Abstract
Objective
To evaluate the major causes of preanalytical errors in medical laboratory of a tertiary care hospital.
Methods
It was a retrospective study in which we analyzed the sample rejection data of hematology and chemical pathology sections from January to December 2018. Number of rejected samples, reason for rejection and type of test ordered on monthly basis were recorded on a platform.
Results
A total of 113,817 samples were received during the study period. Preanalytical errors were found in 1,688 samples, which constitute approximately 1.48% of the total number of samples received.
Conclusion
Our study highlights the magnitude of preanalytical errors in our setup. Preanalytical errors can lead to loss of patient trust in diagnostic services, can dent the laboratory’s reputation, and lead to an increase in the overall operating expenses, both for laboratories as well as the hospitals. Compliance with good laboratory practices can significantly reduce the frequency of pre analytical errors.
Key words: medical errors, clinical laboratory blood specimen collection, phlebotomy, specimen handling
INTRODUCTION
Quality in medical diagnostics is essential to the goal of providing safe health care to patients. Among other clinical disciplines, laboratory medicine assumes a vital role in patient safety (1).
Conventionally, laboratory practice can be divided into three phases, i.e., preanalytical, analytical and post-analytical phase. Preanalytical phase comprises of test selection, patient identification, collection of the sample, handling of the sample, sorting out, pipetting and centrifugation (2, 3). Negligence in any of these steps can lead to erroneous results attributed to preanalytical phase.
Although all three phases are equally important for improving total quality management and should be targeted individually for improving standards of the laboratory, preanalytical phase is considered as the most error prone part of the total testing process. Preanalytical issues have been included in the list of biggest challenges faced by the laboratory professionals in the last two decades (4).
Lippi and colleagues reported that total error rate in laboratory medicine is 0.1% to 3.0% (5). Analytical errors which have been focus of research in the past, account for less than 10% of all the diagnostic mistakes, whereas preanalytical errors are reported to be accounting for 46 to 68.2% (6). Moreover, preanalytical errors constitute 18.5 to 47% of the laboratory errors. Missing patient’s identification, inappropriate containers, missing samples are most commonly encountered preanalytical errors (7).
Worldwide standards relating to blood sampling and standardization are available but compliance to guidelines is very low especially in the background where sampling is done by the nurses/junior doctors without involvement of the laboratory staff (8). Furthermore, there is heterogeneity in criteria for sample rejection from one laboratory to another. Alongside the long road of patient safety, preanalytical phase of laboratory medicine offers a wide room for improvement (9).
There is scarcity of the local data regarding documentation, root cause analysis and preventive strategies for laboratory errors (10). Our study aims to evaluate the major causes of pre analytical errors in medical laboratory of a tertiary care hospital.
MATERIAL AND METHODS
Shalamar Hospital is a teaching hospital in Lahore, Pakistan specializing in various fields like: Surgery, Gynecology & Obstetrics, Cardiology, Gastroenterology, and Psychiatry. It is a 430 bedded hospital providing services to about 376,000 patients per year. Here, phlebotomies of the inpatients are performed by the clinical staff (nurses and junior doctors), whereas blood samples from the outpatients are collected by laboratory personnel.
The samples are collected in evacuated tubes (BD vacutainer), that include purple top ethylenediaminetetraacetic acid di/trisodium salt (EDTA) tubes, blue top sodium citrate tubes, yellow top gel separation tubes, and syringes for arterial blood gas analysis. Upon receiving the samples, they are sorted out for any problem at the reception desk before transporting to concerned sections. In case, any problem exists, it is manually registered in the logbook.
Samples are rejected on the basis of preset rejection criteria, as follows: unlabeled specimen container, specimen without request form, incorrect tube (wrong choice of tube), wrong label/wrong medical record number, incorrect quantity or insufficient sample, hemolysed sample, anticoagulated sample (EDTA and citrated) with clots, improper sample transport, improper container closure, specimen delayed in transit making results invalid, diluted sample. The data generated is viewed periodically (monthly).
It was a retrospective study; we analyzed the sample rejection data of hematology and chemical pathology sections from January to December 2018. Number of rejected samples, and reason for rejection of tests ordered on monthly basis were recorded on a proforma. Data were analyzed on Statistical Package for the Social Sciences version 20 (SPSS V 20). Frequency of each type of preanalytical error was assessed.
RESULTS
A total of 113,817 samples were received during the study period in hematology and chemical pathology sections. Out of these samples, pre analytical errors were found in 1,688 samples, which constituted approximately 1.48% of the total number of samples received. The frequency of errors are show in Table 1.
Table 1.
Frequency of errors on blood samples*
| Type of error | N | Frequency (%) |
|---|---|---|
| Unlabeled sample | 604 | 35.8 |
| Sample clotted (EDTA and sodium citrate) | 252 | 14.9 |
| Sample diluted | 200 | 11.8 |
| Wrong medical record number | 172 | 10.2 |
| Sample hemolysed | 164 | 9.7 |
| Incorrect tube | 148 | 8.8 |
| Incorrect quantity or insufficient sample | 148 | 8.8 |
* Frequency = N / 1688 (total pre analytical errors in a year) ×100.
The most frequent error was “unlabeled samples” which was responsible for 36% of the total preanalytical errors. The total preanalytical errors in the period divided by month is show in Figure 1. Monthly breakup of the pre analytical errors showed that maximum number of errors occurred in the month of October.
Figure 1.
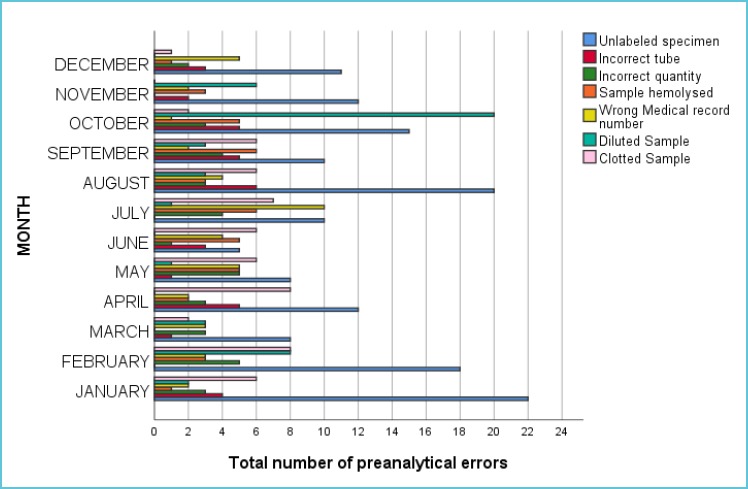
Preanalytical errors per month
DISCUSSION
Our results are comparable to studies that were carried out in other developing countries. In an Indian study by Chawala et al, preanalytical error rate was reported to be 1.52% (11), which is analogous to our result. In another 5 years Spanish study, overall rate of pre analytical errors was reported at 0.047% (12). In previous studies, the variable receiving highest frequency was “hemolysed” sample (11-13). The most frequent pre analytical error in our setting was “unlabeled specimen” (Table 1). Proper labeling of the specimen cannot be overemphasized, as this step is the backbone of a good sampling process (14). Missing slips, wrong labeling/wrong medical record numbers and most important unlabeled specimens can jeopardize the patient outcomes.
Negligence in this domain can lead to delayed diagnosis, additional laboratory testing, or treatment of a patient for a wrong medical condition. Sometimes, these errors may even be fatal (e.g., acute hemolytic reaction after incompatible blood transfusion because of wrong labeling of the EDTA/serum evacuated tubes in which the samples are sent for blood grouping and cross match).
An interesting fact in our study was that majority of the unlabeled samples were sent for analysis of arterial blood gases (ABG). As arterial-blood specimen is used for ABG analysis, the sampling process is relatively cumbersome for the patient as well as for the doctors. However, due to negligence in labeling, it results in unnecessary delays and recollecting of the arterial-blood. Mishandling of the specimen at this step may be attributed to excessive patient workload/ shortage of time in the clinical departments, lack of awareness of the doctors/nursing staff regarding the patient information and lack of bar code system in our setup.
As modern-day laboratory practice is not just confined to “report making”, it is also involved in disseminating this important information to the clinicians. Labeling errors can make this practice delayed and redundant.
After “labeling” the next big problem in our setup was “diluted” samples (Table 1). Majority of the workload of our laboratory comprises of samples from inpatient department, where sampling is being performed by nurses/junior doctors. They sometimes underestimate the importance of drawing blood from the vein without intravenous lines. If intravenous fluid is being given in a patient’s arm, sample should be drawn from the opposite arm (14). If both arms are occupied, blood may be taken after intravenous infusion is turned off for at least two min and tourniquet should be applied below the infusion site before sampling (15).
Anticoagulated samples (EDTA and sodium citrate) with clots constituted around 14% in the present study (Table 1). Gross clots can be easily detected by visual inspection of the sample, however micro clots are sometimes difficult to detect. The presence of clots can be attributed to increased blood to anticoagulant ratio and improper mixing of the blood after dispensing in the tube with anticoagulant (15). In this study, this error could be due to improper mixing of the blood sample and overfilling of the EDTA/citrated vials.
Another significant finding in our study was that maximum number of preanalytical errors occurred in the month of October, and out of them most of the samples were diluted. When we performed the root cause analysis of this issue, we found that neophyte nursing staff was inducted in the month of October. So, this issue can be explained on the basis of inadequate training, poor skills and improper sampling technique of the staff.
Therefore, we conducted in house training sessions for our nursing staff to get them familiarized with the proper phelobotomy technique. After the training session, number of preanalytical errors in the subsequent months had declined to average which was comparable with that of the other months (Figure 1).
Errors in the laboratory are directly proportional to financial constraints and lead to decreased patient satisfaction. Laboratory errors not only affect patient care, including delay in turnaround time, unnecessary redraws, wrong diagnosis, inappropriate treatment but also damages reputation of a laboratory and diminishes confidence of the patient on diagnostic services. Negative impact of laboratory error on patient outcomes is reported to be as high as 24.4% (16). On a broader horizon, they are also adding fuel to the financial constraints of the health system.
A study by Green shows that preanalytical error costs represent between 0.23% and 1.2% of total hospital running budget (17). North American hospitals reported the costs of $337.05 for outpatient (which includes emergency department patients), $162.18 for inpatient (critical), $357.15 for inpatient (other) (17). We lack a comprehensive local data about the effect of preanalytical error on hospital budgets, but for sure we are in a more troublesome situation because of the errors in this phase.
Though improvement in laboratory workflow has significantly reduced the error rate during the analytical phase, preanalytical phase remains the most vulnerable part of laboratory testing, due to the presence of many steps that occur both before and after the specimen reaches the laboratory.
It is evident from the above issues that incorrect sampling technique is the main reason behind the pre analytical errors. This can be attributed to lack of proper training about standard operating procedures of sampling, underestimating the importance of sampling and heavy workloads at the clinical sites. Appropriate training of the staff and proper quality control procedures can help to reduce the pre analytical errors (18,19).
Phlebotomy is considered as a separate domain in most of the developed countries, we could also adopt similar approach for the improvement in quality of our laboratory work.
This whole process demands a holistic approach, including good liaison among the members of the specimen management team, ordering clinicians, phlebotomists, courier who transports the specimen, as well as the laboratory personnel who processes the specimen for testing. Moreover, laboratories should keep a strict record of all the errors observed in the preanalytical phase. Strict compliance to the corrective strategies can gradually reduce the error rates.
CONCLUSION
Compliance with good laboratory practices can significantly reduce the occurrence of preanalytical errors. Management of preanalytical errors needs involvement of the clinical domain for proper patient identification and test requisition, completely filling accompanying slips and sending proper samples for laboratory analysis, since many of the errors fall outside the physical boundaries of the laboratory. We recommend that there should be laboratory policy for error record keeping so that there should be a settlement in “laboratory sentinel events” covering the total testing process.
REFERENCES
- 1.Saurav Patra M, Mukherjee B, Das AK. Preanalytical errors in the clinical laboratory and how to minimize them. Int J Bioassays 2013;02:551-553. [Google Scholar]
- 2.Lima-Oliveira G, Volanski W, Lippi G, Picheth G, Guidi GC. Preanalytical phase management: a review of the procedures from patient preparation to laboratory analysis. Scand J Clin Lab Invest 2017;77:153–163. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Lima-Oliveira G, Brocco G, Bassi A, Salvagno GL. Estimating the intra- and inter-individual imprecision of manual pipetting. Clin Chem Lab Med 2017;55:962–966. [DOI] [PubMed] [Google Scholar]
- 4.Simundic AM, Lippi G. Preanalytical phase-a continuous challenge for laboratory professionals. Biochem Med 2012;22:145-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Plebani M, Simundic AM. Quality in laboratory diagnostics: From theory to practice. Biochem Med 2010;20:126–130. [Google Scholar]
- 6.Hammerling JA. A Review of Medical Errors in Laboratory Diagnostics and Where We Are Today. Lab Med 2012;43:41–44. [Google Scholar]
- 7.Almatrafi AA. Preanalytical Errors: A Major Issue in Medical Laboratory. Acta Sci Med Sci 2019;3:93-95. [Google Scholar]
- 8.Giavarina D, Lippi G. Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin Biochem 2017;50:568-573. [DOI] [PubMed] [Google Scholar]
- 9.Lima-Oliveira G, Lippi G, Salvagno GL, Picheth G, Guidi GC. Laboratory Diagnostics and Quality of Blood Collection. J Med Biochem 2015;34:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima-Oliveira G, Cesare Guidi G, Guimaraes AVP, Abol Correa J, Lippi G. Preanalytical Nonconformity Management Regarding Primary Tube Mixing in Brazil. J Med Biochem 2017;36:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla R, Goswami B, Tayal D, Mallika V. Identification of the types of preanalytical errors in the clinical chemistry laboratory: 1 year study at GB Pant hospital. Lab Med 2010;41:89–92. [Google Scholar]
- 12.Gimenez-Marin A, Rivas-Ruiz F, Perez-Hidalgo Mdel M, Molina-Mendoza P. Preanalytical errors management in the clinical laboratory: a five year study. Biochem Med 2014;24:248-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najat D. Prevalence of Preanalytical Errors in Clinical Chemistry Diagnostic Labs in Sulaimani City of Iraqi Kurdistan. PLoS ONE 2017;12:e0170211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simundic AM, Bölenius K, Cadamuro J, Church S, Cornes MP, van Dongen-Lases EC, Eker P, Erdeljanovic T, Grankvist K, Guimaraes JT, Hoke R, Ibarz M, Ivanov H, Kovalevskaya S, Kristensen GBB, Lima-Oliveira G, Lippi G, von Meyer A, Nybo M, De la Salle B, Seipelt C, Sumarac Z, Vermeersch P, Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI) Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin Chem Lab Med 2018;56:2015-2038. [DOI] [PubMed] [Google Scholar]
- 15.Arul P, Pushparaj M, Pandian K, Chennimalai L, Rajendran K, Selvaraj E, Masilamani S. Prevelence and types of preanalytical error in hematology laboratory of a tertiary care hospital in South India. J Lab Physicians 2018;10:237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, Bassi A, Brocco G, Montagnana M, Salvagno GL, Guidi GC. Preanalytic error tracking in a laboratory medicine department: results of a 1-year experience. Clin Chem 2006;52:1442-1443. [DOI] [PubMed] [Google Scholar]
- 17.Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem 2013;46:1175-1179. [DOI] [PubMed] [Google Scholar]
- 18.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. The effective reduction of tourniquet application time after minor modification of the CLSI H03-A6 blood collection procedure. Biochem Med 2013;23:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Impact of the phlebotomy training based on CLSI/NCCLS H03-a6-procedures for the collection of diagnostic blood specimens by venipuncture. Biochem Med 2012;22:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]


