: 10.1038/srep24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu Y.J., Lee T.H., Chang C.L., Huang Y.T., Yang W.C. Anti-hyperglycemic effects and mechanism of Bidens pilosa water extract. J Ethnopharmacol. 2009;122:379–383. doi: 10.1016/j.jep.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Chien S.C., Young P.H., Hsu Y.J. Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry. 2009;70:1246–1254. doi: 10.1016/j.phytochem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Lai B.Y., Chen T.Y., Huang S.H. Bidens pilosa formulation improves blood homeostasis and beta-cell function in men: a pilot study. Evid Based Complement Alternat Med. 2015;2015:832314. doi: 10.1155/2015/832314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frida L., Rakotonirina S., Rakotonirina A., Savineau J.P. In vivo and in vitro effects of Bidens pilosa L. (Asteraceae) leaf aqueous and ethanol extracts on primed-oestrogenized rat uterine muscle. Afr J Tradit, Complementary Altern Med. 2007;5:79–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Ezeonwumelu J.O.C., Julius A.K., Muhoho C.N. Biochemical and histological studies of aqueous extract of Bidens pilosa leaves from Ugandan Rift Valley in rats. Br J Pharmacol Toxicol. 2011;2:302–309. [Google Scholar]
- 10.Tung H.Y., Chen W.C., Ou B.R. Simultaneous detection of multiple pathogens by multiplex PCR coupled with DNA biochip hybridization. Lab Anim. 2018;52:186–195. doi: 10.1177/0023677217718864. [DOI] [PubMed] [Google Scholar]
- 11.Chang C.L., Yang C.Y., Muthamilselvan T., Yang W.C. Field trial of medicinal plant, Bidens pilosa, against eimeriosis in broilers. Sci Rep. 2016;6:24692. doi: 10.1038/srep24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C.L., Chung C.Y., Kuo C.H., Kuo T.F., Yang C.W., Yang W.C. Beneficial effect of Bidens pilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146141. e0146141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin G.P., Bottomley M.A., Dudley E.S., Schiml P.A., Wyatt C.N., Grobe N. Physiological, behavioral, and histological responses of male C57BL/6N mice to different CO2 chamber replacement rates. J Amer Assoc Lab Anim Sci. 2016;55:451–461. [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim K.E., Al-Mutary M.G., Bakhiet A.O., Khan H.A. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23:1848–1861. doi: 10.3390/molecules23081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi M., Morita T., Kodama Y., Sofuni T., Ishidate M., Jr. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat Res. 1990;245:245–249. doi: 10.1016/0165-7992(90)90153-b. [DOI] [PubMed] [Google Scholar]
- 16.Yang W.C., Tien Y.J., Chung C.Y. Effect of Bidens pilosa on infection and drug resistance of Eimeria in chickens. Res Vet Sci. 2015;98:74–81. doi: 10.1016/j.rvsc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Turner J., Garcés L., Smith W. Compassion in World Farming Trust. 2005. The welfare of broiler chickens in the European Union. Hampshire, UK. [Google Scholar]
- 18.Damerow G. Storey Publishing; North Adams, MA, US.: 1995. Storey's Guide to Raising Chickens. [Google Scholar]
- 19.Sugiharto S., Yudiarti T., Isroli I. Haematological and biochemical parameters of broilers fed cassava pulp fermented with filamentous fungi isolated from the Indonesian fermented dried cassava. Livest Res Rural Dev. 2016;28 [Google Scholar]
- 20.Albokhadaim I. Hematological and some biochemical values of indigenous chickens in Al-Ahsa, Saudi Arabia during summer season. Asian J Poultry Sci. 2012;6:138–145. [Google Scholar]
- 21.Soleimani A.F., Zulkifli I. Effects of high ambient temperature on blood parameters in red jungle fowl, village fowl and broiler chickens. J Anim Vet Adv. 2010;9(8):7. [Google Scholar]
- 22.Silva P., Freitas Neto O., Laurentiz A., Junqueira O., Fagliari J. Blood serum components and serum protein test of Hybro-PG broilers of different ages. Rev Bras Ciência Avícola. 2007;9:229–232. [Google Scholar]
- 23.Bowes V.A., Julian R.J., Stirtzinger T. Comparison of serum biochemical profiles of male broilers with female broilers and White Leghorn chickens. Can J Vet Res. 1989;53:7–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Ubillas R.P., Mendez C.D., Jolad S.D. Antihyperglycemic acetylenic glucosides from Bidens pilosa. Planta Med. 2000;66:82–83. doi: 10.1055/s-0029-1243117. [DOI] [PubMed] [Google Scholar]
- 25.Takamasa I., Hiroshi Y., Toshiyuki H. Adthree Publishing Company Limited; Tokyo: 2001. A Color Atlas of Sectional Anatomy of the Mouse. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.