Abstract
Background
Accumulating evidence points to an association between gut microbial abnormalities and depression disorder. The microbiota-gut-brain axis is an emerging target for treating depression using nutritional strategies, considering the numerous limitations of current pharmacological approaches. Here we studied the effect and probable mechanisms of psychobiotic treatment on depression.
Methods
Chronically stressed C57BL/6J male mice were administered viable Bifidobacterium breve CCFM1025 for 5 weeks prior to behavioral testing. Brain neurological alterations, serum corticosterone, cytokines levels, fecal microbial composition, and short-chain fatty acid (SCFA) content were measured. In addition, the effect of SCFA on 5-hydroxytryptophan (5-HTP) biosynthesis was investigated in an in vitro model of enterochromaffin cells (RIN14B).
Results
CCFM1025 treatment significantly reduced depression- and anxiety-like behaviors. The hyperactive hypothalamic-pituitary-adrenal response, as well as inflammation, were also alleviated, possibly via regulating the expression of glucocorticoid receptors (Nr3c1). Moreover, CCFM1025 also down-regulated the pCREB-c-Fos pathway but increased the expression of brain-derived neurotrophic factor (BDNF). Meanwhile, chronic stress-induced gut microbial abnormalities were restored, accompanied by increased SCFA and 5-HTP levels. The intestinal 5-HTP biosynthesis positively correlated with fecal SCFA and Bifidobacterium breve levels.
Conclusions
In summary, Bifidobacterium breve CCFM1025 showed considerable antidepressant-like and microbiota-regulating effects, which opens avenues for novel therapeutic strategies towards treating depression.
Keywords: Bifidobacterium, 5-Hydroxytryptophan, Stress, Depression, Psychobiotics
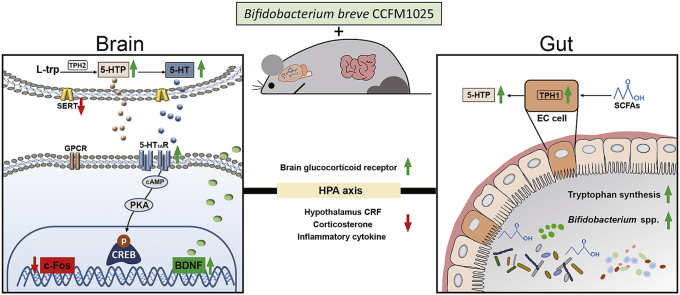
Graphical abstract
Bifidobacterium breve CCFM1025 may exert an antidepressant-like effect via the following pathways: (1) Reshaping gut microbial composition and metagenomic function, and increasing the production of beneficial metabolites. (2) Attenuating the hyperfunction of the hypothalamic-pituitary-adrenal axis and inflammation. (3) Upregulating BDNF expression while downregulating c-Fos expression in the brain. All colored arrows indicate increases (upward green) or decreases (downward red) of the measures. Black lines and arrows connect the elements in the metabolic pathway.
Highlights
-
•
CCFM1025 reshapes the gut microbial composition under the chronic stress.
-
•
CCFM1025 attenuates HPA axis hyperfunction and inflammation.
-
•
CCFM1025 alters the BDNF and c-FOS expression in the brain.
-
•
Antidepressant-like effect of CCFM1025 correlates with gut 5-HTP and SCFA.
1. Introduction
Discovering the role of the microbiota-gut-brain axis is one of the most important advances in the field of gastroenterology and psychology in the past decade (Bercik et al., 2011; Cryan et al., 2019; Foster and Neufeld, 2013; Rhee et al., 2009). Gut bacteria have been found to participate in the regulation of various psychological processes, including mood, cognition, memory, social behavior, and brain development (Erny et al., 2015; Kelly et al., 2019; Sampson et al., 2016; Sharon et al., 2019). These effects may be mediated via immune, nervous or neuroendocrine systems (Bonaz et al., 2018; Fung et al., 2017). The microbiota-gut-brain axis presents as a target for developing novel therapies for brain dysfunction, especially through dietary strategies, such as through the intake of probiotics and/or prebiotics (Burokas et al., 2017; Konturek et al., 2015; Liu et al., 2015).
Depression, which is commonly comorbid with anxiety, is a heterogeneous neuropsychiatric disorder. The total number of people who are currently living with depression is more than 320 million worldwide (Organization, 2017). The serotonin (5-hydroxytryptamine, 5-HT) system in the brain is key for both the development of depression and its treatment (Cryan and Leonard, 2000). Although the first-line treatment for depression remains the selective serotonin reuptake inhibitor (SSRI), only a third of patients receive the emotional benefits (Trivedi et al., 2006). Moreover, chronic SSRI treatment normally works after a delay of 2–4 weeks in conjunction with many reported side effects in the gastrointestinal tract (such as constipation) (Locher et al., 2017; Margolis and Gershon, 2019; Marken and Munro, 2000). As the precursor of 5-HT, 5-hydroxytryptophan (5-HTP) is widely known for its capacity to cross the blood-brain barrier and to yield antidepressant-like function, now becoming the focus of medical and scientific interest (Jacobsen et al., 2016).
Animal studies have shown that the microbiome is sensitive to the effects of depression (Bailey et al., 2011; Bharwani et al., 2016; Foster et al., 2017; Papalini et al., 2019; Partrick et al., 2018). In recent years, emerging clinical data has revealed that the gut microbiota in major depression disorders (MDD) patients is also altered (Hu et al., 2019; Jiang et al., 2015; Kelly et al., 2016; Papalini et al., 2019; Soldi et al., 2019). Besides, transplantation of fecal microbiota from depressed patients into microbiota-deficient rodents resulted in a transfer of the depressive phenotype (Kelly et al., 2016), identifying the essential role of the gut microbiota in the development of depression. Based on the understanding of the microbiota-brain-gut axis, several clinical studies are emerging showing the successful alleviation of depression or stress-related symptomatology with probiotics (Kazemi et al., 2019; Papalini et al., 2019; Wang et al., 2016). Exploration of the above studies opens new avenues for treating depression. However, the mechanisms are not well elucidated (Dinan et al., 2013; Sarkar et al., 2016; Savignac et al., 2014).
Herein, we focused on the interaction between gut microbiota and enterochromaffin cells, using a specific strain of bacteria Bifidobacterium breve CCFM1025, which facilitates 5-HTP synthesis, to explore a novel therapeutic approach for curtailing depression (Tian et al., 2019a). We systematically investigated the impact of CCFM1025 on behaviors, brain neurophysiological alterations, immune status, neuroendocrine responses, as well as gut microbial composition, metabolite production, and functional gene expression.
2. Materials & methods
2.1. Animal experiment
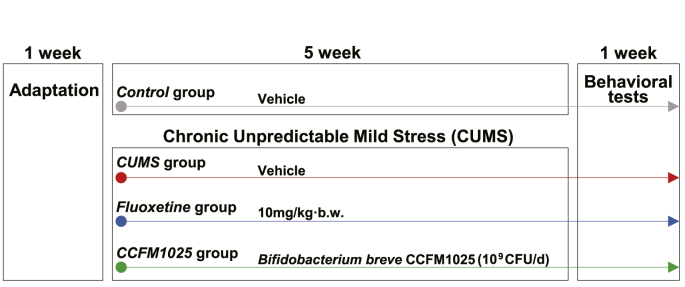
Male adult C57BL/6 mice (6 weeks of age, purchased from SLAC Experimental Animal Co., Ltd, Shanghai, China) were used in the study. The mice were housed in an environmentally controlled (21°C–23 °C, 50%–60% of humidity) room under a 12:12h light-dark cycle with free access to food and water. Animals were allowed to adapt to the environment for one week prior to testing. Forty mice were randomly assigned into four groups (n = 10 in each group). All animal experiments were approved by the Experimental Animal Ethics Committee of Jiangnan University (qualified number: JN. No20181215c0480130 [268]). All procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The experimental schedule is shown in Fig. 1. Protocols of chronic unpredictable mild stress (CUMS) procedures and behavioral tests are described in Supplemental 1.1 and 1.2.
Fig. 1.
Animal experimental schedule. The whole period is seven weeks, including one-week adaptation and six-week treatment. Behavioral tests were performed during the sixth week for all the mice.
2.2. Bacterial treatment
The biological origin of Bifidobacterium breve CCFM1025 is described in Supplemental 1.3. The bacterium was cultured in MRS liquid medium (supplemented with 0.05% L-cysteine) and grown anaerobically at 37 °C. Bacteria for oral administration were prepared by suspending the lyophilized bacteria powder in 10% skimmed cow milk. Colony counting was done to aliquot the concentration of surviving bacteria at 109 CFU/mL. The CCFM1025 treatment group was gavaged at a volume of 0.1 ml/10g body weight daily.
2.3. In vitro cellular experiment
The enterochromaffin cell experiment was carried out as previously described (Tian et al., 2019a; Yano et al., 2015). Briefly, RIN14B cells (ATCC CRL-2059) were pre-treated with 0.1% BSA and 2 μM fluoxetine for 10 s, then cultured in Hank's balanced salt solution containing 10 μM short-chain fatty acids (SCFAs). The bacterial fermented supernatants were passed through 0.2 μm pore syringe filters before added to the cells. At the time point of 0, 15, 30 min, the cells were collected for additional real-time PCR measurement.
2.4. Quantitative real-time PCR (qRT-PCR)
Briefly, RNA was extracted using Trizol reagent (Invitrogen, USA). Complementary DNA was prepared using the PrimeScript RT reagent Kit (Takara Bio Inc., Japan) according to the manufacturer's protocol. Quantitative PCR was carried out on a BioRad-CFX384 system using SYBR Green Supermix (Bio-Rad, USA). Amplification reactions were run with “no template” controls, all in triplicate, for each probe used. Cycle threshold (Ct) values were recorded and normalized to the Gapdh using the 2−ΔΔCt method. Detailed procedures and primer sets are shown in Supplemental Table S1.
2.5. Biochemical measurements
5-HT and 5-HTP levels in tissues and serum were quantified by HPLC with fluorescence detection as described (Tian et al., 2019b). Brain-derived neurotrophic factor (BDNF), Corticotropin-releasing factor (CRF), corticosterone, and inflammatory cytokines (TNF-α, IL-β, IL-6, and IL-17) were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's protocol (R&D Systems, USA). SCFAs were extracted by diethyl ether from fresh cecal contents and qualified on the TSQ 9000 GC-MS system (Thermo Scientific) as previously described (Tian et al., 2019a).
2.6. Western blotting
A detailed procedure is shown in Supplemental 1.5. Primary antibodies used for detection are as follows: cAMP-response element-binding protein (CREB; 1:1000, Cell Signaling Technology, #9197), phosphorylated-CREB (pCREB; Ser133, 1:1000, Cell Signaling Technology, #9198), Tryptophan hydroxylases 1 (TPH1; 1:1000, ABclonal, A1569), and GAPDH (1:3000, ABclonal, AC033). The secondary antibody was horseradish peroxidase (HRP)-conjugated (1:5000, Goat anti-mouse/rabbit IgG-HRP, Absin, abs20001/20002). Protein bands were visualized by chemiluminescence using ECL luminescence reagent (Absin Bioscience Inc., Shanghai, China). Protein expression was quantified using ImageJ software and shown as density relative to GAPDH.
2.7. Immunostaining of brain sections
The detailed procedure is shown in Supplemental 1.6. Briefly, hippocampal sections were made and subjected to immunohistochemical (IHC) detection of proBDNF (1:100, Santa Cruz, sc-65514), and immunofluorescence (IF) detection of c-Fos (1: 200, Cell Signaling Technology, #2250). Immunoreactivities were visualized using 3, 3′-diaminobenzidine-IHC- and fluorescein -IF-Detection Kit (Sangon Biotech, Shanghai, China) according to the manufacturer's instructions. Stained slides were digitized with a confocal laser scanning microscope system (ZEISS, Germany), and analyzed with Image-Pro Plus software (Media Cybernetics, USA) by pathologists blinded to the experimental parameters.
2.8. Metagenomic sequencing and bioinformatic analysis
The procedure of fecal 16S rRNA sequencing is described in detail in Supplemental 1.7. Quantitative Insights into Microbial Ecology (QIIME) platform was used to process the raw data of sequencing (Caporaso et al., 2010). Operational taxonomic units (OTUs) were picked using a criterion of 97% nucleotide identity based on the Greengenes 13.5 database. α-Diversity was measured by species richness and evenness from the rarefied OTU and indicated as Shannon and Simpson index. β-Diversity was estimated by unweighted pairwise UniFrac distances, visualized with principal component analysis (PCA) and principal coordinate analysis (PCoA), and permutational multivariate analysis of variance (perMANOVA) was used to assess differences (Dhariwal et al., 2017; Gloor et al., 2017). Linear discriminant analysis (LDA) effect size (LEfSe) was used to differentiate microbial biomarkers (Wilcoxon rank-sum test, α < 0.05 and logLDA>3.0 were used as the threshold) and visualized by taxonomic cladogram tree (http://huttenhower.org/galaxy). Metagenomes of gut microbiota were computed from 16S rRNA sequences based on Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt), predicted functional pathways were annotated from the Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology (Langille et al., 2013). The network was constructed with Spearman's correlation coefficients and visualized by Gephi (V. 0.9.1). In all cases, recommended parameters were used, unless otherwise mentioned.
2.9. Statistical analysis
Statistical analysis was performed using Prism 6.0 software and SPSS 21.0. Data are presented as means with SEM. For data sets confirmed with a normal distribution (Shapiro-Wilk test), unpaired Student's t-test was performed between the Control and CUMS group, and one-way ANOVA was performed to compare the effects of Fluoxetine and CCFM1025 in CUMS-treated animals, followed by Dunnett's multiple comparisons test against the CUMS group. For data sets that were not normally distributed, the Mann Whitney test was performed between the Control and CUMS group, and the Kruskal-Wallis test was performed for the CUMS, Fluoxetine- and CCFM1025-treated groups with Dunn's post hoc test against the CUMS group. Effect size (Cohen's d) of all comparisons was shown in the Supplemental Table S2-S6. A criterion for significance was set to P < 0.05 in all comparisons. The P-value of multiple comparisons (Padj) was adjusted by family-wise significance and confidence levels of 0.05 (95% confidence interval).
3. Results
3.1. CCFM1025 treatment decreased anxiety- and depressive-like behaviors
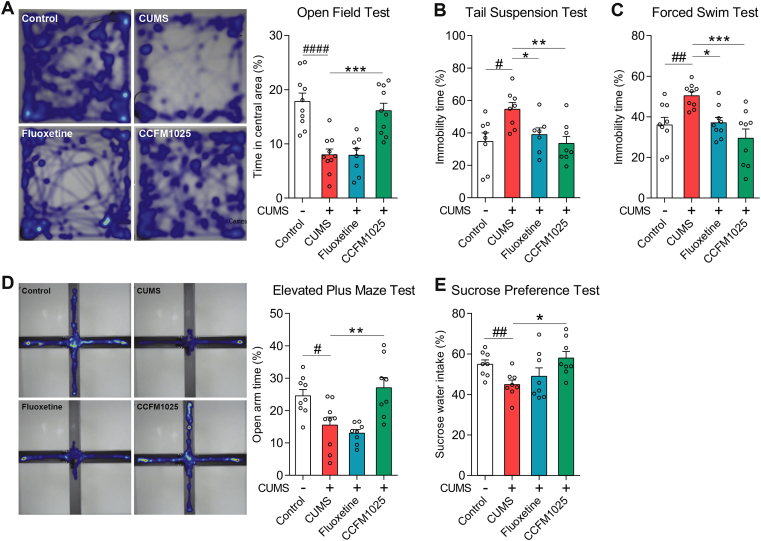
Five-weeks of chronic stress induced significant depressive-like symptoms, including increased immobility (Fig. 2B, T (14) = 2.953, P = 0.011; Fig. 2C, T (16) = 3.424, P = 0.004), and anhedonia (Fig. 2E, T (14) = 3.240, P = 0.006). Anxiety-like symptoms were reflected by the reduced time spent in the center and open areas (Fig. 2A, T (18) = 5.270, P < 0.001; Fig. 2D, T (16) = 2.879, P = 0.011). All behavioral abnormalities were reversed by CCFM1025 treatment (Fig. 2A, F (2, 25) = 14.880, Padj < 0.001; Fig. 2B, F (2, 20) = 6.909, Padj = 0.005; Fig. 2C, F (2, 24) = 10.850, Padj < 0.001; Fig. 2D, F (2, 22) = 9.256, Padj = 0.001; Fig. 2E and F (2, 21) = 4.148, Padj = 0.03).
Fig. 2.
The antidepressant-like and anxiolytic effect of CCFM1025. (A) Open-field test (n = 8–10). The panel displays a representative heatmap of tracking movement, and the histogram indicates time spent in the open area (percentage of the total time). (B) Tail suspension test (n = 9–10). Immobility time presented as a percentage of total time. (C) Forced swim test (n = 9). Immobility time presented as a percentage of total time. (D) Elevated plus maze test (n = 8–9). Representative heatmap of tracking movement and time in open arms (percentage of the total time). (E) Sucrose preference test (n = 8). Percentage of sucrose water intake. Data are means with SEM; Unpaired Student's t-test #P < 0.05, ##P < 0.01, ####P < 0.0001 vs Non-stress Control; One-way ANOVA for CUMS, Fluoxetine- and CCFM1025-treated groups, *P < 0.05, **P < 0.01, ***P < 0.001 vs CUMS vehicle treated.
3.2. CCFM1025 mitigated hypothalamic-pituitary-adrenal (HPA) axis hyperactivity-induced inflammation
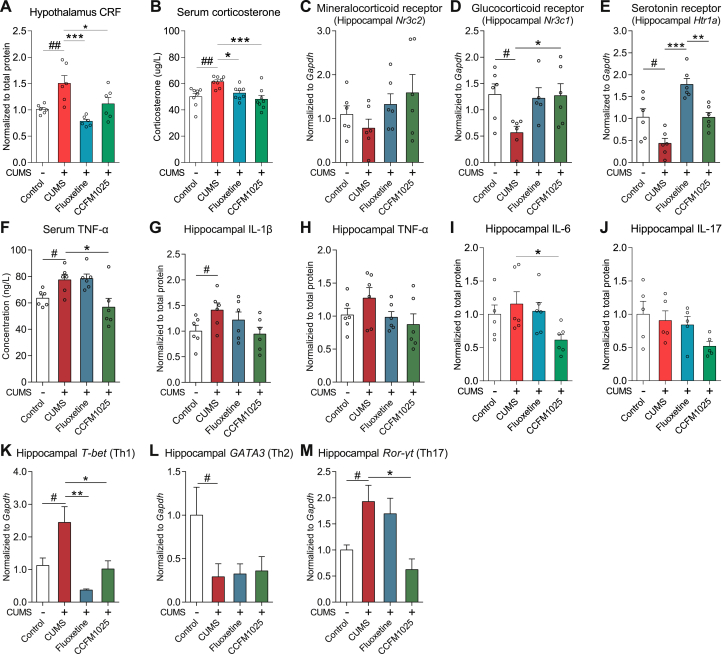
The basal hypothalamus-released CRF (Fig. 3A, T (10) = 3.259, P = 0.009) and serum corticosterone (Fig. 3B, U = 3.0, P = 0.001) levels were elevated in chronically stressed mice. The expression of glucocorticoid receptor (GR) was significantly reduced (Fig. 3D, T (10) = 3.101, P = 0.011), while the level of mineralocorticoid receptor (MR) showed no statistical difference from the control group (Fig. 3C, T (10) = 1.111, P = 0.293). All of the HPA-axis related abnormalities were restored by treatment of CCFM1025 and fluoxetine (Fig. 3A, F (2, 15) = 10.38, Padj = 0.002; Fig. 3B, F (2, 21) = 9.812, Padj < 0.001; Fig. 3D, F (2, 14) = 4.544, Padj = 0.030). Hippocampal 5-Htr1a mRNA level was decreased in chronically stressed mice (Fig. 3E, T (10) = 2.677, P = 0.023) and both fluoxetine and CCFM105 could restore it (F (2, 15) = 30.69, Padj < 0.001). In addition, the hippocampal inflammatory cytokine IL-1β was significantly increased (Fig. 3G, T (10) = 2.277, P = 0.046), but CCFM1025 treatment could only reduce the hippocampal IL-6 levels (Fig. 3I, H = 7.45, Padj = 0.026), whereas fluoxetine treatment showed no obvious anti-inflammatory effects. Gene expression of T-helper specific transcription factors was measured to evaluate the proportion of Th1, Th2, and Th17 cells. CCFM1025 treatment reversed stress-induced overexpression of T-bet (Fig. 3K, T (10) = 2.472, P = 0.033; F (2, 14) = 10.00, Padj = 0.002) and Ror-γt (Fig. 3M, U = 2.5, P = 0.043; H = 8.036, Padj = 0.015), but had no effect on GATA3 (Fig. 3L, U = 1.0, P = 0.03; H = 0.845, Padj > 0.999). CCFM1025 also reduced circulating TNF-α (Fig. 3F, F (2, 15) = 6.146, Padj = 0.011) concentration, while no statistical difference was observed in serum IL-1β, IL-6 and IL-17 concentration (Supplemental Fig. S1).
Fig. 3.
CCFM1025 alleviated HPA-axis hyperactivity and inflammation. (A) Hypothalamus corticotropin-releasing hormone (CRF) (n = 6). (B) Serum corticosterone levels (μg/L) (n = 8). (C) Transcript levels of mineralocorticoid receptor (Nr3c2) in the hippocampus (n = 6). (D) Transcript levels of the glucocorticoid receptor (Nr3c1) in the hippocampus (n = 5–6). (E) Transcript levels of serotonin receptors (Htr1a) in the hippocampus (n = 6). (F) Serum TNF-α levels (ng/L) (n = 6). (G-J) Hippocampal inflammatory cytokines levels (G- I, n = 6; J, n = 5). (K-M) Hippocampal T helper cell-specific transcription factors levels (mRNA) (K, n = 5–6; L, n = 4–6; M, n = 4–6). Data are means with SEM; Unpaired Student's t-test (or Mann Whitney test) #P < 0.05, ##P < 0.01 vs Non-stress Control; One-way ANOVA (or Kruskal-Wallis test) for CUMS, Fluoxetine- and CCFM1025-treated groups, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs CUMS vehicle treated.
3.3. CCFM1025 regulated BDNF and c-Fos expression in brain
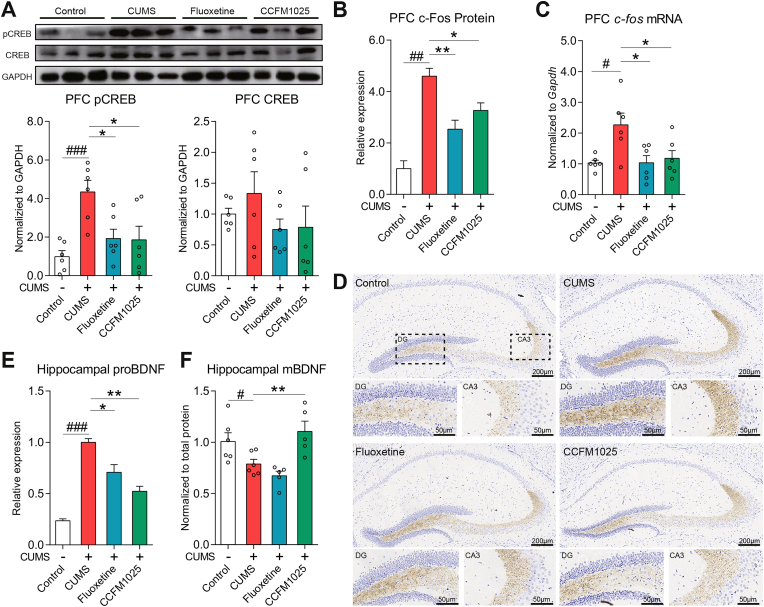
In the CUMS group, pCREB was overexpressed in the prefrontal cortex (PFC) (Fig. 4A, T (10) = 4.964, P = 0.001), while the total CREB showed no statistical difference (Fig. 4A, T (10) = 0.918, P = 0.380; gel photo in Supplemental Fig. S2). The downstream c-Fos was also overexpressed under chronic stress, at both the translation (Fig. 4B, T (4) = 8.332, P = 0.001; Representative sections of immuno-fluorescence staining are in Supplemental Fig. S3) and transcription levels (Fig. 4C, T (10) = 3.139, P = 0.011). CCFM1025 and fluoxetine treatments down-regulated pCREB (Fig. 4A, F (2, 15) = 5.441, Padj = 0.017) and accordingly normalized the expression of c-Fos (Fig. 4B, F (2, 6) = 10.90, Padj = 0.010; Fig. 4C, F (2, 15) = 5.124, P = 0.020). Chronic stress significantly decreased hippocampal mature BDNF level (Fig. 4F, T (10) = 2.277, P = 0.046). In contrast to the alteration of mature BDNF, the level of proBDNF increased (Fig. 4E, T (4) = 8.332, P = 0.022), particularly in the hippocampal regions of the dentate gyrus (DG) and the CA3. CCFM1025 significantly normalized the balance of proBDNF/BDNF levels to that of controls (Fig. 4E and F (2, 6) = 18.80, Padj = 0.003; Fig. 4F, F (2, 13) = 10.65, Padj = 0.002).
Fig. 4.
CCFM1025 treatment regulates the CREB-BDNF/c-Fos pathway. (A) p-CERB/CREB expression in prefrontal cortex (PFC) (n = 6). Representative western blots (three out of six samples) and quantification of band intensities (relative to the expression of GAPDH); (B) c-Fos levels in PFC (n = 3). Representative sections of immuno-fluorescence staining are in Supplemental Fig. S3 The transcript levels of c-fos in PFC (n = 6). (D) Representative immunohistochemical staining of proBDNF in the dorsal hippocampus. DG and CA3 regions are displayed below (E). The histogram indicates the proBDNF levels qualified by staining intensity (n = 3) (F) Mature BDNF (mBDNF) levels in the hippocampus (n = 5–6). Data are means with SEM; Unpaired Student's t-test #P < 0.05, ###P < 0.001, ####P < 0.0001 vs Non-stress Control; One-way ANOVA (or Kruskal-Wallis test) for CUMS, Fluoxetine- and CCFM1025-treated groups, *P < 0.05, **P < 0.01 vs CUMS vehicle treated.
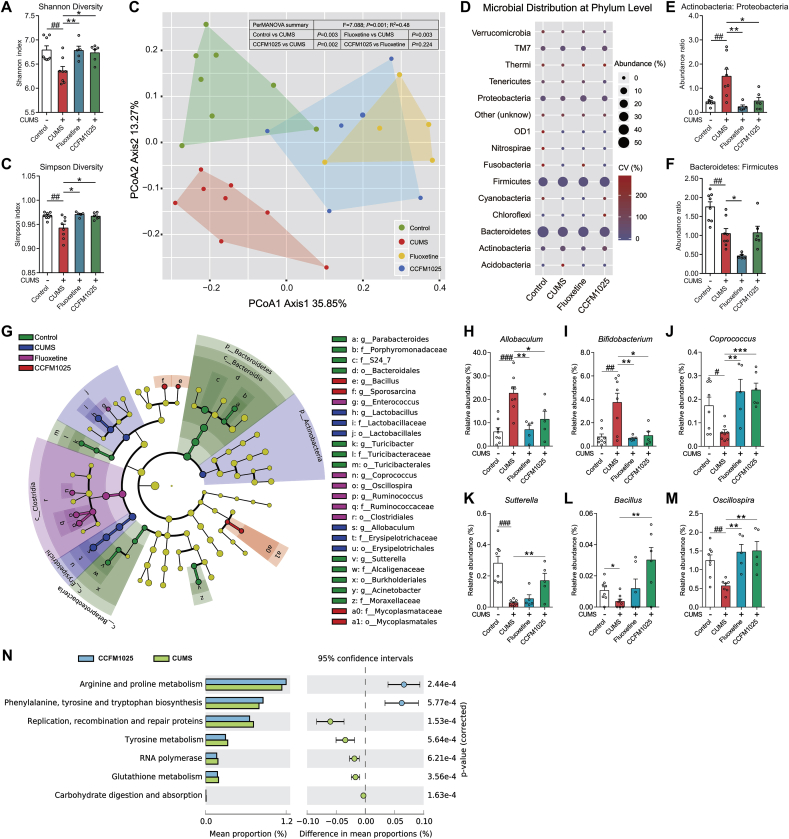
3.4. Stress-induced gut microbiota abnormalities were restored by CCFM1025 treatment
α-diversity was significantly decreased in the chronically stressed group, as determined via Shannon (Fig. 5A, T (14) = 3.414, P = 0.004) and Simpson (Fig. 5B, T (14) = 3.074, P = 0.008) indices. CCFM1025 was able to maintain the α-diversity under chronic stress (Fig. 5A, F (2, 16) = 7.400, Padj = 0.005; Fig. 5B, F (2, 16) = 6.768, Padj = 0.007). A significant difference in β-diversity between the control and CUMS groups was revealed by the PCoA and perMANOVA (Fig. 5B, F = 7.088, P = 0.001; P Control vs CUMS = 0.003). The structure of gut microbiota in CCFM1025-and fluoxetine-treated group showed a trend of separation from the CUMS group (Fig. 5B, P CCFM1025 vs CUMS = 0.002; P Fluoxetine vs CUMS = 0.003). CCFM1025 and fluoxetine treatment normalized the ratio of Actinobacteria: Proteobacteria (Fig. 5E, T (14) = 3.517, P = 0.009; F (2, 16) = 8.541, Padj = 0.003) but failed to restore the ratio of Bacteroidetes: Firmicutes (Fig. 5F, T (14) = 3.729, P = 0.002; F (2, 16) = 5.624, Padj = 0.991), which are two most abundant taxa in gut microbiota. Twelve genera were screened by LEfSe (Fig. 5G, Supplemental Fig. S4), and six of them were significantly affected by both stress and CCFM1025 treatment (Fig. 5H-M). Using the phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt), seven pathways computed from KEGG were identified differential between CCFM1025 and CUMS group (Welch's-t test, P < 0.001; Fig. 5N), among which the arginine and proline metabolism and phenylalanine and tyrosine and tryptophan biosynthesis pathways were significantly upregulated by CCFM1025.
Fig. 5.
Effect of CCFM1025 treatment on gut microbial composition. (A-B) Microbial α-diversity, indicated by the Shannon and Simpson indices (n = 5–8 for each test). (C) Principal coordinates analysis. Contribution vectors (%) of the principal components are labeled on the axes. The difference between groups was analyzed by perMANOVA. (D) The abundance of taxa at the phylum level. The size and color of the circles respectively display the average abundance and coefficient of variance (CV) of the abundance. (E-F) The ratio of Actinobacteria: Proteobacteria and Bacteroidetes: Firmicutes. (G) Linear discriminant analysis (LDA) effect size (LEfSe). Differential taxa are labeled with tags and annotated in the right panel. Data were computed with an LDA score above 3.00 and P value below 0.05 for the factorial Kruskal-Wallis test. (H-M) The relative abundance of taxa found to be significantly altered by stress and CCFM1025 treatment (n = 5–8 for each test). (N) Differential microbial functions between CCFM1025 and depression groups. Metagenomic analysis was performed using PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States), followed by annotation with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Statistical difference of the gene contents between the two groups is screened by Welch's-t test with P < 0.0001. Data are means with SEM; Unpaired Student's t-test (or Mann Whitney test) #P < 0.05, ##P < 0.01, ###P < 0.001 vs Non-stress Control; One-way ANOVA (or Kruskal-Wallis test) for CUMS, Fluoxetine- and CCFM1025-treated groups, *P < 0.05, **P < 0.01, ***P < 0.001 vs CUMS vehicle treated.
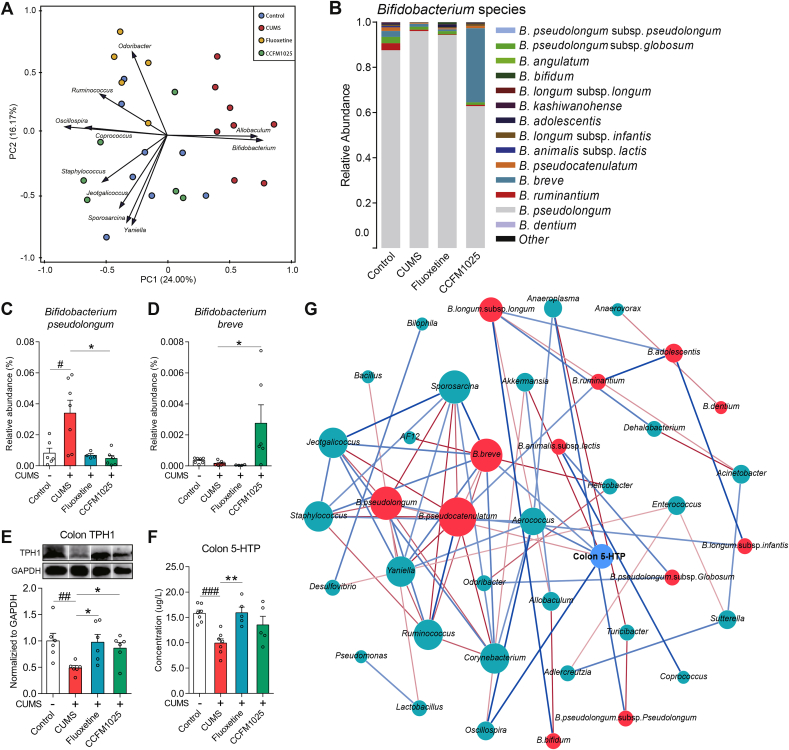
3.5. Bifidobacterium spp. abundance correlated with intestinal 5-HTP levels
Bifidobacterium and Allobaculum were identified as the top microbial contributors which shape the gut microbiota of chronically stressed mice (Fig. 6A). The distribution of Bifidobacterium species was further analyzed (Fig. 6B). The abundance of Bifidobacterium pseudolongum increased (Fig. 6C, T (11) = 2.722, P = 0.020) in chronically stressed mice. CCFM1025 treatment increased the abundance of Bifidobacterium breve (Fig. 6D, F (2, 13) = 4.041, Padj = 0.043), and such competitive colonization reduced the Bifidobacterium pseudolongum abundance (Fig. 6D, H = 8.355, P = 0.011) (the relative abundance of all Bifidobacterium spp. was shown in Supplemental Table S7). The effect of gut microbiota on the biosynthesis of 5-HTP was also investigated. CCFM1025 and fluoxetine normalized the reduced TPH1 (Fig. 6E, T (10) = 3.343, P = 0.008; F (2, 15) = 5.529, Padj = 0.016; gel photo in Supplemental Fig. S5) and 5-HTP (Fig. 6F, T (12) = 5.538, P = 0.001; F (2, 14) = 6.692, Padj = 0.009) levels under stress. A network consisting of 40 nodes and 71 edges was constructed to determine the relationships among core microbial taxa and colonic 5-HTP levels (Fig. 6F). All edges connected two arguments of which the Spearman's coefficient was over 0.6 and the P value below 0.05. Colonic 5-HTP levels had a significant positive correlation with B. breve (r = 0.829), Aerococcus (r = 0.886), and Oscillospira (r = 0.943), but a negative correlation with Akkermansia (r = −0.943) and B. pseudolongum (r = −0.829), which confirmed that the gut microbiota and external excess of B. breve strains indeed affected the biosynthesis of 5-HTP in gut.
Fig. 6.
Bifidobacterium spp. affect gut microbial functions. (A) Principal component analysis. Variables that contribute to the clustering are labeled by arrows. The acute angle between the two arrows indicated a positive correlation between the two variables (P < 0.05). (B) Distribution of Bifidobacterium species in the Bifidobacterium genus. (C-D) The relative abundance of Bifidobacterium pseudolongum and Bifidobacterium breve (n = 4–7 each test). (E) TPH1 expression in the colon (n = 6). (F) 5-HTP levels in colon tissue (n = 5–7). Data are means with SEM; Unpaired Student's t-test #P < 0.05, ##P < 0.01, ###P < 0.001 vs Non-stress Control; One-way ANOVA for CUMS (Chronic unpredictable mild stress), Fluoxetine- and CCFM1025-treated groups, *P < 0.05, **P < 0.01 vs CUMS vehicle treated. (G) Network construction of gut microbial taxa and colon 5-HTP. Each connection between two nodes stands for a strong (Spearman's ρ > 0.6) and a significant (P value < 0.05) correlation. The size of each node is proportional to the number of connections. Blue lines represent positive correlation and the red lines represent a negative correlation. The color depth of the line represents the correlation strength. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
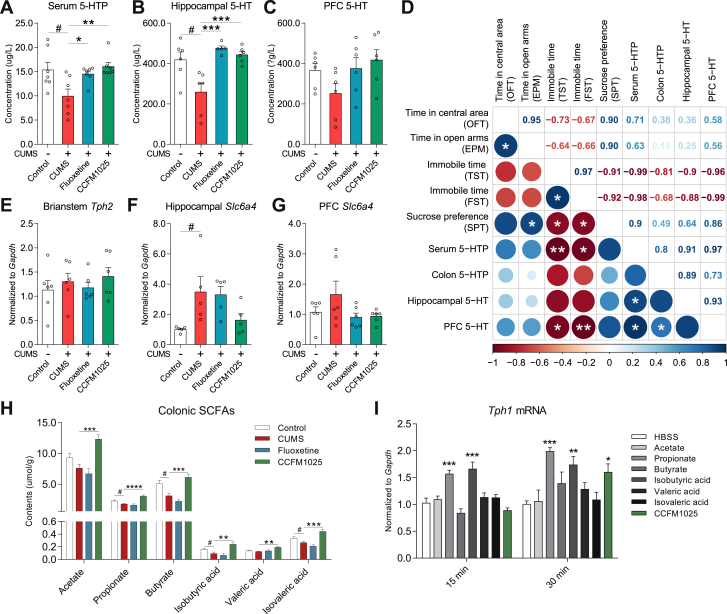
3.6. Gut microbial metabolites mediated 5-HTP facilitated the antidepressant-like effects of psychobiotics
CCFM1025 improved the serum 5-HTP level (Fig. 7A, T (12) = 2.519, P = 0.027; H = 9.506, P = 0.006) and hippocampal 5-HT level (Fig. 7B, U = 4.0, P = 0.026; H = 11.92, P = 0.028). CCFM1025 showed no effect on improving the PFC 5-HT level (Fig. 7C, T (10) = 1.877, P = 0.09; F (2, 15) = 2.773, P = 0.095). Interestingly, a positive association between serum 5-HTP and brain 5-HT was demonstrated by Pearson's correlation coefficient (Fig. 7D, P < 0.05). Besides, colonic 5-HTP and serum 5-HTP (P < 0.05) concentration also negatively correlated with immobility time in TST and FST (Fig. 7D). Notably, tryptophan hydroxylases 2 (Tph2), the key enzyme controlling 5-HT biosynthesis in brain (mainly expressed in the brainstem) (Manoharan et al., 2016), showed no difference among all groups (Fig. 7E, T (10) = 0.664, P = 0.522; H = 1.731, Padj = 0.44). In addition, CCFM1025 and fluoxetine showed no effect on slc6a4 gene expression in hippocampus (Fig. 7F, H = 4.940, Padj = 0.081) and PFC (Fig. 7G and H = 1.979, Padj = 0.391). Furthermore, we measured the SCFA levels in colonic contents. CCFM1025 extensively increased the concentration of SCFAs (Fig. 7H, acetate, F (2, 17) = 18.33, Padj < 0.001; propitiate, F (2, 16) = 26.57, Padj < 0.001; butyrate, F (2, 17) = 24.75, Padj < 0.001; isobutyric acid, F (2, 17) = 10.26, Padj = 0.001; valeric acid, F (2, 17) = 9.803, Padj = 0.002; isovaleric acid, F (2, 17) = 19.32, Padj < 0.001). Lastly, we confirmed the effect of CCFM1025 fermentation products and SCFAs in the biosynthesis of 5-HTP in an in vitro enterochromaffin cell model (RIN14B). After exposure to propionate and isobutyrate for 15 min, gene expression of Tph1 was significantly upregulated (Fig. 7I, F (7, 40) = 13.23, Padj < 0.001). CCFM1025 also enhanced the expression of Tph1 when the treatment was prolonged to 30 min (Fig. 7I, F (7, 40) = 5.537, Padj < 0.001).
Fig. 7.
Gut derived 5-HTP mediated by microbial metabolites which facilitated the antidepressant-like effect of CCFM1025. (A) Serum 5-HTP levels (n = 7). (B-C) 5-HT levels in the hippocampus (n = 5–6) and PFC (n = 6). (D) Correlation analysis of peripheral 5-HTP levels, brain 5-HT levels, and behavioral performance. The color and size of the circle code for the level of correlation calculated by one-tailed Pearson's analyses (*P < 0.05, **P < 0.01). (E) Gene expression of tryptophan hydroxylase 2 (tph2 mRNA) in the brainstem (n = 6). (F-G) Gene expression of the serotonin transporter (slc6a4 mRNA) in the hippocampus (n = 5) and PFC (n = 6). (H) Colonic SCFAs levels (n = 6–8 for each test). Data are means with SEM; Unpaired Student's t-test (or Mann Whitney test) #P < 0.05 vs Non-stress Control; One-way ANOVA (or Kruskal-Wallis test) for CUMS, Fluoxetine- and CCFM1025-treated groups, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs CUMS vehicle treated. (I) Transcript levels of Tph1 in RIN14B when exposure to SCFAs and CCFM1025 fermentation supernatant (n = 6). One-way ANOVA for all groups, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs HBSS.
4. Discussion
Increasing evidence suggests that targeting the microbiota may be a novel strategy to counter the effects of stress (Foster et al., 2017; Long-Smith et al., 2020). Here we show that a specific Bifidobacterium breve strain (CCFM1025) can counter many of the effects of chronic stress at a multitude of levels across the microbiota-gut-brain axis.
Similar to the clinical observations (Hu et al., 2019; Zheng et al., 2016), mice exposed to chronic stress were also found to display alterations in microbial composition (dysbiosis) (Fig. 5). Although the stress-induced changes in α-diversity are restored by CCFM1025 treatment, a new microbial profile, one which was more similar to normal microbiota than that treated with fluoxetine, was formed (Fig. 5C). Interestingly, stress-induced alteration of some taxa was irreversible, the majority of which belonged to the Bacteroidetes and Firmicutes phyla. Bifidobacterium was the leading contributor characterizing the microbiota structure of chronically stressed mice (Fig. 6A), and the abundance of Bifidobacterium was significantly increased under chronic stress but normalized by both the probiotic and the drug. Since Bifidobacterium is the most abundant taxa in Actinobacteria, the phylum showed a similar trend with Bifidobacterium. Until now, only one clinical study has reported a reduction of Bifidobacterium in MDD patients. However, this study failed to control for gender and diet effects (Aizawa et al., 2016). Some groups previously demonstrated an increased Actinobacteria abundance in MDD patients (Jiang et al., 2015; Zheng et al., 2016), which were consistent with our results. Bifidobacterium counts are commonly observed lower in a diseased status, such as with infection and irritable bowel syndrome (Rodiño-Janeiro et al., 2018). It is intriguing that our observation was unconventional, but we believe the physiological role of bifidobacteria should be considered at the species level. Through further identification of Bifidobacterium spp., we confirmed the bloom of Bifidobacterium in depression involved Bifidobacterium pseudolongum specifically. Although B. pseudolongum has been described as one of the most predominant bifidobacteria in the murine intestine, its physiological function remains largely unknown (Centanni et al., 2018). It's worth noting that the abundance of B. pseudolongum negatively correlated with colonic 5-HTP levels while B. breve displayed the opposite effect (Fig. 6G). Moreover, the administration of a B. breve strain improved the metagenomic function of tryptophan biosynthesis, an important branch pathway acting as the precursor for the biosynthesis of 5-HTP and 5-HT (Fig. 5N). This evidence demonstrates that the competitive colonization of B. breve plays a key role in alleviating gastrointestinal and microbial abnormalities in depression. Our results outlined the Bifidobacterium composition in chronically stressed mice, for the first time, which proposes that the role of Bifidobacterium in stress and depression is not straightforward and should be explored at species level if possible.
Apart from Bifidobacterium, another five genus taxa were also normalized from the stress-induced dysbiosis (Fig. 5H, J-M), including Allobaculum and Coprococcus which are typical SCFA-producing bacteria. Coprococcus spp. have been shown to produce butyrate, propionate, and lactate as main fermentation products from non-digestible dietary polysaccharides (Koh et al., 2016), whereas Allobaculum spp. produces acetate via the acetyl-CoA pathway, providing a substrate for butyrate synthesis (La Reau and Suen, 2018). The decrease of Bacillus was also reversed by CCFM1025. Bacillus spp. have been used as medicinal supplements for over 50 years, for their capacity to produce anti-pathogenic compounds (Cutting, 2011; Shafi et al., 2017). A recent clinical study indicated that oral Bacillus coagulans MTCC 5856 efficiently attenuated irritable bowel syndrome and depression symptoms in patients (Majeed et al., 2018). CCFM1025 also significantly increased the abundance of Ruminococcus. Ruminococcus spp. such as R. obeum with propanediol pathway was reported to produce propionate (Louis and Flint, 2017). The alteration of these taxa causally increased the intestinal SCFA levels (Fig. 7H). SCFAs are well known as energy substrates and signaling molecules presenting a variety of distinct physiological effects, such as improving barrier function, maintaining immune homeostasis, and stimulating the secretion of gut peptides (Larraufie et al., 2018; Rooks and Garrett, 2016; Zhao et al., 2018). Our results demonstrated the potential capacity of propionate and isobutyrate to improve the 5-HTP levels through upregulating the Tph1 expression in vitro (Figs. 6F and 7I). As the key neurotransmitter which can cross the BBB, 5-HTP links the bidirectional communication between gut and brain, making it possible for the brain and gut to jointly maintain a host's health. Over 90% of the endogenous 5-HTP is produced by enterochromaffin cells using dietary L-tryptophan, and gut microbiota is essential during this process (Amireault et al., 2013; Yano et al., 2015). Gastrointestinal derived 5-HTP (oral administration) has been demonstrated to enter the brain and participate in 5-HT synthesis (Gijsman et al., 2002). Notably, the slow release of exogenous 5-HTP significantly delays the elimination of plasma 5-HTP, thus potentially maintaining consistently high precursor level for 5-HT synthesis and enhance the antidepressant-like effects of SSRI (Jacobsen et al., 2016, 2019). In stressed mice, CCFM1025 treatment increased the hippocampal 5-HT levels without significantly affecting the expression of tph2 and slc6a4 (Fig. 7B, E & F). This evidence may suggest that an extra-brain compensation pathway exists. It is tempting to speculate that it is the gut-derived 5-HTP that participates in the synthesis of brain 5-HT, because of the statistically positive correlation among gut 5-HTP, serum 5-HTP and brain 5-HT levels (Fig. 7D). This is the first time, to our knowledge, that we provide indirect evidence from both in vivo and in vitro studies that probiotics improve the 5-HTP biosynthesis of enterochromaffin cells via an SCFA-mediated way, and as such gut-derived 5-HTP may facilitate the improvement of brain 5-HT then show the antidepressant-like effects.
The mechanisms involved in stress-induced behavioral changes include HPA axis dysregulation and increased inflammation (Pereira et al., 2019). Here we show, as expected, that chronic stress impaired the negative feedback of corticosterone in the HPA axis by downregulating the glucocorticoid receptors (Fig. 3D). The alteration in glucocorticoid receptor gene expression can lead to glucocorticoid resistance and is also coincident with a high level of inflammation, consistent with previous reports (Cohen et al., 2012; Dendoncker and Libert, 2017; Keller et al., 2017; Pariante, 2017; Pariante and Lightman, 2008). Interestingly, CCFM1025 intervention normalized the stress-induced expression of brain Nr3c1, as well as serum proinflammatory cytokine measures and in turn, led to the alleviation of depression-related behaviors. However, more studies are needed to assess the relative causal contribution of the HPA axis and inflammation to the behavioral effects of CCFM1025.
In addition, pretreatment with CCFM1025 was able to counteract these effects of stress on key neuroplasticity related pathways. These include attenuating the pCREB-mediated overexpression of proBDNF and c-Fos, but increasing mBDNF levels (Fig. 4). proBDNF, the precursor of mature BDNF, binds to p75 neurotrophin receptor with high affinity, which triggers a long-term depression and neuronal apoptosis in the brain (Licznerski and Jonas, 2018; Sartori et al., 2011). However, the imbalance between BDNF/proBDNF in depression was still largely unknown. Clinical studies found that depression patients showed a decreased serum BDNF level, while the proBDNF was not affected, or even up-regulated (Qiao et al., 2017; Yoshida et al., 2012; Zhou et al., 2013). Increased conversion of proBDNF to BDNF within the hippocampus was also observed in rats under an enriched living condition with less environmental stress, which in turn reduced their depression- and anxiety-like behaviors (Cao et al., 2014).
There are several limitations that needed to be addressed. First, the present results were all generated from male mice. Since the prevalence of depression in humans is much greater in female individuals, future studies should assess the potential for antidepressant-like effects of CCFM1025 in female rodents before further confirmation in clinical trials. Second, it is still unclear which metabolites produced by CCFM1025 contribute to its antidepressant-like effect. A metabolomics-based analysis of CCFM1025 fermented products should be investigated. It is also helpful to investigate the gut-brain related metabolomics, for elucidating the metabolic kinetics of gut enterochromaffin cell-derived 5-HTP trafficking from the gut into the brain. At last, although we have shown a number of plausible mechanisms underpinning the behavioral effects of CCFM1025, future work is needed to prove the causality of these pathways and temporality of such effects.
In summary, the present study demonstrated a marked antidepressant-like effect of Bifidobacterium breve CCFM1025. The potential mechanisms involved may include the mitigation of HPA-axis hyperactivity and inflammation, up-regulation of BDNF coupled with down-regulation of c-Fos levels, enhancing the serotonergic system in both gut and brain, as well as the modification on gut microbial composition and metagenome. This study further supports the importance of targeting the microbiota-gut-brain axis in regulating mood disorder and offers a strategy for testing the potential psychobiotic basis for novel therapeutic strategies for depression.
CRediT authorship contribution statement
Peijun Tian: Conceptualization, Investigation, Formal analysis, Visualization, Writing - original draft, Funding acquisition. Kenneth J. O'Riordan: Writing - original draft, Validation. Yuan-kun Lee: Writing - review & editing. Gang Wang: Conceptualization, Investigation, Validation, Writing - review & editing, Supervision, Project administration, Funding acquisition. Jianxin Zhao: Validation, Resources, Data curation. Hao Zhang: Conceptualization, Methodology, Validation, Resources. John F. Cryan: Conceptualization, Supervision, Writing - review & editing. Wei Chen: Resources, Supervision, Funding acquisition.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgment
We thank Kieran Rea and Thomaz F. S. Bastiaanssen (APC Microbiome Ireland, Cork, Ireland) for revising the data interpretation. We thank Simon Spichak (APC Microbiome Ireland, Cork, Ireland) for editing and proofreading the manuscripts. We thank Qingmin Kong, Botao Wang, Renying Zou (Jiangnan University, China) for technically experimental support. This work was supported by the National Natural Science Foundation of China (No. 31972052, 31671839, 31820103010), the Fundamental Research Funds for the Central Universities (JUSRP51501), the National First-class Discipline Program of Food Science and Technology (JUFSTR20180102), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1810), the Open Research Funds Projects of State of Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics (T151808). This work is also supported by a scholarship from the China Scholarship Council while the first author studying in University College Cork (File NO. 201906790026).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100216.
Contributor Information
Gang Wang, Email: wanggang@jiangnan.edu.cn.
John F. Cryan, Email: J.Cryan@ucc.ie.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aizawa E., Tsuji H., Asahara T., Takahashi T., Teraishi T., Yoshida S., Ota M., Koga N., Hattori K., Kunugi H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016;202:254–257. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Amireault P., Sibon D., Côté F. Life without peripheral serotonin: insights from tryptophan hydroxylase 1 knockout mice reveal the existence of paracrine/autocrine serotonergic networks. ACS Chem. Neurosci. 2013;4:64–71. doi: 10.1021/cn300154j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bharwani A., Mian M.F., Foster J.A., Surette M.G., Bienenstock J., Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217–227. doi: 10.1016/j.psyneuen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Bonaz B., Bazin T., Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatr. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- Cao W., Duan J., Wang X., Zhong X., Hu Z., Huang F., Wang H., Zhang J., Li F., Zhang J., Luo X., Li C.-Q. Early enriched environment induces an increased conversion of proBDNF to BDNF in the adult rat's hippocampus. Behav. Brain Res. 2014;265:76–83. doi: 10.1016/j.bbr.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni M., Lawley B., Butts C.A., Roy N.C., Lee J., Kelly W.J., Tannock G.W. Bifidobacterium pseudolongum in the ceca of rats fed Hi-Maize starch has characteristics of a keystone species in bifidobacterial blooms. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00547-18. e00547-00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D., Doyle W.J., Miller G.E., Frank E., Rabin B.S., Turner R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Leonard B.E. 5‐HT1A and beyond: the role of serotonin and its receptors in depression and the antidepressant response. Hum. Psychopharmacol. Clin. Exp. 2000;15:113–135. doi: 10.1002/(SICI)1099-1077(200003)15:2<113::AID-HUP150>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., O'Riordan K.J., Cowan C.S., Sandhu K.V., Bastiaanssen T.F., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Cutting S.M. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Dendoncker K., Libert C. Glucocorticoid resistance as a major drive in sepsis pathology. Cytokine Growth Factor Rev. 2017;35:85–96. doi: 10.1016/j.cytogfr.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Dhariwal A., Chong J., Habib S., King I.L., Agellon L.B., Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Stanton C., Cryan J.F. Psychobiotics: a novel class of psychotropic. Biol. Psychiatr. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Erny D., Hrabe de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermohlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.A., Neufeld K.-A.M. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiology of stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20:145. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsman H.J., van Gerven J.M., de Kam M.L., Schoemaker R.C., Pieters M.S., Weemaes M., de Rijk R., van der Post J., Cohen A.F. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J. Clin. Psychopharmacol. 2002;22:183–189. doi: 10.1097/00004714-200204000-00012. [DOI] [PubMed] [Google Scholar]
- Gloor G.B., Macklaim J.M., Pawlowsky-Glahn V., Egozcue J.J. Microbiome datasets are compositional: and this is not optional. Front. Microbiol. 2017;8:2224. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Li A., Huang T., Lai J., Li J., Sublette M.E., Lu H., Lu Q., Du Y., Hu Z. Gut microbiota changes in patients with bipolar depression. Adv. Sci. 2019 doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J.P.R., Krystal A.D., Krishnan K.R.R., Caron M.G. Adjunctive 5-Hydroxytryptophan slow-release for treatment-resistant depression: clinical and preclinical rationale. Trends Pharmacol. Sci. 2016;37:933–944. doi: 10.1016/j.tips.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J.P.R., Oh A., Bangle R., Roberts W.L., Royer E.L., Modesto N., Windermere S.A., Yi Z., Vernon R., Cajina M. Slow-release delivery enhances the pharmacological properties of oral 5-hydroxytryptophan: mouse proof-of-concept. Neuropsychopharmacology. 2019;44:2082–2090. doi: 10.1038/s41386-019-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kazemi A., Noorbala A.A., Azam K., Eskandari M.H., Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 2019;38:522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Keller J., Gomez R., Williams G., Lembke A., Lazzeroni L., Murphy G.M., Jr., Schatzberg A.F. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatr. 2017;22:527. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J.R., Borre Y., O'Brien C., Patterson E., El Aidy S., Deane J., Kennedy P.J., Beers S., Scott K., Moloney G. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., O'Keane V., Cryan J.F., Clarke G., Dinan T.G. Mood and microbes: gut to brain communication in depression. Gastroenterol. Clin. 2019;48:389–405. doi: 10.1016/j.gtc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Konturek P., Haziri D., Brzozowski T., Hess T., Heyman S., Kwiecien S., Konturek S., Koziel J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. 2015;66:483–491. [PubMed] [Google Scholar]
- La Reau A.J., Suen G. The Ruminococci: key symbionts of the gut ecosystem. J. Microbiol. 2018;56:199–208. doi: 10.1007/s12275-018-8024-4. [DOI] [PubMed] [Google Scholar]
- Langille M.G.I., Zaneveld J., Caporaso J.G., Mcdonald D., Dan K., Reyes J.A., Clemente J.C., Burkepile D.E., Thurber R.L.V., Knight R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraufie P., Martin-Gallausiaux C., Lapaque N., Dore J., Gribble F., Reimann F., Blottiere H. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licznerski P., Jonas E.A. BDNF signaling: harnessing stress to battle mood disorder. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115:3742–3744. doi: 10.1073/pnas.1803645115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Cao S., Zhang X. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015;63:7885–7895. doi: 10.1021/acs.jafc.5b02404. [DOI] [PubMed] [Google Scholar]
- Locher M.C., Koechlin M.H., Zion M.S.R., Werner M.C., Pine D.S., Kirsch I., Kessler R.C., Kossowsky J. Efficacy and safety of SSRIs, SNRIs, and placebo in common psychiatric disorders: a comprehensive meta-analysis in children and adolescents. JAMA Psychiatr. 2017;74:1011. doi: 10.1001/jamapsychiatry.2017.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Smith C., O'Riordan K.J., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota-gut-brain Axis: new therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020;60:477–502. doi: 10.1146/annurev-pharmtox-010919-023628. [DOI] [PubMed] [Google Scholar]
- Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- Majeed M., Nagabhushanam K., Arumugam S., Majeed S., Ali F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018;62 doi: 10.29219/fnr.v62.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan A., Shewade D.G., Rajkumar R.P., Adithan S. Serotonin transporter gene (SLC6A4) polymorphisms are associated with response to fluoxetine in south Indian major depressive disorder patients. Eur. J. Clin. Pharmacol. 2016;72:1215–1220. doi: 10.1007/s00228-016-2099-9. [DOI] [PubMed] [Google Scholar]
- Margolis, K.G., Gershon, M.D., 2019. Prevention of ssri-induced gastrointestinal dysfunction with a 5-ht4 receptor antagonist. Google Patents.
- Marken P.A., Munro J.S. Selecting a selective serotonin reuptake inhibitor: clinically important distinguishing features. Prim. Care Companion J. Clin. Psychiatry. 2000;2:205. doi: 10.4088/pcc.v02n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. World Health Organization; 2017. Depression and Other Common Mental Disorders: Global Health Estimates. [Google Scholar]
- Papalini S., Michels F., Kohn N., Wegman J., van Hemert S., Roelofs K., Arias-Vasquez A., Aarts E. Stress matters: randomized controlled trial on the effect of probiotics on neurocognition. Neurobiology of stress. 2019;10 doi: 10.1016/j.ynstr.2018.100141. 100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C.M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur. Neuropsychopharmacol. 2017;27:554–559. doi: 10.1016/j.euroneuro.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Partrick K.A., Chassaing B., Beach L.Q., McCann K.E., Gewirtz A.T., Huhman K.L. Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behav. Brain Res. 2018;345:39–48. doi: 10.1016/j.bbr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J.d.C., Rea K., Nolan Y.M., O'Leary O.F., Dinan T.G., Cryan J.F. Depression's unholy trinity: dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 2019;71 doi: 10.1146/annurev-psych-122216-011613. [DOI] [PubMed] [Google Scholar]
- Qiao H., An S.-C., Xu C., Ma X.-M. Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res. 2017;1663:29–37. doi: 10.1016/j.brainres.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6:306. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodiño-Janeiro B.K., Vicario M., Alonso-Cotoner C., Pascua-García R., Santos J. A review of microbiota and irritable bowel syndrome: future in therapies. Adv. Ther. 2018;35:289–310. doi: 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori C., Vieira A., Ferrari E., Langone F., Tongiorgi E., Parada C. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Savignac H., Kiely B., Dinan T., Cryan J. Bifidobacteria exert strain‐specific effects on stress‐related behavior and physiology in BALB/c mice. Neuro Gastroenterol. Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Shafi J., Tian H., Ji M. Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol. Biotechnol. Equip. 2017;31:446–459. [Google Scholar]
- Sharon G., Cruz N.J., Kang D.-W., Gandal M.J., Wang B., Kim Y.-M., Zink E.M., Casey C.P., Taylor B.C., Lane C.J. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177:1600–1618. doi: 10.1016/j.cell.2019.05.004. e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldi S., Tagliacarne S.C., Valsecchi C., Perna S., Rondanelli M., Ziviani L., Milleri S., Annoni A., Castellazzi A. Effect of a multistrain probiotic (Lactoflorene® Plus) on inflammatory parameters and microbiota composition in subjects with stress-related symptoms. Neurobiology of stress. 2019;10 doi: 10.1016/j.ynstr.2018.11.001. 100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Wang G., Zhao J., Zhang H., Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019;66:43–51. doi: 10.1016/j.jnutbio.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Tian P., Zou R., Song L., Zhang X., Jiang B., Wang G., Lee Y.-k., Zhao J., Zhang H., Chen W. Ingestion of Bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019;10:7588–7598. doi: 10.1039/c9fo01630a. [DOI] [PubMed] [Google Scholar]
- Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am. J. Psychiatr. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Wang H., Lee I.-S., Braun C., Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J. Neurogastroenterol. Motil. 2016;22:589. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Ishikawa M., Niitsu T., Nakazato M., Watanabe H., Shiraishi T., Shiina A., Hashimoto T., Kanahara N., Hasegawa T. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PloS One. 2012;7 doi: 10.1371/journal.pone.0042676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chen F., Wu W., Sun M., Bilotta A.J., Yao S., Xiao Y., Huang X., Eaves-Pyles T.D., Golovko G. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., Zeng L., Chen J., Fan S., Du X. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatr. 2016;21:786. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zhou L., Xiong J., Lim Y., Ruan Y., Huang C., Zhu Y., Zhong J.-h., Xiao Z., Zhou X.-F. Upregulation of blood proBDNF and its receptors in major depression. J. Affect. Disord. 2013;150:776–784. doi: 10.1016/j.jad.2013.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.