Abstract
The immunoassays methods need avoiding interferences that can influence result interpretation. Main sources of interference arise from either patient status, preparation and physiology or laboratory process and procedures.
The aim of this non-systematic critical review is to highlight the preanalytical interferences on laboratory immunoassays.
Blood hormone profile changes according with age and depending on sex: these are important variables, mainly in newborn, during both sexual maturation and childbearing. Gonadotropins FSH and LH show a sharp increase with age in females, whereas in males LH appears rather stable. With age both males and females show progressive decay of the hormone profile. Stress causes variations, as it influences GH, prolactin, Cortisol and the total/free ratio of thyroid hormone. Diurnal variations, day of cycle, influence by estrogens on thyroid hormone are relevant for result variability. Paraproteins and autoantibodies can interfere in some assays particularly drug, vitamin D and thyroid hormone. As regards the variables due to sample matrix, and to evacuated tubes components, some additives and anticoagulants have been reported to influence specific assays, e.g. thyroid hormone. Hemolysis, lipemia and bilirubin cause interferences on specific techniques/tests, e.g. ferritin, TSH, Vitamin B12, progesterone and folic acid. Nicotine and cocaine addictions interfere with some hormones. Thus, laboratory professionals should be aware of preanalytical problems particularly important when dealing with the immunoassays, by taking appropriate actions to avoid any relevant interferences.
Key words: diagnostic error, reproducibility of results, total quality management, blood specimen collection, patient safety
INTRODUCTION
The immunoassays methods employ antibodies showing high affinity for epitopes on antigens in order to both detect them with high specificity and make the immune reaction detectable and measurable, using a range of indicator reactions or labels. Due to the nature of immune reactions, well-controlled conditions are needed in order to avoid non-specific binding. When analyzing biological samples such as serum or plasma (usual laboratory matrices), interferences are also possible due to similar compounds, such as other proteins presenting like epitopes (1).
All the above analytical interferences can account for several technical problems. Moreover, pathophysiological variables can also confound the interpretation of e.g. the pituitary-thyroid axis interaction (2). Generally, TSH-independent states are observed when the disorder affects primarily the thyroid gland, but there are non-thyroid conditions that can alter the interaction and confound the diagnosis, such as trophoblastic tumors, struma ovarii, and generalized resistance to thyroid hormone or selective organ resistance to thyroid hormone. Besides, there are TSH-dependent variables due to inappropriate TSH secretion or lack of secretion such as pituitary adenoma, resistance states to thyroid hormone (generalized or pituitary), psychiatric states, smoking, and malabsorption. Besides, there are physiological variables, as follows: circadian rhythm, seasonal influences, environmental influences, exercise, posture, and pregnancy.
Furthermore, dealing with thyroid, Iatrogenic causes should always be considered, taking into account: prior thyroid treatment (surgical or medical), drug therapy (systemic or local), and plasmapheresis. Regarding pituitary function, some physiological variables can induce modifications to the pituitary secretion, as follows: age, travel (that influences both pituitary and adrenal functions), circadian rhythm and pulsatile release, seasonal influences, environmental influences, exercise, stress, and posture (3).
Moreover, there are pathophysiological variables acting on pituitary, such as malnutrition, starvation, lifestyle (e.g. smoking increases secretion; whereas alcohol reduces secretion), blindness (that causes loss of hypothalamic pituitary axis stimulation), and drugs (4).
Adding to the above issues, clinical laboratories should be aware of preanalytical problems that are particularly relevant when dealing with the immunoassays. The preanalytical phase is the major source of laboratory variability.
The most common preanalytical variables (Figure 1) could be classified in:
patient status and physiology (5);
patient preparation (6-11); and
laboratory process/procedures (12-18)
Figure 1.
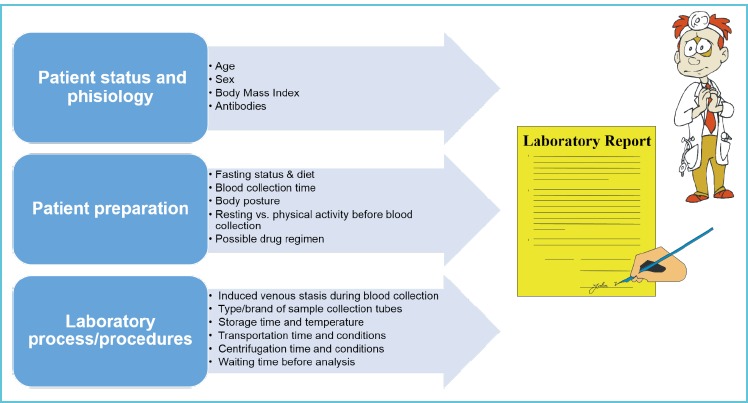
Preanalytical sources of laboratory interferences
The collection of liquid biological specimens by evacuated tubes is usual practice in healthcare and in veterinary care settings (19). The aim of this non-systematic critical review is to highlight the preanalytical interferences on laboratory immunoassays. To enrich the argument, we also provide an additional evacuated tube verification.
CAUSES OF PREANALYTICAL INTERFERENCES ON LABORATORY ASSAYS
Lipemia, bilirubin, and hemolysis interference
Lipemia may interfere the antigen-antibody precipitation leading to falsely high results, whereas high bilirubin per se can cause spectral interferences in immunoassays with absorbance measurement readings at about 450-460 nm.
Hasanato et al., assessed the interference of progressively increasing amounts of hemoglobin, bilirubin and lipids added to sera on a series of immunoassays. The following immunoassays: ferritin, TSH, Vitamin B12, progesterone and folic acid, all showed variable degrees of interference. It was observed that ferritin and TSH levels were overestimated in direct relationship with hemolysis, whereas Vitamin B12 progressively decreased. Progesterone levels decreased with increasing lipemia. Folic acid levels decreased with increasing bilirubin (20). Free hemoglobin, at different concentrations (g/L), has shown various levels of interference on different methods for anti-HIV-1 antibody detection, either immunochromatography (at 5.7 g/L), or enzyme-immunoassay (at 86 g/L), or chemiluminescence (at 115 g/L) based methods. Lipemia did not show any interference (21). The effects of hemolysis (H index range 0-1000), icterus (I index range 0-40) and lipemia (L index range 0-1000) were evaluated on three acetaminophen assays, respectively the Syva® EMIT®, the Microgenics DRI® assay, and the Roche assay on a Roche cobas® c501 or an Integra 800 analyzer. No interference was observed due to hemolysis and icterus on Syva® EMIT® and DRI® assay, whereas interference was marked on Roche assays; whereas lipemia was less evident on Roche than on Syva® EMIT® and DRI® assay (22).
Saracevic et al. reported interferences on resistin and myeloperoxidase immunosorbent assays from BioVendor. Both resistin and myeloperoxidase levels showed significant biases at 1 g/L of free hemoglobin (58.7% and 66.7%, respectively) and at 4.66 mmol/L triglycerides (33.8% and 12.2%, respectively) (23).
Variables regarding patient status and physiology
Age and sex are particularly important for interpreting the results of hormones, mostly when considering the ample range of variations shown by several hormones during the life, from new-born to adult age. Each newborn shows the influence of the maternal hormones that mainly pass through placenta during pregnancy and can influence some physiological responses in babies, particularly as regards thyroid function (24). Even during the breast feeding there is passage of hormones from mother to newborn through the milk, particularly of hormones that are needed for regulating infant growth (25, 26).
With age progression the blood hormone profile changes accordingly and depending on sex, showing the effects both of sexual maturation by the relevant hormone levels and of the stature lengthening by the growth hormones. Gonadotropins FSH and LH show sharp increase with age in females, whereas in males LH appears rather stable. FSH is far higher in females than in males, particularly during puberty (27). Moreover, serum concentrations of thyroid hormones are reportedly different between males and females and for age groups in white children/adolescents (28).
During maturity, the main hormone changes are shown at menopause by the sexual hormones in females. The hormone replacement therapy is used as a prevention treatment for women at the onset of menopause (29). The parallel andropause is a condition due to low plasma testosterone in conjunction with symptoms of reduced sexual function (30). With age both males and females are implicated by a progressive decay of the hormone profile, with some aspects regarding particular hormones such as the thyroid ones that should be carefully considered, since critical for several metabolic functions of the body (31).
The female sex hormones show variations during the menstrual cycle depending on the length of the cycle (32). Important information to be gathered before blood collection regards exact day of cycle and/or intake of hormone replacement therapy and/or birth control pill. Moreover, estrogens influence thyroglobulin during the cycle, thus influencing fT4 levels. With menopause the cessation of ovarian production of estrogens and progesterone is preceded by irregular menstrual cycles (33). During this period estrogen, in particular, and progesterone decline, whereas FSH rises and high levels of this hormone are typically observed at the beginning of menopause, although with wide oscillations.
Measurement of serum testosterone level is important for the assessment of hypogonadism in men and androgen excess in women, where it vary mainly according to the phase of the menstrual cycle. Yet serum testosterone levels can vary widely, even between samples from the same patient and among different laboratory assays, due to a multitude of factors, such as diurnal variation, systemic illnesses, and seasonal variation, as well as assay-specific factors (34).
Kisspeptins (Kps) are peptide hormones, generators of both sex steroid negative and positive feedback signals for GnRH secretion in male and female at puberty, preserve male fertility, and govern the pre-ovulatory LH surge in females (35). Kps show very low values (<2 pmol/L) in male and in non-pregnant female; however, increase dramatically during pregnancy. There are some preanalytical issues to be considered: i) rapid degradation is observed in serum, therefore sample must be processed immediately after collection (freezing-thawing does not significantly affect results); ii) either heparin with 2000 U trasylol or EDTA should be used as anticoagulant, whereas citrate causes lowering of results.
The main hormone that determines the height growth at each stage of development is growth hormone (GH), together with its mediator, insulin-like growth factor 1 (IGF-1). The presence of an alteration in the GH/IGF-1 axis in the pediatric population results in impaired growth (36). The assays of random serum GH concentrations are of no clinical value as GH secretion is pulsatile and most of GH pulses occur overnight, showing very low hormone concentrations between pulses. Thus, provocative tests of GH secretion using appropriate stimuli are employed to test for GH deficiencies (GHD). GH stimulation tests use a defined cut-off concentration for peak GH to distinguish deficient from non-deficient GH subjects. Serum IGF-I is synthesized in the liver under the control of GH and circulates bound to the IGF binding proteins (IGFBPs). There are six known IGFBPs of which IGFBP-3 is the major serum carrier of IGF-I. Unlike GH, serum concentrations of IGF-I and IGFBP-3 are stable. Total IGF-I concentrations in serum are mainly measured using assays that dissociate IGF-I from its binding proteins (37). Serum IGF-I concentrations vary with age and it must be considered that the normal range for serum IGF-I concentrations in young children superimposes on the range observed in children with GH deficiency. Moreover IGF-I concentrations are reduced in malnourished children, and/or with hypothyroidism, chronic disease, renal failure and diabetes (37). They also rise intensely during puberty. IGFBP-3 concentrations were thought to be potentially superior to measurement of IGF-I alone as IGFBP-3 is less nutritionally sensitive than IGF-I. However, multiple studies have found no difference in IGFBP-3 concentrations between GHD and non-GHD subjects (38), with a poor sensitivity at 50% and no advantage over measurement of IGF-I alone (39).
Body mass index increase (BMI), waist circumference and hip circumference have demonstrated association with increased levels of several sex hormones, in particular obese individuals show increased blood concentrations of estrone, estradiol and free estradiol with negative association with Sex Hormone Binding Globulin (SHBG) (40). Moreover, TSH has shown significant direct association with BMI, which has been explained as due to TSH directly stimulating preadipocyte differentiation that results in adipogenesis (41); whereas even leptin can be involved (42). Other studies have shown association between adiposity and prolactin, although more variables might be involved such as alcohol intake and sedentary lifestyle (43). Therefore, each country should define reference intervals and clinical decision limits to minimize the impact of age, sex and BMI on laboratory assays based in their population (44). High concentrations of paraproteins can interfere with various laboratory immunoassays, and laboratory personnel should be aware of this when the laboratory findings are discordant with the clinical findings (45). Possible false negative results have been reported as regards the assay of phenytoin in a patient with high levels of IgM. The interference caused no detection of the drug in a Particle-Enhanced Turbidimetric Inhibition Immunoassay (PETINIA) method notwithstanding, based on dosage, the patient trough plasma concentration was estimated between 5 and 10 mg/L (46). Moreover, false negative results for vancomycin were reported in a patient with high IgM levels, despite assuming appropriate dosage of the drug. The high IgM caused interference on a PETINIA method for vancomycin that was not evident when an enzyme multiplied immunoassay (EMIT) was employed (47). Very high, near to toxicity, 25-OH vitamin D level (327 nmol/L) was assayed on ARCHITECT. After recalling the patient to confirm the result, 49 nmol/L of 25-OH vitamin D was measured by mass spectrometry; whereas 289 nmol/L was measured by ARCHITECT. Briefly, the patient had high circulating IgG paraproteins with a clinical history of rheumatoid arthritis with rheumatoid factor (RF) and myeloma. Thus, the interference on immunoassay was due to IgG paraprotein, but the contribution of rheumatoid factor could not be ruled out (48). As regards RF, falsely elevated results were reported in serum (but not in plasma) samples spiked with patient RF pool when vancomycin immune assays were performed (49).
The patient’s blood itself can cause pre-analytical errors. Briefly, some patients have circulating antibodies that can strongly modify the results of the immunoassays and simulate a disease.
There are reports regarding:
the interference of heterophile antibody affecting fT4 Roche immunoassay, indicating a suspect hyperthyroidism which revealed false after retesting on another platform (Centaur, Siemens) (50);
cases of anti-streptavidin antibodies mimicking heterophilic antibodies in thyroid tests (51).
cases of apparent hyperthyroidism due to Biotin-like interference from IgM anti-streptavidin, a pattern that mimics the excess biotin ingestion (52);
Moreover, drugs over the counter, high-dose biotin supplements are accompanied by observations of analytical interference by exogenous biotin (53) in the immunoassays used to evaluate endocrine function (54). Best practices have been proposed to avoid such interferences, comprising sample dilutions, retesting after biotin clearance, assessment of biotin presence (55).
Variables due to patient preparation
Thyroid hormones appear influenced by fasting status or diet composition (6, 56). On the other hand, either diet composition, meal frequency or eating time, all can influence GH, prolactin, and cortisol levels (57). Moreover, treatments or drugs that delay gastric emptying and motility, such as omeprazole can act on the levels of some hormones. Fasting induces enhancement of GH release with a concomitant reduction of somatomedin C. The GH release is achieved through combined frequency and amplitude modulation (57). Ghrelin is controlled by food intake, being higher during fasting or before meals then decreasing after meal. It is known as the “hunger hormone” since after being released mainly by gastric cells – and also by gut, pancreas and brain cells – it stimulates appetite and consequent food intake and fat accumulation acting on hypothalamus. However, ghrelin is more than that, since it regulates glucose homeostasis by inhibiting insulin secretion. Moreover, it shows a series of positive effects on heart, muscle and bone (58).
To increase accuracy, testosterone in men should be measured with a fasting morning sample and repeated if the level is found to be low; in women, the laboratory must perform the measurements at the follicular phase of the cycle. In both cases, borderline results may be clarified by the assessment of free testosterone. Thus, interpretation of testosterone results is challenging. Presently the reference assay method for measuring blood testosterone levels is based on mass spectrometry (59). Moreover, as avoidance of fasting time could jeopardize result accuracy and thus patient safety (60), 12h overnight fasting before blood sampling for laboratory immunoassays are recommended (6, 8, 61).
Short-term studies have shown that caffeine increases insulin levels, reduces insulin sensitivity and increases cortisol levels (62). Coffee has been considered to influence the energy intake possibly acting on appetite hormones. On this note, it has been shown that although coffee and caffeine ingested 0.5-4 h before a meal can suppress acute energy intake, the influence on appetite hormones appears equivocal (63). Moreover, habitual coffee intake is related to effects on either glycerophospholipid metabolism (64) or several metabolic pathways (65). All the above suggest that before blood collection, patients should be requested to inform about their drinking coffee habit, either short-term or long-term.
The nicotine from smoking increases cortisol, GH, prolactin, LH, ACTH and DHEA, in males; similar effects are observed with intravenous cocaine abuse (66, 67). On the contrary, nicotine decreases prolactin in pregnant (68) and in lactating women (69) (without effect on fetal prolactin).
Many hormones show diurnal variations (e.g. ACTH, cortisol, GH) and pulsatile secretion is reported for some hormones with short time variations, up to 25%. The pituitary hormones show a circadian cycle that is linked to the sleep-wake rhythm. Cortisol shows diurnal variation, with the peak value at about 8 am in the morning and the lowest levels in the late night until early morning, from 12 pm to 4 am. Individuals affected by Cushing’s syndrome show incongruously high ACTH and cortisol levels during late night hours. Thus, the sample collection time should be planned consequently. Cortisol can be assayed either in serum or in saliva (70). The salivary cortisol assay was described several years ago. It has the advantages of being simple to collect, non-invasive and the sample can be easily stored for repetition. The advantages of salivary cortisol assays have been evidenced in patients with Addison disease and appear more adequate than serum in the screening for Cushing disease after assaying salivary samples obtained during night time (71). When collecting saliva samples, it is important to instruct the patient to avoid hydrocortisone containing creams or ointments in order to prevent analytical interference (72). Salivary melatonin assay can be a reliable non-invasive biomarker of the circadian secretive rhythm of the pineal gland (73). Prolactin is another hormone with diurnal variation and with higher levels at night (74). Prolactin levels in samples taken in the morning can sometimes show increased levels thus indicating a hyperprolactinemia status. Since prolactin diurnal variation is associated with night peak that sometimes declines steadily and prolongs in the first morning hours, there are individuals showing late reduction of prolactin to the reference values observed in the morning values. Besides a true hyperprolactinemia, elevated morning levels can be due to stress conditions, medications or spurious hyperprolactinemia. Indeed, in most cases after sample retaking a normal value is observed. For these reasons and for avoiding unnecessary additional tests, it has been suggested to delay late in the morning the sample collection for prolactin assays, i.e. around 11 am (75). The measure of PTH is a second-level examination, requested by physicians with other tests: e.g., calcium to elucidate a hyperparathyroidism hypothesis (76). It is recommended that blood samples for PTH measurement should be taken, ideally between 10:00 and 16:00, and plasma separated within 24 h of blood collection. Plasma samples should be stored at +4°C and analyzed within 72 h from collection (77). Therefore, considering that for several hormones the diurnal variations are critical for result interpretation, we suggest that blood withdrawal hours should be appropriately defined to guarantee suitable laboratory results and thus patient safety.
Variables due to sample matrix and evacuated tube
Different laboratory results, including hormones, were reported using different brands of serum- and plasma-evacuated tubes (13, 78, 79). The reasonable cause for these divergences was ascribed to interaction between blood and components in the evacuated tubes, e.g. surfactant(s), stopper(s), stopper-lubricant(s), separator gel(s), and additive(s) (80, 81). The commonly used tube surfactant – Silwet L-720 – has been reported to cause the desorption of antibodies from the solid phase (82). Moreover, samples collected on SST tubes (BD Vacutainer) have been reported with values of total triiodothyronine, measured by the Immulite 2000, significantly higher than samples collected in glass or Vacuette tubes (Greiner Bio-One). Alterations in concentrations of triiodothyronine, thyroxine and cortisol in either quality control materials or in serum specimens have been reported after collecting or pouring in different blood-collection tube types (83). Discrepancies have been observed in C-reactive protein (CRP) assays measured in serum samples from SST tubes, in particular lower values in SST serum than in serum from plain tubes. It appears due to SST adsorption of some CRP macromolecules that form complexes with SST gel (84). Therefore, the need to verify the suitability of the evacuated tubes employed in sample collection for immunoassays is mandatory in order to avoid unwanted outcomes and to warrant the safety of the patients’ results (8, 85, 86).
Plasma vs. serum samples reveal different hormone results due to interferences from lithium heparin, EDTA, sodium fluoride, or potassium oxalate (87). Parathormone (PTH) levels decrease after blood collection in either EDTA or serum samples, with divergent results. A greater stability has been reported for PTH collected in potassium EDTA (88).
The Clinical and Laboratory Standards Institute recommend to follow the instructions from evacuated tubes manufacturer regarding: sample mixing, resting time before centrifugation, centrifugation time, g-force (89). As far as we know, Vacumed ® (FL Medical, Torreglia) lacks a formal specification regarding sample processing and handling. Thus, after having checked other brands information (90-92), we decided to verify this evacuated tubes (Table 1). A sometimes-overlooked fact is that the accuracy of blood drawing with evacuated tube systems is influenced by altitude, because of variations of the atmospheric pressure. It is well known that reduced blood volumes are collected into the tubes in altitude settings (90), thus, altering the blood/additive ratio (91) (Table 2).
Table 1.
Verification of Vacumed ®
| Evacuated tubes | Additives | Mix by gently inversion | Centrifugation | |
|---|---|---|---|---|
| g-force | time (min) | |||
| 42010; 42011; 42012 | K2EDTA | 2 X | 1200 | 15 |
| 43016 | ||||
| 44019 | ||||
| 42110; 42111; 42112 | K3EDTA | 2 X | 1200 | 15 |
| 43116 | ||||
| 44119 | ||||
| 42210; 42211; 42212 | Potassium Fluoride + K3EDTA |
3 X | 1000 | 15 |
| 43216 | ||||
| 44219 | ||||
| 42310; 42311; 42312 | Lithium Heparin | 2 X | 1200 | 15 |
| 43316 | ||||
| 44319 | ||||
| 43350; 43351; 43352 | Sodium Heparin | 3 X | 1200 | 15 |
| 43356 | ||||
| 44359 | ||||
| 42410; 42411; 42412 | Sodium Citrate 3.2% | 2 X | 1500 NA NA NA |
15 |
| 42508 | ||||
| 42510 | ||||
| 42512 | ||||
| 42611; 42612 | Clot activator | 2 X | 1200 | 15 |
| 43616 | ||||
| 44619 | ||||
| 42691 | Clot activator + sferes separator |
3 X | 1000 | 15 |
| 43696 | ||||
| 44698 | ||||
| 42711 | Clot activator + gel separator |
2 X | 2000 | 10 |
| 43716 | ||||
| 44718 | ||||
| 42811 | gel separator + Lithium Heparin |
2 X | 1800 | 10 |
| 43816 | ||||
| 44818 | ||||
| 42851 | K2EDTA + gel separator |
2 X | 1600 | 10 |
| 43856 | ||||
| 44858 | ||||
| 42910; 42911; 42912 | without additive | NA | 2200 for whole blood | 10 |
| 43916 | ||||
| 44919 | ||||
Note: Serum tubes should rest at least 45 min in upright position after blood collection and before centrifugation. However, should be centrifuged within 2 h after blood collection. All tubes including tubes with gel separator should be transported in upright position after centrifugation process. The results reported in this table is part of the project 2451/15 of University of Verona.
Table 2.
Mean volume filling regarding different altitudes
| Altitude (m) | Mean volume filling of evacuated tubes | |||
|---|---|---|---|---|
| 4.5 mL | 3.5 mL | 3 mL | 2 mL | |
| 0 | 4.50 | 3.50 | 3.00 | 2.00 |
| 250 | 4.37 | 3.40 | 2.91 | 1.94 |
| 500 | 4.24 | 3.30 | 2.83 | 1.89 |
| 1000 | 4.00 | 3.11 | 2.67 | 1.78 |
| 1250 | 3.89 | 3.02 | 2.59 | 1.73 |
| 1500 | 3.78 | 2.94 | 2.52 | 1.68 |
| 1750 | 3.67 | 2.86 | 2.45 | 1.63 |
| 2000 | 3.57 | 2.78 | 2.38 | 1.59 |
Note: Results are presented as mean volume from five evacuated tubes producers – BD Vacutainer®, Becton, Dickinson and Company; Vacuette®, Greiner Bio-One; Vacumed®, FL Medical; Vacutest®, Kima; and S-Monovette, Sarstedt using the vacuum technique – filled with a solution with density and viscosity like whole blood. Volumes (ml) reported by the evacuated tube manufacturer at sea level (0 m) are presented in bold. Altitudes are referred in meters above sea level. All tubes used were 13×75 mm.
CONCLUSIONS
In immunochemistry assays the results can be significantly and positively influenced by the appropriateness of the procedures adopted along the process starting from test prescription and ending with result interpretation. Particular attention should be addressed separately to variables that on the contrary could be viewed as a collective whole in the pre-analytical phase, therefore resulting in less influence on the outcomes and possibly misleading in the interpretation. In this context it should be summarized that:
results can be significantly influenced by the techniques adopted, that in turn can be affected by some preanalytical conditions;
age and gender are important variables, particularly in newborn, during sexual maturation and childbearing;
stress can influence GH, prolactin cortisol and the total/free ratio of thyroid hormone;
diurnal variations, day of cycle, influence by estrogens on thyroid hormone are to be accounted for result variability;
in adults/elders some results can be interfered by autoantibodies;
some additives and anticoagulants of collection tubes can influence specific assays;
hemolysis, lipemia and bilirubin cause interferences on specific techniques/tests;
paraproteins mainly cause interferences;
specific issues should be considered as regards thyroid, pituitary and sex hormones;
nicotine and cocaine addictions interfere with some hormones.
Therefore, in providing the results to the patients, laboratory professionals have the responsibility of ascertaining that all the above have been checked and duly evaluated and the appropriate actions have been accomplished to avoid any relevant interferences.
REFERENCES
- 1.Koivunen ME, Krosgrud RL. Principles of Immunochemical Techniques Used in Clinical Laboratories. Lab Med 2006;37:490-497. [Google Scholar]
- 2.Keffer JH. Preanalytical considerations in testing thyroid function. Clin Chem 1996;42:125-134. [PubMed] [Google Scholar]
- 3.Sam S, Frohman LA. Normal physiology of hypothalamic pituitary regulation. Endocrinol Metab Clin North Am 2008;37:1-22. [DOI] [PubMed] [Google Scholar]
- 4.Veldhuis JD. Changes in pituitary function with ageing and implications for patient care. Nat Rev Endocrinol 2013;9:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayanan S, Guder WG. Preanalytical Variables and Their Influence on the Quality of Laboratory Results. Ejifcc 2001;13:9-12. [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaña W, Aranda E, Arredondo ME, Brennan-Bourdon LM, Campelo MD, Espinoza E, Flores S, Ochoa P, Vega V, Varela B, Lima-Oliveira G. Impact of an Andean breakfast on biochemistry and immunochemistry laboratory tests: an evaluation on behalf COLABIOCLI WG-PRE-LATAM. Biochem Med 2019;19:020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arredondo ME, Aranda E, Astorga R, Brennan-Bourdon LM, Campelo MD, Flores S, Manriquez I, Ochoa P, Varela B, Salinas CV, Lima-Oliveira G. Breakfast can Affect Routine Hematology and Coagulation Laboratory Testing: An Evaluation on Behalf of COLABIOCLI WG-PRE-LATAM. TH open 2019;3:e367-e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simundic AM, Bolenius K, Cadamuro J, Church S, Cornes MP, van Dongen-Lases EC, Eker P, Erdeljanovic T, Grankvist K, Guimaraes JT, Hoke R, Ibarz M, Ivanov H, Kovalevskaya S, Kristensen GBB, Lima-Oliveira G, Lippi G, von Meyer A, Nybo M, De la Salle B, Seipelt C, Sumarac Z, Vermeersch P, Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI) Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin Chem Lab Med 2018;56: 2015-2038. [DOI] [PubMed] [Google Scholar]
- 9.Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemot 2017;39:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ide BN, Souza-Junior TP, McAnulty SR, de Faria MAC, Costa KA, Nunes LAS. Immunological Responses to a Brazilian Jiu-Jitsu High-Intensity Interval Training Session. J Hum Kinet 2019;70:115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao H, Rayburn ER, Shi Q, Gao L, Hu W, Li H. FDA-approved drugs that interfere with laboratory tests: A systematic search of US drug labels. Crit Rev Clin Lab Sci 2017;54: 1-17. [DOI] [PubMed] [Google Scholar]
- 12.Lima-Oliveira G, Lippi G, Salvagno GL, Gaino S, Poli G, Gelati M, Picheth G, Guidi GC. Venous stasis and whole blood platelet aggregometry: a question of data reliability and patient safety. Blood Coagul Fibrinolysis 2015;26: 665-668. [DOI] [PubMed] [Google Scholar]
- 13.Lima-Oliveira G, Salvagno GL, Lippi G, Brocco G, Voi M, Montagnana M, Picheth G, Guidi GC. Quality management of preanalytical phase: impact of lithium heparin vacuum tubes changes on clinical chemistry tests. Accred Qual Assur 2013;18:429-434. [Google Scholar]
- 14.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Poli G, Solero GP, Picheth G, Guidi GC. K(3)EDTA Vacuum Tubes Validation for Routine Hematological Testing. ISRN hematolog 2012;2012:875357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grankvist K, Gomez R, Nybo M, Lima-Oliveira G, von Meyer A. Preanalytical aspects on short- and long-term storage of serum and plasma. Diagnosis 2019;6:51-56. [DOI] [PubMed] [Google Scholar]
- 16.Lippi G, Lima-Oliveira G, Nazer SC, Moreira ML, Souza RF, Salvagno GL, Montagnana M, Scartezini M, Picheth G, Guidi GC. Suitability of a transport box for blood sample shipment over a long period. Clin Biochem 2011;44: 1028-1029. [DOI] [PubMed] [Google Scholar]
- 17.Lima-Oliveira G, Lippi G, Salvagno GL, Gelati M, Bassi A, Contro A, Pizzolo F, Guidi GC. Abnormal gel flotation caused by contrast media during adrenal vein sampling. Biochem Med 2016;26:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi G, Salvagno GL, Danese E, Lima-Oliveira G, Brocco G, Guidi GC. Inversion of lithium heparin gel tubes after centrifugation is a significant source of bias in clinical chemistry testing. Clin Chim Acta 2014;436:183-187. [DOI] [PubMed] [Google Scholar]
- 19.Lima-Oliveira G, Cesare Guidi G, Guimaraes AVP, Abol Correa J, Lippi G. Preanalytical Nonconformity Management Regarding Primary Tube Mixing in Brazil. J Med Biochem 2017;36:39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasanato R, Brearton S, Alshebani M, Bailey L, Aldugashim S, Alothaim A, Tamimi W. Effects of serum indices interference on hormonal results from the Abbott Architect i2000 immunoassay analyser. Br J Biomed Sci 2015;72:151-155. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza-Benitez PS, Aguilar-Ruiz SR, Romero-Tlalolini MD, Torres-Aguilar H. Evaluation of the Interference of Lipemia and Hemolysis in the Detection Limit of Anti-HIV-1 Antibodies. Clin Lab 2019;65. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YV, Fan SL, Kellogg MD. Effect of hemolysis, icterus, and lipemia on three acetaminophen assays: Potential medical consequences of false positive results. Clin Chim Acta 2018;487:287-292. [DOI] [PubMed] [Google Scholar]
- 23.Saracevic A, Dukic L, Simundic AM. Haemolysis and lipemia interfere with resistin and myeloperoxidase Bio-Vendor ELISA assays. Biochem Med 2019;29:020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consortium on Thyroid and Pregnancy-Study Group on Preterm Birth. Korevaar TIM, Derakhshan A, Taylor PN, Meima M, Chen L, Bliddal S, Carty DM, Meems M, Vaidya B, Shields B, Ghafoor F, Popova PV, Mosso L, Oken E, Suvanto E, Hisada A, Yoshinaga J, Brown SJ, Bassols J, Auvinen J, Brames WM, Lopez-Bermejo A, Dayan C, Boucai L, Vafeiadi M, Grineva EN, Tkachuck AS, Pop VJM, Vrijkotte TG, Guxens M, Chatzi L, Sunyer J, Jimenez-Zabala A, Riano I, Murcia M, Lu X, Mukhtar S, Delles C, Feldt-Rasmussen U, Nelson SM, Alexander EK, Chaker L, Mannisto T, Walsh JP, Pearce EN, Steegers EAP, Peeters RP. Association of Thyroid Function Test Abnormalities and Thyroid Autoimmunity With Preterm Birth: A Systematic Review and Meta-analysis. Jama 2019;322:632-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner CL, Baatz JE, Newton D, Hollis BW. Analytical considerations and general diagnostic and therapeutic ramifications of milk hormones during lactation. Best Pract Res Clin Endocrinol Metab 2018;32:5-16. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Rong SS, Sun X, Ding G, Wan W, Zou L, Wu S, Li M, Wang D. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: a longitudinal study. Brit J Nutr 2018;120: 1380-1387. [DOI] [PubMed] [Google Scholar]
- 27.Soldin OP, Hoffman EG, Waring MA, Soldin SJ. Pediatric reference intervals for FSH, LH, estradiol, T3, free T3, cortisol, and growth hormone on the DPC IMMULITE 1000. Clin Chim Acta 2005;355:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunapalasingham G, Frithioff-Bojsoe C, Lund MAV, Hedley PL, Fonvig CE, Dahl M, Pedersen O, Christiansen M, Hansen T, Lausten-Thomsen U, Holm JC. Reference values for fasting serum concentrations of thyroid-stimulating hormone and thyroid hormones in healthy Danish/North-European white children and adolescents. Scand J Clin Lab Invest 2019;79:129-135. [DOI] [PubMed] [Google Scholar]
- 29.Lobo RA, Pickar JH, Stevenson JC, Mack WJ, Hodis HN. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 2016;254:282-290. [DOI] [PubMed] [Google Scholar]
- 30.Huhtaniemi IT. Andropause--lessons from the European Male Ageing Study. Ann Endocrinol 2014;75:128-131. [DOI] [PubMed] [Google Scholar]
- 31.Duntas LH. Thyroid Function in Aging: A Discerning Approach. Rejuvenation Res 2018;21:22-28. [DOI] [PubMed] [Google Scholar]
- 32.Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online 2014;28:714-722. [DOI] [PubMed] [Google Scholar]
- 33.El Khoudary SR, Thurston RC. Cardiovascular Implications of the Menopause Transition: Endogenous Sex Hormones and Vasomotor Symptoms. Obst Gynecol Clin North Am 2018;45:641-661. [DOI] [PubMed] [Google Scholar]
- 34.Herati AS, Cengiz C, Lamb DJ. Assays of Serum Testosterone. Urol Clin North Am 2016;43:177-184. [DOI] [PubMed] [Google Scholar]
- 35.Chianese R, Cobellis G, Chioccarelli T, Ciaramella V, Migliaccio M, Fasano S, Pierantoni R, Meccariello R. Kisspeptins, Estrogens and Male Fertility. Curr Med Chem 2016;23:4070-4091. [DOI] [PubMed] [Google Scholar]
- 36.Esposito S, Leonardi A, Lanciotti L, Cofini M, Muzi G, Penta L. Vitamin D and growth hormone in children: a review of the current scientific knowledge. J Transl Med 2019;17:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray PG, Dattani MT, Clayton PE. Controversies in the diagnosis and management of growth hormone deficiency in childhood and adolescence. Arch Dis Child 2016;101: 96-100. [DOI] [PubMed] [Google Scholar]
- 38.Phillip M, Chalew SA, Kowarski AA, Stene MA. Plasma IGFBP-3 and its relationship with quantitative growth hormone secretion in short children. Clin Endocrinol 1993;39: 427-432. [DOI] [PubMed] [Google Scholar]
- 39.Nunez SB, Municchi G, Barnes KM, Rose SR. Insulinlike growth factor I (IGF-I) and IGF-binding protein-3 concentrations compared to stimulated and night growth hormone in the evaluation of short children--a clinical research center study. J Clin Endocrinol Metab 1996;81: 1927-1932. [DOI] [PubMed] [Google Scholar]
- 40.McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY; Women’s Health Initiative Investigators. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity 2006;14:1662-1677. [DOI] [PubMed] [Google Scholar]
- 41.Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab 2002;87:2352-2358. [DOI] [PubMed] [Google Scholar]
- 42.Feldt-Rasmussen U. Thyroid and leptin. Thyroid 2007; 17:413-419. [DOI] [PubMed] [Google Scholar]
- 43.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst 1995;87: 1297-1302. [DOI] [PubMed] [Google Scholar]
- 44.Ozarda Y, Sikaris K, Streichert T, Macri J; IFCC Committee on Reference intervals and Decision Limits (C-RIDL). Distinguishing reference intervals and clinical decision limits-A review by the IFCC Committee on Reference Intervals and Decision Limits. Crit Rev Clin Lab Sci 2018;55: 420-431. [DOI] [PubMed] [Google Scholar]
- 45.LeGatt DF, Higgins TN. Paraprotein interference in immunoassays. Ther Drug Monit 2015;37:417. [DOI] [PubMed] [Google Scholar]
- 46.Hirata K, Saruwatari J, Enoki Y, Iwata K, Urata Y, Aizawa K, Ueda K, Shirouzono T, Imamura M, Moriuchi H, Ishima Y, Kadowaki D, Watanabe H, Hirata S, Maruyama T, Fukunaga E. Possible false-negative results on therapeutic drug monitoring of phenytoin using a particle enhanced turbidimetric inhibition immunoassay in a patient with a high level of IgM. Ther Drug Monit 2014;36:553-555. [DOI] [PubMed] [Google Scholar]
- 47.Cooper AA, Cowart K, Clayton A, Paul J. Undetectable Vancomycin Concentrations Utilizing a Particle Enhanced Turbidimetric Inhibition Immunoassay in a Patient with an Elevated IgM Level. Clin Lab 2017;63:1527-1532. [DOI] [PubMed] [Google Scholar]
- 48.Ong MW, Salota R, Reeman T, Lapsley M, Jones L. Artefactual 25-OH vitamin D concentration in multiple myeloma. Ann Clin Biochem 2017;54:716-720. [DOI] [PubMed] [Google Scholar]
- 49.LeGatt DF, Blakney GB, Higgins TN, Schnabl KL, Shalapay CE, Dias VC, Wesenberg JC. The effect of paraproteins and rheumatoid factor on four commercial immunoassays for vancomycin: implications for laboratorians and other health care professionals. Ther Drug Monit 2012;34:306-311. [DOI] [PubMed] [Google Scholar]
- 50.Serei VD, Marshall I, Carayannopoulos MO. Heterophils antibody interference affecting multiple Roche immunoassays: A case study. Clin Chim Acta 2019;497: 125-129. [DOI] [PubMed] [Google Scholar]
- 51.Favresse J, Lardinois B, Nassogne MC, Preumont V, Maiter D, Gruson D. Anti-streptavidin antibodies mimicking heterophilic antibodies in thyroid function tests. Clin Chem Lab Med 2018;56:e160-e163. [DOI] [PubMed] [Google Scholar]
- 52.Lam L, Bagg W, Smith G, Chiu WW, Middleditch MJ, Lim JC, Kyle CV. Apparent Hyperthyroidism Caused by Biotin-Like Interference from IgM Anti-Streptavidin Antibodies. Thyroid 2018;28:1063-1067. [DOI] [PubMed] [Google Scholar]
- 53.Bowen RA, Chan Y, Cohen J, Rehak NN, Hortin GL, Csako G, Remaley AT. Effect of blood collection tubes on total triiodothyronine and other laboratory assays. Clin Chem 2005;51:424-433. [DOI] [PubMed] [Google Scholar]
- 54.Samarasinghe S, Meah F, Singh V, Basit A, Emanuele N, Emanuele MA, Mazhari A, Holmes EW. Biotin Interference with Routine Clinical Immunoassays: Understand the Causes and Mitigate the Risks. Endocr Pract 2017;23: 989-998. [DOI] [PubMed] [Google Scholar]
- 55.Bowen R, Benavides R, Colon-Franco JM, Katzman BM, Muthukumar A, Sadrzadeh H, Straseski J, Klause U, Tran N. Best practices in mitigating the risk of biotin interference with laboratory testing. Clin Biochem 2019;74:1-11. [DOI] [PubMed] [Google Scholar]
- 56.Basolo A, Begaye B, Hollstein T, Vinales KL, Walter M, Santini F, Krakoff J, Piaggi P. Effects of Short-Term Fasting and Different Overfeeding Diets on Thyroid Hormones in Healthy Humans. Thyroid 2019;29:1209-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, Thorner MO. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest 1988;81: 968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pradhan G, Samson SL, Sun Y. Ghrelin:much more than a hunger hormone. Curr Opin Clin Nutr Metab Care 2013;16:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanakis GA, Tsametis CP, Goulis DG. Measuring testosterone in women and men. Maturitas 2019;125:41-44. [DOI] [PubMed] [Google Scholar]
- 60.Guidi GC, Simundic AM, Salvagno GL, Aquino JL, Lima-Oliveira G. To avoid fasting time, more risk than benefits. Clin Chem Lab Med 2015;53:e261-e264. [DOI] [PubMed] [Google Scholar]
- 61.Lima-Oliveira G, Valentin CD, Guidi GC. Lipid profile, changes in laboratory prescriptions are necessary. J Clin Lipidol 2017;11:768-769. [DOI] [PubMed] [Google Scholar]
- 62.MacKenzie T, Comi R, Sluss P, Keisari R, Manwar S, Kim J, Larson R, Baron JA. Metabolic and hormonal effects of caffeine: randomized, double-blind, placebo-controlled crossover trial. Metabolism 2007;56:1694-1698. [DOI] [PubMed] [Google Scholar]
- 63.Schubert MM, Irwin C, Seay RF, Clarke HE, Allegro D, Desbrow B. Caffeine, coffee, and appetite control: a review. Int J Food Sci Nutr 2017;68:901-912. [DOI] [PubMed] [Google Scholar]
- 64.Kuang A, Erlund I, Herder C, Westerhuis JA, Tuomilehto J, Cornelis MC. Lipidomic Response to Coffee Consumption. Nutrients 2018;10:E1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornelis MC, Erlund I, Michelotti GA, Herder C, Westerhuis JA, Tuomilehto J. Metabolomic response to coffee consumption: application to a three-stage clinical trial. J Intern Med 2018;283:544-557. [DOI] [PubMed] [Google Scholar]
- 66.Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology 1998;23:103-113. [DOI] [PubMed] [Google Scholar]
- 67.Mello NK. Hormones, nicotine, and cocaine: clinical studies. Horm Behav 2010;58:57-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersen AN, Ronn B, Tjonneland A, Djursing H, Schioler V. Low maternal but normal fetal prolactin levels in cigarette smoking pregnant women. Acta Obstet Gynecol Scand 1984;63:237-239. [DOI] [PubMed] [Google Scholar]
- 69.Andersen AN, Lund-Andersen C, Larsen JF, Christensen NJ, Legros JJ, Louis F, Angelo H, Molin J. Suppressed prolactin but normal neurophysin levels in cigarette smoking breast-feeding women. Clin Endocrinol 1982;17:363-368. [DOI] [PubMed] [Google Scholar]
- 70.Bastin P, Maiter D, Gruson D. Salivary cortisol testing: preanalytic and analytic aspects. Ann de Biol Clin 2018;76: 393-405. [DOI] [PubMed] [Google Scholar]
- 71.Restituto P, Galofre JC, Gil MJ, Mugueta C, Santos S, Monreal JI, Varo N. Advantage of salivary cortisol measurements in the diagnosis of glucocorticoid related disorders. Clin Biochem 2008;41:688-692. [DOI] [PubMed] [Google Scholar]
- 72.Raff H, Singh RJ. Measurement of late-night salivary cortisol and cortisone by LC-MS/MS to assess preanalytical sample contamination with topical hydrocortisone. Clin Chem 2012;58:947-948. [DOI] [PubMed] [Google Scholar]
- 73.Lewy AJ, Sack RL, Blood ML, Bauer VK, Cutler NL, Thomas KH. Melatonin marks circadian phase position and resets the endogenous circadian pacemaker in humans. Ciba Found Symp 1995;183:303-321. [DOI] [PubMed] [Google Scholar]
- 74.Samperi I, Lithgow K, Karavitaki N. Hyperprolactinaemia. J Clin Med 2019;8:2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewandowski KC, Skowronska-Jozwiak E, Szosland K, Lewinski A. Effect of timing of prolactin sampling on the incidence of spurious hyperprolactinaemia. Endocr Abstracts 2005;9:223. [Google Scholar]
- 76.Lima-Oliveira G, Lippi G, Salvagno GL, Brocco G, Guidi GC. In vitro diagnostic company recalls and medical laboratory practices: an Italian case. Biochem Med 2015;25:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanon EA, Sturgeon CM, Lamb EJ. Sampling and storage conditions influencing the measurement of parathyroid hormone in blood samples: a systematic review. Clin Chem Lab Med 2013;51:1925-1941. [DOI] [PubMed] [Google Scholar]
- 78.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Preanalytical management: serum vacuum tubes validation for routine clinical chemistry. Biochem Med 2012;22:180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zungun C, Yilmaz FM, Boru EG, Topcuoglu C. Comparison of Improvacuter tubes with BD Vacutainer tubes for various hormones in the aspects of stability and influence of gel separators. Clin Chem Lab Med 2015;53:231-238. [DOI] [PubMed] [Google Scholar]
- 80.Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med 2014;24:31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bowen RA, Adcock DM. Blood collection tubes as medical devices: The potential to affect assays and proposed verification and validation processes for the clinical laboratory. Clin Biochem 2016;49:1321-1330. [DOI] [PubMed] [Google Scholar]
- 82.Bowen RA, Chan Y, Ruddel ME, Hortin GL, Csako G, Demosky SJ, Jr, Remaley AT. Immunoassay interference by a commonly used blood collection tube additive, the organosilicone surfactant silwet L-720. Clin Chem 2005;51: 1874-1882. [DOI] [PubMed] [Google Scholar]
- 83.Bowen RA, Sattayapiwat A, Gounden V, Remaley AT. Blood collection tube-related alterations in analyte concentrations in quality control material and serum specimens. Clin Biochem 2014;47:150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang CY, Lu JY, Chien TI, Kao JT, Lin MC, Shih PC, Yan SN. Interference caused by the contents of serum separator tubes in the Vitros CRP assay. Ann Clin Biochem 2003;40: 249-251. [DOI] [PubMed] [Google Scholar]
- 85.Giavarina D, Banfi G, Daves M, Dolci A, Farci Santarcangeli D, Lima-Oliveira G, Miconi D, Milanesi B, Montagnana M, Morandini M, Piva E, Salvano GL, Troiano T, Lippi G. Blood collection systems in clinical laboratories: local adaptation of the EFLM guidelines. Biochim Clin 2016;40:347-352. [Google Scholar]
- 86.International Organization for Standardization. Medical laboratories-Requirements for quality and competence ISO document 15189. Geneva, Switzerland: International Organization for Standardization; 2012. [Google Scholar]
- 87.Evans MJ, Livesey JH, Ellis MJ, Yandle TG. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem 2001;34: 107-112. [DOI] [PubMed] [Google Scholar]
- 88.English E, McFarlane I, Taylor KP, Halsall DJ. The effect of potassium EDTA on the stability of parathyroid hormone in whole blood. Ann Clin Biochem 2007;44:297-299. [DOI] [PubMed] [Google Scholar]
- 89.Clinical Laboratory Standards Institute. Procedures for the handling and processing of blood specimens for common laboratory tests. CLSI H18-A4 document. 4th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2010. [Google Scholar]
- 90.BD Diagnostics - Preanalytical Systems. Product Catalogue https://www.bd.com/resource.aspx?IDX=10155 [Accessed June 10th 2019].
- 91.Greiner Bio-One Product Catalog. Vacuette blood collection tubes. https://www.gbo.com/fileadmin/user_upload/1729004RN_Joint_Catalog_2017_Web.pdf [Accessed June 10th 2019].
- 92.Sarstedt full product catalogue. https://www.sarstedt.com/fileadmin/user_upload/Katalog/2019/45_200_catalogue_en_eu_eu_2019_2020_hp.pdf [Accessed June 10th 2019].
- 93.MacNutt MJ, Sheel AW. Performance of evacuated blood collection tubes at high altitude. High Alt Med Biol 2008;9(3):235-237. [DOI] [PubMed] [Google Scholar]
- 94.Chuang J, Sadler MA, Witt DM. Impact of evacuated collection tube fill volume and mixing on routine coagulation testing using 2.5-ml (pediatric) tubes. Chest. 2004;126(4): 1262-1266. [DOI] [PubMed] [Google Scholar]


