Abstract
Objective
The mechanism underlying the fatigue of football players is closely related to the energy depletion and accumulation of metabolites; the present study tries to explore the metabolic mechanism in teenage football players during exercise-induced fatigue.
Methods
12 teenage football players were subjected to three groups of combined training by using a cycle ergometer, with the subjective Rating of Perceived Exertion (RPE) as a fatigue criterion. The following indicators were measured in each group after training: maximum oxygen uptake (VO2max), anaerobic power, and average anaerobic power. Urine samples were collected before and after the training. Gas chromatography-mass spectrometry (GC-MS) was performed for the metabonomics analysis of the samples. The metabolism data was analyzed by using principal component analysis (PCA) and orthogonal partial least squares analysis (OPLS-DA), through the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to confirm the potential differences between metabolites, and the MetPA database was used to analyze the related metabolic pathways.
Results
There was no significant difference between the maximal oxygen uptakes among the three groups. Compared with group 1, the maximum and average anaerobic power in group 3 significantly decreased (p < 0.05) at the end of training. GC-MS detected 635 metabolites in the urine samples. Through PCA, OPLS-DA analysis, and KEGG matching, 25 different metabolites (3↑22↓) that met the conditions were finally selected. These different metabolites belonged to 5 metabolic pathways: glycine-serine-threonine metabolism, citrate cycle, tyrosine metabolism, nitrogen metabolism, and glycerophospholipid metabolism.
Conclusions
During the combined exercise of aerobic and anaerobic metabolism, teenage football players show a significant decrease in anaerobic capacity after fatigue. The metabolic mechanism of exercise fatigue was related to disorders in amino acid and energy metabolism.
1. Introduction
Exercise-induced fatigue refers to the inability of the body to maintain the predetermined exercise intensity, causing a temporary decline of exercise capacity. But, after appropriate rest and adjustment, the body can return to the original exercise capacity [1]. The improvement of an athlete's training level is the virtuous circle of “fatigue-recovery.” If exercise-induced fatigue cannot well recover, then the athlete will experience overfatigue or extreme fatigue, which can affect exercise ability and health. Therefore, monitoring fatigue is important. Achieving the desired state of fatigue after exercise is needed for scientific training. Additionally, among the commonly used methods of detecting exercise-induced fatigue, recent studies have used metabonomics to examine the metabolic characteristics of human exercise [2–4]. Metabolites can be produced at every level of an organism's cells, organelles, tissues, organs, body fluids, etc. [5] Thus, every physiological activity in the body has to be regulated and influenced by metabolites. Metabolomics is a systematic biological approach to the study of metabolites in the body, focusing on the metabolism of small molecules in biological organisms. Metabonomics research can screen differential markers and examine metabolic pathways and mechanisms. For the metabonomics sample selection, blood and urine are typically used in human tests because of the convenience and noninvasiveness of sampling [6, 7].
Football match is a long-time high-intensity exercise. From the energy metabolism perspective, the energy metabolism of aerobic exercise and anaerobic exercise was combined. Therefore, the mechanism underlying the fatigue of football players is closely related to the energy depletion and accumulation of metabolites. For teenage athletes, their bodies have exuberant metabolism but easily get fatigued. Therefore, the monitoring of fatigue in this age group has practical use in guiding scientific training. The use of a treadmill and a cycle ergometer is appropriate for handling training and establishing a scientific research model. Many studies have used this method as a training model for studying the various exercise intensities of children and adolescents [8–10]. However, there have been several reports on metabolomics studies of adolescents after exercise-induced fatigue. Therefore, in the present study, teenage football players with a certain training background were subjected to a combination of aerobic and anaerobic exercises through a cycle ergometer. After exercise-induced fatigue was reached, urine samples were collected and tested by GC-MS to identify metabolic differences and explore the metabolic mechanism of exercise-induced fatigue.
2. Methods
2.1. Training Methods
12 male teenage football players from Shaanxi Sports School volunteered to participate in the experiment, and the written informed consents were obtained from their guardians. The tests were performed at 3:00 pm on March 6, 2018. There was no fatigue accumulation on the athletes. 30 min of warming up was permitted. According to the age of the participants, the experimental training model refers to the relevant high-intensity interval training of children and adolescents, and appropriate adjustments were made [8–10].
An alternating exercise of aerobic cycle ergometer (Ergoselect 100 k, Germany) and anaerobic cycle ergometer (MAX-VII, Japan) was used. Each training group included aerobic exercise→rest for 1 min→anaerobic exercise→rest for 3 min, performed in a total of three training times; the aerobic cycle ergometer using the Astrand-Ryhming aerobic exercise mode: 55~60 rpm/min speed for 6 min, and the load was 150 w [11]; and Wingate anaerobic exercise mode: each subject ridded the anaerobic cycle ergometer at fastest speed for 30 s, and each load was set according to their weight according to the direction of Wingate exercise mode [12]. The real-time heart rate was detected by wearing a polar heart rate monitor (RS800sd, Finland). VO2max was counted according to the heart rate at 5 min and 6 min after aerobic exercise, while the maximum anaerobic power, average anaerobic power, and RPE were recorded immediately after training. Fatigue criteria: RPE grades 16 to 19 as the fatigue range, with positive urine protein and urinary gallbladder at 3 h after exercise, and urine protein and urinary gallstones content recovery the next morning to determine exercise-induced fatigue [13–15]. This study was reviewed and approved by Special Committee on Scientific Ethics of Shaanxi Normal University.
2.2. Urine Samples
To eliminate interference, such as diet and physical activity, on the day before the experiment, the 12 subjects were provided unified diets and were not permitted to take drugs, tobacco, or alcohol. Dietary standards follow the nutrient intake standard for Chinese young athletes. Mealtime: 7:00-8:00 (breakfast), 12:00-13:00 (lunch), and 18:00-19:00 (dinner). Routine activities and nonintensive exercise were permitted. Urine sampling occurred at two times. The first sampling occurred at 30 min before exercise (preexercise), while the second sampling occurred at 3 h after exercise (postexercise). The urine samples were collected in a 5 mL lidded centrifuge tube, at a sample size of no less than 2 mL. Subsequently, the urine samples were placed in liquid nitrogen cryogenic storage.
2.3. GC-MS Assay
A 100 μL aliquot of the urine sample was added to 20 μL of urease and incubated at 37°C for 1 h. Subsequently, 0.35 mL of methanol and 20 μL of L-2-chlorophenylalanine (internal standard) were added, followed by vortexing and centrifugation (13000 rpm, 15 min) at 4°C. Then, 0.39 mL of the supernatant was removed and placed in a 2 mL vial, dried in a vacuum concentrator, mixed with 80 μL of methoxyamine salt reagent, and incubated at 80°C for 30 min; 100 μL of N, O-bis (trimethylsilyl) trifluoroacetamide (BSTFA) was rapidly added to the sample, and the mixture was incubated at 70°C for 120 min. After cooling to room temperature, 10 μL of saturated fatty acid methyl ester standard mixture was added, and the sample was mixed well. After machine test, the Agilent 7890 GC-TOF-MS, equipped with an Agilent DB-5MS capillary column (30 m × 250 μm × 0.25 μm, J & W Scientific, Folsom, CA, USA), was used. The following conditions were implemented for the GC-TOF-MS analysis: injection volume 1 μL; splitless mode; forward sample flow purge flow rate 3 mL/min−1; column flow rate 1 mL/min, at a rate of 10°C per minute up to 300°C for 7 minutes; an inlet temperature of 280°C; an ion source temperature of 220°C; and a scanning mode of 50-500 m/z.
2.4. Multidimensional Statistical Analysis
The data of height, weight, and BMI were detected by Shapiro-Wilk test of the SPSS 20.0 to confirm normal distribution, and repeated analysis of general linear model (GLM) was performed on the data of the VO2max, maximum anaerobic power, and average anaerobic power. The results were expressed as the means ± standard deviation (−x ± s) with a significance level of p < 0.05.
The Chroma TOF4.3X software (LECO) was used to identify, match, filter out noise, correct baseline, peak alignment, deconvoluted spectra, peak quantify, and quantify the mass spectra of the samples. Internal standard normalization was adopted to reduce the variation in the sample concentrations. Subsequently, multivariate pattern recognition analysis of the normalized data was performed by using SIMCA software (V14, Umetrics AB, Umea, Sweden). Principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were used to process the complex data obtained between the discrete degrees of the score chart to achieve statistical analysis of the data of each group. All compounds were screened for potential differential metabolites through the Kyoto Encyclopedia of Genes and Genomes (KEGG). The three screening criteria were performed as the previous report: (1) the similarity of the metabolites was greater than 700 when compared to the corresponding peak in KEGG database [16]. (2) The variable importance in the projection (VIP value) of the metabolite was greater than 1. (3) p value of the metabolite content was less than 0.05 after Paired-Samples t-test. Finally, the selected differential metabolites were entered into the MetPA database (http://www.metaboanalyst.ca) to analyze the influence weights of the corresponding metabolic pathways on screening with a threshold value of more than 0.05, and filtered out after exercise fatigue main metabolic pathways.
3. Results
3.1. Comparison of Some Physiological Indicators
The basic information of the participants was shown in Table 1. The teenage football players were trained for 3 to 4 years, and their physical qualities were better. The data of height, weight, and BMI were normal distribution after Shapiro-Wilk test, and there were no outliers and extreme values in them.
Table 1.
Basic information of the exercise participants (n = 12).
| Age (years) | Training time (years) | Height (m) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| 14−16 | 3−4 | 1.68 ± 0.04 | 55.31 ± 2.97 | 19.65 ± 1.01 |
The changes in the physiological indicators of teenage football players during training were shown in Figure 1; the maximum anaerobic power and average anaerobic power were significantly decreased in group 3 compared with those in group 1 (p < 0.05). The changes in other indicators were not obvious. No significant difference was found between the other groups. At the end of each training, the individual RPE reports for each subject ranged from 16 to 19 levels. In addition, urinary protein and urobilinogen showed positive or weak positive results at 3 h after exercise. However, the results of the morning urine test showed that 11 subjects returned to negative results and were determined to have exercise-induced fatigue. One subject did not recover, which may lead to overfatigue. Therefore, in the GC-MS test, the postexercise urine samples for 11 members were tested.
Figure 1.
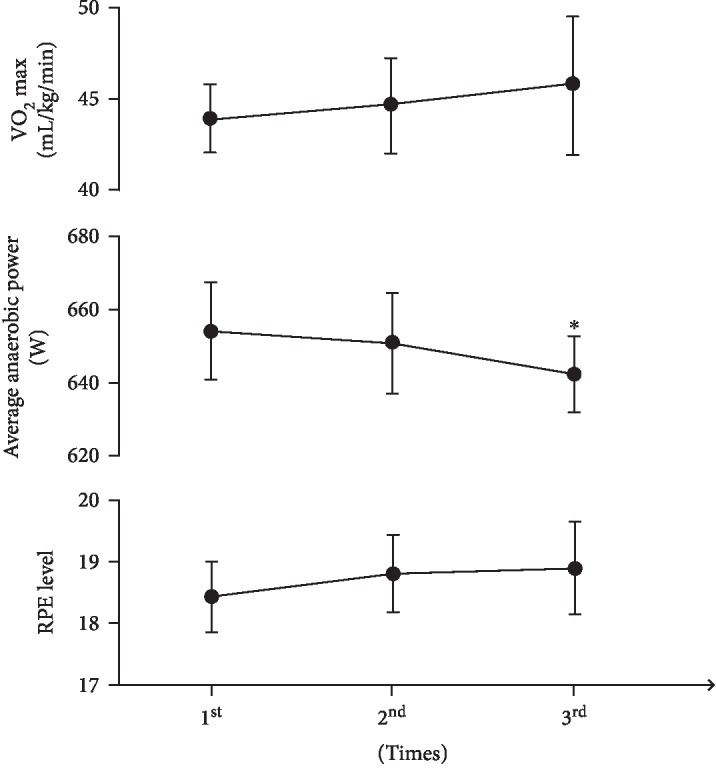
Results of some physiological indices of teenage football players in training (n = 12, ∗compared with the 1st group; p < 0.05).
3.2. Test Results by GS-MS
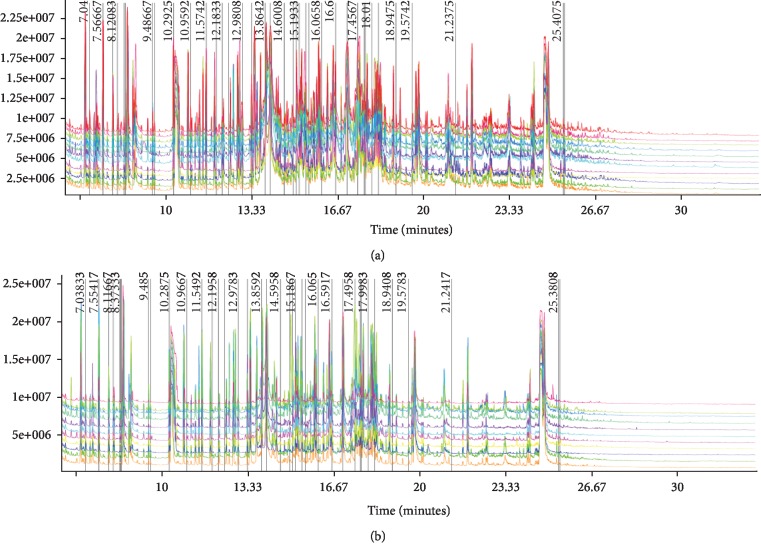
Urine samples were processed and analyzed by GC-MS to obtain a total ion chromatogram (TIC) as shown in Figure 2. The results showed the total ion chromatogram for the urine samples. A total of 635 peaks were detected before and after training. There is a marked difference between the two groups, indicating that teenage football players had more differences in metabolites before and after training. Therefore, further analysis is necessary. In addition, the chromatograms of total ions in the two groups are neat, and the reproduction of the retention time is good, indicating that the GC-MS instrument used in the present study has high stability and reliability.
Figure 2.
Total ion chromatograms of GC-MS detection. (a) Preexercise. (b) Postexercise.
3.3. PCA and OPLS-DA Analyses of the Urine Metabolites
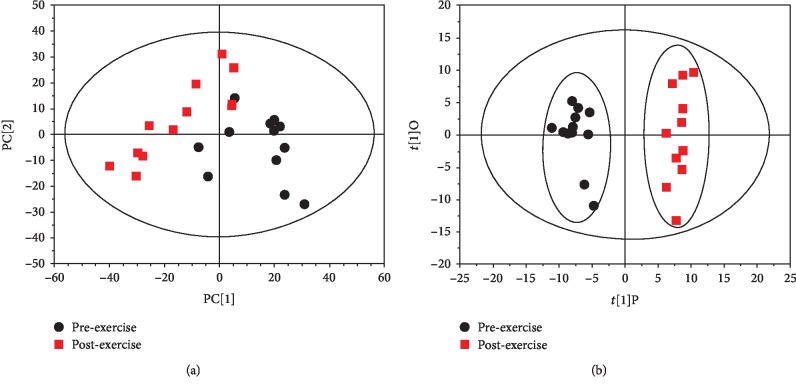
The 635 peaks obtained above were normalized and filtered. The PCA analysis and OPLS-DA analysis of the normalized data by SIMCA software are shown in Figure 3. The PCA score is an overall presentation of the sample distribution of the raw data. Each point represents a sample, and the coordinates of the sample in the figure are determined by the sample composition, that is, the differences in sample distribution are determined by the differences of composition. The score chart (Figure 3(a)) shows that the scores of preexercise samples are distributed in quadrants I, III, and IV. The scores of postexercise are distributed in quadrants I, II and III. A crossover phenomenon exists between the two samples, and the samples should be further subjected to OPLS-DA analysis to filter out the irrelevant orthogonal signal since the resulting differential metabolites were more reliable. The present study used the VIP (Variable Importance in the Projection) value (threshold > 1) of the first principal component of the OPLS-DA model to determine the differential expression combined with the p value of the t-test (threshold 0.05) to mark the different metabolites. As shown in the OPLS-DA score chart, the preexercise scores are distributed in quadrants II and III, while the OPLS-DA scores after exercise are distributed in quadrants I and IV (Figure 3(b)). The separation is clearly obvious. The OPLS-DA score can well reflect the similarity of the urine in the group and the sample differences between the groups, indicating a marked difference in the urine metabolites before and after training. Additionally, the model was validated using a permutation test. As shown in Figure 4, R2 Y and Q2 of the OPLS-DA model were 0.889 and 0.607, respectively, indicating that the model was both reliable and predictive.
Figure 3.
PCA and OPLS-DA analyses of urine metabolites. (a) PCA score. (b) OPLS-DA score.
Figure 4.
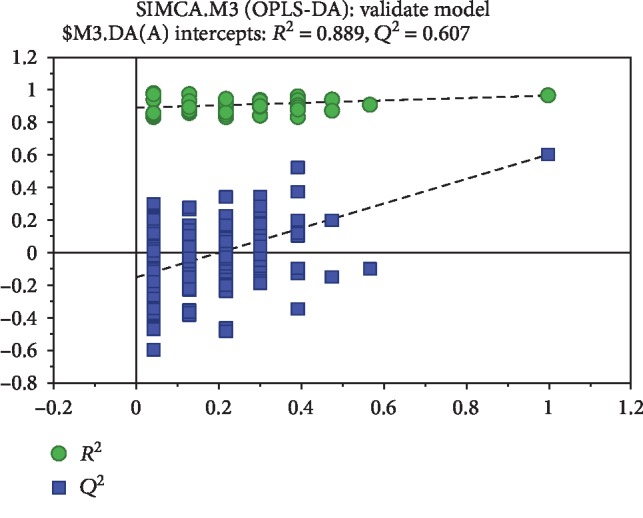
Validation of OPLS-DA model by permutation test.
3.4. Potential Differences in the Screening of Metabolites
By using OPLS-DA analysis, all compounds were screened for differential metabolites through the KEGG database. 25 differential metabolites were identified (VIP > 1, p < 0.05, and Similarity > 700). As shown in Table 2, these differential metabolites are mainly involved in amino acid metabolism and energy metabolism. After fatigue, hydroxylamine, citric acid, and sorbitol showed a significant increase (p < 0.05 or p < 0.01) compared with that before training. These three metabolites are related to nitrogen metabolism, tricarboxylic acid metabolism, and galactose metabolism. The other remaining 22 differential metabolites were significantly decreased after exercise.
Table 2.
Information of the selected differential metabolites after training.
| No. | t R (min) | Similarity | VIP | KEGG | Metabolites | Molecular formula | p value | Fold changea | Trendb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.04 | 901 | 1.6 | C02502 | 2-Hydroxypyridine | C5H5NO | 9.334E-03 | 1.387 | ↓∗∗ |
| 2 | 7.56 | 939 | 1.3 | C00160 | Glycolic acid | C2H4O3 | 3.388E-02 | 1.584 | ↓∗ |
| 3 | 8.12 | 942 | 1.5 | C00192 | Hydroxylamine | NH3O | 6.903E-03 | 0.756 | ↑∗∗ |
| 4 | 8.44 | 869 | 1.6 | C00213 | Sarcosine | C3H7NO2 | 2.891E-03 | 1.510 | ↓∗∗ |
| 5 | 10.29 | 867 | 1.7 | C00189 | Ethanolamine | C2H7NO | 1.148E-03 | 1.804 | ↓∗∗ |
| 6 | 10.97 | 822 | 2.0 | C00042 | Succinic acid | C4H6O4 | 7.766E-05 | 3.262 | ↓∗∗∗ |
| 7 | 11.56 | 907 | 1.2 | C00065 | Serine | C3H7NO3 | 3.582E-02 | 1.575 | ↓∗ |
| 8 | 11.96 | 886 | 1.7 | C05519 | L-Allothreonine | C4H9NO3 | 1.076E-03 | 2.272 | ↓∗∗ |
| 9 | 12.18 | 747 | 1.2 | C00489 | Glutaric acid | C5H8O4 | 1.358E-04 | 4.344 | ↓∗∗∗ |
| 10 | 12.98 | 834 | 1.0 | C00872 | Aminomalonic acid | C3H5NO4 | 1.485E-04 | 3.351 | ↓∗∗∗ |
| 11 | 13.87 | 772 | 1.5 | C01108 | Pyrogallol | C6H6O3 | 2.651E-02 | 2.296 | ↓∗ |
| 12 | 14.07 | 797 | 1.8 | C00300 | Creatine | C4H9N3O2 | 4.041E-03 | 3.987 | ↓∗∗ |
| 13 | 14.60 | 892 | 1.8 | C03761 | 3-Hydroxy-3-methylglutaric acid | C6H10O5 | 3.848E-04 | 2.108 | ↓∗∗∗ |
| 14 | 14.95 | 855 | 1.7 | C00156 | 4-Hydroxybenzoic acid | C7H6O3 | 3.561E-03 | 2.170 | ↓∗∗ |
| 15 | 15.08 | 792 | 1.7 | C00642 | 4-Hydroxyphenylacetic acid | C8H8O3 | 1.853E-03 | 2.369 | ↓∗∗ |
| 16 | 15.44 | 871 | 1.6 | C08353 | Ribose | C5H10O5 | 8.630E-04 | 1.790 | ↓∗∗∗ |
| 17 | 15.57 | 736 | 1.8 | C03139 | Guanidinosuccinic acid | C5H9N3O4 | 1.532E-04 | 2.247 | ↓∗∗∗ |
| 18 | 16.07 | 882 | 1.8 | C03722 | Quinolinic acid | C7H5NO4 | 5.011E-04 | 2.031 | ↓∗∗∗ |
| 19 | 16.60 | 746 | 1.6 | C11527 | 4-Hydroxymandelic acid | C8H8O4 | 4.214E-03 | 1.823 | ↓∗∗ |
| 20 | 17.03 | 867 | 1.3 | C00158 | Citric acid | C6H8O7 | 4.131E-02 | 0.518 | ↑∗ |
| 21 | 17.70 | 895 | 1.2 | C00198 | Gluconic lactone | C6H10O6 | 2.088E-02 | 1.383 | ↓∗ |
| 22 | 17.75 | 910 | 1.8 | C00031 | Glucose | C6H12O6 | 3.691E-03 | 3.907 | ↓∗∗ |
| 23 | 18.01 | 724 | 1.3 | C00159 | Mannose | C6H12O6 | 1.309E-02 | 1.435 | ↓∗ |
| 24 | 18.26 | 797 | 1.6 | C00794 | Sorbitol | C6H14O6 | 4.359E-02 | 0.392 | ↑∗ |
| 25 | 21.24 | 758 | 1.5 | C02470 | Xanthurenic acid | C10H7NO4 | 7.551E-06 | 5.567 | ↓∗∗∗ |
Fold changea refers to the ratio of the average metabolite level in postexercise group relative to that in preexercise group.
Trendb refers to the changed trend of the average metabolite level in postexercise group relative to that in preexercise group. ↑: increase; ↓: decrease; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
3.5. Metabolic Pathway Analysis
The above results showed 25 different metabolites involved in many metabolic pathways. In order to identify possible metabolic pathways affected by intensive training, the biomarkers mentioned above were analyzed through MetPA (http://www.metaboanalyst.ca/). Subsequently, their pathway impact values were calculated via a pathway topology analysis. The selected criterion of different metabolites were the impact scores > 0.05 and −log (p) > 1 [17, 18]. The selected metabolic pathway is a potential target pathway for exercise-induced fatigue in teenage soccer players. As shown in Table 3 and Figure 5, in accordance with the screening criteria, 5 metabolic pathways were detected: glycine-serine-threonine metabolism, tricarboxylic acid cycle, tyrosine metabolism, nitrogen metabolism, and glycerophosphate metabolism. This finding suggests that the exercise-induced fatigue of teenage football players is due to the disorder among the five metabolic pathways described above.
Table 3.
Impact scores for metabolic pathways after exercise.
| Pathway name | Match status | p | −log(p) | FDR | Impact | Resultsa |
|---|---|---|---|---|---|---|
| Glycine, serine, and threonine metabolism | 4/48 | 0.001 | 6.97 | 0.07 | 0.187 | √ |
| Glyoxylate and dicarboxylate metabolism | 3/50 | 0.011 | 4.49 | 0.29 | 0.010 | |
| Citrate cycle (TCA cycle) | 2/20 | 0.015 | 4.20 | 0.29 | 0.078 | √ |
| Pentose phosphate pathway | 2/32 | 0.036 | 3.31 | 0.58 | 0.043 | |
| Galactose metabolism | 2/41 | 0.057 | 2.86 | 0.76 | 0.002 | |
| Tyrosine metabolism | 2/76 | 0.163 | 1.81 | 1.00 | 0.059 | √ |
| Arginine and proline metabolism | 2/77 | 0.166 | 1.79 | 1.00 | 0.038 | |
| Methane metabolism | 1/34 | 0.280 | 1.27 | 1.00 | 0.017 | |
| Propanoate metabolism | 1/35 | 0.287 | 1.24 | 1.00 | 0.001 | |
| Ubiquinone and other terpenoid-quinone biosynthesis | 1/36 | 0.294 | 1.22 | 1.00 | 0.030 | |
| Glycerophospholipid metabolism | 1/39 | 0.314 | 1.15 | 1.00 | 0.056 | √ |
| Nitrogen metabolism | 1/39 | 0.314 | 1.15 | 1.00 | 0.060 | √ |
| Butanoate metabolism | 1/40 | 0.321 | 1.13 | 1.00 | 0.017 | |
| Nicotinate and nicotinamide metabolism | 1/44 | 0.347 | 1.05 | 1.00 | 0.024 | |
| Lysine degradation | 1/47 | 0.366 | 1.00 | 1.00 | 0.065 | |
| Fructose and mannose metabolism | 1/48 | 0.372 | 0.98 | 1.00 | 0.008 | |
| Starch and sucrose metabolism | 1/50 | 0.384 | 0.95 | 1.00 | 0.017 | |
| Cysteine and methionine metabolism | 1/56 | 0.419 | 0.86 | 1.00 | 0.012 | |
| Aminoacyl-tRNA biosynthesis | 1/75 | 0.519 | 0.65 | 1.00 | 0.056 |
aSelected criterion was the impact scores > 0.05 and −log (p) > 1.
Figure 5.
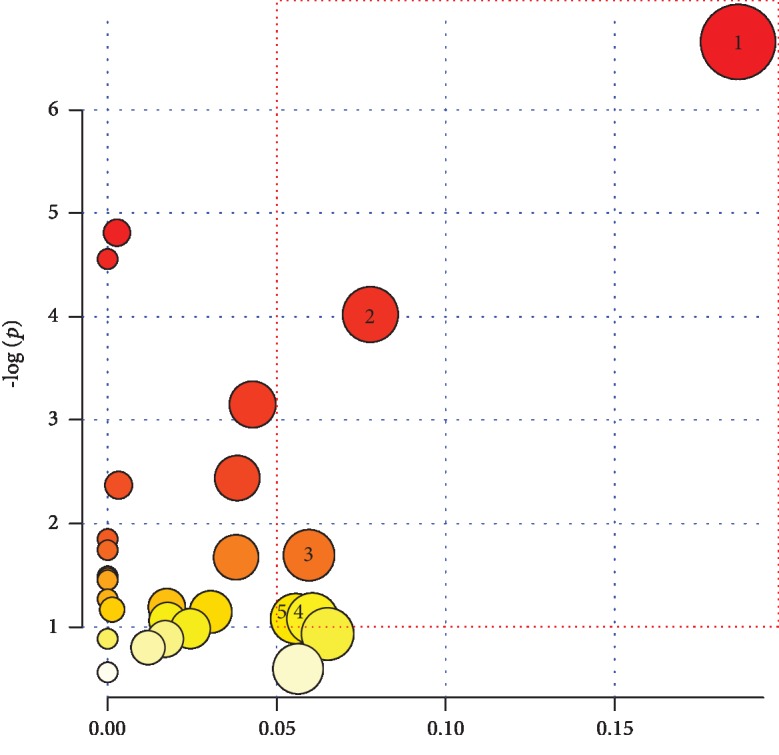
The impact score of the metabolic pathways (1: glycine, serine, and threonine metabolism; 2: citrate cycle; 3: tyrosine metabolism; 4: nitrogen metabolism; 5: glycerophospholipid metabolism).
4. Discussion
From an energy metabolism perspective, football is a combination exercise of aerobic metabolism and anaerobic metabolism. Football has high requirements on the aerobic capacity and anaerobic capacity of an athlete. Therefore, it is important to strengthen the comprehensive metabolic ability of the athlete in training. To explore the variation characteristics of differential metabolites and metabolic pathways after exercise-induced fatigue in teenage soccer players, the present study designed a cycle ergometer training model combining aerobic exercise and anaerobic exercise. The results showed that the VO2max of teenage football players remained at a constant level during the three training sessions, but the maximum anaerobic power and average anaerobic power showed a decreasing trend. The third group significantly differs from the first group. The heart rate and RPE reports showed that the athletes had achieved a tired level. According to urinary protein and urobilinogen tests, 11 athletes reached the state of exercise-induced fatigue. Because 1 person experienced extreme fatigue, only 11 samples were used for the urine metabolomics GC-MS detection.
Metabolomics is currently at the forefront of biological detection methods, and many studies use metabolism in sports training, including aerobic exercise [19, 20], anaerobic exercise [21, 22], and physical activity in the general population [23, 24]. From the perspective of the kinetics model, relevant metabolic pathways, HIIT, and changes in bioenergetic metabolic pathways, such as lipid metabolism, protein metabolism, and tricarboxylic acid cycling, are analyzed [25]. However, moderate-intensity continuous training does not show any changes in these metabolic pathways [26]. Therefore, exercise intensity may be the key factor that causes changes in the metabolic pathways of the athletes. The exercise model designed in the present study is a combination of aerobic exercise and anaerobic exercise. This model belongs to high-intensity gap training. After training, the athlete experiences fatigue. The experimental results show that there are 5 metabolic pathways in teenage football players after exercise: glycine-serine-threonine metabolism, tricarboxylic acid metabolism, tyrosine metabolism, nitrogen metabolism, and glycerol phospholipid metabolism. These results can be summarized as three types of metabolism: protein metabolism, tricarboxylic acid cycle metabolism, and lipid metabolism. This result is similar to that of Radom-Aizik et al. [27]
In high-intensity training, sugar and glycogen metabolism is the main energy sources for body movement, but with the extension of exercise time, some variable proteins and amino acids are also involved in energy metabolism to maintain constant exercise intensity [1]. Some studies have found that the urinary levels of histidine and glycine were decreased after exercise in weightlifters, and the levels of histidine and tyrosine in middle- and long-distance runners were also decreased [28]. After 14-18 years of age in power cycling, the contents of glycine-serine-threonine, branched chain amino acids, arginine-proline, glutamic acid-alanine-aspartic acid, etc. were decreased in the urine [27]. The above studies show that different items, different ages, and other factors have different effects on the amino acid metabolism after exercise. These results also showed that in the glycine-serine-threonine metabolic pathway, the four metabolites creatine, L-threonine, creatine, and serine significantly decreased after exercise. In the tyrosine metabolic pathway, amber acid and 4-hydroxyphenylacetic acid were significantly decreased. In the nitrogen metabolism pathway, the hydroxylamine content was significantly increased. These results indicate that amino acid and protein metabolism are increased in athletes during the high-intensity gap training in this experiment. The results of urinary protein and urobilinogen tests and the results of nitrogen metabolism after exercise showed a certain degree of “negative nitrogen” in the athletes, resulting from fatigue after exercise.
The tricarboxylic acid cycle is essential for the catabolism of carbohydrates, fats, and protein. This metabolic pathway not only provides the energy needed for body movement but also provides intermediates for the biosynthesis of many substances [29]. A study conducted by Suzhou University found that the succinate content was decreased and that the citric acid content was increased in athletes after long-distance running, while these parameters did not significantly change in athletes after weight-lifting exercise [28]. The results of the present study showed that succinic acid, a significant metabolite associated with the tricarboxylic acid cycle, was significantly decreased and that citric acid was significantly increased, indicating that exercise-induced fatigue led to the disorder of the tricarboxylic acid cycle in the body, consistent with the results of the above study.
Fatty acid metabolism is the main mechanism of fat metabolism during exercise. Fatty acids are important energy substances in the body. Fatty acid metabolism is closely related to long-term exercise and moderate and low exercise intensity [30]. However, the results of the present study only detected a significant decrease in the content of ethanolamine, a metabolite related to the metabolic pathway of glycerophosphate. Glycerophospholipids are the most abundant types of phospholipids in the body. These compounds constitute biofilms are components of bile and membrane surface-active substances and participate in the recognition and signal transduction of proteins by the cell membrane [31]. A previous study found that acute high-intensity exercise can cause changes in glycerophospholipid metabolism, thus affecting the function of the cell membrane [32]. The experiment showed that disorders of metabolic pathway of glycerol ester are related to the high-intensity training.
5. Conclusions
The present study used a cycle ergometer to establish a combined training model of aerobic exercise and anaerobic exercise to ultimately achieve the body fatigue state of teenage football players after exercise. Differential metabolites and related metabolic pathways showed that the metabolic mechanism of exercise-induced fatigue in teenage football players was related to the metabolism of glycine-serine-threonine, tricarboxylic acid cycle, tyrosine metabolism, nitrogen metabolism, and glycerophosphate metabolism. In addition, a variety of the differential metabolites detected here can be used as urinalysis biomarkers for exercise-induced fatigue in teenage football players. The above findings have practical applications in managing the exercise-induced fatigue of football players in this age group and monitoring scientific training.
Acknowledgments
We thank Huanhuan Qin (Bio-tree Biotech Co. Ltd., Shanghai, China) for her valuable suggestions. This research was supported by the National Natural Science Foundation of China (No. 31871209).
Data Availability
All data generated or used during the study appear in the submitted article. Some raw data generated or used during the study are available from the corresponding author by request. (List items: Table 2 and Figure 1)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.St Clair Gibson A., Swart J., Tucker R. The interaction of psychological and physiological homeostatic drives and role of general control principles in the regulation of physiological systems, exercise and the fatigue process - the Integrative Governor theory. European Journal of Sport Science. 2018;18(1):25–36. doi: 10.1080/17461391.2017.1321688. [DOI] [PubMed] [Google Scholar]
- 2.Alves R. D. A. M., Dane A. D., Harms A., et al. Global profiling of the muscle metabolome: method optimization, validation and application to determine exercise-induced metabolic effects. Metabolomics. 2015;11(2):271–285. doi: 10.1007/s11306-014-0701-7. [DOI] [Google Scholar]
- 3.Jang H. J., Kim D. M., Kim K. B., et al. Analysis of metabolomic patterns in thoroughbreds before and after exercise. Asian-Australasian Journal of Animal Sciences. 2017;30(11):1633–1642. doi: 10.5713/ajas.17.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messier F. M., Le Moyec L., Santi C., et al. The impact of moderate altitude on exercise metabolism in recreational sportsmen: a nuclear magnetic resonance metabolomic approach. Applied Physiology, Nutrition, and Metabolism. 2017;42(11):1135–1141. doi: 10.1139/apnm-2016-0717. [DOI] [PubMed] [Google Scholar]
- 5.Lindon J. C., Holmes E., Bollard M. E., Stanley E. G., Nicholson J. K. Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers. 2004;9(1):1–31. doi: 10.1080/13547500410001668379. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Lorenzo M., Gonzalez-Calero L., Ramos-Barron A., et al. Urine metabolomics insight into acute kidney injury point to oxidative stress disruptions in energy generation and H2S availability. Journal of Molecular Medicine. 2017;95(12):1399–1409. doi: 10.1007/s00109-017-1594-5. [DOI] [PubMed] [Google Scholar]
- 7.Dykstra M. A., Switzer N., Eisner R., et al. Urine metabolomics as a predictor of patient tolerance and response to adjuvant chemotherapy in colorectal cancer. Molecular and Clinical Oncology. 2017;7(5):767–770. doi: 10.3892/mco.2017.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baquet G., Gamelin F. Ç.-X., Mucci P., Thévenet D., Van Praagh E., Berthoin S. Continuous vs. interval aerobic training in 8- to 11-year-old children. Journal of Strength and Conditioning Research. 2010;24(5):1381–1388. doi: 10.1519/JSC.0b013e3181d1575a. [DOI] [PubMed] [Google Scholar]
- 9.Mann T., Lamberts R. P., Lambert M. I. Methods of prescribing relative exercise intensity: physiological and practical considerations. Sports Medicine. 2013;43(7):613–625. doi: 10.1007/s40279-013-0045-x. [DOI] [PubMed] [Google Scholar]
- 10.Mazurek K., Zmijewski P., Krawczyk K., et al. High intensity interval and moderate continuous cycle training in a physical education programme improves health-related fitness in young females. Biology of Sport. 2016;33(2):139–144. doi: 10.5604/20831862.1198626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namgoong H., Lee D., Hwang M. H., Lee S. The relationship between arterial stiffness and maximal oxygen consumption in healthy young adults. Journal of Exercise Science and Fitness. 2018;16(3):73–77. doi: 10.1016/j.jesf.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stastny P., Tufano J., Kregl J., et al. The role of visual feedback on power output during intermittent Wingate testing in ice hockey players. Sports. 2018;6(2):p. 32. doi: 10.3390/sports6020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorpe R. T., Strudwick A. J., Buchheit M., Atkinson G., Drust B., Gregson W. Tracking morning fatigue status across in-season training weeks in elite soccer players. International Journal of Sports Physiology and Performance. 2016;11(7):947–952. doi: 10.1123/ijspp.2015-0490. [DOI] [PubMed] [Google Scholar]
- 14.Kerhervé H. A., Millet G. Y., Solomon C. The dynamics of speed selection and psycho-physiological load during a mountain ultramarathon. PLoS One. 2015;10(12, article e0145482) doi: 10.1371/journal.pone.0145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faude O., Hecksteden A., Hammes D., et al. Reliability of time-to-exhaustion and selected psycho-physiological variables during constant-load cycling at the maximal lactate steady-state. Applied Physiology, Nutrition, and Metabolism. 2017;42(2):142–147. doi: 10.1139/apnm-2016-0375. [DOI] [PubMed] [Google Scholar]
- 16.Li N., Song Y. p., Tang H., Wang Y. Recent developments in sample preparation and data pre-treatment in metabonomics research. Archives of Biochemistry and Biophysics. 2016;589:4–9. doi: 10.1016/j.abb.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Chi A., Shen Z., Zhu W., Sun Y., Kang Y., Guo F. Characterization of a protein-bound polysaccharide from _Herba Epimedii_ and its metabolic mechanism in chronic fatigue syndrome. Journal of Ethnopharmacology. 2017;203:241–251. doi: 10.1016/j.jep.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Met P. A. Database. https://www.metaboanalyst.ca/
- 19.Danaher J., Gerber T., Wellard R. M., Stathis C. G., Cooke M. B. The use of metabolomics to monitor simultaneous changes in metabolic variables following supramaximal low volume high intensity exercise. Metabolomics. 2016;12(1):p. 7. doi: 10.1007/s11306-015-0883-7. [DOI] [Google Scholar]
- 20.Reinehr T., Wolters B., Knop C., et al. Changes in the serum metabolite profile in obese children with weight loss. European Journal of Nutrition. 2015;54(2):173–181. doi: 10.1007/s00394-014-0698-8. [DOI] [PubMed] [Google Scholar]
- 21.Glynn E. L., Piner L. W., Huffman K. M., et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015;58(10):2324–2335. doi: 10.1007/s00125-015-3705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berton R., Conceição M. S., Libardi C. A., et al. Metabolic time-course response after resistance exercise: a metabolomics approach. Journal of Sports Sciences. 2016;35(12):1211–1218. doi: 10.1080/02640414.2016.1218035. [DOI] [PubMed] [Google Scholar]
- 23.Huffman K. M., Koves T. R., Hubal M. J., et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. 2014;57(11):2282–2295. doi: 10.1007/s00125-014-3343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauschert S., Uhl O., Koletzko B., Hellmuth C. Metabolomic biomarkers for obesity in humans: a short review. Annals of Nutrition & Metabolism. 2014;64(3-4):314–324. doi: 10.1159/000365040. [DOI] [PubMed] [Google Scholar]
- 25.Zafeiridis A., Chatziioannou A. C., Sarivasiliou H., et al. Global metabolic stress of isoeffort continuous and high intensity interval aerobic exercise: a Comparative1H NMR metabonomic study. Journal of Proteome Research. 2016;15(12):4452–4463. doi: 10.1021/acs.jproteome.6b00545. [DOI] [PubMed] [Google Scholar]
- 26.Enea C., Seguin F., Petitpas-Mulliez J., et al. 1H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Analytical and Bioanalytical Chemistry. 2010;396(3):1167–1176. doi: 10.1007/s00216-009-3289-4. [DOI] [PubMed] [Google Scholar]
- 27.Radom-Aizik S., Haddad F., Lu K., et al. Plasma metabolomics in response to an acute bout of exercise in adolescents boys and Girls. Medicine & Science in Sports & Exercise. 2017;49(5s):282–283. doi: 10.1249/01.mss.0000517632.86766.89. [DOI] [Google Scholar]
- 28.Yue X. Study of urine metabonomics based on GC-MS on middle-distance race athletes and weight-lifting athletes [PhD Thesis] Suzhou China: Suzhou University; 2011. [DOI] [Google Scholar]
- 29.Mukherjee K., Edgett B. A., Burrows H. W., et al. Whole blood transcriptomics and urinary metabolomics to define adaptive biochemical pathways of high-intensity exercise in 50-60 year old masters athletes. Plos One. 2014;9(3, article e92031) doi: 10.1371/journal.pone.0092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen M. D. Fate of fatty acids at rest and during exercise: regulatory mechanisms. Acta Physiologica Scandinavica. 2003;178(4):385–390. doi: 10.1046/j.1365-201X.2003.01167.x. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Baro M. R., Coleman R. A. Mitochondrial acyltransferases and glycerophospholipid metabolism. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 2017;1862(1):49–55. doi: 10.1016/j.bbalip.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Schindler J., Kanhere A., Edwards L., et al. Exercise and high-fat feeding remodel transcript-metabolite interactive networks in mouse skeletal muscle. Scientific Reports. 2017;7(1, article 13485) doi: 10.1038/s41598-017-14081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or used during the study appear in the submitted article. Some raw data generated or used during the study are available from the corresponding author by request. (List items: Table 2 and Figure 1)