Abstract
Background
Preventing and reducing substance use disorders, sexually transmitted infections (STIs)/HIV, and teen pregnancy, and the associated risk behaviors (ie, substance use and sexual risk behaviors) among youth remain public health priorities in the United States. Equally important is improving the uptake of STI/HIV testing among the youth. Mobile health (mHealth) apps may be a solution to ameliorate these public health concerns; however, few mHealth preventive interventions have demonstrated efficacy in reducing substance use or sexual risk behaviors or improving the uptake of STI/HIV testing among the youth, particularly in clinic settings.
Objective
This small-scale study aimed to examine the feasibility of conducting a pilot randomized controlled trial (RCT). We evaluated the effects of Storytelling 4 Empowerment (S4E), relative to enhanced usual practice, on the potential mechanisms by which behavior change occurs, namely clinician-youth risk communication, prevention knowledge, and substance use and sexual risk refusal self-efficacy. We also assessed the ability to measure targeted outcomes of past 30-day substance use (ie, alcohol, tobacco, and other drug use), condomless sex, and alcohol or drug use before sex, as well as the uptake of STI/HIV testing.
Methods
Employing community-based participatory research principles, 50 youths aged 13 to 21 years were recruited from a youth-centered community health clinic in Southeast Michigan, randomized sequentially to either S4E or enhanced usual practice, and assessed at baseline, immediately postintervention, and 30 days postintervention. S4E consists of 3 modules, including alcohol and drug use, tobacco, and STI/HIV.
Results
Relative to youth in the enhanced usual practice group, S4E participants demonstrated higher youth-clinician risk communication (mean 3.22, SD 1.67) and increases in prevention knowledge (∆ score mean 0.36, SD 0.51) and self-efficacy (∆ score mean 0.16, SD 0.47). In addition, youth in the S4E group showed reductions in the proportions of past 30-day overall substance use (Cohen h=0.71, 95% CI 0.15 to 1.27), as well as past 30-day alcohol (Cohen h=0.71, 95% CI 0.15 to 1.27), tobacco (Cohen h=0.17, 95% CI −0.39 to 0.73), and drug use (Cohen h=1.28, 95% CI 0.72 to 1.84). The results also suggest a reduction in the proportion of youths who reported past 30-day condomless sex (Cohen h=0.18, 95% CI −0.38 to 0.74) and alcohol use before sex (Cohen h=0.44, 95% CI −0.12 to 1.00). Finally, the findings also demonstrated an increase in the proportion of youths who reported STI/HIV testing over time (Cohen h=0.16, 95% CI −0.39 to 0.72).
Conclusions
The findings suggest the feasibility of a small-scale pilot RCT. S4E demonstrated shifts in the hypothesized direction, reducing substance use, sexual risk behaviors, and improving the uptake of STI/HIV testing among youth in a clinic setting. The findings suggest that a larger RCT may be warranted.
Trial Registration
ClinicalTrails.gov NCT03855410, https://clinicaltrials.gov/ct2/show/NCT03855410.
Keywords: youth, mHealth, illicit drugs, sex behavior, HIV, primary care
Introduction
The Prevalence of Sexually Transmitted Infections/HIV and Associated Risk Behaviors Among the Youth
Sexually transmitted infections (STIs), including HIV, and teen pregnancy, remain significant public health concerns among youth in the United States [1-4]. STIs and HIV infection have been linked to infertility, cancer, and increasing vulnerability to opportunistic infections [5,6]. In addition, teen pregnancy has been linked to low income, poverty, and low educational attainment [7]. Therefore, preventing and reducing STIs, HIV, and teen pregnancy, as well as associated risk behaviors such as substance use and sexual risk behaviors, remain critical public health priorities.
Substance use and sexual risk behaviors may directly or indirectly increase the risk of STIs, HIV, and teen pregnancy. These behaviors often increase during adolescence, highlighting the need to intervene in these behaviors throughout this developmental period of increased vulnerability [8-10]. National epidemiologic data suggest that substance use behaviors, including alcohol, tobacco, and other drug use, are widespread among the youth [11]. Substance use behaviors often parallel other risk behaviors [12] and have been linked to increased sexual risk behaviors among the youth [8,13,14]. In addition to preventing and reducing substance use and sexual risk behaviors, STI and HIV testing are key strategies to reduce the high rates of STI/HIV infection among the youth [15,16]. Despite the Centers for Disease Control and Prevention guidelines, many youths are not routinely screened for STIs [17], and 90.7% of 9th- to 12th-grade students report having never been tested for HIV [11]. Therefore, there remains an urgent need to identify settings and tools that may be leveraged to improve the uptake of STI/HIV testing among youth.
Leveraging Youth-Centered Community Health Clinics and Mobile Health Apps to Prevent Sexually Transmitted Infections/HIV Among the Youth
Youth-centered community health clinics may be an ideal setting to engage youth in prevention services. Evidence supports that compared with adult-focused clinics and AIDS service organizations, youth are more likely to seek substance use and sexual risk prevention and risk reduction services from youth-centered community health clinics [18,19]. However, relatively few interventions have been developed and tested in clinic settings [20,21]. Leveraging clinic settings, in combination with technology, may have great utility in identifying substance use, sexual risk behaviors, and STI/HIV testing solutions for youth.
Mobile health (mHealth) refers to medical or public health initiatives and practices supported by mobile devices such as tablets and the internet [22]. Among a limited yet growing body of research, mHealth interventions have been pilot-tested and demonstrated positive shifts in reducing substance use, sexual risk behaviors, or increasing STI/HIV testing among youth [23-28]. For example, researchers have shown that brief mHealth interventions reduce marijuana use among youths aged 15 to 24 years at 3 months postintervention [24] and heavy alcohol consumption among young bisexual men at 3 months postintervention [26]. Other research has shown that brief mHealth interventions can improve the uptake of HIV [23,25,28,29] and STI testing [23] and decrease the frequency of condomless sex [25].
Limitations of Scientific Knowledge on Mobile Health Preventive Interventions
Although scientific advancements on mHealth preventive interventions have been made, several important limitations exist. First, few mHealth preventive interventions focus on substance use and concurrent risk behaviors (ie, sexual risk behaviors) in younger adolescents (aged <18 years) [25,30], missing the opportunity to affect a key developmental period of enhanced risk-taking [31]. Second, interventions targeting sexual risk behaviors and uptake of STI/HIV testing have focused primarily on young men who have sex with men [23,25,29], with few interventions focused on other vulnerable populations. Indeed, stark HIV disparities among men who have sex with men exist, accounting for 87% of new HIV diagnoses among youths aged 13 to 24 years [32]. Also important are racial and ethnic minority youth and adolescent women who constitute additional vulnerable populations of youth [32-34]. Third, to date, interventions have focused primarily on linking the youth to STI/HIV testing sites [23,29], with relatively few preventive interventions focused on the youth once they arrive at the clinic. Although drawing youth to the clinic is an important first step, it does little good if effective prevention services are not provided once the youth arrive at the clinic. Simply focusing on drawing the youth to clinics may create missed opportunities for engaging the youth in additional prevention strategies, particularly among those who are unaware of their engagement in risky behaviors [30]. To address these limitations, we conducted a small-scale randomized controlled trial (RCT) to pilot-test the feasibility of a multilevel mHealth preventive intervention among a diverse sample of youth in a clinic setting.
The Storytelling 4 Empowerment Mobile Health Preventive Intervention App
Employing community-based participatory research (CBPR) principles [35], we developed Storytelling 4 Empowerment (S4E) [30,36]. Guided by ecodevelopmental [37] and empowerment theories [38], S4E aims to reduce substance use, sexual risk behaviors, and improve uptake of STI/HIV testing through improving clinician-youth risk communication, prevention knowledge, and self-efficacy [30]. This is accomplished through a multilevel mHealth app that provides interactive, targeted, and tailored content focused on the prevention of substance use and sexual risk behaviors. This mHealth app is then followed up with a clinician-initiated prevention and risk reduction face-to-face encounter, providing clinicians the opportunity to reinforce content provided to youth during their interaction with the mHealth app. Because S4E has been shown to have a positive user experience, an effective user interface, and high feasibility and acceptability among both youth and clinicians [30,39], a next important step is to conduct a small-scale pilot RCT to determine the feasibility of S4E and examine shifts in potential mechanism of change and the ability to measure substance use, sexual risk behaviors, and uptake of STI/HIV testing among a diverse sample of youth. We believe our S4E multilevel approach may offer advantages over other approaches for several reasons. First, our intervention was developed with and for the targeted community. For example, youth helped steer the development of S4E with regard to the user experience and user interface [36]. Researchers affirm that community-engaged research employing CBPR principles may lead to enhanced uptake of, and optimally efficacious, preventive interventions [35]. Second, our intervention is developmentally and culturally congruent, utilizing spaces and tools that align with youth perspectives. Specifically, youth-centered clinics are safe spaces for many youths, thereby providing a potentially high-impact context to improve public health. Furthermore, approximately 95% of the youth report having access to mobile devices [40], which may be leveraged to deliver risk behavior solutions to this vulnerable population. Finally, our S4E approach was grounded in decades of science and informed by prevention principles [41]. For example, S4E is theory driven, targets multiple levels, and focuses on multiple risk behaviors that often co-occur [30,36,39].
Purpose of the Study
The purpose of this study was to conduct a small-scale pilot RCT to determine the feasibility of S4E, relative to enhanced usual practice. We evaluated changes in the potential mechanisms of change, namely clinician-youth risk communication, prevention knowledge, and self-efficacy over time. We also assessed the ability to measure substance use, sexual risk behaviors, and uptake of STI/HIV testing over time among a diverse sample of youth in a clinic setting. Given the small-scale pilot nature of our study and sample size, statistical significance was deemphasized. Rather, our goal was to assess feasibility and establish the critical parameters necessary to inform a larger future RCT.
Methods
Participants
We recruited youth and clinicians between October 2016 and July 2017 from a youth-centered community health clinic located in Southeast Michigan that offers a full range of health care, mental health, and supportive services to young people as they transition to adulthood.
Youth recruitment occurred during the clinic’s health appointment reminder phone calls. To be eligible for this study, youth had to (1) be aged between 13 and 21 years, (2) live in Southeast Michigan without plans to move out of the area during the study period, (3) have a scheduled appointment with a participating clinician, and (4) report no prior history of psychiatric hospitalization. Of the 277 youths who were screened for eligibility, 211 met the inclusion criteria. Of these, 109 youths did not return calls or messages left by the study team, 23 canceled or did not show for their scheduled health appointment, 11 had disconnected or incorrect phone numbers, 10 reported conflicting schedules with school or work, and 8 refused to participate. Therefore, of the 211 eligible youths, 50 were successfully recruited (see Figure 1).
Figure 1.
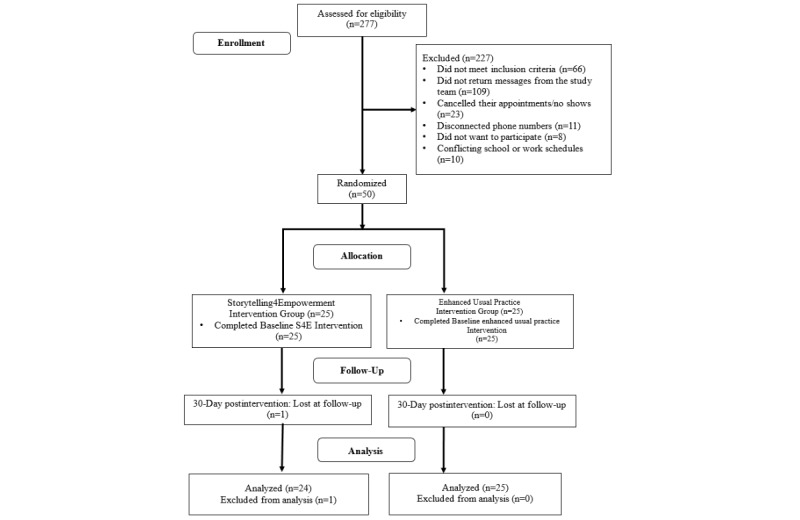
Consolidated Standards of Reporting Trials table.
Clinician recruitment occurred during weekly clinic staff meetings, and all clinicians at the health clinic were eligible to participate if they (1) worked in Southeast Michigan and (2) worked with our target population. The study team approached 8 clinicians, of which 7 agreed to participate. Both youths and clinicians who expressed interest in participating in the study were contacted by the study team to screen for eligibility, to enroll, and to complete study consent protocols. To protect the confidentiality of the youth aged between 13 and 17 years, a waiver of parental permission was obtained. This waiver was in accordance with the state of Michigan’s Title X Program and Public Health Code, MCL 333.6121, which states that a minor aged 17 years or younger can consent to sexual and reproductive health and substance use services without parental knowledge. Both participating youths older than 18 years and clinicians were presented with consent through a comprehensive written waiver of documentation that did not require a signature from the participant or legally authorized representative while containing all the elements of informed consent required by the Health and Human Services’ regulations and policy.
Sample Characteristics
In line with other research pilot testing mHealth preventive interventions [24,26], the sample size for this study was 50, with 25 youth participants randomized to the S4E group and 25 randomized to the enhanced usual practice group. Of the 50 youths, 41 (82%) identified as female, followed by 4 (8%) males, and 4 (8%) transmales, and 1 refused to respond. The mean age of the youths was 18.82 years (SD 2.1, range 13-21). The racial composition of these 50 youths consisted of 23 (46%) non-Hispanic white, 21 (42%) black, 1 (2%) Native American, 4 (8%) ascribing to more than one race, and 1 (2%) selecting Other. Regarding the youths’ educational attainment, 36% (18/50) of youths reported having completed some college, whereas 30% (15/50) reported having completed high school. The remaining 34% (17/50) of youths reported having completed a grade between 7th and 11th.
Among the 7 clinicians who agreed to participate, 6 (86%) identified as female, with a mean age of 43.14 years (SD 7.95, range 34-56), and 5 (71%) were non-Hispanic white, followed by 1 (14%) Hispanic or Latino and 1 (14%) Asian. They reported an average of 10.86 years (SD 7.45, range 1-22) of medical practice in their respective specialties: 71% (5/7) practiced family medicine and 29% (2/7) were pediatricians. Finally, 71% (5/7) of the clinicians reported having lived in the area where they work for more than 10 years, and 29% (2/7) reported having lived in the area for fewer than 10 years.
Study Design
This study employed community-based participatory research principles [35]. A youth leadership council, clinic director, and staff were involved in all aspects of this research, including preparing and submitting the proposal to fund this study, identifying the target population, developing the study design, and disseminating the study findings (eg, publications). This study consisted of a 2 (group) × 2 (time) small-scale pilot RCT. Youth participants were randomly assigned to either the S4E experimental group or enhanced usual practice control group via sequential randomization [42]. Data were collected on tablets and captured using Research Electronic Data Capture, a Health Insurance Portability and Accountability Act–compliant, web-based app that is hosted on secure servers at the University of Michigan Medical School. To reduce potential bias, eligible participants completed health surveys that included questions regarding substance use, sexual risk behaviors, and STI/HIV testing practices, before randomization. All youths arrived 1 hour before their scheduled health care appointment to have the study explained to them in detail, to provide consent, and to complete baseline assessments in a reserved room, all of which took approximately 30 min. Youths participated in the intervention (S4E or enhanced usual practice) in a reserved room with internet connection for approximately 30 min, while they waited for their health appointment. Participants completed the S4E intervention on tablets provided to them by the research team. The S4E mHealth version tested in this study was developed for Apple’s operating system (iOS). Because this was a phase I/II pilot study, we had participants complete the S4E intervention in the clinic to have a more controlled environment. Youth participants were assessed at baseline before their health appointment, immediately postintervention, and 30 days postbaseline. Clinicians were assessed at baseline and immediately postintervention for each health appointment.
We retained 49 participants at our 30-day follow-up (49/50, 98% retention rate). Youth participants received a total of US $60 in incentives, corresponding to US $20 at baseline and US $40 at the 30-day follow-up. Through a collaborative process, the clinic and research team decided to provide a US $2000 incentive to benefit the entire community health clinic for providing us with meeting space and for the clinicians’ time on the project, rather than give individual incentives to the participating clinicians.
Study Groups
Storytelling 4 Empowerment Group
The S4E intervention content was generated through community-university research involving youth-led groups in conjunction with scientific prevention principles [30]. Theory-driven, S4E takes a multilevel approach and is guided by an ecodevelopmental [37] and empowerment framework [38]. Youths in the S4E group received targeted, tailored prevention content based on their responses to the S4E risk behavior assessment, which includes the Car, Relax, Alone, Forget, Friends, Trouble screener [43]. This assessment is intended to identify the youths’ specific risk behaviors based on the past year and lifetime reports of substance use, sexual risk behaviors, and past 6-month STI/HIV testing practices (Multimedia Appendix 1). From an empowerment perspective, the scores prompt risk-specific interactive prevention content (eg, short animated storytelling scenarios, interactive diagram of body health activities) for the S4E youth (Figure 2). This interactive content is delivered via 3 modules (ie, alcohol and drug use, tobacco, and STI/HIV) and aims to improve prevention knowledge, self-efficacy, and refusal skills while linking youth to important adult figures. From an ecodevelopmental perspective, the clinician-facing app contained the participants’ health appointment information, their assigned group, and their S4E risk assessment responses. Both the youth and clinician S4E apps work synergistically, providing clinicians access to the youths’ risk responses, motivational interviewing scripts to facilitate clinician-youth communication, and resources to local services that are based on the youths’ risk behaviors (Figure 3). Overall, the S4E intervention aims to improve substance use and sexual risk prevention knowledge, self-efficacy, and refusal skills, as well as to facilitate clinician-youth risk communication. By focusing on these malleable factors, the overarching goal of the S4E intervention is disease prevention (ie, substance use disorders, STI/HIV) and health promotion (ie, STI/HIV testing) through reductions in substance use, sexual risk behaviors, and improvements in STI/HIV testing among youth.
Figure 2.

Storytelling 4 Empowerment animated storytelling scenario.
Figure 3.
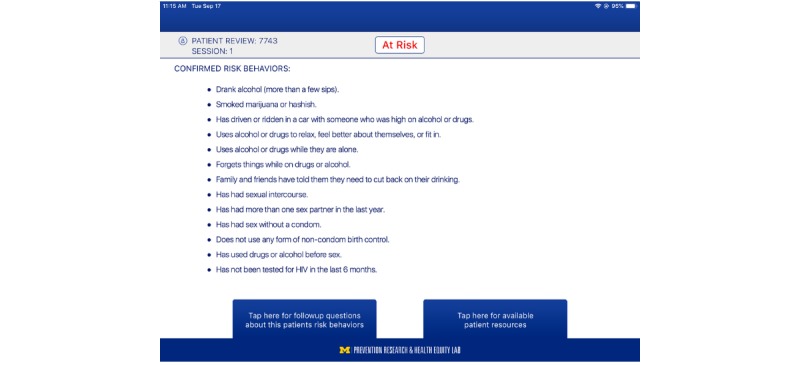
Clinician-facing Storytelling 4 Empowerment application highlighting youth risk behaviors.
Enhanced Usual Practice Group
The participating youth-centered community health clinic’s usual practice consists of primary care, mental health, sexual and reproductive health, substance use prevention, support, and education (eg, Women, Infants, and Children Supplemental Food Program), and gender-affirming health care to youths aged 12 to 25 years. Participants in both the S4E group and enhanced usual practice received the clinic’s usual services. In addition, participants in the enhanced usual practice group received a binder with a printed PDF version of the S4E tobacco module content to view before their health care appointment. The materials consisted of epidemiologic statistics and prevention health information related to tobacco and cigarette use. The Youth Leadership Council strongly recommended an enhanced usual practice control group so that all youth participants in the trial would receive some form of additional prevention services.
Measures
Demographics
Youth and clinicians completed a demographic survey that asked their age, ethnicity, race, and gender identity. In addition, youth reported educational attainment, and clinicians reported their medical specialty, years of clinical experience, and length of time residing in the area where they practiced.
Clinician-Youth Communication (Immediately Postintervention)
Both clinicians (Cronbach alpha=.81, 9-item) and youth (Cronbach alpha=.95, 9-item) interactions during the clinic visit were assessed via items extracted and adapted from the matched pair instrument (MPI) [44] immediately postintervention. These items assessed the process and content of the communication, including the language used and behaviors performed by clinicians related to substance use and sexual risk behaviors services. A sample statement for clinicians is as follows: Encouraged the patient to express his or her thoughts concerning drug use and/or sexual risk behaviors. A sample statement for youths is as follows: My doctor encouraged me to express my thoughts concerning drug use and/or sexual risk behaviors. Both clinicians and youth responded to items in the measure using a 6-point Likert-type scale (0=not applicable, 5=strongly agree). To make the MPI sum scores more interpretable, the scores were rescaled to their original 6-point metric by dividing the sum total by the total number of items before significance testing.
Self-Efficacy Outcomes
Substance Use Refusal Skills
Youths’ substance use refusal skills were assessed through 2 separate items on a 4-point scale (1=very hard to 4=not very hard). Sample questions included the following: Pretend your best friend offered you a drink of beer or wine and you did not want it. How hard would it be to say no? and Pretend your best friend offered you some marijuana and you did not want it. How hard would it be to say no?
Sexual Risk Behavior Refusal Skills
One item was used to assess youths’ sexual risk behavior refusal skills. The statement read the following: I can say no to sex if my partner and I do not have a condom. Responses were on a 5-point agreement scale (1=strongly disagree, 5=strongly agree).
Prevention Knowledge Outcomes
Substance Use Prevention Knowledge
Youths’ knowledge about alcohol or drugs and tobacco products was assessed through 2 separate items. Items included the following: If I use alcohol or drugs, I will have more health problems than other people and If I use tobacco products, I will have more health problems. Responses were on a 4-point agreement scale (1=disagree a lot to 4=agree a lot).
Sexual Risk Prevention Knowledge
Sexual risk prevention knowledge was measured through 2 separate items related to the effectiveness of condom use. Items included the following: Condoms help prevent pregnancy, and If I have sex without a condom, I am likely to get HIV/STIs. Responses were on a 5-point agreement scale (1=strongly disagree, to 5=strongly agree).
Behavioral Outcomes
Substance use behaviors, sexual risk behaviors, and HIV/STI testing were dichotomized for analysis, whereby 0 was used for No (ie, no risk present), and 1 indicated Yes (ie, risk was present) for the item in question.
Substance Use Behaviors (Baseline and 30-Day Postbaseline)
Youths’ lifetime and past 30-day substance use behaviors were assessed using items adapted from the Monitoring the Future study [45,46]. Sample items included the following: Have you ever had any beer, wine, wine cooler, or liquor to drink? and Have you had more than a few sips of alcohol on more than one occasion during the past 30 days?
Sexual Risk Behaviors (Baseline and 30-Day Postbaseline)
Participants’ lifetime and past 30-day sexual risk behaviors were assessed using items extracted from the Sexual Behavior Instrument [47]. Sample items include Have you ever had vaginal, anal, or oral sex without using a condom? and In the past 30 days, about how often have you had vaginal, anal, or oral sex without using a condom?
Sexually Transmitted Infection and HIV Testing (Baseline, Immediately Postintervention, 30-Day Postbaseline)
We assessed youths’ lifetime and most recent STI and HIV testing. Example questions included the following: Have you ever been tested for HIV? and Did you receive an STI test?
Analytic Strategy
Given the modest sample size and goals of a pilot RCT, significance testing by group was deemphasized [23]. Rather, we determined whether outcomes shifted in the hypothesized direction and gathered the necessary parameters to use in a larger RCT in the future [48]. The data analytic strategy consisted of 4 steps. First, we conducted a descriptive statistical analysis on demographic and outcome variables at baseline and used chi-square tests and analysis of variance to test for significant group differences at baseline. Second, we conducted chi-square tests and 2-tailed t tests to determine if there were significant differences by group in attrition and elapsed time between baseline and 30-day follow-up assessments. Third, we determined group differences in change of potential mechanisms of change (ie, self-efficacy, prevention knowledge, clinician-youth risk communication) over time. Finally, we assessed the ability to measure between-group differences (ie, S4E vs control) in the change of reported substance use, sexual risk behaviors, and STI/HIV testing over time. We also assessed the ability to measure within-group differences in outcomes among participants in the S4E intervention group. Differences were determined using proportion change (∆ %) for categorical outcomes and mean change (∆ score, absolute net gains) over time for continuous outcomes. We report the observed effect sizes for the outcome change difference scores by group using Cohen d for continuous outcomes and Cohen h for binary outcomes, which measure the difference between 2 proportions (h=2arcsin × (sqrt P1) − 2arcsin × (sqrt P2), where P1=proportion 1 and P2=proportion 2). Effect sizes were estimated as small (d/h<.20), medium (.20≥d/h≤.45), and large (d/h>.45) to observe the magnitude of differences [49]. All analyses were performed in SPSS version 24 [50], with the exception of Cohen h power calculations, which were performed in R’s version 3.5.2 PWR package [51].
Results
Comparability of Groups
As shown in Table 1, chi-square tests and analyses of variance results suggest no significant S4E vs control group differences at baseline on any demographic characteristic (eg, race), lifetime or past 30-day substance use, sexual risk behaviors, and lifetime STI/HIV testing. The absence of significant differences in these variables at baseline suggests that our trial’s randomization procedures were successful. The median number of days between baseline and follow-up was 31 (mean 32.63, SD 8.62); no significant differences by group in the number of days between baseline and 30-day follow-up assessments were observed using a 2-tailed t test, t46.36=0.42, P=.68. In addition, no significant differences in attrition by group were observed, χ21=0.0, P=.92.
Table 1.
Baseline comparisons by group on demographic and behavioral outcomes (N=50).
| Outcomes | S4Ea (n=25) | Control (n=25) | t test/chi-square (df) | P value | |
| Age (years), mean (SD) | 18.6 (2.15) | 19.0 (2.19) | −0.58 (47.98)b | .56 | |
| Gender, n (%) | |||||
|
|
Female | 21 (84) | 20 (80) | 1.0 (2) | .61 |
|
|
Male | 2 (8) | 2 (8) | 1.0 (2) | .61 |
|
|
Transmale | 1 (4) | 3 (12) | 1.0 (2) | .61 |
|
|
Transfemale | —c | — | 1.0 (2) | .61 |
|
|
Refuse to answer | 1 (4) | — | 1.0 (2) | .61 |
| Race, n (%) | |||||
|
|
Black | 11 (44) | 10 (40) | 2.4 (4) | .66 |
|
|
White | 10 (40) | 13 (52) | 2.4 (4) | .66 |
|
|
Native American | 1 (4) | — | 2.4 (4) | .66 |
|
|
More than one race | 2 (8) | 2 (8) | 2.4 (4) | .66 |
|
|
Other | 1 (4) | — | 2.4 (4) | .66 |
| Lifetime substance use, n (%) | |||||
|
|
Lifetime alcohol use | 21 (84) | 21 (84) | — | — |
|
|
Lifetime tobacco use | 16 (64) | 17 (68) | 0.9 (1) | .77 |
|
|
Lifetime other drug use | 14 (56) | 13 (52) | 0.1 (1) | .78 |
| Past 30-day substance use, n (%) | |||||
|
|
Past 30-day substance use (ATODd) | 15 (60) | 16 (64) | 0.1 (1) | .77 |
|
|
Past 30-day alcohol use | 10 (40) | 14 (56) | 1.3 (1) | .26 |
|
|
Past 30-day tobacco use | 7 (28) | 11 (44) | 1.4 (1) | .23 |
| Past 30-day other drug use | 12 (48) | 7 (28) | 1.3 (1) | .26 | |
| Lifetime sexual risk behaviors, n (%) | |||||
|
|
Lifetime condomless sex | 20 (80) | 20 (80) | — | — |
|
|
Lifetime alcohol use before sex | 9 (36) | 14 (56) | 2.0 (1) | .16 |
| Life drug use before sex | 7 (28) | 8 (32) | 0.2 (1) | .69 | |
| Past 30-day sexual risk behaviors, n (%) | |||||
|
|
Past 30-day condomless sexe | 13 (62) | 13 (62) | 0.2 (1) | .67 |
|
|
Past 30-day alcohol use before sexe | 2 (10) | 3 (14) | 0.2 (1) | .67 |
|
|
Past 30-day drug use before sexe | 3 (14) | 4 (19) | 0.1 (1) | .73 |
|
|
Lifetime HIV/STIf testing | 22 (88) | 20 (80) | 0.6 (1) | .44 |
aS4E: Storytelling 4 Empowerment.
bt test for age; rest are chi-square values.
cData are not applicable.
dATOD: alcohol, tobacco, other drug use.
eAmong sexually active youth.
fSTI: sexually transmitted infection.
Intervention Effects on Clinician-Youth Risk Communication
Immediately postintervention, youths in the S4E group reported higher levels of clinician-youth risk communication (mean 3.22, SD 1.67), relative to the youths in the control group (mean 2.96, SD 1.63; t46.77=0.56; P=.58). Although these group differences were not statistically significant, the estimated effect size (Cohen d=0.16, 95% CI −0.41 to 0.72) yielded a small effect size. Similarly, S4E clinicians reported higher levels of clinician-youth risk communication (mean 3.47, SD 1.13), relative to clinicians in the control group immediately postintervention (mean 3.23, SD 1.02; t45.98=0.75; P=.45). Although these differences were not statistically significant, the estimated effect size (Cohen d=0.22, 95% CI −0.34 to 0.79) yielded a small value.
Intervention Effects on Self-Efficacy
As shown in Table 2, youths in the S4E group reported greater change scores in substance use self-efficacy alcohol refusal (∆ score mean 0.22, SD 0.67), relative to the youths in the control group (∆ score mean 0.16, SD 0.55; t42.79=0.32; P=.75; Cohen d=0.10) and for drug refusal (∆ score mean 0.09, SD 0.68), relative to the youths in the control group (∆ score mean 0.08, SD 0.70; t44.51=0.05; P=.96; Cohen d=0.01). Yet, both the S4E group (∆ score mean 0.08, SD 0.78) and the control group (∆ score mean 0.13, SD 0.92) showed an increase in sex self-efficacy (t44.06=−0.19; P=.85; Cohen d=−0.06). In contrast to the between-group effects, intervention effects within the S4E group (Table 3) for both substance use items, alcohol refusal (∆ score mean 0.21; t22=1.55, P=.14; Cohen d=0.38) and drug use refusal (∆ score mean 0.09; t21=0.62; P=.54; Cohen d=0.12), yielded small to medium effect sizes. Similarly, sexual risk self-efficacy change scores within the S4E group (∆ score mean 0.08; t23=0.53; P=.60; Cohen d=0.10) yielded small effect sizes.
Table 2.
Self-efficacy and prevention knowledge by group.
| Outcomes | S4Ea, ∆ score, mean (SD) | Control, ∆ score, mean (SD) | Independent t test (df) | Cohen’s d (S4E vs control) | Cohen d 95% CI | |
| Substance use self-efficacy | ||||||
|
|
Alcohol refusal | 0.22 (0.67) | 0.16 (0.55) | 0.32 (42.79) | 0.10 | −0.47 to 0.66 |
|
|
Drug refusal | 0.09 (0.68) | 0.08 (0.70) | 0.05 (44.51) | 0.01 | −0.56 to 0.59 |
| Sexual risk self-efficacy | ||||||
|
|
Condomless sex | 0.08 (0.78) | 0.13 (0.92) | −0.19 (44.06) | −0.06 | −0.61 to 0.50 |
| Substance use prevention knowledge | ||||||
|
|
Use of tobacco products | 0.30 (0.77) | 0.16 (0.80) | 0.64 (45.93) | 0.18 | −0.40 to 0.76 |
|
|
Use of alcohol or drugs | 0.35 (0.65) | 0.20 (1.00) | 0.61 (41.46) | 0.18 | −0.39 to 0.74 |
| Sexual risk prevention knowledge | ||||||
|
|
Pregnancy prevention | 0.08 (0.65) | 0.00 (0.41) | 0.53 (38.29) | 0.15 | −0.41 to 0.71 |
|
|
STIb/HIV prevention | 0.25 (1.22) | −0.17 (1.74) | 0.96 (41.35) | 0.28 | −0.28 to 0.84 |
aS4E: Storytelling 4 Empowerment.
bSTI: sexually transmitted infection.
Table 3.
Storytelling 4 Empowerment intervention effects on self-efficacy and prevention knowledge (n=25).
| Outcomes | S4Ea baseline, mean (SD) | S4E follow-up, mean, (SD) | ∆ score mean | Paired t test (df) | Cohen d | Cohen d 95% CI | |
| Substance use self-efficacy | |||||||
|
|
Alcohol refusal | 3.57 (0.59) | 3.78 (0.52) | 0.21 | 1.55 (22) | 0.38 | −0.94 to 0.19 |
|
|
Drug refusal | 3.50 (0.86) | 3.59 (0.67) | 0.09 | 0.62 (21) | 0.12 | −0.68 to 0.44 |
| Sexual risk self-efficacy | |||||||
|
|
Condomless sex | 3.25 (0.85) | 3.33 (0.76) | 0.08 | 0.53 (23) | 0.10 | −0.66 to 0.46 |
| Substance use prevention knowledge | |||||||
|
|
Use of tobacco products | 3.09 (0.79) | 3.43 (0.59) | 0.34 | 2.58 (22) | 0.49 | −0.10 to 1.07 |
|
|
Use of alcohol or drugs | 3.30 (0.56) | 3.61 (0.58) | 0.31 | 1.91 (22) | 0.50 | −0.07 to 1.07 |
| Sexual risk prevention knowledge | |||||||
|
|
Pregnancy prevention | 3.50 (0.72) | 3.58 (0.50) | 0.08 | 0.62 (23) | 0.10 | −0.43 to 0.69 |
|
|
STIb/HIV prevention | 2.67 (1.24) | 2.92 (1.10) | 0.25 | 1.00 (23) | 0.21 | −0.35 to 0.77 |
aS4E: Storytelling 4 Empowerment.
bSTI: sexually transmitted infection.
Intervention Effects on Prevention Knowledge
As shown in Table 2, youths in the S4E group reported higher overall gains in substance use prevention knowledge for tobacco use (∆ score mean 0.30, SD 0.77), relative to the youths in the control group (∆ score mean 0.16, SD 0.80; t45.93=0.64; P=.53; Cohen d=0.18). The S4E group reported similar gains of prevention knowledge for alcohol or drug use (∆ score mean 0.35, SD 0.65), relative to the youths in the control group (∆ score mean 0.20, SD 1.00; t41.46=0.61; P=.54; Cohen d=0.18). In addition, S4E youths reported overall gains for sexual risk prevention knowledge (pregnancy prevention, ∆ score mean 0.08, SD 0.65; STI/HIV prevention, ∆ score mean 0.25, SD 1.22), relative to the control group (pregnancy prevention, ∆ score mean 0.00, SD 0.41; t38.29=0.53; P=.60; Cohen d=0.15 and STI/HIV prevention, ∆ score mean −0.17, SD 1.74; t43.35=0.96; P=.34; Cohen d=0.28). Both outcomes yielded small to medium effect sizes. In contrast to the between-group effects, intervention effects within the S4E group (Table 3) for both substance use prevention knowledge (tobacco use ∆ score mean 0.34; t22=2.58; P=.02; Cohen d=0.49 and alcohol or drug use ∆ score mean 0.31; t22=1.91; P=.07; Cohen d=0.50) and the sexual risk prevention knowledge-pregnancy prevention (∆ score mean 0.08; t23=0.62; P=.54; Cohen d=0.10) and STI/HIV prevention (∆ score mean 0.25; t23=1.00; P=.32; Cohen d=0.21) yielded small to medium effect sizes.
Between-Group Intervention Effects on Substance Use Behaviors
Overall, participant reports of substance use at baseline were not significantly different (Table 1). However, the S4E group reported a greater reduction in any substance use (ie, ATOD) relative to the control group (3/25, 12% vs 0/25, 0%; Table 4). Although chi-square testing (χ22=4.5; P=.10) suggests that these proportion differences between groups were marginally significant, the estimated proportion change effect size difference between groups (Cohen h=0.71) yielded a large effect size. We then deconstructed past 30-day substance use into past 30-day alcohol, tobacco, and other drug use to determine between-group intervention effects on each of these outcomes.
Table 4.
Past 30-day behavior outcome proportion change scores by group.
| Outcomes | ∆ S4Ea | ∆ Control | Cohen h (S4E vs control) | Cohen h, 95% CI | |
| Substance use, n (%) | |||||
|
|
Substance use (ATODb) | 3 (−12) | 0 (0) | 0.71 | 0.15 to 1.27 |
|
|
Alcohol use | 3 (−12) | 0 (0) | 0.71 | 0.15 to 1.27 |
|
|
Tobacco use | 2 (−8) | 1 (−4) | 0.17 | −0.39 to 0.73 |
|
|
Other drug use | 3 (−12) | 2 (+8) | 1.28 | 0.72 to 1.84 |
| Sexual risk behaviors, n (%)c | |||||
|
|
Condomless sex | 2 (−10) | 1 (−5) | 0.18 | −0.38 to 0.74 |
|
|
Alcohol use before sex | 1 (− 5) | 0 (0) | 0.44 | −0.12 to 1.00 |
|
|
Drug use during sex | 2 (−10) | 2 (−10) | N/Ad | N/A |
aS4E: Storytelling 4 Empowerment.
bATOD: alcohol, tobacco, other drug use.
cSexual risk behaviors are based on responses from sexually active participants (n=42).
dNot applicable.
Alcohol Use
Overall, 84% (42/50) of participants reported lifetime alcohol use. Relative to participants in the control group, S4E group participants reported a greater reduction in past 30-day alcohol use at 30-day follow-up (3/25, 12% vs. 0/25, 0%). Although chi-square testing (χ22=3.9; P=.14) suggests that this proportion change difference was not statistically significant between groups, the estimated proportion change effect size between groups (Cohen h=0.71) yielded a large effect size (Table 4).
Tobacco Use
Overall, 66% (33/50) of participants reported lifetime tobacco use. Participants in the S4E group reported a greater decrease in tobacco use, as compared with participants in the control group at 30-day follow-up (2/25, 8% vs 1/25, 4%). Although chi-square testing (χ21=0.4; P=.50), suggests that these proportion differences were not statistically significant, the estimated effect size difference across groups (Cohen h=0.17) yielded a small effect size (Table 4).
Other Drug Use
Overall, 54% (27/50) of participants reported lifetime drug use. Participants in the S4E group reported a reduction (3/25, 12%) in drug use at 30-day follow-up, relative to an increase in drug use (2/25, 8%) among participants in the control group. Although chi-square testing (χ22=2.9; P=.23) suggests that these proportion differences were not statistically significant, the estimated effect size difference across groups (Cohen h =1.28) yielded a large effect size (Table 4).
Between- Group Intervention Effects on Sexual Risk Behaviors
Condomless Sex
Overall, 80% (40/50) of participants reported engaging in lifetime condomless sex. Relative to sexually active participants in the control group (n=21), sexually active S4E group participants (n=21) reported a greater reduction in past 30-day condomless sex at 30-day follow-up (2/21, 10% vs 1/21, 5%). Although chi-square testing (χ22=0.2; P=.91) suggests that these proportion differences were not statistically significant, the estimated effect size difference between groups (Cohen h=0.18) yielded a small effect size (Table 4).
Alcohol Use Before Sex
Overall, 46% (23/50) of participants reported lifetime alcohol use before sex. Relative to sexually active participants in the control group (n=21) who reported no change, sexually active S4E group (n=21) participants reported a reduction in alcohol use before sex at 30-day follow-up (1/21, 5% vs 0/21, 0%). Although chi-square testing (χ22=2.3; P=.32) suggests that these proportion differences were not statistically significant, the estimated effect size difference between groups (Cohen h=0.44) yielded a medium effect size (Table 4).
Drug Use Before Sex
Overall, 15 (30%) participants reported lifetime drug use before sex. Both the control and S4E group participants reported similar reductions in drug use before sex at 30-day follow-up (2/21, 10% vs 2/21, 10%; Table 4).
Within-Group Intervention Effects on Substance Use Behaviors
As shown in Table 5, of the 25 youths in the S4E group, 12% (3/25) reported a decrease of any substance use from baseline assessment to 30-day follow-up (χ21=10.9; P<.001; Cohen h=0.24). We then separated past 30-day substance use into past 30-day alcohol, tobacco, and other drug use to determine within-group intervention effects on each of these outcomes. S4E intervention effects for alcohol use from baseline assessment to 30-day follow-up showed a 12% (3/25) decrease in alcohol use (χ21=3.6; P=.06; Cohen h=0.24). Similarly, 8% (2/25) of the S4E participants reported a decrease in tobacco use from baseline assessment to 30-day follow-up (χ21=14.6; P<.001; Cohen h=0.19). In addition, 12% (3/25) of S4E youth reported a decrease in other drug use from baseline assessment to 30-day follow-up (χ21=17.0; P<.001; Cohen h=0.24). Although chi-square significance was deemphasized, S4E intervention effects on substance use behaviors yielded small to medium effect sizes.
Table 5.
Past 30-day Storytelling 4 Empowerment intervention effects on behaviors from baseline to follow-up (n=25).
| Outcomes | S4Ea baseline, n (%) | S4E follow-up, n (%) | ∆ score, n (%) | Cohen h | Cohen h, 95% CI | |||||
| Substance use behavior | ||||||||||
|
|
Substance use (ATODb) | 15 (60) | 12 (48) | 3 (−12) | 0.24 | −0.32 to 0.80 | ||||
|
|
Alcohol use | 10 (40) | 7 (28) | 3 (−12) | 0.24 | −0.31 to 0.81 | ||||
|
|
Tobacco use | 7 (28) | 5 (20) | 2 (−8) | 0.19 | −0.37 to 0.74 | ||||
|
|
Other drug use | 12 (48) | 9 (36) | 3 (−12) | 0.24 | −0.32 to 0.80 | ||||
| Sexual risk behaviorsc | ||||||||||
|
|
Condomless sex | 13 (62) | 11 (52) | 2 (−10) | 0.19 | −0.37 to 0.75 | ||||
|
|
Alcohol use before sex | 2 (10) | 1 (5) | 1 (−5) | 0.18 | −0.38 to 0.74 | ||||
|
|
Drug use before sex | 3 (14) | 1 (5) | 2 (−10) | 0.33 | −0.23 to 0.89 | ||||
aS4E: Storytelling 4 Empowerment.
bATOD: alcohol, tobacco, other drug use.
cSexual risk behaviors are based on responses from sexually active participants (n=21).
Within-Group Intervention Effects on Sexual Risk Behaviors
As shown in Table 5, within-group intervention effects on S4E sexual risk behaviors from baseline assessment to 30-day follow-up had small to medium effect sizes that helped establish differences. Specifically, S4E reports of condomless sex decreased by 10% (2/21) from baseline assessment to 30-day follow-up (χ21=2.9; P=.09; Cohen h =0.19). Moreover, decreases in alcohol use before sex (1/25, 5%; χ21=8.97; P=.003; Cohen h=0.24) and drug use before sex (2/25, 10%; χ21=8.47; P=.004; Cohen h =0.33) were observed.
Intervention Effects on Sexually Transmitted Infections/HIV Testing
We sought to determine whether STI/HIV testing behaviors varied by group over time, independently of participants’ prior lifetime STI/HIV testing behaviors. At baseline, of the 50 youths, 42 (84%) reported having been tested for STI/HIV during their lifetime (Table 1). Moreover, no baseline lifetime STI/HIV testing differences were found by group. Relative to the control group, participants in the S4E group reported an overall higher uptake of STI/HIV testing across the trial (11/25, 44% vs 13/25, 52%). Although chi-square testing (χ21=0.3; P=.57) suggests these differences were not statistically significant, the estimated effect size (Cohen h=0.16, 95% CI −0.39 to 0.72) yielded a small value.
Discussion
Principal Findings
The findings suggest the feasibility of a small-scale pilot RCT and demonstrated hypothesized shifts in reducing substance use, condomless sex, and alcohol use before sex, as well as improving uptake of STI/HIV testing among a diverse sample of youth. The estimated proportion change effect sizes of our behavioral outcomes (ie, substance use, sexual risk behaviors, STI/HIV testing) and potential mechanisms of change (ie, clinician-youth communication, prevention knowledge, self-efficacy) between the S4E and enhanced usual practice control groups yielded small to large effect sizes. Drawing on previous literature, the findings provide evidence for the promise of S4E in preventing and reducing substance use and sexual risk behaviors [23,27]. Reducing substance use and sexual risk behaviors and improving uptake of STI/HIV testing have been identified as key strategies to improve the health of young people in the United States [10,15,16]. Pilot testing S4E advances the scientific knowledge on mHealth preventive interventions and has important public health implications.
We demonstrated the feasibility of measuring intermediate outcomes. The potential mechanisms underlying the hypothesized shifts in substance use, sexual risk behaviors, and STI/HIV testing among youth in the S4E group may be partially explained by improvements in clinician-youth risk communication, substance use refusal self-efficacy, and prevention knowledge. Specifically, relative to youth in the control group, S4E participants demonstrated higher levels of clinician-youth communication immediately postintervention, as well as higher levels of substance use refusal self-efficacy and prevention knowledge at 30-day follow-up. The present design precludes us from formal mediation testing. However, these findings build on previous research indicating that clinician-youth communication, self-efficacy, and STI/HIV prevention knowledge may be pathways through which S4E has an effect on substance use, sexual risk behaviors, and STI/HIV testing [39]. Future research should include at least 3 time points to allow for formal mediation analysis [52], especially because few pathways by which mHealth preventive interventions affect behavioral outcomes have been identified [53].
We demonstrated the ability to measure outcomes targeted by S4E. Evaluation of the S4E intervention suggests reductions in overall licit and illicit substance use behaviors among youth. When we separated substance use behaviors, S4E helped decrease the proportion of youths who engaged in alcohol, tobacco, or other drug use at 30-day follow-up. These findings have important public health implications because reducing substance use behaviors among the youth aligns with the nation’s prevention goals and strategies to ameliorate substance use–related morbidity and mortality [15]. Furthermore, our findings are similar to those of other researcher’s pilot testing preventive interventions and lend support to the promise that mHealth strategies have in preventing and reducing youth risk behaviors [24-26].
The findings that S4E demonstrated hypothesized shifts in reducing condomless sex and alcohol use before sex have important public health implications because these risk behaviors are widespread among youth [11]. Therefore, the findings that S4E participants show reductions in the proportion of youth who engage in condomless sex or alcohol use before sex have important public health implications, as these risk behaviors are linked to increased vulnerability to STIs, HIV, and unplanned pregnancy—outcomes that disproportionately affect the youth [54]. Contrary to what we hypothesized, we did not find a between-group effect on drug use before sex; however, the findings suggest a statistically significant within-group change among S4E group participants. It may be that the 30-day follow-up time period is not sufficient in duration to capture the long-term effects of S4E relative to the control group on drug use before sex. Therefore, future research should examine the effects of S4E on drug use before sex over a longer period. Importantly, findings also suggest that S4E demonstrated shifts in improving uptake of STI/HIV testing among youth. This is especially important in the realm of public health, as improving STI and HIV testing uptake among youth is a key strategy to reducing the burden of STI and HIV infection among this vulnerable population [16,54,55]. Taken together, our small-scale pilot RCT suggests the feasibility of S4E, including determining intermediate outcomes, ability to measure behavioral outcomes, as well as demonstrated hypothesized shifts in reducing substance use, sexual risk behaviors, and STI/HIV testing among youth over 30 days, which aligns with other research pilot testing technology-based interventions on these behaviors [23-28]. It is also important to note that some researchers have found that technology-based interventions have a moderate health impact on exercise over 6 weeks [56]. Thus, in general, an important future research direction is to examine both the short and long-term effects of mHealth preventive interventions. In addition, this study focused on a sample of youths seeking care in a youth-centered community health clinic. Youths who are currently not in care may be more vulnerable to substance use and sexual risk behaviors, and future research could target this population.
Our findings have important future research implications and suggest that examining the efficacy of S4E in a larger RCT may be warranted. Implementation science designs might offer other alternatives to the traditional RCT, including stepped-wedge design and type 1 hybrid studies, which may increase the practicality of exploring intervention effects within real-life contexts [57,58]. Furthermore, given the multilevel approach of S4E, a sequential multiple assignment randomized trial (SMART) may lead to an optimally efficacious preventive strategy through the optimization of dose based on response (or lack thereof) to the intervention [59-62]. SMART is an innovative design that provides evidence for individualized decision making through adaptive interventions for the prevention of substance use and sexual risk behaviors [59,60]. In addition, our findings have important clinical implications. Our previous research establishing high feasibility and acceptability of S4E among both clinicians and youth [30,39] in conjunction with the findings of this pilot RCT endorses the implementation of strategies that support the youth-focused clinical health care workforce, especially if we are to achieve the nation’s public health goals laid out by initiatives such as the Substance Abuse and Mental Health Services Administration Strategic Plan for years 2019-2023 [63], National Prevention Strategy for increasing HIV/STI testing [64], ending the HIV Epidemic in the United States by 2030 [65], and teen pregnancy objectives in healthy people by 2020 [66].
Study Limitations
Several limitations are worth noting. First, the sample in this study is not representative of the clinic population, limiting the generalizability of our findings. However, we demonstrated hypothesized shifts in prominent substance use, sexual risk behaviors, and STI/HIV testing in S4E youth, the majority of whom identified as racial and ethnic minorities. Second, the reliance on self-reported risk behavior outcomes is a limitation. Future research should consider access to medical charts as part of the study design. Third, our control group received a printed version of the S4E tobacco module content. Future research should use an attention- and time-matched control group design [67,68]. That is, an mHealth app focused on youth risk behaviors with similar intended dosage as S4E may be used in a future RCT to examine the differential effects of mHealth apps. Another limitation is that of the 211 potential participants eligible to participate in the trail; we could only reach 91 youth (43.1%). Of these, we successfully enrolled 50 youth (54.9%); however, these enrollment rates are similar to other research focused on mHealth preventive interventions with vulnerable populations [26]. The sample size of 50 is a study limitation; however, the sample size is akin to other research focused on pilot testing mHealth preventive interventions [24,26]. Finally, clinicians were not randomly assigned and provided usual practice to participants in both the experimental and control groups. Thus, there is potential for contamination between groups; however, contamination is highly unlikely given that the 2 groups are vastly different. Importantly, any potential bias would bias the findings toward the null, because clinicians could deliver S4E prevention strategies to youth in the control group.
Conclusions
In summary, this study’s findings suggest the feasibility of a small-scale pilot RCT. S4E may have the potential as an mHealth strategy to reduce substance use and sexual risk–related outcomes such as STIs, HIV, and unplanned pregnancy among youth. In addition, the findings suggest that S4E demonstrated hypothesized shifts in improving uptake of STI/HIV testing, which is important for reducing the transmission and acquisition of STIs and HIV infection among youth. These findings advance scientific knowledge on mHealth preventive interventions and contribute toward improving public health through the identification of potential technology-based, youth substance use, and sexual risk behavior solutions.
Acknowledgments
All study procedures were approved by the University of Michigan Institutional Review Board (HUM00097290). A certificate of confidentiality (CC-CA-17-048-AI) was obtained from the National Institutes of Health/National Cancer Institute. This study is registered with ClinicalTrails.gov (NCT03855410). The Youth Leadership Council consisted of Ian Stewart, Erika Riano-Mojica, Bishop Warford, Franco Machado, Kiristen Hubbard, Brie Widian, Sakinah Rahman, Zakiyyah Rahman, Zeaira Chestang, and Katheryne Messer. This research was supported by pilot funding from the University of Michigan Rogel Cancer Center to David Cordova. Preparation of this manuscript was supported, in part, by grants from the National Institute of Mental Health to Torsten B Neilands (R25 MH067127), and the National Institute on Drug Abuse (R03DA04189101A1) to David Cordova.
Abbreviations
- ATOD
alcohol, tobacco, other drug use
- CBPR
community-based participatory research
- mHealth
mobile Health
- MPI
Matched Pair Instrument
- RCT
randomized controlled trial
- S4E
Storytelling For Empowerment
- SMART
Sequential Multiple Assignment Randomized Trial
- STI
sexually transmitted infection
Appendix
S4E youth risk behavior assessment.
CONSORT-eHEALTH checklist (V 1.6.1).
Footnotes
Conflicts of Interest: None declared.
References
- 1.Centers for Disease Control and Prevention (CDC) 2019. Jul 30, [2019-07-12]. STDs in Adolescents and Young Adults https://www.cdc.gov/std/stats18/adolescents.htm.
- 2.Ocfemia MC, Dunville R, Zhang T, Barrios LC, Oster AM. HIV diagnoses among persons aged 13-29 years - United States, 2010-2014. MMWR Morb Mortal Wkly Rep. 2018 Feb 23;67(7):212–5. doi: 10.15585/mmwr.mm6707a2. doi: 10.15585/mmwr.mm6707a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep. 2018 Nov;67(8):1–50. [PubMed] [Google Scholar]
- 4.Sedgh G, Finer LB, Bankole A, Eilers MA, Singh S. Adolescent pregnancy, birth, and abortion rates across countries: levels and recent trends. J Adolesc Health. 2015 Feb;56(2):223–30. doi: 10.1016/j.jadohealth.2014.09.007. https://linkinghub.elsevier.com/retrieve/pii/S1054-139X(14)00387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) 2018. Jul 24, [2019-08-15]. STDs in Women and Infants https://www.cdc.gov/std/stats17/womenandinf.htm.
- 6.Centers for Disease Control and Prevention (CDC) 2019. Aug 6, [2019-08-15]. AIDS and Opportunistic Infections https://www.cdc.gov/hiv/basics/livingwithhiv/opportunisticinfections.html.
- 7.National Conference of State Legislatures (NCSL) 2018. Oct 11, [2019-08-15]. Teen Pregnancy Prevention http://www.ncsl.org/research/health/teen-pregnancy-prevention.aspx.
- 8.Ritchwood TD, Ford H, DeCoster J, Sutton M, Lochman JE. Risky sexual behavior and substance use among adolescents: a meta-analysis. Child Youth Serv Rev. 2015 May;52:74–88. doi: 10.1016/j.childyouth.2015.03.005. http://europepmc.org/abstract/MED/25825550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) 2019. Aug 13, [2019-09-10]. Sexual Risk Behaviors Can Lead to HIV, STDs, & Teen Pregnancy https://www.cdc.gov/healthyyouth/sexualbehaviors.
- 10.Centers for Disease Control and Prevention (CDC) 2019. Jul, [2019-09-10]. Substance Use and Sexual Risk Behaviors Among Youth https://www.cdc.gov/healthyyouth/substance-use/pdf/dash-substance-use-fact-sheet.pdf.
- 11.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Queen B, Lowry R, Chyen D, Whittle L, Thornton J, Lim C, Bradford D, Yamakawa Y, Leon M, Brener N, Ethier KA. Youth Risk Behavior Surveillance - United States, 2017. MMWR Surveill Summ. 2018 Jun 15;67(8):1–114. doi: 10.15585/mmwr.ss6708a1. http://europepmc.org/abstract/MED/29902162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordova D, Huang S, Arzon M, Freitas D, Malcolm S, Prado G. The role of attitudes, family, peer and school on alcohol use, rule breaking and aggressive behavior in Hispanic delinquent adolescents. Open Fam Stud J. 2011;4(Suppl 1-M4):38–45. doi: 10.2174/1874922401104010038. http://europepmc.org/abstract/MED/22473467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clayton HB, Lowry R, August E, Jones SE. Nonmedical use of prescription drugs and sexual risk behaviors. Pediatrics. 2016 Jan;137(1) doi: 10.1542/peds.2015-2480. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=26668299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Córdova D, Heinze JE, Hsieh H, Mistry R, Salas-Wright CP, Cook SH, Zimmerman MA. Are trajectories of a syndemic index in adolescence linked to HIV vulnerability in emerging and young adulthood? AIDS. 2018 Feb 20;32(4):495–503. doi: 10.1097/QAD.0000000000001717. http://europepmc.org/abstract/MED/29239889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Office of Disease Prevention and Health Promotion Healthy People 2020. 2020. [2019-09-02]. Sexually Transmitted Diseases https://www.healthypeople.gov/node/3523/objectives#5267.
- 16.Centers for Disease Control and Prevention (CDC) 2018. Apr, [2019-09-02]. HIV Infection, Risk, Prevention, and Testing Behaviors Among Heterosexuals at Increased Risk for HIV Infection - National HIV Behavioral Surveillance 17 US Cities, 2016 https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-number-19.pdf.
- 17.Centers for Disease Control and Prevention (CDC) 2013. Apr, [2019-09-02]. Sexually Transmitted Infections Among Young Americans https://www.cdc.gov/std/products/youth-sti-infographic.pdf.
- 18.Buzi RS, Madanay FL, Smith PB. Integrating routine HIV testing into family planning clinics that treat adolescents and young adults. Public Health Rep. 2016;131(Suppl 1):130–8. doi: 10.1177/00333549161310S115. http://europepmc.org/abstract/MED/26862238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bensussen-Walls W, Saewyc EM. Teen-focused care versus adult-focused care for the high-risk pregnant adolescent: an outcomes evaluation. Public Health Nurs. 2001;18(6):424–35. doi: 10.1046/j.1525-1446.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 20.Shafii T, Benson SK, Morrison DM. Brief motivational interviewing delivered by clinician or computer to reduce sexual risk behaviors in adolescents: acceptability study. J Med Internet Res. 2019 Jul 10;21(7):e13220. doi: 10.2196/13220. https://www.jmir.org/2019/7/e13220/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jemmott JB, Jemmott LS, Braverman PK, Fong GT. HIV/STD risk reduction interventions for African American and Latino adolescent girls at an adolescent medicine clinic: a randomized controlled trial. Arch Pediatr Adolesc Med. 2005 May;159(5):440–9. doi: 10.1001/archpedi.159.5.440. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . mHealth: New Horizons For Health Through Mobile Technologies: Second Global Survey On eHealth (Global Observatory For eHealth) Geneva: World Health Organization; 2011. [Google Scholar]
- 23.Bauermeister JA, Pingel ES, Jadwin-Cakmak L, Harper GW, Horvath K, Weiss G, Dittus P. Acceptability and preliminary efficacy of a tailored online HIV/STI testing intervention for young men who have sex with men: the Get Connected! program. AIDS Behav. 2015 Oct;19(10):1860–74. doi: 10.1007/s10461-015-1009-y. http://europepmc.org/abstract/MED/25638038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrier LA, Burke PJ, Kells M, Scherer EA, Sarda V, Jonestrask C, Xuan Z, Harris SK. Pilot randomized trial of MOMENT, a motivational counseling-plus-ecological momentary intervention to reduce marijuana use in youth. Mhealth. 2018;4:29. doi: 10.21037/mhealth.2018.07.04. doi: 10.21037/mhealth.2018.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biello KB, Marrow E, Mimiaga MJ, Sullivan P, Hightow-Weidman L, Mayer KH. A mobile-based app (MyChoices) to increase uptake of HIV testing and pre-exposure prophylaxis by young men who have sex with men: protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2019 Jan 7;8(1):e10694. doi: 10.2196/10694. https://www.researchprotocols.org/2019/1/e10694/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lelutiu-Weinberger C, Manu M, Ionescu F, Dogaru B, Kovacs T, Dorobantescu C, Predescu M, Surace A, Pachankis JE. An mHealth intervention to improve young gay and bisexual men's sexual, behavioral, and mental health in a structurally stigmatizing national context. JMIR Mhealth Uhealth. 2018 Nov 14;6(11):e183. doi: 10.2196/mhealth.9283. https://mhealth.jmir.org/2018/11/e183/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ybarra ML, Prescott T, Mustanski B, Parsons J, Bull SS. Feasibility, acceptability, and process indicators for Guy2Guy, an mHealth HIV prevention program for sexual minority adolescent boys. J Adolesc Health. 2019 Sep;65(3):417–22. doi: 10.1016/j.jadohealth.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauermeister JA, Tingler RC, Demers M, Harper GW. Development of a tailored HIV prevention intervention for single young men who have sex with men who meet partners online: Protocol for the myDEx project. JMIR Res Protoc. 2017 Jul 19;6(7):e141. doi: 10.2196/resprot.7965. https://www.researchprotocols.org/2017/7/e141/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu A, Coleman K, Bojan K, Serrano PA, Oyedele T, Garcia A, Enriquez-Bruce E, Emmanuel P, Jones J, Sullivan P, Hightow-Weidman L, Buchbinder S, Scott H. Developing a mobile app (LYNX) to support linkage to HIV/sexually transmitted infection testing and pre-exposure prophylaxis for young men who have sex with men: protocol for a randomized controlled trial. JMIR Res Protoc. 2019 Jan 25;8(1):e10659. doi: 10.2196/10659. https://www.researchprotocols.org/2019/1/e10659/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordova D, Alers-Rojas F, Lua FM, Bauermeister J, Nurenberg R, Ovadje L, Fessler K, Delva J, Salas-Wright CP, Council YL. The usability and acceptability of an adolescent mHealth HIV/STI and drug abuse preventive intervention in primary care. Behav Med. 2018;44(1):36–47. doi: 10.1080/08964289.2016.1189396. http://europepmc.org/abstract/MED/27223646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. http://europepmc.org/abstract/MED/18688292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) 2019. Apr, [2019-08-19]. HIV and Youth https://www.cdc.gov/hiv/pdf/group/age/youth/cdc-hiv-youth.pdf.
- 33.Forhan SE, Gottlieb SL, Sternberg MR, Xu F, Datta SD, McQuillan GM, Berman SM, Markowitz LE. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics. 2009 Dec;124(6):1505–12. doi: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) 2019. [2019-08-20]. HIV Surveillance – Adolescents and Young Adults https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-adolescents-young-adults-2017.pdf.
- 35.Israel BA, Eng E, Schulz AJ, Parker EA. Methods For Community-Based Participatory Research For Health. Hoboken, NJ: Wiley; 2005. Introduction to methods for CBPR for health. [Google Scholar]
- 36.Cordova D, Bauermeister JA, Fessler K, Delva J, Nelson A, Nurenberg R, Lua FM, Alers-Rojas F, Salas-Wright CP, Youth Leadership Council A community-engaged approach to developing an mHealth HIV/STI and drug abuse preventive intervention for primary care: a qualitative study. JMIR Mhealth Uhealth. 2015 Dec 18;3(4):e106. doi: 10.2196/mhealth.4620. https://mhealth.jmir.org/2015/4/e106/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szapocznik J, Coatsworth JD. An ecodevelopmental framework for organizing the influences on drug abuse: a developmental model of risk and protection. In: Glantz MD, Hartel CR, editors. Drug abuse: Origins & interventions. Washington, DC: American Psychological Association; 1999. pp. 331–66. [Google Scholar]
- 38.Zimmerman MA, Israel BA, Schulz A, Checkoway B. Further explorations in empowerment theory: an empirical analysis of psychological empowerment. Am J Commun Psychol. 1992;20(6):707–27. doi: 10.1007/bf00942234. [DOI] [Google Scholar]
- 39.Cordova D, Lua FM, Muñoz-Velázquez J, Street K, Bauermeister JA, Fessler K, Adelman N, Youth Leadership Council. Neilands TB, Boyer CB. A multilevel mHealth drug abuse and STI/HIV preventive intervention for clinic settings in the United States: a feasibility and acceptability study. PLoS One. 2019;14(8):e0221508. doi: 10.1371/journal.pone.0221508. http://dx.plos.org/10.1371/journal.pone.0221508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaeffer K. Pew Research Center. 2019. Aug 23, [2019-11-12]. Most US Teens Who Use Cellphones Do It to Pass Time, Connect With Others, Learn New Things https://www.pewresearch.org/fact-tank/2019/08/23/most-u-s-teens-who-use-cellphones- do-it-to-pass-time-connect-with-others-learn-new-things/
- 41.Robertson EB, David SL, Rao SA. Preventing Drug Use Among Children And Adolescents. Bethesda, MD: National Institute on Drug Abuse; 2004. [Google Scholar]
- 42.Kidwell KM, Postow MA, Panageas KS. Sequential, multiple assignment, randomized trial designs in immuno-oncology research. Clin Cancer Res. 2018 Feb 15;24(4):730–6. doi: 10.1158/1078-0432.CCR-17-1355. http://clincancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=28835379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oesterle TS, Hitschfeld MJ, Lineberry TW, Schneekloth TD. CRAFFT as a substance use screening instrument for adolescent psychiatry admissions. J Psychiatr Pract. 2015 Jul;21(4):259–66. doi: 10.1097/PRA.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 44.Campbell C, Lockyer J, Laidlaw T, Macleod H. Assessment of a matched-pair instrument to examine doctor-patient communication skills in practising doctors. Med Educ. 2007 Feb;41(2):123–9. doi: 10.1111/j.1365-2929.2006.02657.x. [DOI] [PubMed] [Google Scholar]
- 45.Johnston LD, Miech RA, O'Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Deep Blue. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2018. [2020-03-03]. Monitoring the Future National Survey Results On Drug Use, 1975-2017: Overview, Key Findings on Adolescent Drug Use https://deepblue.lib.umich.edu/bitstream/handle/2027.42/148123/Overview%202018%20FINAL%20print%201-30.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 46.Johnston LD, O'Malley RA, Miech RA, Bachman JG, Schulenberg JE. Education Resources Information Center. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2018. [2020-03-03]. Demographic Subgroup Trends among Adolescents in the Use of Various Licit and Illicit Drugs, 1975-2017 https://eric.ed.gov/?id=ED589759. [Google Scholar]
- 47.Jemmott JB, Jemmott LS, Fong GT. Abstinence and safer sex HIV risk-reduction interventions for African American adolescents: a randomized controlled trial. J Am Med Assoc. 1998 May 20;279(19):1529–36. doi: 10.1001/jama.279.19.1529. [DOI] [PubMed] [Google Scholar]
- 48.Kendall JM. Designing a research project: randomised controlled trials and their principles. Emerg Med J. 2003 Mar;20(2):164–8. doi: 10.1136/emj.20.2.164. http://emj.bmj.com/cgi/pmidlookup?view=long&pmid=12642531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen J. A power primer. Psychol Bull. 1992 Jul;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 50.IBM Corp. Armonk, NY: IBM Corp; 2016. [2020-03-03]. IBM SPSS Statistics 24.0 https://www.ibm.com/support/pages/release-notes-ibm-spss-statistics-240. [Google Scholar]
- 51.Champely S, Ekstrom C, Dalgaard P, Gill J, Weibelzahl S, Anandkumar A, Ford C, Volcic R, de Rosario H. CRAN - R Project. 2018. Mar 3, [2020-03-03]. Package 'pwr' https://cran.r-project.org/web/packages/pwr/pwr.pdf.
- 52.Carper MM, Makover HB, Kendall PC. Future directions for the examination of mediators of treatment outcomes in youth. J Clin Child Adolesc Psychol. 2018;47(2):345–56. doi: 10.1080/15374416.2017.1359786. http://europepmc.org/abstract/MED/28841335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Korte E, Wiezer N, Roozeboom MB, Vink P, Kraaij W. Behavior change techniques in mHealth apps for the mental and physical health of employees: systematic assessment. JMIR Mhealth Uhealth. 2018 Oct 3;6(10):e167. doi: 10.2196/mhealth.6363. https://mhealth.jmir.org/2018/10/e167/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC) 2013. [2019-08-21]. Sexually Transmitted Infections among Young Americans Infographic https://www.cdc.gov/nchhstp/newsroom/2013/sam-2013.html.
- 55.Conserve DF, Jennings L, Aguiar C, Shin G, Handler L, Maman S. Systematic review of mobile health behavioural interventions to improve uptake of HIV testing for vulnerable and key populations. J Telemed Telecare. 2017 Feb;23(2):347–59. doi: 10.1177/1357633X16639186. http://europepmc.org/abstract/MED/27056905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howe KB, Suharlim C, Ueda P, Howe D, Kawachi I, Rimm EB. Gotta catch'em all! Pokémon GO and physical activity among young adults: difference in differences study. Br Med J. 2016 Dec 13;355:i6270. doi: 10.1136/bmj.i6270. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=27965211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernet AC, Willens DE, Bauer MS. Effectiveness-implementation hybrid designs: implications for quality improvement science. Implementation Sci. 2013;8(Suppl 1):S2. doi: 10.1186/1748-5908-8-S1-S2. [DOI] [Google Scholar]
- 58.Hemming K, Taljaard M, McKenzie JE, Hooper R, Copas A, Thompson JA, Dixon-Woods M, Aldcroft A, Doussau A, Grayling M, Kristunas C, Goldstein CE, Campbell MK, Girling A, Eldridge S, Campbell MJ, Lilford RJ, Weijer C, Forbes AB, Grimshaw JM. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. Br Med J. 2018 Nov 9;363:k1614. doi: 10.1136/bmj.k1614. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=30413417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A 'SMART' design for building individualized treatment sequences. Annu Rev Clin Psychol. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. http://europepmc.org/abstract/MED/22224838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kidwell KM, Hyde LW. Adaptive Interventions and SMART Designs: application to child behavior research in a community setting. Am J Eval. 2016 Sep;37(3):344–63. doi: 10.1177/1098214015617013. http://europepmc.org/abstract/MED/28239254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014 Sep;4(3):260–74. doi: 10.1007/s13142-014-0265-0. http://europepmc.org/abstract/MED/25264466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vock DM, Almirall D. Wiley Online Library. 2018. Aug, [2020-03-03]. Wiley StatsRef: Statistics Reference Online - Sequential Multiple Assignment Randomized Trial (SMART) https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118445112.stat08073.
- 63.Substance Abuse and Mental Health Services Administration (SAMHSA) 2018. Nov 2, [2019-09-12]. SAMHSA Strategic Plan FY2019-FY2023 https://www.samhsa.gov/about-us/strategic-plan.
- 64.US Department of Health and Human Services HIV.gov. 2015. Aug 2, [2019-09-20]. National HIV/AIDS Strategy: Updated to 2020. 5 Major Changes Since 2010 https://files.hiv.gov/s3fs-public/nhas-update-5-things.pdf. [DOI] [PubMed]
- 65.UNAIDS The Joint United Nations Programme on HIV/AIDS (UNAIDS) 2014. Oct, [2019-09-15]. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. [PubMed]
- 66.US Department of Health and Human Services Healthy People 2020. 2020. [2019-09-02]. Family Planning https://www.healthypeople.gov/2020/topics-objectives/topic/family-planning/objectives.
- 67.Rose S, Laan MJ. Why match? Investigating matched case-control study designs with causal effect estimation. Int J Biostat. 2009 Jan 6;5(1):Article 1. doi: 10.2202/1557-4679.1127. http://europepmc.org/abstract/MED/20231866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aycock DM, Hayat MJ, Helvig A, Dunbar SB, Clark PC. Essential considerations in developing attention control groups in behavioral research. Res Nurs Health. 2018 Jun;41(3):320–8. doi: 10.1002/nur.21870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S4E youth risk behavior assessment.
CONSORT-eHEALTH checklist (V 1.6.1).


