Abstract
Prenatal stress (PNS) can influence behaviors associated with cognition, reward and emotional regulation, which are controlled by brain areas such as the cortex, hippocampus, hypothalamus, midbrain and cerebellum. Allopregnanolone in these regions modulates behavioral and parasympathetic effects. The current study tested whether exposing pregnant dams to 5 days of resident-intruder stress on prenatal days 15–20 for 10 min altered the levels of allopregnanolone in cortex, hypothalamus, hippocampus, midbrain, and cerebellum of male and female juvenile offspring. In cortex, hypothalamus, and midbrain of male rats exposed to prenatal stress, levels of allopregnanolone were significantly lower compared to all other groups. In the hippocampus and cerebellum, among females exposed to prenatal stress levels were significantly higher compared to all other groups. These differences in allopregnanolone levels varying by prenatal stress, sex and brain regions provide insight in potential mechanism of stress regulation and etiopathophysiology of stress-related disorders.
Keywords: Chronic stress, Corticosterone, Dopamine, Microdialysis, Spatial memory, Rat
Highlights
-
•
In cortex, hypothalamus and midbrain, prenatal stress decreased allopregnanolone in males compared to all other groups.
-
•
In hippocampus and cerebellum, prenatal stress females had higher levels of allopregnanolone compared to all other groups.
-
•
Allopregnanolone levels caused by resident intruder stress were similar to levels seen in prenatal stress due to other means.
1. Introduction
Steroid hormones play fundamental roles in the development and/or function of the central nervous system (CNS). Androgens, typically genetically signaled by a gene on the Y chromosome, begin secretion by the gonads as early in development as postnatal day 6–8 in rodents (Kellogg and Frye, 1999) or weeks 6–8 in people. This gives rise to organize other hormonal and phenotypic sex-dependent patterns of steroids in the perinatal period. They also contribute to organizing effects of hormones across the lifespan at critical periods later in life. Among them is the pattern that at puberty males have a daily pattern in steroid hormone secretion of androgens and females will have a monthly secretion in steroids hormones, such as estradiol (E2), progesterone (P4) and its metabolites, such as allopregnanolone. This is a normative pattern; however, exposure to stress during pregnancy aka prenatal stress can alter this pattern and result in life-long effects on offspring.
Although acute stress can be adaptive in adults through enhancing autonomic, neuroendocrine, and behavioral outputs that subserve species-typical adaptive responses (e.g., fight-or-flight), developmental stress can be pathological and detrimental to mothers and offspring. Typically, during late gestation, maternal stress responding is dampened. This stress hyporesponsive period, which is well-documented in rodents, may limit the deleterious developmental programming effects of prenatal stress. The period of stress hypo-responsiveness coincides with elevations in allopregnanolone (Paris and Frye, 2008; Brunton et al., 2009), which can be protective to the offspring (Frye and Bayon, 1998; Yawno et al., 2009), and dampen hypothalamic-pituitary-adrenal (HPA) axis reactivity (Patchev et al., 1994, 1996; Patchev and Almeida, 1996; Paris and Frye, 2008; Frye, 2009). There are prenatal stress-induced alterations in neurosteroid levels, which may play a direct role in programming the developing fetus (Kehoe et al., 2000; McCormick et al., 2002). Allopregnanolone's role in attenuating stress axis responses extends to maintaining gestation, protection of the fetus, and/or possible organizing effects on the later function of the nervous system.
Among pregnant women and rodents, circulatory allopregnanolone is elevated throughout gestation and its decline precedes parturition (Concas et al., 1999; Gilbert-Evans et al., 2005; Paris and Frye, 2008). During early pregnancy, progestogens are secreted from the corpora lutea until the placenta becomes a source of progestogens (Concas et al., 1998 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3195272/). Circulating levels of E2, P4 and allopregnanolone co-vary and increase together during the first half of gestation. However, E2 levels decline prior to P4, and then allopregnanolone, later in pregnancy. Among rats, E2 and P4 levels peak between gestational day (GD)10–15; whereas, allopregnanolone asymptotes later in pregnancy (GD19; Concas et al., 1998) and declines to estrous levels around GD21 (Concas et al., 1998). In the developing offspring, central allopregnanolone is observed as early as embryonic day 14 in rats (Kellogg and Frye, 1999), while postnatal allopregnanolone increases in response to stressors on day post-natal day 6 (Kehoe et al., 2000), and such isolation, also alters stress hormones and mesolimbic dopamine release (McCormick et al., 2002). Inhibiting allopregnanolone formation with a 5α-reductase inhibitor, finasteride, in late pregnancy restores HPA activation of adrenocorticotropic hormone (ACTH), corticosterone, corticotropin releasing factor (CRF), and oxytocin, which can result in termination of pregnancy (Antonijevic et al., 2000; Brunton and Russell, 2008a,b, 2010; Brunton et al., 2009; Chanrachakul et al., 2005; Grobin and Morrow, 2001; Russell et al., 2003). Thus, during gestation allopregnanolone influences stress responding and gestational outcomes (see Frye et al., 2011 for review).
Inhibiting the formation of 5α-reduced steroids during late gestation in rats reduces gestational length, the number of viable pups per litter, and impairs cognitive and neuroendocrine function in the juvenile offspring. Immune stress due to injection of a cytokine interleukin 1 beta (IL-1B) or an endotoxin, lipopolysaccharide, from postnatal days s 17–21 caused female rats to give birth significantly earlier than vehicle controls and the number of viable pups was significantly reduced. Exposure to IL1B reduced investigation of a novel object of both males and female rats when in puberty. In investigation of a novel open field, females made fewer central entries than did control males but female offspring of dams that experienced prenatal stress with IL-1B reversed the effect and increased their investigation. Notably, prenatal stress via cytokine exposure reduced levels of 5α-reduced steroids (allopregnanolone and its precursor dihydroprogesterone) but increased estradiol in a sex-dependent manner in the hippocampus of female rats (Paris, Brunton, Russell and Frye et al., 2011; stress). Estradiol levels were lowered by IL1b treatment in the cortex, hypothalamus, of both females and males (Paris, Brunton, Russell and Frye et al., 2011; stress).
The purpose of this paper was to examine the effects of a more naturalistic prenatal stress stressor, which involves resident intruder stress during gestation, compared to the effects of administration of a 5α-reductase inhibitor or immune challenge that we have investigated previously to produced behavior affects in juvenile outcomes.
2. Methods
2.1. Subjects and housing
Female Sprague-Dawley rats from Charles River Laboratories were 255–305 g upon arrival. They were group housed, 4–6 rats, in each open-top cage in a pathogen-free rodent facility, under standard conditions of temperature (20–21 °C), humidity (50–55%), and lighting (12 h light/dark cycle, lights on at 08:00 h) with food and water ad libitum. At least one week later, female rats were paired overnight with a sexually experienced male (from the in-house colony). The presence of a semen plug in the breeding cage the following morning confirmed pregnancy and was considered day 1, expected parturition was day 22. Pregnant rats were caged individually from gestational day 14. All procedures were performed with approval from the University of Edinburgh Ethical Committee and in accordance with current UK Home Office legislation.
Pregnant “intruder” rats (days 16–20 of gestation, n = 24) were placed in the cage of an unfamiliar lactating “resident” rat (between days 2–8 of lactation, where day 1 was the day of parturition. The intruders remained for 10 min/a day on 5 consecutive days between 09:00 and 11:30 h. Resident rats, particularly those lactating, exhibit consistent and extreme aggressive behavior toward intruders, which show defensive behavior and HPA axis responses. Following the last social stress exposure (on day 20) pregnant rats were returned to their home cage and left undisturbed, except for routine husbandry, through parturition and lactation until weaning. Pregnant controls (n = 24) remained in their home cage throughout, except for daily weighing and routine husbandry.
On the day of parturition, litter size, birth weights, male–female ratio were recorded. Litters remained with dams until weaning on postnatal days 21–22. Following weaning, offspring were housed in groups of same-sex littermates (litter sizes were not adjusted).
To measure allopregnanolone, brains were collected on postnatal days 21–22 from non-stressed and dams that were stressed. All pups were rapidly decapitated between 10:00–11:00 a.m. (4–5 h into light). The brain of pups were then rapidly removed from skull on ice and placed in a labeled weigh boat and covered with a labeled piece of tin foil. The frozen brains of pups were sent on dry ice to the laboratory of Dr. Cheryl Frye from the laboratory of Dr. Paula Brunton for dissection into brain regions of interest and radioimmunoassay of allopregnanolone.
2.2. Brain dissections
The brain was positioned ventral side up for gross dissection of the midbrain and hypothalamus. The optic chiasm was utilized as the border between these regions. The pontine regions were utilized as the posterior border, the cerebral aqueduct was the ventral border for the midbrain. The borders were ~1.5 mm from the anterior, posterior, and midline as previously reported (Frye et al., 2007). For the hypothalamus, a small region above where the pituitary stalk remained was taken in a 2 mm cube. The brain was then turned over and the corpus callosum was cut. The brain was splayed open and the hippocampus peeled out. The cortex above was taken on each side. The cerebellum was taken without hindbrain structures.
2.3. Allopregnanolone measures
Brain allopregnanolone was measured using radioimmunoassay as previously described (Frye and Duncan, 1996). Allopregnanolone was extracted from brain with 50% methanol and acetic acid and centrifuged at 3000×g for 10 min. The supernatant was chromatagraphed on Sepak-cartridges equilibrated with 50% methanol and acetic acid. Steroids were eluted with increasing concentrations of methanol (50% and 100%). Solvents were removed using a Savant speed drier and samples were reconstituted to 300 μL assay buffer.
Allopregnanolone antibody #921412-5 (from Dr. Robert Purdy from La Jolla, CA, USA used in a ratio of 1:5000 dilution bound between 40 and 60% of [3Allop] and bound 52% in the present study. Following incubation for 15 min and the addition of dextran-coated, samples were centrifuge at 3000 x G. The supernatant was pipetted in glass scintillation vials with 5 mL of scintillation cocktail. Unknowns were interpolated from the standard curve using Assay Zap. The minimum level of detection with the assay is 15 pg/tube and the inter- and intra-assay reliability co-efficients were 0.05 and 0.08, respectively. All samples were assayed together.
2.4. Statistical analyses
Two-way analyses of variance, with sex as one factor (female or male) and stress condition (prenatal stress or control) as another revealed highly-statistically significant interactions for each brain area examined. Specific differences between groups were revealed using Student-Newman–Keuls multiple-comparison post hoc tests.
3. Results
3.1. Cortex
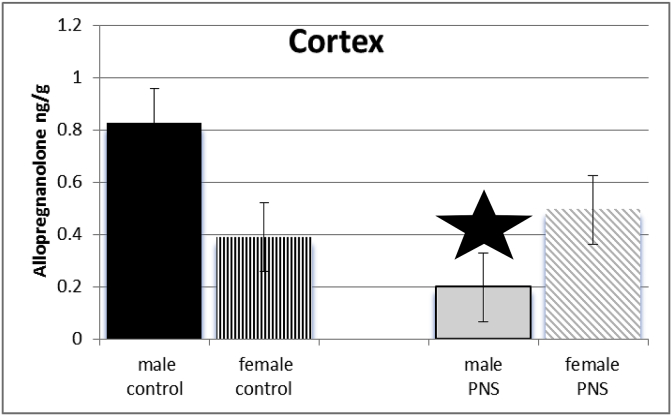
Exposure of dams to prenatal stress and sex of juvenile offspring interacted to influence levels of allopregnanolone among offspring in cortex (F(3,64) = 271.45; p ≤ 0.005). Post-hoc test revealed levels of allopregnanolone were significantly lower in cortex of prenatal stress male juvenile offspring compared to controls. See Fig. 1.
Fig. 1.
There was a significant interaction between sex and PRENATAL STRESS. Male juvenile offspring whose dams had been exposed to stress (grey bars) had significantly lower levels of allopregnanolone in cortex, compared to male offspring of control dams (black bars), prenatal stress female rats (grey diagonal bars), and female control rats (black vertical stripes).
3.2. Hypothalamus
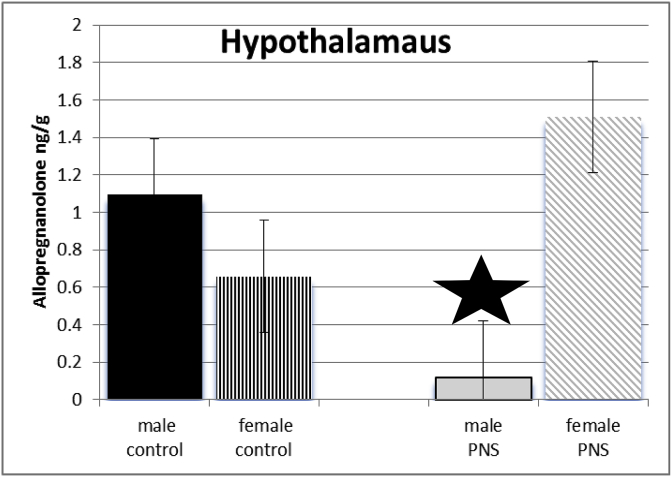
Exposure of dams to prenatal stress and sex of juvenile offspring interacted to influence levels of allopregnanolone of offspring in hypothalamus (see Fig. 2). Post-hoc test revealed levels of allopregnanolone were significantly lower in hypothalamus of prenatal stress male juvenile offspring compared to controls. (F(3,64) = 755.65; p ≤ 0.005).
Fig. 2.
There was a significant interaction between sex and PRENATAL STRESS. Male juvenile offspring whose dams had been exposed to stress (grey bars) had significantly lower levels of allopregnanolone in cortex, compared to male offspring of control dams (black bars), prenatal stress female rats (grey diagonal bars) and female control rats (black vertical stripes).
3.3. Midbrain
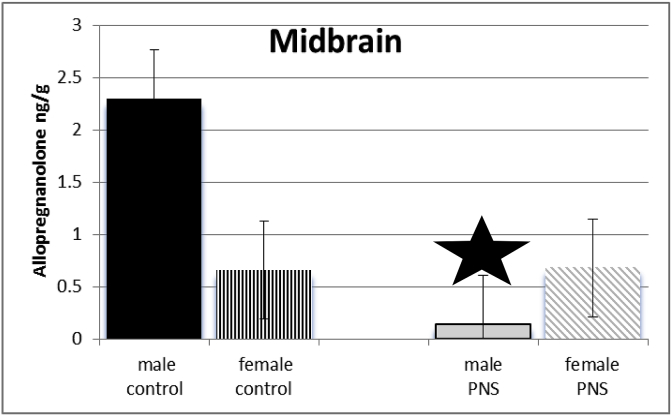
Exposure of dams to prenatal stress and sex of juvenile offspring interacted to influence levels of allopregnanolone of offspring in midbrain (F(3,64) = 1296.1; p ≤ 0.005). Post-hoc test revealed levels of allopregnanolone were reduced in midbrain of prenatal stress male juvenile offspring compared to controls. See Fig. 3.
Fig. 3.
There was a significant interaction between sex and PRENATAL STRESS. Male juvenile offspring whose dams had been exposed to stress (grey bars) had significantly lower levels of allopregnanolone in midbrain, compared to male offspring of control dams (black bars), prenatal stress female rats (grey diagonal bars) and female control rats (black vertical stripes).
3.4. Hippocampus
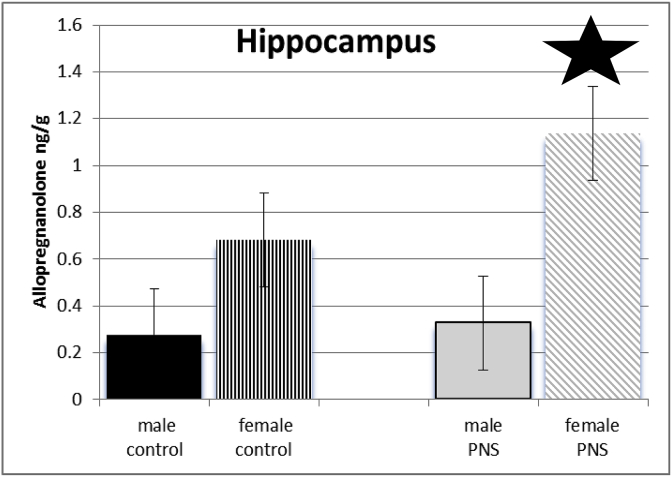
Exposure of dams to prenatal stress and sex of juvenile offspring interacted to influenced levels of allopregnanolone of offspring in hippocampus (F(3,64) = 476.5; p ≤ 0.005). Post-hoc tests indicate female offspring of dams that had been exposed to prenatal stress had significantly higher levels of allopregnanolone than did female controls, as well as all males. See Fig. 4.
Fig. 4.
There was a significant interaction between sex and PRENATAL STRESS. Prenatal stress females (grey diagonal bars) had significantly higher levels of allopregnanolone in hippocampus compared to female control rats (black vertical stripes), male juvenile offspring of control dams (black bars), and those whose dams had been exposed to stress (grey bars).
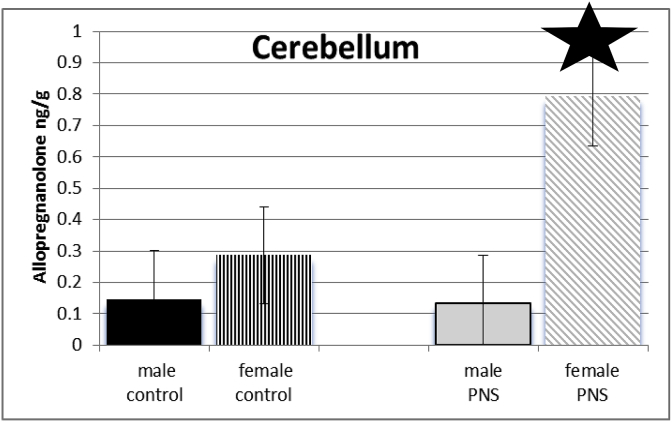
3.5. Cerebellum
Exposure of dams to prenatal stress and sex of juvenile offspring interacted to influence the levels of allopregnanolone of offspring in cerebellum (F(3,64) = 703.915; p ≤ 0.005). Post-hoc tests show females, but not males, of dams that had been exposed to prenatal stress had significantly higher levels of allopregnanolone than did female controls. See Fig. 5.
Fig. 5.
There was a significant interaction between sex and PRENATAL STRESS. Prenatal stress females (grey diagonal bars) had significantly higher levels of allopregnanolone in cerebellum compared to female control rats (black vertical stripes), male juvenile offspring of control dams (black bars), and those whose dams had been exposed to stress (grey bars).
4. Discussion
There were three findings of this study. First, allopregnanolone levels of the non-stressed control rats were consistent with previous studies (Paris, Brunton, Russell and Frye et al., 2011). Second, in the cortex, hypothalamus, and midbrain, exposure to prenatal stress decreased allopregnanolone levels in males but had little effects on females. Third, in the hippocampus and cerebellum, allopregnanolone levels were increased among female rats exposed to prenatal stress but had little effects on male rats. In general, this prenatal stress regimen has a greater effect on allopregnanolone levels of male juvenile offspring in the cortex, hypothalamus and midbrain and among female prenatal stress effects were greater in the hypothalamus and cerebellum.
This study confirms and extends many of our own conducted over the last 20 plus years, and that of others. For example, perturbations of pregnant dams for as minimal as 45 min on GD 18, to as extended over the entire inclusive period in which the hippocampus, and related structures would be developing, produces similar behavioral, neuroendocrine, and neuroanatomical sequelae. Most relevant for this paper, 45 min of restraint exposure on gestational day 17 results in a significantly smaller size hippocampus of female offspring of the prenatal stress dam (Schmitz et al., 2002). Second, neonatal isolation of pups (equivalent to 3rd trimester) resulted in altered stress responses of juvenile rats, as indicated by elevation in corticosterone, allopregnanolone, and dopamine levels, compared to controls) (Kehoe et al., 2000; McCormick et al., 2002). Third, we also observed offspring of prenatal stress females had higher levels of allopregnanolone in the hippocampus, as was previously observed with prenatal stress exposed to IL1-B, a regimen that reversed the well known sex difference for males to have more exploratory behavior in the center of an open field (Paris and Frye, 2008). Hence, prenatal and early life exposure has not only effects on the allostatic stress load but also on the parasympathetic nervous system, which may be involved in the susceptibility to depression.
The efficacy of some therapeutics to treat anxiety and/or depressive disorders are associated with their capacity to increase levels of progestogens (Griffin and Mellon, 1999; Uzunov et al., 1996; Uzunova et al., 1998). Recent clinical findings indicate that P4's anti-depressant effects may involve actions of allopregnanolone. Some patients who have depressive disorders have reduced plasma concentrations and/or cerebrospinal fluid levels of allopregnanolone (Romeo et al., 1998; Stahl, 1997; Uzunova et al., 1998). Administration of antidepressants, such as fluoxetine or fluvoxamine, normalize decreased allopregnanolone concentrations concomitant with reducing depressive symptomatology (Uzunova et al., 2004, 2006). Allopregnanolone may also play a role in the therapeutic treatment of depression.
Various treatments of depression alter allopregnanolone. Anti-depressants (Dubrovsky, 2006; Uzunova et al., 2004), sleep deprivation (Schüle et al., 2003), electroconvulsive therapy (Baghai et al., 2005) and transcranial magnetic stimulation (Padberg et al., 2002) have been investigated for their capacity to alter neurosteroids and depression. Notably, measurable changes in plasma levels of neurosteroids are not as readily observable with non-pharmacological treatments of depression. However, common effects of therapeutic treatments may include direct central changes in neurosteroidogenesis or alterations in peripheral HPA function that may change steroid biosynthesis and thereby alter core symptoms of depression, such as anxiety, memory, sleep, sexual function (Dubrovsky, 2006). Although, the ability to assess central levels of neurosteroids in people limits the capacity to elucidate this, in animal models, allopregnanolone has effects in adult rodents to decrease depressive behavior (Khisti et al., 2000, discussed further below). Thus, allopregnanolone may contribute to the antidepressant effects of some therapeutics.
Mechanisms underlying common effects of E2 and P4 may involve allopregnanolone. P4 is converted by 5α-reductase (5α-R) to dihydroprogesterone, which is then metabolized to allopregnanolone by 3α-hydroxysteroid dehydrogenase (3α-HSD). E2 increases activity of these enzymes and allopregnanolone levels (Frye and Rhodes, 2006; Frye and Duncan, 1996). If allopregnanolone underlies changes in affective behavior this has implications for its mechanisms. E2 and P4 have effects via specific cognate receptors (estrogen receptors (ERs); progestin receptors (PRs), but allopregnanolone does not. Allopregnanolone has actions via the HPA and actions at CRF to mediate parasympathetic activity. These effects can have profound actions for physiological, cognitive and affective responses in areas such as the cortex, hippocampus, hypothalamus, midbrain and cerebellum. We believe that E2 and P4 may have beneficial effects for physiological, cognitive and affective behavior through formation of allopregnanolone and its PR-independent actions in these brain regions to increase parasympathetic responses and thereby decrease HPA reactivity (Goder et al., 2019).
There have also been studies that suggest allopregnanolone can be anxiogenic in certain conditions, such as premenstrual dysphoric disorder (Smith et al., 2006). Pregnant women with lower levels of allopregnanolone have a higher chance of being diagnosed with postpartum depression (PPD) (Frye et al., 2011). It has also been suggested that women who have boys are more likely to have PPD than women who have a girl baby (Myers and Johns, 2019).
Most current pharmaceuticals for postpartum depression usually take four to six weeks to begin working. This lack of efficacy may be due to progesterone working through its metabolite, allopregnanolone, in the hippocampus, to alter trophic factors and rebuild and enhance neurogenesis. One model of postpartum depression states that experiences which happen to the mother will have epigenetic effects on the offspring, in which a depressive phenotype is passed on. It is difficult to determine if changes in behavior or hormone levels in the offspring of prenatally stressed animals are due to the fact the offspring experienced stress in the womb or because the mother developed PPD due to prenatal stress and was less attentive to the pups. In the past we have looked at the behavioral effects of prenatal stress on the offspring (Frye et al., 2011). We aimed, in this research, to look at allopregnanolone levels in brain areas that are associated with the parasympathetic stress response, basic cognitive and emotional function, as we expected that allopregnanolone may be altered in these levels in a sex dependent manner of those exposed to prenatal stress. Research into the effects of E2 and progesterone levels is vital because it can be used to create pharmaceuticals that take effect immediately, effectively moderating as postpartum depression. There are some obvious limitation of this study. There could be prenatal stress- or sex-dependent effects when considering the entire juvenile period. Allopregnanolone could be measured in plasma or other brain regions that may demonstrate a different effect of prenatal stress. These were beyond the scope of this study.
5. Conclusion
In conclusion, prior exposure to prenatal stress, decreased allopregnanolone levels in the cortex, hypothalamus and midbrain in males and increased allopregnanolone levels in the hippocampus and cerebellum in females compared to controls. Allopregnanolone has actions via the HPA and actions at CRF to mediate parasympathetic activity. These effects can have profound actions for physiological, cognitive and affective responses to stress. We believe that E2 and P4 may have beneficial effects for physiological, cognitive and affective behavior through formation of allopregnanolone and its PR-independent actions in these brain regions to increase parasympathetic responses and thereby decrease HPA reactivity. One proposed function of the stress-induced hippocampal and cerebellar increase among prenatal stress females is to moderate appropriate coping behaviors (Berridge and Robinson, 2003; Sullivan, 2004). Further examination of the effects of prenatal stress on allopregnanolone function in the brain may provide a greater understanding of the pathological and adaptive consequences of stress. That being said, it is a profound accomplishment and leap forward in the treatment of peripueral depression to have medications available now that work immediately, have low rates of being treatment refractory, and have actions through steroid systems.
CRediT authorship contribution statement
Jennifer K. Torgersen: Formal analysis. Rose Petitti: Writing - original draft. Sedric Tello: Writing - original draft. Vincent F. Lembo: Formal analysis. Cheryl A. Frye: Funding acquisition, Supervision.
Declaration of competing interest
No conflicts of interest declared.
Acknowledgments
Source of funding: National Institute of General Medical Sciences (8P20GM103395-12). Its contents are the sole responsibility of the authors and do not necessarily represent the official view of NIGMS. Dr. Paula Brunton graciously provided the tissues.
References
- Antonijevic I.A., Russell J.A., Bicknell R.J., Leng G., Douglas A.J. Effect of progesterone on the activation of neurons of the supraoptic nucleus during parturition. J. Reprod. Fertil. 2000;120(2):367–376. doi: 10.1530/jrf.0.1200367. Nov. [DOI] [PubMed] [Google Scholar]
- Baghai T.C., di Michele F., Schüle C., Eser D., Zwanzger P., Pasini A., Romeo E., Rupprecht R. Plasma concentrations of neuroactive steroids before and after electroconvulsive therapy in major depression. Neuropsychopharmacol. 2005;30(6):1181–1186. doi: 10.1038/sj.npp.1300684. Jun. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. Parsing reward.(2003) Trends Neurosci. 2003 Sep;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. Review. Erratum in: Trends Neurosci. Nov;26(11):581. PMID: 12948663. [DOI] [PubMed] [Google Scholar]
- Brunton P.J., McKay A.J., Ochedalski T., Piastowska A., Rebas E., Lachowicz A., Russell J.A. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J. Neurosci. 2009;29:6449–6460. doi: 10.1523/JNEUROSCI.0708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton P.J., Russell J.A. Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: the neurosteroid opioid connection. J. Physiol. 2008;586:369–375. doi: 10.1113/jphysiol.2007.146233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton P.J., Russell J.A. Keeping oxytocin neurons under control during stress in pregnancy. Prog. Brain Res. 2008;170:365–377. doi: 10.1016/S0079-6123(08)00430-5. [DOI] [PubMed] [Google Scholar]
- Brunton P.J., Russell J.A. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J. Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Concas A., Follesa P., Barbaccia M.L., Purdy R.H., Biggio G. Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur. J. Pharmacol. 1999;375(1–3):225–235. doi: 10.1016/s0014-2999(99)00232-0. Jun 30. [DOI] [PubMed] [Google Scholar]
- Concas A., Mostallino M.C., Porcu P., Follesa P., Barbaccia M.L., Trabucchi M., Purdy R.H., Grisenti P., Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. U. S. A. 1998;95(22):13284–13289. doi: 10.1073/pnas.95.22.13284. Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanrachakul B., Broughton Pipkin F., Warren A.Y., Arulkumaran S., Khan R.N. Progesterone enhances the tocolytic effect of ritodrine in isolated pregnant human myometrium. Am. J. Obstet. Gynecol. 2005;192:458–463. doi: 10.1016/j.ajog.2004.07.077. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol. Biochem. Behav. 2006;84(4):644–655. doi: 10.1016/j.pbb.2006.06.016. Epub 2006 Sep. 7. [DOI] [PubMed] [Google Scholar]
- Frye C.A. Neurosteroids-from basic research to clinical perspectives. In: Rubin R.T., Pfaff D.W., editors. Hormones/Behavior Relations of Clinical Importance. Academic Press; San Diego: 2009. pp. 395–416. [Google Scholar]
- Frye C.A., Bayon L.E. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3α,5α-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye C.A., Duncan J.E. Estradiol benzoate potentiates neuroactive steroids' effects on pain sensitivity. Pharmacol. Biochem. Behav. 1996;53(1):27–32. doi: 10.1016/0091-3057(95)00194-8. Jan. [DOI] [PubMed] [Google Scholar]
- Frye C.A., Paris J.J., Osborne D.M., Campbell J.C., Kippin T.E. Prenatal stress alters progestogens to mediate susceptibility to sex-typical, stress-sensitive disorders, such as drug abuse: a review. Front. Psychiatr. 2011;2:52. doi: 10.3389/fpsyt.2011.00052. 2011 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C.A., Rhodes M.E. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83(5–6):336–347. doi: 10.1159/000096051. Epub 2006 Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert Evans S.E., Ross L.E., Sellers E.M., Purdy R.H., Romach M.K. 3alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol. Endocrinol. 2005;21(5):268–279. doi: 10.1080/09513590500361747. Nov. [DOI] [PubMed] [Google Scholar]
- Goder S.C., Cadeddu R., Floris G., MosherLJ Mi Z., Jarmolowicz D.P., Scheggi S., Walf A.A., Konnce C.J., Frye C.A., Muma N.A., Bortolato M. The sterodiogenesis inhibitor finasteride reduces the response to both stressful and rewarding stimuli. Biomolecules. 2019;9(11):749. doi: 10.3390/biom9110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin L.D., Mellon S.H.S. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc. Natl. Acad. Sci. U. S. A. 1999;96(23):13512–13517. doi: 10.1073/pnas.96.23.13512. 1999 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin A.C., Morrow A.L. 3α-hydroxy-5α-pregnan-20-one levels and GABA(A) receptor-mediated 36Cl(−) flux across development in rat cerebral cortex. Brain Res. Dev. Brain Res. 2001;131:31–39. doi: 10.1016/s0165-3806(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Kehoe P., Mallinson K., McCormick C.M., Frye C.A. Central allopregnanolone is increased in rat pups in response to repeated, short episodes of neonatal isolation. Brain Res. Dev. Brain Res. 2000;124:133–136. doi: 10.1016/s0165-3806(00)00106-1. [DOI] [PubMed] [Google Scholar]
- Kellogg C.K., Frye C.A. Endogenous levels of 5 alpha-reduced progestins and androgens in fetal vs. adult rat brains. Brain. Res. Dev. Brain Res. 1999;115(1):17–24. doi: 10.1016/s0165-3806(99)00041-3. Jun 8. [DOI] [PubMed] [Google Scholar]
- Khisti R.T., Chopde C.T., Jain S.P. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol. Biochem. Behav. 2000;67(1):137–143. doi: 10.1016/s0091-3057(00)00300-2. Sep. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Kehoe P., Mallinson K., Cecchi L., Frye C.A. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol. Biochem. Behav. 2002;73:77–85. doi: 10.1016/s0091-3057(02)00758-x. [DOI] [PubMed] [Google Scholar]
- Myers S., Johns S.E. Male infants and birth complications are associated with increased incidence of postnatal depression. Soc. Sci. Med. 2019;220:56–64. doi: 10.1016/j.socscimed.2018.10.008. Jan. [DOI] [PubMed] [Google Scholar]
- Padberg F., di Michele F., Zwanzger P., Romeo E., Bernardi G., Schüle C., Baghai T.C., Ella R., Pasini A., Rupprecht R. Plasma concentrations of neuroactive steroids before and after repetitive transcranial magnetic stimulation (rTMS) in major depression. Neuropsychopharmacol. 2002;27(5):874–878. doi: 10.1016/S0893-133X(02)00355-X. Nov. [DOI] [PubMed] [Google Scholar]
- Paris J.J., Frye C.A. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev V.K., Almeida O.F. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J. Neurosci. 1996;16:7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev V.K., Shoaib M., Holsboer F., Almeida O.F. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Patchev V.K., Hassan A.H., Holsboer D.F., Almeida O.F. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Romeo E., Ströhle A., Spalletta G., di Michele F., Hermann B., Holsboer F., Pasini A., Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatr. 1998;155(7):910–913. doi: 10.1176/ajp.155.7.910. Jul. [DOI] [PubMed] [Google Scholar]
- Russell J.A., Leng G., Douglas A.J. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front. Neuroendocrinol. 2003;24:27–61. doi: 10.1016/s0091-3022(02)00104-8. [DOI] [PubMed] [Google Scholar]
- Schmitz C.1, Rhodes M.E., Bludau M., Kaplan S., Ong P., Ueffing I., Vehoff J., Korr H., Frye C.A. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol. Psychiatr. 2002;7(7):810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- Schüle C., di Michele F., Baghai T., Romeo E., Bernardi G., Zwanzger P., Padberg F., Pasini A., Rupprecht R. Influence of sleep deprivation on neuroactive steroids in major depression. Neuropsychopharmacol. 2003;28(3):577–581. doi: 10.1038/sj.npp.1300084. Mar. [DOI] [PubMed] [Google Scholar]
- Smith S.S., Ruderman Y., Frye C., Homanics G., Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology (Berlin) 2006;186(3):323–333. doi: 10.1007/s00213-005-0168-3. 2006 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. Exaggerated pain behavior: by what standard? Clin. J. Pain. 2004 Nov-Dec;20(6):433–439. doi: 10.1097/00002508-200411000-00008. Review. PMID: 15502687. [DOI] [PubMed] [Google Scholar]
- Stahl S.M. Sex therapy in psychiatric treatment has a new partner: reproductive hormones. J. Clin. Psychiatr. 1997;58(11):468–469. doi: 10.4088/jcp.v58n1101. Nov. [DOI] [PubMed] [Google Scholar]
- Uzunov D.P., Cooper T.B., Costa E., Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc. Natl. Acad. Sci. U. S. A. 1996;93(22):12599–12604. doi: 10.1073/pnas.93.22.12599. Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V., Sampson L., Uzunov D.P. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacol. (Berlin) 2006;186(3):351–361. doi: 10.1007/s00213-005-0201-6. Jun. [DOI] [PubMed] [Google Scholar]
- Uzunova V., Sheline Y., Davis J.M., Rasmusson A., Uzunov D.P., Costa E., Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Nat. Acad. Sci. U S A. 1998;95(6):3239–3244. doi: 10.1073/pnas.95.6.3239. Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V., Wrynn A.S., Kinnunen A., Ceci M., Kohler C., Uzunov D.P. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur. J. Pharmacol. 2004;486(1):31–34. doi: 10.1016/j.ejphar.2003.12.002. Feb 13. [DOI] [PubMed] [Google Scholar]
- Yawno T., Hirst J.J., Castillo-Melendez M., Walker D.W. Role of neurosteroids in regulating cell death and proliferation in the late gestation fetal brain. Neuroscience. 2009;163:838–847. doi: 10.1016/j.neuroscience.2009.07.009. [DOI] [PubMed] [Google Scholar]