Abstract
Background/purpose
Enterococcus faecalis (E. faecalis) is considered a predominant pathogen for persistent periapical infections. Antisense walR (ASwalR) RNA was reported to inhibit the biofilm formation and sensitized E. faecalis to calcium hydroxide medication. The aims of this study were to investigate whether the graphene oxide (GO) nanosheets could be used to enhance antibacterial activity of ASwalR RNA for E. faecalis in periapical periodontitis.
Materials and methods
We developed a graphene-based plasmid transformation system by loading antisense walR plasmid with GO-polyethylenimine (PEI) complexes (GO-PEI-ASwalR). The particle size distributions and zeta-potential of the GO-PEI-ASwalR were evaluated. Then, ASwalR plasmids were labeled with gene encoding enhanced green fluorescent protein (ASwalR-eGFP). The transformation efficiencies and the bacterial viability of E. faecalis were evaluated by confocal laser scanning microscopy. Quantitative real-time PCR assays were used to investigate the expressions of E. faecalis virulent genes after transformed by GO-PEI-ASwalR. Also, the antibacterial properties of the GO-PEI-ASwalR were validated in the rat periapical periodontitis model.
Results
We showed that GO-PEI could efficiently deliver the ASwalR plasmid into E. faecalis cell. GO-PEI-ASwalR significantly reduced virulent-associated gene expressions. Furthermore, GO-PEI-ASwalR suppressed biofilm aggregation and improved bactericidal effects using infected canal models in vitro. In four-weeks periapical infective rat models, the GO-PEI-ASwalR strains remarkably reduced the periapical lesion size.
Conclusion
Transformation efficiency and antibacterial prosperity of ASwalR can be marked improved by GO-PEI based delivery system for E. faecalis infections.
Keywords: Enterococcus faecalis, walR, Graphene oxide, Antisense RNA, Periapical periodontitis
Introduction
Enterococcus faecalis (E. faecalis), a gram-positive coccus, is considered a predominant pathogen for persistent periapical infections.1,2 E. faecalis can survive in a wide range of temperature, pH values, oxygen tension, humidity and harsh nutrient availability.3 Also, it is able to tolerate to conventional calcium hydroxide medication and alkaline challenge.4,5 Two-component signal transduction systems (TCSs) play a key role in bacterial adaption to environmental challenges.6 The more often related virulent factors of E. faecalis include ace (collagen binding protein), epaA (glycosyltransferase) and gelE (gelatinase).7,8 However, attempts to inactivate the walRK locus in E. faecalis were unsuccessful, indicating the WalRK TCS is essential for E. faecalis viability.9
Antisense RNAs (asRNAs) which regulatory activity is based on complementary mechanisms to and base-pairing to the mRNA, and this interaction results in blocking the translation to functional protein.10 Antisense RNAs have been applied to identify and investigate novel antibacterial targets for clinical applications.11 We previously reported that antisense walR (ASwalR) RNA interference reduced the transcripts of the virulent genes, alkaline stress tolerance ability and biofilm aggregation.12 However, one of the biggest obstacles for the use of those therapeutic antisense oligonucleotides is their limited uptake by bacterial cells without a suitable and effective carrier system.13 A common transformation method is to conjugate an antisense RNA with a cell-penetrating peptide which can facilitate access process.12,14 Nevertheless, the shortcomings of these peptides included dubious biocompatibility, risk of adverse immunogenicity to animal cells and sensitivity to protease.15 Additionally, some bacteria that acquire mutations in genes encoding translocation proteins also resist the uptake of cell-penetrating peptides.16 Hence, a novel and effective vector with favorable biocompatibility for delivering antisense RNAs has been a hot area of research focus.
Graphene oxide (GO) nanosheets could be used to deliver nucleic acid efficiently when ionically bonded to cationic polymers.17,18 Polyethylenimine (PEI) is known as a kind of cationic polymer for gene transfer because of its strongly binding to DNA, effectively uptake by cells and helping delivered nucleic acids escape from the lysosomal pathways.19 When electrostatic interacted with GO, the cytotoxicity of GO-PEI complex is largely reduced.20,21 Through oxidation reaction the surface of GO obtains large quantities of functional groups,22 GO preferentially interacts with nucleic acids on its surface. In this way, the adsorbed nucleic acids are effectively prevented from enzymatic nucleases.23 Until recently, new generations of graphene-oxide nanocomposites which decreased bacterial adhesion to this nanomaterial surface of have increasing attention in antibacterial properties.24,25 The aim of this study was to develop a graphene-based plasmid transformation system using electrostatic interacted GO-PEI complexes loaded with antisense walR plasmid (GO-PEI-AS walR) and its antibacterial efficacy against E. faecalis was investigated. Furthermore, we validated the role of the GO-PEI-ASwalR in the pathogenicity using a rat periapical periodontitis model. It was hypothesized that nano-graphene oxide loaded with antisense walR RNA will be a more effective strategy in treating E. faecalis infections in periapical periodontitis.
Materials and methods
Preparation of GO-PEI-ASwalR and cytotoxicity evaluation
Bacterial strains and plasmids were listed in Table 1. The synthesis of GO-PEI complexes was proceeded as previously described.21,26 The optional concentration of GO-PEI based ASwalR was determined by cytotoxicity measurements. We seeded 3T3 fibroblasts cell lines in 96-well plates at a density of 1000 cells/well with GO-PEI-ASwalR at a range of concentrations from 1000 μg/mL to 0 μg/mL with Dulbecco Modified Eagle Medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% of fetal bovine serum (FBS; Hyclone). After 48 h or 72 h incubation (37 °C, 5% CO2), the culture medium was removed. Then the cells were washed with phosphate buffer solution (PBS, pH = 7.4; Hyclone) twice and evaluated with a cell counting kit (CCK-8; Dojindo Laboratories, Kumamoto, Japan) for the cellular vitality. Ten microliters of CCK-8 was added in each well and the absorbance at 540 nm was detected using a microplate reader (ELX800, Gene, Hong Kong, China) after 2 h culture.
Table 1.
Bacterial strains and plasmids used in this study.
| Strains and plasmids | Relevant characteristics/purpose | Source |
|---|---|---|
| Strains | ||
| E. faecalis | ||
| E. faecalis V583 | Specs | ATCC |
| AS walR | pDL278 (Specr) | This study |
| E.coli | ||
| DH5-α | General cloning and plasmid propagation | Invitrogen |
| Plasmids | ||
| pDL278 | (Specr) Vector for recombinant generating | Novagen |
| pDL278ASwalR | (Specr) Vector for AS walR expression | |
| pDL278ASwalR-eGFP | (Specr) Vector for AS walR-eGFP expression | This study |
Particle size distribution, zeta potential and atomic force microscopy measurements
The particle size distribution and zeta-potential of the GO-PEI based ASwalR samples were evaluated using Dynamic Light Scattering (DLS) and zeta-potential of the GO-PEI based ASwalR samples were evaluated in triplicate by a Nano ZS Malvern Zetasizer (Malvern Instruments, Worcestershire, UK).27 The prepared sample solution (50μL/drop) was dropped at the coverslip, dried in room temperature and characterized by atomic force microscope (AFM) using SPM-9500J2 (Shimadzu, Tokyo, Japan) with the contact mode.
Bacterial culture and transformation
The E. faecalis standard strain V583 was used and grown in Brain Heart Infusion (BHI; Becton Dickinson, Franklin Lakes, NJ, USA) at 37 °C in a 5% CO2 atmosphere. The bacterial culture and transformation were conducted as previously described.14,28
Evaluation the morphology of biofilm cells
All the E. faecalis biofilms were imaged by scanning electron microscopy (SEM; FEI Company, Hillsboro, OR, USA), crystal violet (CV) assays and the confocal laser scanning microscopy (CLSM; TSP SP2, Leica, Solms, Germany) as previously described.29,30
Transfection efficiency of GO-PEI-ASwalR in vitro
Recombinant ASwalR plasmids were labeled with gene encoding enhanced green fluorescent protein (ASwalR-eGFP). ASwalR-eGFP and GO-PEI-ASwalR-eGFP strains were constructed according to the transformation procedures previously described.12 The expression levels of eGFP were determined by CLSM and the transfection efficiency was determined by comparing the green fluorescent intensities. The expression of ASwalR, walR and walK as well as virulent factors genes including ace, epaA, and gelE of all E. faecalis strains were examined using real-time polymerase chain reaction (RT-PCR). The primers were listed in Table 2.
Table 2.
Oligonucleotide primers used in this study.
| Primers | Sequence 5′-3′ (Forward/Reverse) | Resource |
|---|---|---|
| QRT-PCR | ||
| 16s | 5′- AGCAACGCGAAGAACCTTAC-3′/5′- ATTTGACGTCATCCCCACCT-3′ | Sangon Biotech |
| ace | 5′- GGCGACTCAACGTTTGAC -3′/5′- TCCAGCCAAATCGCCTAC -3′ | Sangon Biotech |
| gelE | 5′- GGAACAGACTGCCGGTTTAG -3′/5′- TTCTGGATTAGATGCACCCG -3′ | Sangon Biotech |
| walR | 5′ -CATGGTCTCAAAACGGGGTG -3′/5′-AATAACCAACCCCACGACGA-3′ | Sangon Biotech |
| walK | 5′- CGCGTGTATGTGAATGTCCA -3′/5′- GCTTCGTGATTGATCGTGCA -3′ | Sangon Biotech |
| epaA | 5′- GCCTATGATGCACCAGGAGA -3′/5′- CAACCATTCCACCAGCCAAA -3′ | Sangon Biotech |
| QRT-PCR for ASwalR | ||
| PCR1 | 5′-ATGGTGACTGCCAAAGATTCT-3′ | First strand cDNA synthesis |
| AS2 | 5′- CGGCTTCTTTCGCATTGGTT -3′ | QRT-PCR analysis |
Protein extraction and western blotting analysis
The expression of WalR protein in E. faecalis strains after planktonic growth was quantified by Western blotting analysis as previously described.14
Infection of root canal
For dentinal infections, a three-week old biofilm model was established as previously described.12 Briefly, the root canal specimens were incubated in E. faecalis V583, ASwalR strain, E. faecalis + GO strain, and E. faecalis + GO-PEI-ASwalR strain respectively for 3 weeks.
Pulp-exposed periapical periodontitis lesions in rats
Animal experiments were approved by approved by The West China Hospital of Sichuan University Biomedical Research Ethics Committee (No. 2018039A) and all the procedures were conducted as previously described,31 following the guidelines for animal care and use of laboratory animals. Six-week-old female Sprague–Dawley rats (260–280 g) were anesthetized with ketamine/xylazine (100 and 5 mg/kg body weight, respectively) by intraperitoneal injection. An access opening was made on the occlusal surface of the left mandible first molars. Then, 0.1 mL of log-phased E. faecalis V583 and ASwalR bacterial suspensions were inoculated into pulp chamber and covered with light-cure flowable resin. Four weeks post-operation, the rats were scarified and the imaging of the rats was taken using the Quantum GX Micro-CT System (PerkinElmer, Waltham, MA). Rats were sedated using isoflurane (1–5%) and oxygen (2 L/min) mixture during imaging procedures. The scanning conditions were used as following: kV = 90; CT μA = 72; 360° scan time = 8 s. We analyzed the reconstructed images with Analyze 12.0 (PerkinElmer, Waltham, MA). The values of relative periapical cavity were calculated when compared to the control groups.
Data analysis
The Bartlett's test was performed to assess the homogeneity of data variances and Shapiro–Wilk test was conducted to determine the normal distribution of data. One-way analysis of variance was used to compare the data, followed by pairwise multiple comparisons.
Results
Cytotoxicity and characterization of GO-PEI-ASwalR
After 48 h or 72 h of incubation with GO-PEI-ASwalR, the viability of 3T3 fibroblasts cells significantly decreased at concentrations of 60 μg/mL or higher. The results showed that synthesized GO-PEI-ASwalR was not toxic until the concentration reached to 60 μg/mL compared with the control group (Fig. 1A).
Figure 1.
Cytotoxicity and characterization of GO-PEI-ASwalR. (A) The cytotoxicity of GO-PEI-ASwalR was assessed and the cell viability was determined with CCK-8 after 48 h or 72 h of incubation with GO-PEI-ASwalR (Dulbecco Modified Eagle Medium supplemented with 10% of fetal bovine serum as the blank control) (B) The particle size distributions was measured using Dynamic Light Scattering (DLS) (C) The zeta-potential of the GO-PEI based ASwalR were evaluated by a Malvern Zetasizer (D) AFM confirmed the roughness parameters of GO, GO-PEI and GO-PEI-ASwalR films (n = 10,*P < 0.05, when compared to GO control group); AFM results confirmed the roughness parameters of GO, GO-PEI and GO–PEI–ASwalR films to be 3.83 ± 0.46 nm, 2.23 ± 0.22 nm, and 9.99 ± 2.24 nm, with maximum heights of 22.40 ± 2.25 nm, 16.40 ± 3.30 nm, and 74.00 ± 20.30 nm (E) AFM images of GO, GO-PEI and GO-PEI-ASwalR complex (upper lane for 2-D view and lower lane for 3D view) (F) ASwalR plasmids were labeled with gene encoding enhanced green fluorescent protein (ASwalR-eGFP) and CLSM was applied to determine the expression level of eGFP, scale bars, 100 μm (G) The transfection efficiency was determined by comparing the green fluorescent intensities (n = 10, *P < 0.05, when compared to ASwalR-eGFP control group).
Dynamic Light Scattering (DLS) measurements indicated that the Z-average size of the GO were 280 nm. For GO-PEI and GP-PEI-ASwalR, Z-average sizes of 95 nm and 290 nm were obtained respectively indicating that the size of GO-PEI-ASwalR was slightly larger than GO (Fig. 1B). Using zeta potential measurement, the surface charge of GO showed negative values of approximately −21.7 mV. However, GO-PEI and GO-PEI-ASwalR complex demonstrated the positive surface charges of approximately 13.1 mV and 33.7 mV respectively (Fig. 1C). The AFM results revealed that GO-PEI-ASwalR nanosheets increased the roughness and height of the membranes compared to GO and GO-PEI membrane (n = 10, P < 0.05; Fig. 1D and E).
GO-PEI-ASwalR increased ASwalR transformation and significantly reduced virulent-associated gene expressions
Using CLSM, higher levels of GFP-expression were observed in samples induced with GO-PEI-ASwalR when compared with the ASwalR (Fig. 1F). Quantitatively, a 200% increase in GFP-expression transcripts in GO-PEI-ASwalR transformed strains was found when compared with that of ASwalR cells (P < 0.05; Fig. 1G). Quantitative RT-PCR showed expressions of ASwalR RNA in ASwalR and GO-PEI-ASwalR strains significantly increased by 2.8 and 6.5 folds respectively when compared to E. faecalis V583 strain (n = 10, P < 0.05; Fig. 2A). Correspondingly, the levels expressions of walR and walK mRNA were significantly decreased in ASwalR and GO-PEI-ASwalR strains (n = 10, P < 0.05). Moreover, the expressions of ace, gel and epaA genes were the lowest in the GO-PEI-ASwalR strain (n = 10, P < 0.05) (Fig. 2A). Western blotting probed with anti-WalR antibody showed that the level of WalR protein was the lowest in the GO-PEI-ASwalR cells (Fig. 2B and C).
Figure 2.
GO-PEI-ASwalR increased ASwalR transformation and suppressed the E. faecalis biofilm aggregation. (A) Quantitative RT-PCR analysis showed the gene transcripts in E. faecalis, ASwalR, GO and GO-PEI-ASwalR strains. E. faecalis gene expression was relatively quantified using 16S as an internal control and calculated based on the E. faecalis V583 expression, which was set as 1.0. Experiments were performed in triplicate and are presented as the mean ± standard deviation (n = 10, *P < 0.05, when compared to E. faecalis V583 control group) (B) WalR production was quantified in Western blots probed with anti-WalR antibody (C) Coomassie-stained SDS-PAGE gel supporting equal loading of samples (D) SEM of E. faecalis, ASwalR, GO and GO-PEI-ASwalR strains. Biofilm developed in BHI for 24 h. Scale bar for 2000 magnifications, 50 μm. Scale bars for 20000 magnifications, 5 μm.
GO-PEI-ASwalR suppressed biofilm viability in infected canal and reduced periapical lesion size
SEM observation demonstrated that E. faecalis V583 cells were densely packed with extracellular matrix, whereas the GO, ASwalR and GO-PEI-ASwalR strains showed the reduced extracellular matrix in the biofilms interspersed among “blank” areas (Fig. 2D). Particularly, the very few small microcolonies were found to be randomly distributed in the GO-PEI-ASwalR strain biofilms compared to the other groups (Fig. 2D). By double staining of CLSM observation, we found that silencing of walR by GO-PEI-ASwalR as delivery system markedly decreased the biomass of the biofilms (Fig. 3A). The quantitative data showed that the proportions of viable bacteria were significantly decreased in GO, ASwalR and GO-PEI-ASwalR strains when compared to the E. faecalis V583 strains (Fig. 3B). The lowest percentage of live bacteria was detected in the GO-PEI-ASwalR strain to be 14.83 ± 0.5% (n = 10, P < 0.05). Crystal violet microtiter assays demonstrated that GO-PEI-ASwalR strain exhibited the lowest optical density (OD) values of biofilm biomass (n = 10, P < 0.05, Fig. 3C).
Figure 3.
GO-PEI-ASwalR improved bactericidal effects in biofilm growth. (A) Double labeling of the biofilms in the E. faecalis, ASwalR, GO and GO-PEI-ASwalR strains. Green, viable bacteria (SYTO 9); red, dead bacteria (PI); scale bars, 100 μm. The three-dimensional reconstruction of the biofilms was performed using Imaris 7.0.0 (B) Percentage (%) of viable E. faecalis cells in biofilm growth (n = 10, *P < 0.05 when compared to E. faecalis V583 control group; proportion of viable bacteria in the E. faecalis V583 was set as the 100% to estimate the live bacteria rations) (C) Biomass was quantified by crystal violet staining (n = 10, *P < 0.05 when compared to E. faecalis V583 control group) (D) Number of CFUs after the E. faecalis, ASwalR, GO and GO-PEI-ASwalR infections [n = 10, *P < 0.05 when compared to E. faecalis V583 control group; log (CFU/mL)].
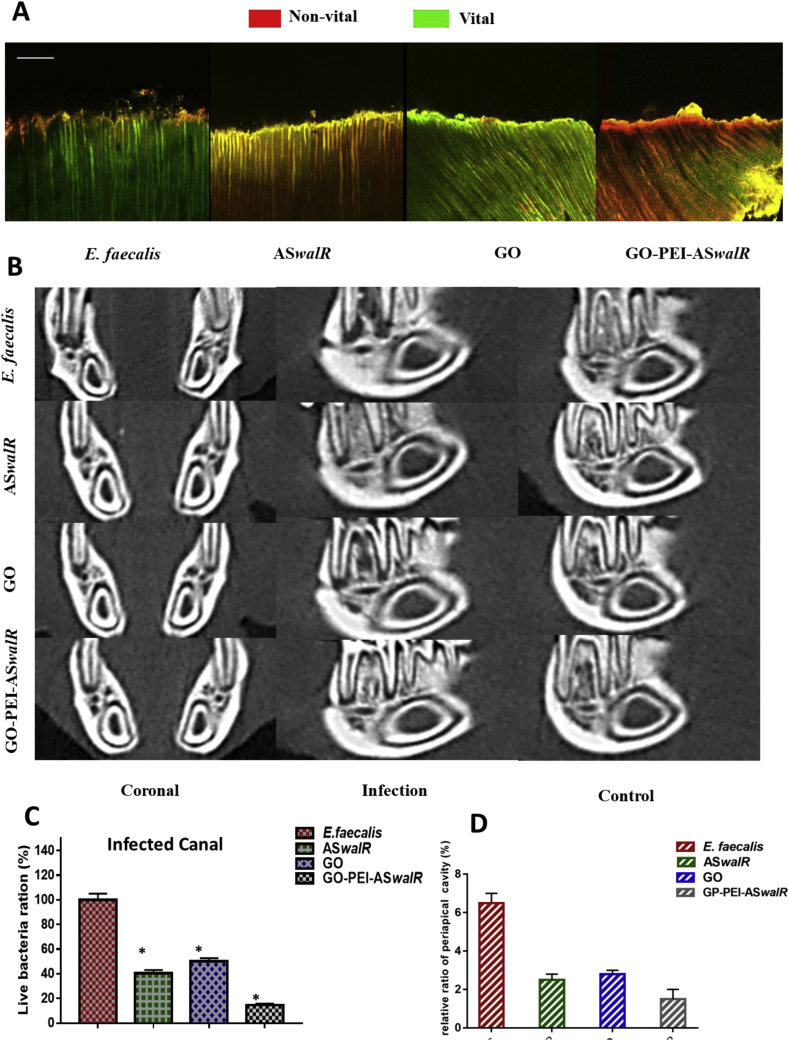
In general, colony-forming unit (CFU) counts significantly decreased in the GO, ASwalR and GO-PEI-ASwalR strains indicating antibacterial properties in root canals. Particularly, the average CFU counts in the GO-PEI-ASwalR strain were the lowest (n = 10, P < 0.05; Fig. 3D). Correspondingly, CLSM observation showed mixtures of bacterial cells inside the dentin (Fig. 4A). The lowest proportion of viable bacteria in the GO-PEI-ASwalR strain was 14.5 ± 0.43% (n = 10, P < 0.05; Fig. 4C), whereas the E. faecalis V583 strain exhibited the highest ratio (n = 10, P < 0.05; Fig. 4C). At 4 weeks post-operation, the GO, ASwalR and GO-PEI-ASwalR strains presented remarkably reduced periapical lesion size compared to the E. faecalis V583 group (Fig. 4B). The micro-computed tomography (μCT) analysis was used to quantify the relative ratios of periapical cavity which indicated that GO-PEI-ASwalR group presented the lowest periapical lesion ratio while the largest periapical lesion size was observed in the E. faecalis V583 group (Fig. 4D).
Figure 4.
GO-PEI-ASwalR inhibited the bacterial viability in infected canal and reduced periapical lesion size. (A) Three-dimensional reconstruction of CLSM images of E. faecalis viability in dentinal tubules. Green, viable bacteria (SYTO 9); red, dead bacteria (PI), scale bars, 100 μm (B) The reconstructed images for micro-CT scanning of the rat periapical lesions (C) Percentage (%) of viable E. faecalis cells in infected canal (n = 10, *P < 0.05 when compared to E. faecalis V583 control group; proportion of viable bacteria in the E. faecalis V583 was set as the 100% to estimate the live bacteria rations) (D) The values of relative periapical cavity (%) were calculated (n = 10, *P < 0.05 when compared to E. faecalis V583 control group).
Discussion
Conventional antibiotics may not be effective against all bacteria, especially those that develop resistance.7,32,33 Therefore, supplementary therapies heighten the natural defenses against the susceptible organisms and lessen use of antibiotics for the periapical infection management. It has been suggested that the overexpression of ASwalR reduced the transcripts of the virulent genes, inhibited extracellular polymeric substances (EPS) synthesis, and suppressed biofilm organization of E. faecalis which could be explored as a potential therapeutic strategy for the management of persistent root canal infections.12 Due to the RNA instability, antisense RNAs are difficult for preservation, therefore we used recombinant pDL278 ASwalR overexpression plasmid for transformation. In the preliminary study, pDL278 plasmids have been transformed into E. faecalis as the empty vector control and did not affect the growth of E. faecalis cells.12 Hence, there is a need to develop effective and stable vectors that can deliver nucleic acid molecules without detrimental effects to normal eukaryotic cells.32
It is reported that graphene oxide (GO) can be used to deliver genes efficiently when ionically bonded to cationic polyethyleneimine (PEI) polymers.16 Therefore, we used these stable GO-PEI complexes to load antisense RNA to enhance transformation efficiency in bacteria and acquired a more efficiency than a traditional competence stimulating peptide method. These positive surface charges can favor the interaction with the negatively charged cell surface and facilitate cellular transfection.26 It has been showed that the concentrations of GO-PEI lower than 50 μg/mL had no significant effects on cell apoptosis rate.21 In current study, our results showed that synthesized GO-PEI-ASwalR was not toxic until the concentration reached to 60 μg/mL (Fig. 1A). Therefore, we adopted GO-PEI-ASwalR of 30 μg/mL as the working concentration for the subsequent studies.
The successful construction of the GO-PEI-ASwalR delivery system carrying this ASwalR was evidenced by the efficient uptake by bacterial cells and quantitative ASwalR expressions of E. faecalis.34 To evaluate the vector transformation efficiencies, ASwalR recombinant plasmids were labeled with gene encoding enhanced green fluorescent protein (ASwalR-eGFP). The levels of GFP-expression indicated ASwalR transcripts, which revealed higher transformation efficiencies induced by GO-PEI-ASwalR when compared to pure ASwalR plasmids. Correspondingly, the quantitative RT-PCR showed expressions of ASwalR RNA in GO-PEI-ASwalR strain significantly increased by 6.5 folds compared to E. faecalis V583 strain (Fig. 2A). Particularly, the fold change of GO-PEI-ASwalR strain was about three times in ASwalR transformed by competence stimulating peptide indicating the GO-PEI-ASwalR probably act more efficiently than the conventional competence stimulating peptide strategy as delivery system. AFM observations revealed that the surface roughness of GO-PEI-ASwalR nanosheets increased compared to GO and GO-PEI membrane films. Due to the thicker the multilayers, the surface parameter of material films likely increased the roughness which resulted in enhanced adhesion force.35 On the other hand, altered surface charge of GO-PEI demonstrated its ability to strengthen DNA adsorption.17,18 Taken together, we speculated that improved delivery efficiency of the GO-PEI-ASwalR may be attributed to altered surface charge and higher surface roughness.36
After 24 h biofilm establishment, the results revealed that GO-PEI-ASwalR most suppressed biofilm aggregation and reduced the cellular viability (Fig. 3A). Our previous report confirmed that ASwalR sensitized E. faecalis in infected canals to calcium hydroxide medication using a 3-week old biofilm model.12 The 3-week old biofilm would be more appropriate to reflect the conditions of the biofilm during the long starvation phase because the root canal infections are likely several weeks or months old before the treatment is started.37 GO sharp edge to disrupt membranes physically and GO can interfere with cellular metabolism and lead to cell necrosis/apoptosis by inducing oxidative stress. GO can form as a blanket over bacteria and isolate them from external environment, which will inhibit cells proliferation and nutrients achievement.38 In the current study, the GO-PEI-ASwalR strain exhibited the lowest viable cell proportion of E. faecalis in dentin tubules (Fig. 4A). Moreover, the GO-PEI-ASwalR remarkably reduced periapical lesion size at 4 weeks after root canal treatment compared to the ASwalR group and the E. faecalis V583 group presented the large periapical lesions (Fig. 4B). In the present study, restricting the excessive in periapical lesions was considered an important step of the stimulating periapical repair process.39 It was speculated that the pathogenicity of E. faecalis was markedly decreased by ASwalR interference and GO-PEI-ASwalR improved bactericidal effects in periapical periodontitis. Graphene oxide is a kind of non-biodegradable martial, which is similar to other carbon-based material. However, its biocompatibility turned out to be safer than most other types of nanomaterials, which may have an important role in it potential for clinical application.40,41 Future directions needed to extend the applications of GO-PEI-ASwalR strategy as a potential substitutive therapy for root canal infections.
In summary, we developed a graphene-based plasmid transformation system using electrostatic interacted GO-PEI complexes loaded with antisense walR plasmid (GO-PEI-ASwalR). GO-PEI could efficiently deliver ASwalR plasmid into E. faecalis cells with excellent transcription of ASwalR. GO–PEI–ASwalR significantly reduced virulent-associated gene expressions, suppressed biofilm aggregation, improved bactericidal effects in infected canal and reduced periapical lesion size. The results of present study revealed that preserving nano-graphene oxide with antisense walR RNA will be a more effective and stable strategy in treating E. faecalis infections in periapical periodontitis.
Declaration of Competing Interest
None.
Acknowledgments
This study was supported by Natural Science Foundation of China 81800964, Sichuan Provincial Natural Science Foundation of China 2018SZ0125, and Sichuan Provincial Natural Science Foundation of China 2019YFS0270, Chinese Postdoctoral Science Foundation 2018M633380.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2019.09.006.
Contributor Information
Hui Zhang, Email: caesarzh@163.com.
Lei Lei, Email: leilei@scu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gomes B.P., Pinheiro E.T., Gadê-Neto C.R. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19:71–76. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 2.Pinheiro E.T., Gomes B.P., Ferraz C.C., Sousa E.L., Teixeira F.B., Souza-Filho F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 3.Colomer-Winter C., Flores-Mireles A.L., Baker S.P. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J., Tong Z., Ling J., Liu H., Wei X. The effects of sodium hypochlorite and chlorhexidine irrigants on the antibacterial activities of alkaline media against Enterococcus faecalis. Arch Oral Biol. 2015;60:1075–1081. doi: 10.1016/j.archoralbio.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Kayaoglu G., Erten H., Bodrumlu E., Ørstavik D. The resistance of collagen-associated, planktonic cells of Enterococcus faecalis to calcium hydroxide. J Endod. 2009;35:46–49. doi: 10.1016/j.joen.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Fukuchi K., Kasahara Y., Asai K., Kobayashi K., Moriya S., Ogasawara N. The essential two-component regulatory system encoded by yycF and yycG modulates expression of the ftsAZ operon in Bacillus subtilis. Microbiol. 2000;146:1573–1583. doi: 10.1099/00221287-146-7-1573. [DOI] [PubMed] [Google Scholar]
- 7.Dale J.L., Cagnazzo J., Phan C.Q., Barnes A.M., Dunny G.M. Multiple roles for Enterococcus faecalis glycosyltransferases in biofilm-associated antibiotic resistance, cell envelope integrity, and conjugative transfer. Antimicrob Agents Chemother. 2015;59:4094–4105. doi: 10.1128/AAC.00344-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa-Ribeiro M., De-Jesus-Soares A., Zaia A.A., Ferraz C.C., Almeida J.F., Gomes B.P. Antimicrobial susceptibility and characterization of virulence genes of Enterococcus faecalis isolates from teeth with failure of the endodontic treatment. J Endod. 2016;42:1022–1028. doi: 10.1016/j.joen.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Hancock L.E., Perego M. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J Bacteriol. 2004;186:7951–7958. doi: 10.1128/JB.186.23.7951-7958.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomason M.K., Storz G. Bacterial antisense RNAs: how many are there, and what are they doing? Annu Rev Genet. 2010;44:167–188. doi: 10.1146/annurev-genet-102209-163523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil S.D., Sharma R., Srivastava S., Navani N.K., Pathania R. Downregulation of yidC in Escherichia coli by antisense RNA expression results in sensitization to antibacterial essential oils eugenol and carvacrol. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S.Z., Liu Y.J., Zhang H., Lei L. The susceptibility to calcium hydroxide modulated by the essential walR gene reveals the role for Enterococcus faecalis biofilm aggregation. J Endod. 2019;45:295–301. doi: 10.1016/j.joen.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Bessa L.J., Ferreira M., Gameiro P. Evaluation of membrane fluidity of multidrug-resistant isolates of Escherichia coli and Staphylococcus aureus in presence and absence of antibiotics. J Photochem Photobiol B. 2018;181:150–156. doi: 10.1016/j.jphotobiol.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Lei L., Stipp R.N., Chen T., Wu S.Z., Hu T., Duncan M.J. Activity of streptococcus mutans VicR is modulated by antisense RNA. J Dent Res. 2018;97:1477–1484. doi: 10.1177/0022034518781765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosal A., Vitali A., Stach J.E., Nielsen P.E. Role of SbmA in the uptake of peptide nucleic acid (PNA)-peptide conjugates in E. coli. ACS Chem Biol. 2013;8:360–367. doi: 10.1021/cb300434e. [DOI] [PubMed] [Google Scholar]
- 16.Quijano E., Bahal R., Ricciardi A., Saltzman W.M., Glazer P.M. Therapeutic peptide nucleic acids: principles, limitations, and opportunities. Yale J Biol Med. 2017;90:583–598. [PMC free article] [PubMed] [Google Scholar]
- 17.Yin D., Li Y., Lin H. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology. 2013;24 doi: 10.1088/0957-4484/24/10/105102. [DOI] [PubMed] [Google Scholar]
- 18.Kim H., Kim W.J. Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small. 2014;10:117–126. doi: 10.1002/smll.201202636. [DOI] [PubMed] [Google Scholar]
- 19.Putnam D., Gentry C.A., Pack D.W., Langer R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc Natl Acad Sci USA. 2001;98:1200–1205. doi: 10.1073/pnas.031577698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Z., Zhang Z., Ma H., Chen Y. In vitro hemocompatibility and toxic mechanism of graphene oxide on human peripheral blood T lymphocytes and serum albumin. ACS Appl Mater Interfaces. 2014;6:19797–19807. doi: 10.1021/am505084s. [DOI] [PubMed] [Google Scholar]
- 21.Dou C., Ding N., Luo F. Graphene-based MicroRNA transfection blocks preosteoclast fusion to increase bone formation and vascularization. Adv Sci. 2017;5 doi: 10.1002/advs.201700578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andre Mkhoyan K., Contryman A.W., Silcox J. Atomic and electronic structure of graphene-oxide. Nano Lett. 2009;9:1058–1063. doi: 10.1021/nl8034256. [DOI] [PubMed] [Google Scholar]
- 23.Park J.S., Na H.K., Min D.H., Kim D.E. Desorption of single-stranded nucleic acids from graphene oxide by disruption of hydrogen bonding. Analyst. 2013;138:1745–1749. doi: 10.1039/c3an36493c. [DOI] [PubMed] [Google Scholar]
- 24.Tang J., Chen Q., Xu L. Graphene oxide-silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl Mater Interfaces. 2013;5:3867–3874. doi: 10.1021/am4005495. [DOI] [PubMed] [Google Scholar]
- 25.Yousefi M., Dadashpour M., Hejazi M. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater Sci Eng C Mater Biol Appl. 2017;74:568–581. doi: 10.1016/j.msec.2016.12.125. [DOI] [PubMed] [Google Scholar]
- 26.Feng L., Zhang S., Liu Z. Graphene based gene transfection. Nanoscale. 2011;3:1252–1257. doi: 10.1039/c0nr00680g. [DOI] [PubMed] [Google Scholar]
- 27.Valencia C., Valencia C.H., Zuluaga F., Valencia M.E., Mina J.H., Grande-Tovar C.D. Synthesis and application of scaffolds of chitosan-graphene oxide by the freeze-drying method for tissue regeneration. Molecules. 2018;23 doi: 10.3390/molecules23102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei L., Liu H., Cai Y., Wei X. MTAD combined with endosonic irrigation as a new approach for the disinfection of Enterococcus faecalis biofilm. J Dent Sci. 2015;10:437–443. [Google Scholar]
- 29.Liu H., Wei X., Ling J., Wang W., Huang X. Biofilm formation capability of Enterococcus faecalis cells in starvation phase and its susceptibility to sodium hypochlorite. J Endod. 2010;36:630–635. doi: 10.1016/j.joen.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Lei L., Shao M., Yang Y., Mao M., Yang Y., Hu T. Exopolysaccharide dispelled by calcium hydroxide with volatile vehicles related to bactericidal effect for root canal medication. J Appl Oral Sci. 2016;24:487–495. doi: 10.1590/1678-775720160014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah A., Lee D., Song M., Kim S., Kang M.K., Kim R.H. Clastic cells are absent around the root surface in pulp-exposed periapical periodontitis lesions in mice. Oral Dis. 2018;24:57–62. doi: 10.1111/odi.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegarty J.P., Stewart D.B., Sr. Advances in therapeutic bacterial antisense biotechnology. Appl Microbiol Biotechnol. 2018;102:1055–1065. doi: 10.1007/s00253-017-8671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alekshun M.N., Levy S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Ma W., Zhu Y. Inhibiting methicillin-resistant staphylococcus aureus by tetrahedral DNA nanostructure-enabled antisense peptide nucleic acid delivery. Nano Lett. 2018;18:5652–5659. doi: 10.1021/acs.nanolett.8b02166. [DOI] [PubMed] [Google Scholar]
- 35.Wong J.E., Zastrow H., Jaeger W., von Klitzing R. Specific ion versus electrostatic effects on the construction of polyelectrolyte multilayers. Langmuir. 2009;25:14061–14070. doi: 10.1021/la901673u. [DOI] [PubMed] [Google Scholar]
- 36.Gribova V., Auzely-Velty R., Picart C. Polyelectrolyte multilayer assemblies on materials surfaces: from cell adhesion to tissue engineering. Chem Mater. 2012;24:854–869. doi: 10.1021/cm2032459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Shen Y., Haapasalo M. Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2012;38:1376–1379. doi: 10.1016/j.joen.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 38.Palmieri V., Papi M., Conti C., Ciasca G., Maulucci G De Spirito M. The future development of bacteria fighting medical devices: the role of graphene oxide. Expert Rev Med Devices. 2016;13:1013–1019. doi: 10.1080/17434440.2016.1245612. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui Y.D., Omori K., Ito T. Resolvin D2 induces resolution of periapical inflammation and promotes healing of periapical lesions in rat periapical periodontitis. Front Immunol. 2019;10:307. doi: 10.3389/fimmu.2019.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T.H., Lee T., El-Said W.A., Choi J.W. Graphene-based materials for stem cell applications. Materials. 2015;8:8674–8690. doi: 10.3390/ma8125481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin S.R., Li Y.C., Jang H.L. Graphene-based materials for tissue engineering. Adv Drug Deliv Rev. 2016;105:255–274. doi: 10.1016/j.addr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.