Abstract
Background/purpose
Oral metastatic carcinomas are rarely found in oral soft tissues. This study reported the clinicopathological features of 13 intraoral soft tissue metastatic carcinomas.
Materials and methods
A total of 13 intraoral soft tissue metastatic carcinomas were included in this study. The clinicopathological features of the 13 cases including the primary cancer site, metastatic intraoral soft tissue region, clinical presentation, and histopathological diagnoses were examined and reported.
Results
The 13 intraoral soft tissue metastatic carcinomas occurred in 13 patients (11 males and 2 females) with a mean age of 59.4 (range, 39–78) years. Nine cases originated from the liver (69.2%), and one each from the colon (7.7%), pancreas (7.7%), thyroid (7.7%), and kidney (7.7%). The histopathological diagnoses of the metastatic lesions were hepatocellular carcinoma in 9 cases, adenocarcinomas in 2 cases (one each from the colon and pancreas), clear cell carcinoma of the kidney in one case, and follicular thyroid carcinoma in one case. The gingiva and alveolar mucosa were the major metastatic sites (10 cases, 76.9%), followed by the buccal mucosa (two cases, 15.4%), and soft palate (one case, 7.7%). Twelve metastatic lesions manifested as ulcerated, easy-bleeding, and pyogenic granuloma-like lesions.
Conclusion
The results of our series of 13 cases indicate that intraoral soft tissue metastatic carcinomas have a male predilection with a male to female ratio of 11:2, are commonly found in the gingiva and alveolar mucosa (76.9%), present frequently as an easy-bleeding pyogenic granuloma-like lesion (92.3%). In addition, the most common primary cancer site is the liver.
Keywords: Oral metastatic carcinoma, Oral soft tissue
Introduction
Metastatic neoplasms of the mouth generally indicate a disseminated disease, with a mean survival duration of merely approximately seven months.1 Metastasis to the oral cavity is rare, representing approximately 1% of all oral malignant neoplasms, the primary origins of which may be anywhere.1 Metastases can involve either the jawbones, where most are found in the mandibular molar region, or more rarely the oral soft tissues (less than 0.1% of all oral malignancies).1 It has been suggested that the prevalence of oral metastases differs owing to differences in primary sites and between various geographic areas.1 Despite two large case series of metastatic neoplasms of oral soft tissue having been documented,2,3 a review of the English literature revealed that, to the best of our knowledge, no such study has been performed in Taiwan. The current study reports an analysis of a series of patients with metastatic tumors of the intraoral soft tissues (eliminating lymph node and/or tonsil tumors).
Materials and methods
A total of 13 cases of intraoral soft tissue metastasis were confirmed between January of 2000 and August of 2019 in the Department of Oral Pathology of our Institution. These 13 histopathologically-diagnosed cases were evaluated with regards to the primary site, metastatic intraoral region, clinical presentation, and histopathological diagnosis (Table 1).
Table 1.
Summary of the 13 intraoral soft tissue metastatic cases in the current study.
| Case no. | Age (years) | Gender | Site of primary malignancy | Histopathological type | Site of intraoral metastasis |
|---|---|---|---|---|---|
| 1 | 68 | Female | Liver | Hepatocellular carcinoma | Gingiva |
| 2 | 58 | Male | Liver | Hepatocellular carcinoma | Gingiva |
| 3 | 52 | Male | Liver | Hepatocellular carcinoma | Gingiva |
| 4 | 73 | Male | Liver | Hepatocellular carcinoma | Alveolar mucosa |
| 5 | 39 | Male | Liver | Hepatocellular carcinoma | Gingiva |
| 6 | 61 | Male | Liver | Hepatocellular carcinoma | Soft palatal mucosa |
| 7 | 51 | Male | Liver | Hepatocellular carcinoma | Buccal mucosa |
| 8 | 58 | Male | Liver | Hepatocellular carcinoma | Gingiva |
| 9 | 67 | Male | Liver | Hepatocellular carcinoma | Gingiva |
| 10 | 62 | Male | Pancreas | Adenocarcinoma | Alveolar mucosa |
| 11 | 43 | Male | Colon | Adenocarcinoma | Gingiva |
| 12 | 78 | Female | Thyroid | Follicular thyroid carcinoma | Buccal mucosa |
| 13 | 62 | Male | Kidney | Clear cell renal cell carcinoma | Gingiva |
Results
Gender and age
There were 11 male and 2 female patients in this study. The age of the 13 patients ranged from 39 to 78 years with a mean age of 59.4 years. The mean age of the male patients was 56.9 years, while that of the two female patients was 73.0 years (Table 1).
Primary sites
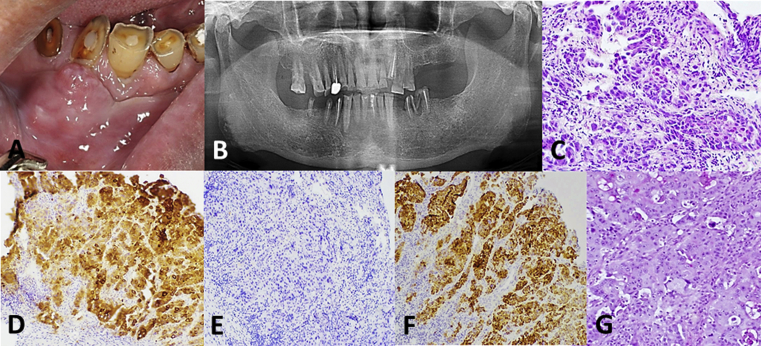
The primary site of malignancy had already been identified prior to discovery of the intraoral metastatic lesion in all patients. Representative clinical, radiographic, and histopathological images (case no. 8, Table 1) are presented in Fig. 1. Nine intraoral metastatic carcinomas originated from the liver (69.2%), and one each (7.7%) from the colon, pancreas, thyroid, and kidney. In the presence of the known malignancies, the histopathological diagnoses of the intraoral soft tissue metastases in the current series were established according to the similarity between the oral metastatic lesion and the original primary tumor, with the assistance of immunohistochemical staining using a panel of antibodies to different tumor markers (Fig. 1). The histopathological diagnoses of the 13 intraoral soft tissue metastatic lesions were hepatocellular carcinoma in 9 cases, adenocarcinomas in 2 cases (one each from the colon and pancreas), clear cell carcinoma of the kidney in one case, and follicular thyroid carcinoma in one case (Table 1).
Figure 1.
Clinical, radiographic, and microscopic photographs of case 8, a primary hepatocellular carcinoma with metastasis to the gingiva. (A) An exophytic, partially ulcerated mass at the buccal gingiva of teeth 43 to 45. (B) Panoramic radiography revealed no bony destruction at the mandibular body between teeth 43 and 45. (C) The metastatic carcinoma composed of islands of hyperchromatic and pleomorphic tumor cells (hematoxylin and eosin stain; magnification, 200×). (D, E and F) The tumor cells were positive for CK7 (D, magnification, 100×), negative for CK20 (E, magnification, 100×), and positive for HepaPar1 (F, magnification, 100×). (G) Microscopic features of the gingival metastatic lesion are consistent with a primary hepatocellular carcinoma (magnification, 100×).
Intraoral soft tissue sites with metastatic carcinomas
The gingiva and alveolar mucosa were the predominant metastatic sites (10 cases, 76.9%), followed by the buccal mucosa (two cases, 15.4%) and soft palatal mucosa (one case, 7.7%) (Table 1).
Signs and symptoms
Besides the metastatic thyroid carcinoma (case no. 12, Table 1), which presented as a painful ulceration, all the remaining 12 metastatic intraoral lesions manifested as ulcerated, easy-bleeding masses, with a clinical impression of pyogenic granuloma.
Discussion
The mean age of the patients in the present study was approximately 60 years, which was consistent with the two previous large case series studies conducted by Hirshberg et al.;2,3 additionally, it was compatible with four other series studies performed in Japanese (6 cases of oral soft tissue metastasis),4 Korean (18 cases of oral soft tissue metastasis),5 Dutch (24 cases of oral soft tissue metastasis),6 and Spanish patients (16 cases of oral soft tissue metastasis).7 In the present study, the rate of metastatic tumors was higher in males (male-to-female ratio of 5:1), which was compatible with the report of Murillo et al.7 (male-to-female ratio of 4.3:1) and much higher than that reported by Hirshberg et al.2 (male-to-female ratio of 1.6:1), but differed from the report of van der Waal et al.,6 who documented an equal sex distribution.
It has been reported that in up to one-third of patients with oral metastasis, this is the first clinical sign of an unrecognized primary malignancy,2 suggesting that detection of an oral metastasis could lead to finding of an occult primary malignancy elsewhere in other organs. The primary malignancies had already been identified prior to the appearance of the oral soft tissue metastatic lesions in the patients in the current study. Nevertheless, keeping oral metastasis as a differential diagnosis is important for dentists when examining patients suffering from primary cancers in other organs. In a Korean study, in 26.8% of the patients, metastatic oral lesions were the first sign of an unknown malignancy at a distant site.5
The liver was the most common primary site in our patient series, perhaps at least partly reflecting the high prevalence of carcinoma of the liver in Taiwan, and this was consistent with a previous study conducted in a Korean population.5 In other series, such as those of Hirshberg et al.2 and Murillo et al.,7 the lung was the most common site of the primary tumor causing metastases in the soft tissues of the mouth. On the other hand, the gingiva was the most commonly affected site in the present patient series, consistent with two other studies of Dutch and Korean populations.5,6 Regarding the clinical appearance, gingival metastatic lesions tend to ulcerate and bleed, and are painful, and so these lesions, as demonstrated in the current study, mimic benign hyperplastic and reactive lesions, such as pyogenic granuloma, hemangioma, peripheral giant cell granuloma and peripheral fibroma, leading to erroneous diagnoses.8,9 However, gingival metastatic lesions vary from reactive lesions due to the fact that they display rapid and progressive growth; confirmative diagnosis relies on biopsy and the alertness of the dentist who may be the first to encounter the oral lesion. The role of inflammation in increasing the affinity of metastatic cells in the gingiva has been speculated to be part of the pathogenesis of oral metastases.2
Despite their rarity, metastatic neoplasms should be considered in the differential diagnoses of an intraoral malignant neoplasm, particularly if there is a known primary malignancy that the histopathologic features resemble, especially as this could be the first indication of the existence of a primary malignancy in some patients.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2019.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hirshberg A., Berger R., Allon I., Kaplan H. Metastatic tumors to the jaws and mouth. Head Neck Pathol. 2014;8:463–474. doi: 10.1007/s12105-014-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirshberg A., Leibovich P., Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. 1993;22:385–390. doi: 10.1111/j.1600-0714.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirshberg A., Buchner A. Metastatic tumors to the oral region. An overview. Eur J Cancer B Oral Oncol. 1995;31B:355–360. doi: 10.1016/0964-1955(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura Y., Yakata H., Kawasaki T., Nakajima T. Metastatic tumors of the mouth and jaws. A review of the Japanese literature. J Maxillofac Surg. 1982;10:253–258. doi: 10.1016/s0301-0503(82)80050-7. [DOI] [PubMed] [Google Scholar]
- 5.Lim S.Y., Kim S.A., Ahn S.G. Metastatic tumours to the jaws and oral soft tissues: a retrospective analysis of 41 Korean patients. Int J Oral Maxillofac Surg. 2006;35:412–415. doi: 10.1016/j.ijom.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 6.van der Waal R.I., Buter J., van der Waal I. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. 2003;41:3–6. doi: 10.1016/s0266-4356(02)00301-7. [DOI] [PubMed] [Google Scholar]
- 7.Murillo J., Bagan J.V., Hens E., Diaz J.M., Leopoldo M. Tumors metastasizing to the oral cavity: a study of 16 cases. J Oral Maxillofac Surg. 2013;71:1545–1551. doi: 10.1016/j.joms.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Peris K., Cerroni L., Paoloni M. Gingival metastasis as first sign of an undifferentiated carcinoma of the lung. J Dermatol Surg Oncol. 1994;20:407–409. doi: 10.1111/j.1524-4725.1994.tb02626.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez J.R., Seoane J., Montero J., Esparza Gómez G.C., Cerero R. Isolated gingival metastasis from hepatocellular carcinoma mimicking a pyogenic granuloma. J Clin Periodontol. 2003;30:926–929. doi: 10.1034/j.1600-051x.2003.00391.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.