Introduction
Drug-induced lupus erythematosus is a rare adverse event of biological treatment, mainly observed with tumor necrosis factor–α antagonists and also known as lupus-like syndrome. Recently, it was also described with interleukin (IL) 17 inhibition. Here, we report the case of a 39-year-old man with onset of cutaneous discoid lupus erythematosus during subcutaneous administration of the IL-17A inhibitor secukinumab, which was initiated for the treatment of chronic plaque psoriasis.
Case report
Treatment with secukinumab 300 mg subcutaneously was initiated in a 39-year-old male patient with long-known, histologically confirmed, severe, plaque-type psoriasis and inadequate response and poor tolerability to previous conventional therapy (methotrexate and psoralen plus ultraviolet A). Approximately 2 months after beginning treatment and with many of the psoriatic plaques cleared, the patient developed new skin lesions on the face and right elbow. He also reported occasional arthralgia, abdominal cramping, and fatigue. Gastroesophageal reflux disease was the only known preexisting condition and had been treated with pantoprazole for several years. The personal and family medical history was free of autoimmune diseases.
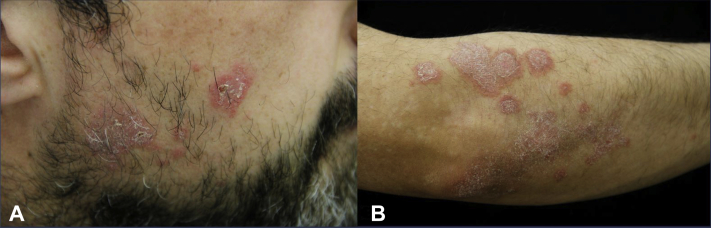
Skin examination revealed multiple, 0.5- to 2.0-cm2, sharply defined, annular, slightly elevated, scaly, erythematous plaques on both cheeks and the right elbow (Fig 1). Psoriatic well-demarcated red plaques with fine scales were apparent on the elbows and knees, and postinflammatory hyperemic hyperpigmented patches were observed on the ventral aspects of both tibiae. Hair, nails, and skin-neighboring mucous membranes were unaffected.
Fig 1.
Secukinumab-induced chronic discoid lupus erythematosus. A, Sharply defined, annular, scaly, erythematous plaques on the patient's right cheek. B, Annular, scaly, erythematous plaques on radial and diffuse, scaly, erythematous, psoriatic plaques on the dorsal aspects of the patient's right elbow.
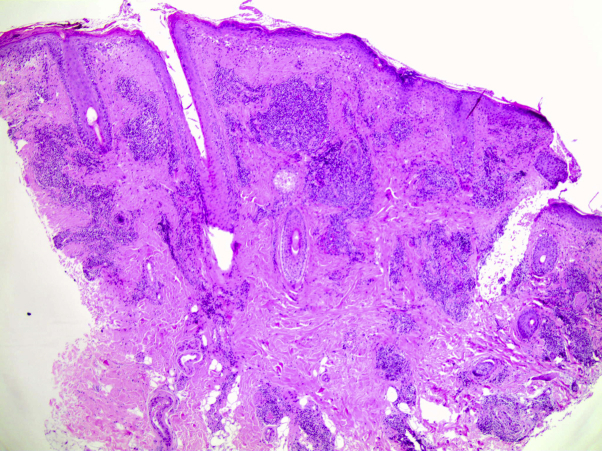
Lesional skin biopsies from the left cheek and right elbow showed compact orthokeratosis and parakeratosis; basal vacuolization; a lymphocytic periadnexal cufflike infiltrate in the dermis with extension into the subcutaneous fat tissue; and mucin between the collagen fibers in the superficial and deep dermis (Fig 2). These findings were compatible with chronic discoid lupus erythematosus.
Fig 2.
Secukinumab-induced chronic discoid lupus erythematosus. Biopsy specimen from the radial aspect of the right elbow. Interface dermatitis with vacuolar degeneration of the basal keratinocytes. Lymphocytic periadnexal infiltrate and mucin deposits in superficial and deep dermis. (Hematoxylin-eosin stain.)
An extensive laboratory and imaging evaluation (chest radiograph, abdomen sonography, and echocardiography) revealed no abnormalities. The antinuclear antibodies were slightly elevated, at 1:160 (normal <1:80). Anti–double-stranded DNA, antihistone, anti-Ro/Sjögren's syndrome A, anti-La/Sjögren's syndrome B, and anti-extractable nuclear antigen antibodies showed negative results, and no depletion of complement C3 and C4 was observed.
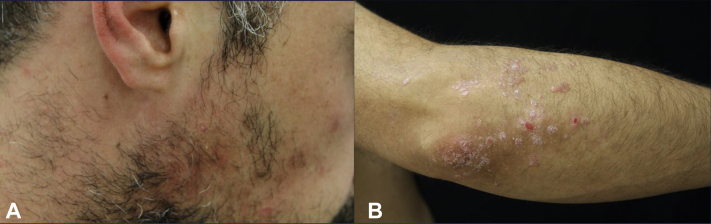
From the clinical, histopathologic, and laboratory findings, as well as the patient's medical history, we concluded a diagnosis of a secukinumab-induced chronic discoid lupus erythematosus. Secukinumab was discontinued and topical treatment with mometasone furoate cream at 1 mg/g for lesions on the body and prednicarbate cream at 2.5 mg/g for those on the facial areas was initiated. The patient was advised to avoid sun exposure. Because a few reports suggest that proton-pump inhibitors1,2 may trigger lupus erythematosus, switching of pantoprazole to ranitidine was also recommended. Approximately 8 weeks later, the lupus lesions cleared but an increase in psoriatic plaques on the elbows, knees, and lower portion of the legs was observed (Fig 3). One more skin biopsy was taken from the patient's left knee. The histologic finding was compatible with psoriasis and showed no signs of lupus erythematosus.
Fig 3.
Secukinumab-induced chronic discoid lupus erythematosus. A, Resolution of the lupus lesions on the right cheek. B, Improvement of lupus lesions and aggravation of psoriatic plaques on the patient's right elbow after secukinumab discontinuation.
Discussion
Like idiopathic lupus, drug-induced lupus erythematosus is clinically classified into systemic and cutaneous lupus erythematosus.1,2 Because there are no established diagnostic criteria for drug-induced lupus erythematosus, temporal relationship with the drug (eg, onset of symptoms after drug initiation, continuous drug exposure, resolution of symptoms on drug discontinuation) and absence of an autoimmune disease, especially lupus erythematosus, are crucial for the diagnosis.1,2 Drugs most commonly associated with drug-induced lupus erythematosus include hydralazine, procainamide, quinidine, isoniazid, minocycline, and targeted immunotherapy.2
Recently, there has been an increase in the use of biologics for different indications. Although relatively rare, tumor necrosis factor–α antagonists are found to be associated with drug-induced lupus erythematosus, also referred to as tumor necrosis factor–α antagonist-induced lupuslike syndrome in medical literature.1,2
To our knowledge, there has been only 1 previous case report on secukinumab-induced subacute cutaneous lupus erythematosus.3 The exact pathomechanism of this type of lupus erythematosus is unknown. T-helper–type 17 cells can promote inflammation through IL-17A, IL-17F, tumor necrosis factor–α, IL-6, IL-21, and IL-22 secretion.4 These cytokines may lead to manifestation of lupus erythematosus because they serve as local inflammatory and tissue-damaging factors with stimulatory effects on B cells.4 Therefore, drug-induced lupus erythematosus using IL-17 inhibition may be regarded as an unexpected paradoxic reaction. On the other hand, recent data demonstrate that IL-17A may also have a protective effect against inflammation.5 One hypothesis is that the IL-17 inhibition is causing changes to the antimicrobial peptides and microbiome, as well as skin and mucosal immunity,6 with consequently increased incidence of infections. This may induce polyclonal B lymphocyte activation and promote autoantibody production.2 Also, a genetic predisposition for drug-induced lupus erythematosus may be considered.
In summary, drug-induced lupus erythematosus can also occur in psoriasis patients receiving treatment with an IL-17 inhibitor. In these cases, the implicated drug should be discontinued. The role of the IL-17 pathway and its inhibition in the pathogenesis of drug-induced lupus erythematosus will need further research.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
Presented at the 113th meeting of the Schleswig-Holstein Dermatologists, Lübeck, Germany, May 22, 2019.
References
- 1.Marzano A., Vezzoli P., Crosti C. Drug-induced lupus: an update on its dermatologic aspects. Lupus. 2009;18:935–940. doi: 10.1177/0961203309106176. [DOI] [PubMed] [Google Scholar]
- 2.Vedove C.D., Simon J.C., Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents. J Dtsch Dermatol Ges. 2012;10:889–897. doi: 10.1111/j.1610-0387.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wehrmann C., Sondermann W., Körber A. Secukinumab-induzierter subakut-kutaner Lupus erythematodes. Hautarzt. 2018;69:64–66. doi: 10.1007/s00105-017-4071-8. [DOI] [PubMed] [Google Scholar]
- 4.Nalbandian A., Crispín J.C., Tsokos G.C. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol. 2009;157(2):209–215. doi: 10.1111/j.1365-2249.2009.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor W., Kamanaka M., Booth C.J. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolls J.K., McCray P.B., Chan Y.R. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8(11):829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]