Summary
Human mononuclear phagocytes comprise several subsets of dendritic cells (DCs), monocytes, and macrophages. Distinguishing one population from another is challenging, especially in inflamed tissues, owing to the promiscuous expression of phenotypic markers. Using a synthetic library of humanized llama single domain antibodies, we identified a novel surface marker for human naturally occurring monocyte-derived DCs. Our antibody targets an extra-cellular domain of LSP-1, specifically on monocyte-derived DCs, but not on other leukocytes, in particular monocytes, macrophages, classical DCs, or the recently described blood DC3 population. Our findings will pave the way for a better characterization of human mononuclear phagocytes in pathological settings.
Subject Areas: Molecular Biology
Graphical Abstract
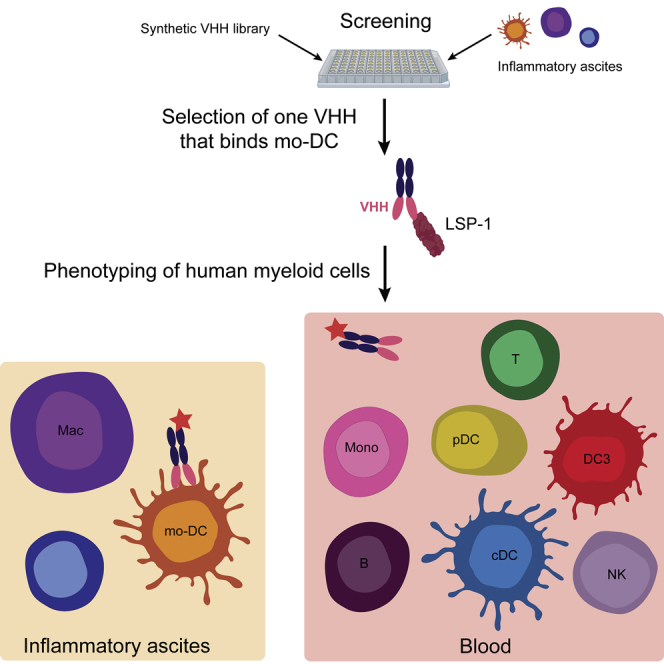
Highlights
-
•
Identification of a humanized llama single chain antibody recognizing surface LSP-1
-
•
Surface LSP-1 is a specific marker of monocyte-derived dendritic cells
-
•
Classical dendritic cells or blood “DC3” are not stained by the antibody
-
•
Surface LSP-1 may be a useful marker for analyzing myeloid cells in human disease
Molecular Biology
Introduction
Mononuclear phagocytes play a central role in the initiation and resolution of innate and adaptive immune responses. They comprise several populations of monocytes, macrophages, and dendritic cells (DCs), which are classically defined by their phenotype and ontogeny (Guilliams et al., 2014). Monocytes are very plastic cells and can differentiate into cells displaying typical features of macrophages or DCs, referred to as monocyte-derived macrophages (mo-Mac) and monocyte-derived DCs (mo-DC). This phenomenon has been described in vitro and in vivo, in both mouse and human (Jakubzick et al., 2017, Coillard and Segura, 2019, Ginhoux and Guilliams, 2016). Recently, high-resolution single-cell technologies have revealed a new subset of blood DCs, termed DC3, displaying a mixed transcriptomic profile of classical DC (cDC) and monocyte genes (Bakdash et al., 2016, Dutertre et al., 2019, Villani et al., 2017), reminiscent of that of mo-DC (Segura et al., 2013, Goudot et al., 2017). Whether blood DC3 represent circulating mo-DC has been unclear.
Accumulating evidence suggests that monocyte-derived cells are involved in the pathogenesis of autoimmune and chronic inflammatory diseases (Coillard and Segura, 2019). However, their characterization in inflamed tissues is complicated by the promiscuous expression of numerous phenotypic markers, shared in particular with macrophages and cDCs. More specific markers are needed in order to advance our understanding of the respective properties of macrophages, cDCs, and mo-DC, and ultimately to allow the manipulation of these cells for therapeutic strategies.
To identify novel markers for human mo-DC, we have used a synthetic library of humanized llama single domain antibodies. We identified one antibody, recognizing surface LSP-1, that stains specifically mo-DC, but not monocytes, macrophages, cDCs, or DC3.
Results
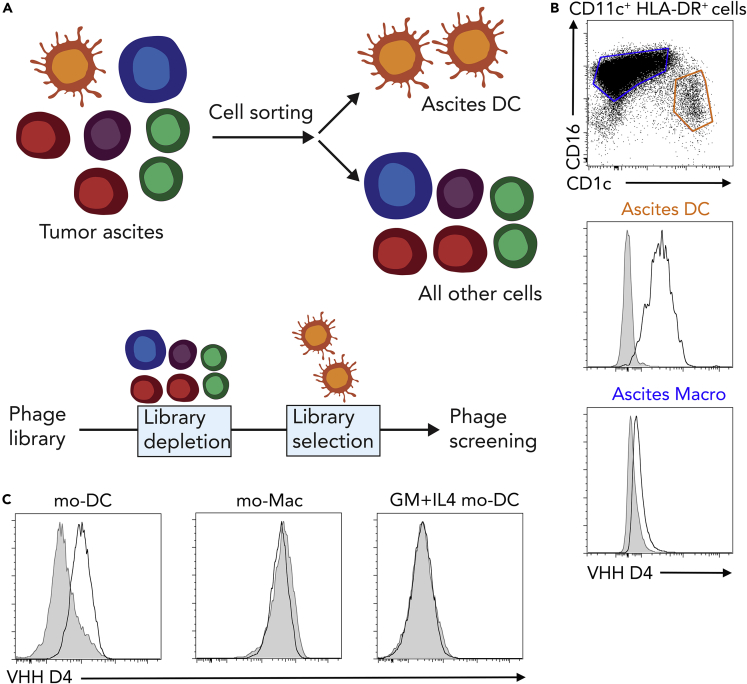
In order to identify novel surface markers for human naturally occurring mo-DC, we set up a phage display screen using a synthetic library of humanized llama single domain antibodies (termed VHH) (Moutel et al., 2016). We have previously shown that peritoneal ascites from patients with cancer contain a population of mo-DC (Segura et al., 2013, Tang-Huau et al., 2018). Cells from tumor ascites were separated into DCs and all other cells (non-DCs), including tumor cells, macrophages, T cells, and other immune cells. The library was first depleted for phages binding to non-DCs, then phages binding to ascites DCs were screened (Figure 1A). For subsequent studies, we produced the VHH of interest with a human Fc region containing a streptavidin-binding peptide. Using this strategy, we identified a VHH antibody, termed “D4,” that stains ascites DCs but not macrophages from the same samples (Figure 1B). We have recently reported a culture model allowing the generation of in vitro counterparts of naturally occurring mo-DC and mo-Mac, by culturing CD14+ monocytes with M-CSF, IL-4, and TNFα (Goudot et al., 2017). In this model, the VHH D4 stained mo-DC but not mo-Mac (Figure 1C). We have also shown that the classical model of culturing monocytes with GM-CSF and IL-4 generates mo-DC that do not closely resemble the ones found in vivo in inflammatory fluids (Goudot et al., 2017). Consistent with this, the VHH D4 did not stain mo-DC generated with GM-CSF and IL-4 (Figure 1C).
Figure 1.
Identification of a VHH Specific for Monocyte-Derived Dendritic Cells
(A) Strategy for identifying novel surface markers for ascites dendritic cells (DCs).
(B) Ascites cells were stained with VHH D4. Stainings for DC and macrophages (Macro) are shown (representative of five individual donors). Gray shaded histograms are fluorescence-minus-one controls.
(C) Monocytes were cultured with M-CSF, IL-4, and TNFa to generate DC (mo-DC) and macrophages (mo-Mac) or with GM-CSF and IL-4 to generate DC (GM + IL4 mo-DC). Cells were stained with VHH D4. Gray shaded histograms are fluorescence-minus-one controls. Representative of four individual donors.
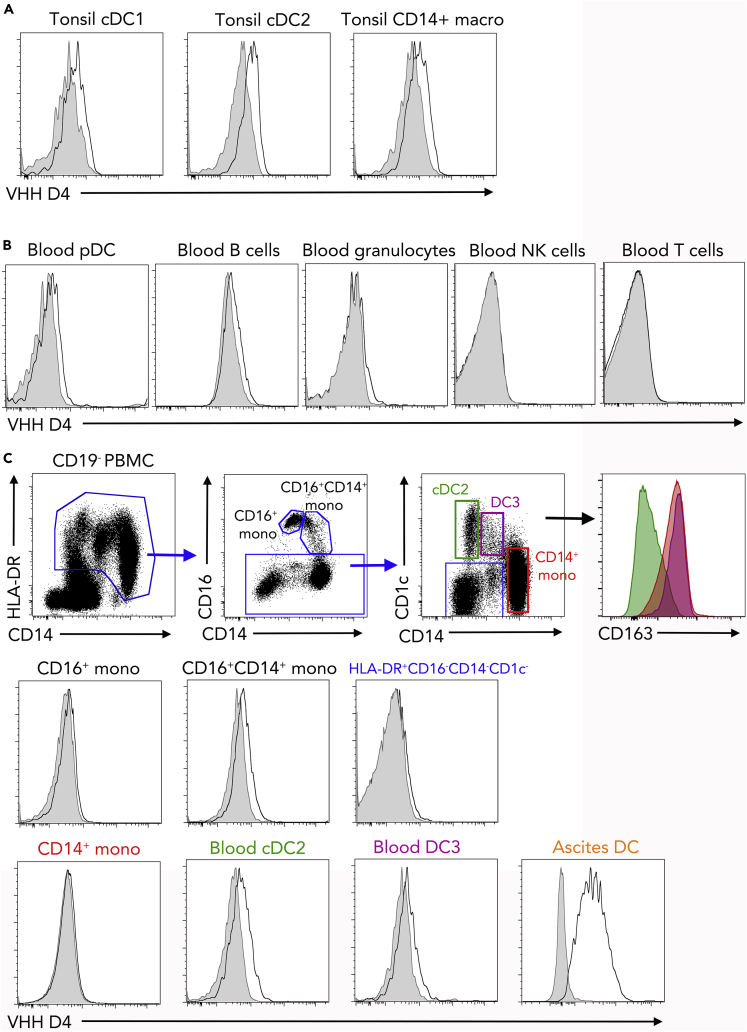
We then sought to address whether the VHH D4 could also stain other myeloid cells, in particular cDCs. We first used the VHH D4 on cells isolated from human tonsils. We have previously shown using single-cell RNA sequencing (RNA-seq) analysis that tonsils contain cDC1 (CD141highCD1c− DCs) and cDC2 (CD141lowCD1c+ DCs), but no population of mo-DC (Durand et al., 2019). There was no significant staining of VHH D4 on cDC1, cDC2, or tonsil macrophages (Figure 2A). This result suggests that the VHH D4 is specific of mo-DC and does not stain cDCs.
Figure 2.
VHH D4 Does Not Stain Classical Dendritic Cell Populations
(A) Tonsil DC and macrophages were stained with VHH D4. Gray shaded histograms are fluorescence-minus-one controls. Representative of two individual donors. Peripheral blood cells (B and C) or ascites cells (C) were stained with VHH D4. Gray shaded histograms are fluorescence-minus-one controls. Representative of five (blood) or four (ascites) individual donors. (C) Gating strategy for analyzing HLA-DR+ cells, including CD14+ monocytes, cDC2, and DC3, is shown. Staining of ascites DC is shown as a positive control.
To extend these observations, we stained peripheral blood cells. There was no significant staining on plasmacytoid DCs (pDC), B cells, granulocytes, NK cells, or T cells (Figure 2B). We further dissected VHH D4 staining on myeloid cells (Figure 2C). Blood monocytes comprise three subpopulations defined by their expression of CD16 and CD14. In addition, it has recently been shown that blood CD1c+ DCs are heterogeneous and comprise CD1c+CD14−CD163− DCs (bona fide cDC2) and CD1c+CD14+CD163+ DCs, termed DC3 (Alcantara-Hernandez et al., 2017, Bakdash et al., 2016, Dutertre et al., 2019, Villani et al., 2017). The VHH D4 did not show a significant staining on blood monocytes, cDC2, DC3, or other CD19−HLA-DR+ cells (Figure 2C). These results confirm that VHH D4 does not recognize cDCs or other blood leukocyte populations.
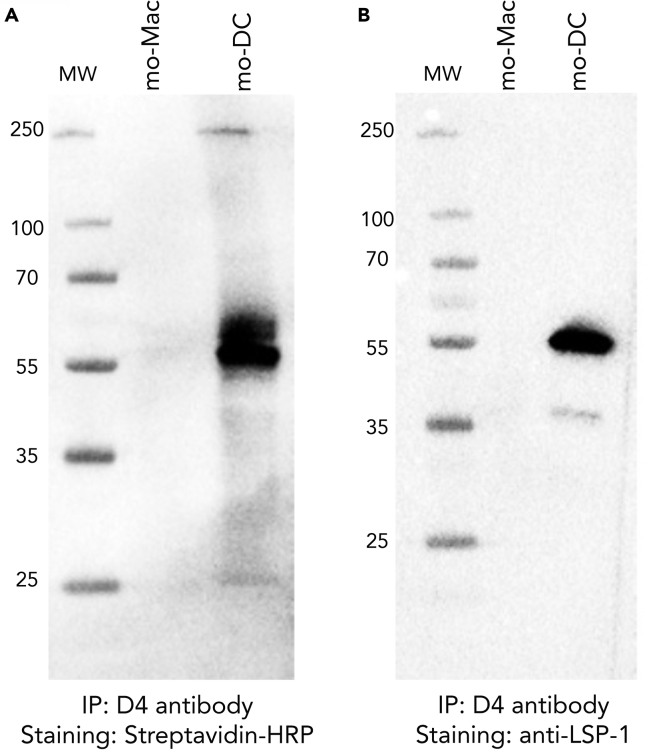
To determine the target of VHH D4 on the surface of mo-DC, we performed immuno-precipitation followed by proteomics mass spectrometry, using in vitro-generated mo-DC (cultured with M-CSF, IL-4, and TNFα) as a source of material. Mass spectrometry identified only one protein: Lymphocyte-Specific Protein 1 (LSP-1), a protein of predicted molecular weight of 60 kDa. To validate this result, we performed immuno-precipitation with VHH D4 on in vitro-generated mo-DC and mo-Mac and revealed the immuno-precipitated proteins using streptavidin (Figure 3A) or a commercial anti-LSP-1 antibody (Figure 3B). Both methods showed the same band around 55 kDa, only in immuno-precipitated material from mo-DC. This observation confirms that the VHH D4 binds LSP-1.
Figure 3.
Identification of LSP-1 as the Target of VHH D4
In vitro-generated mo-DC or mo-Mac were incubated with VHH D4, then lysed. Immuno-precipitation was performed on cell lysate using the streptavidin-binding peptide tag. MW, molecular weight. Immuno-precipitated material was analyzed by western blot. Staining was performed using streptavidin-HRP (A) or a commercial anti-LSP-1 antibody (B).
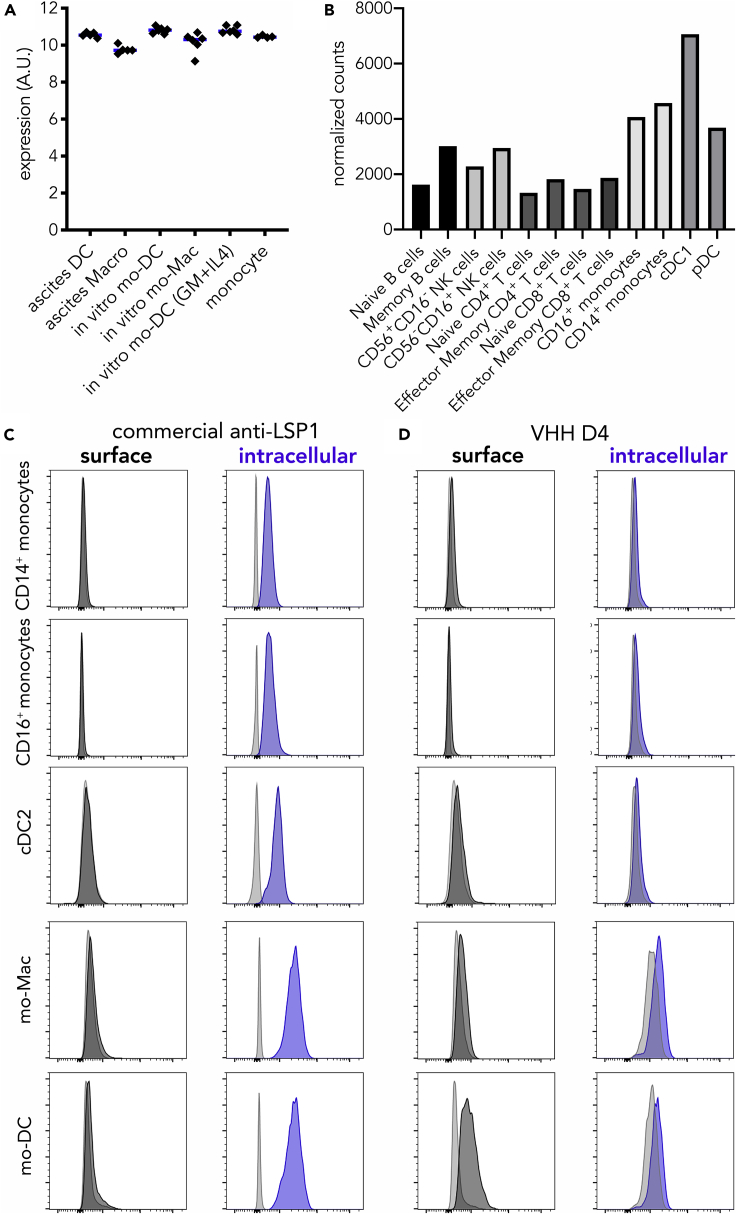
LSP-1 is an F-actin binding protein reported to be expressed in all leukocytes (Pulford et al., 1999). Using our previously generated transcriptomic data (Goudot et al., 2017), we confirmed that LSP1 was expressed at similar levels in ascites mo-DC; ascites macrophages; in vitro mo-DC and mo-Mac generated with M-CSF, IL-4, and TNFα; in vitro mo-DC generated with GM-CSF and IL-4; and blood monocytes (Figure 4A). Using transcriptomic data from the Human Cell Atlas, we also observed that LSP1 was expressed in all blood leukocytes, with myeloid cells displaying the highest levels (Figure 4B). To confirm the intracellular expression of LSP-1, we performed flow cytometry on permeabilized cells using either a commercial anti-LSP1 antibody or VHH D4. Although the commercial anti-LSP1 did not show any surface staining (including on mo-DC), it detected intracellular LSP1 in blood myeloid cells and in vitro-generated mo-DC and mo-Mac (Figure 4C). By contrast, VHH D4 did not stain intracellular LSP1 in blood myeloid cells or mo-Mac and mo-DC (Figure 4D). These results suggest that VHH D4 recognizes a surface epitope of LSP-1 that is specifically expressed by mo-DC.
Figure 4.
VHH D4 Recognizes a Surface Epitope of LSP-1 Specifically Expressed by mo-DC
(A) Expression levels (arbitrary units) of LSP1 in ascites DC; ascites macrophages; in vitro mo-DC and mo-Mac generated with M-CSF, IL-4, and TNFα; in vitro mo-DC generated with GM-CSF and IL-4; and blood CD14+ monocytes. Each dot represents an individual donor. Median is shown. Affymetrix data from dataset GSE102046.
(B) Normalized counts of LSP1 expression in selected human blood cells. RNA-seq data from the Human Cell Atlas (http://immunecellatlas.net).
(C and D) Monocytes were cultured with M-CSF, IL-4, and TNFα to generate DC (mo-DC) and macrophages (mo-Mac). Peripheral blood myeloid cells were also analyzed (monocytes and cDC2). Cells were stained with a commercial anti-LSP-1 antibody (C) or VHH D4 (D), with membrane permeabilization (intracellular) or not (surface). Gray shaded histograms are fluorescence-minus-one controls. Representative of five (blood) or six (in vitro-generated) individual donors.
Discussion
Using a synthetic library of humanized llama single domain antibodies, we have identified one antibody, recognizing LSP-1, that is specific for human mo-DC and distinguishes them from monocytes, macrophages, cDCs, or DC3.
LSP-1 has been shown so far to reside on the cytoplasmic side of the plasma membrane (Klein et al., 1989). After membrane permeabilization, LSP-1 can be detected by flow cytometry, consistent with previous work (Pulford et al., 1999). Because VHH D4 stains mo-DC without membrane permeabilization, our results suggest the existence of an extra-cellular domain of LSP-1 specifically in mo-DC. The VHH D4 stains both mo-DC found in vivo in tumor ascites and generated in vitro with M-CSF, IL-4, and TNFα, indicating that the expression of a surface domain of LSP-1 is not due to the inflammatory micro-environment, but rather could be the result of a mo-DC core transcriptional program. Whether surface LSP-1 results from alternative splicing or another post-transcriptional mechanism remains open for future investigation.
DC3 express a mixed transcriptional program with hallmark cDC2 genes (such as FCER1A, CD1C, CLEC10A) as well as typical monocyte genes (such as S100A8, S100A9, VCAN) (Bakdash et al., 2016, Dutertre et al., 2019, Villani et al., 2017), raising the question of their ontogeny, in particular whether DC3 correspond to circulating mo-DC. Our results add to different lines of evidence suggesting that DC3 are distinct from mo-DC. Blood cDC2 and DC3, but not monocytes, were increased upon injection of Flt3-ligand to patients with lymphoma (Dutertre et al., 2019). In addition, in vitro differentiation assays and analysis of an allelic series of human IRF8 deficiency suggested that DC3 and monocytes derive from a common progenitor but along distinct differentiation pathways (Cytlak et al., 2019). Here, we did not observe a significant staining of VHH D4 on blood DC3, showing that the phenotype of DC3 is distinct from that of ascites mo-DC. This is consistent with the hypothesis that DC3 do not represent circulating blood mo-DC.
Together with our previous phenotypic characterization of mo-DC (Goudot et al., 2017) and recent single-cell analysis of blood cDC2, DC3, and monocytes (Dutertre et al., 2019, Villani et al., 2017), our work enables a more precise phenotypic definition of human DC subsets. cDC2 can be defined as CD88−CD14−CD1c+FcεRI+CD226−CD163−sLSP-1-, DC3 as CD88−CD14+CD1c+FcεRI+CD226−CD163+sLSP-1−, and mo-DC as CD88lowCD14+CD1c+FcεRI+CD226+CD163−sLSP-1+.
Mo-DC have been identified in several inflammatory contexts in human tissues (Coillard and Segura, 2019). In cancer, the picture is less clear as DCs displaying a phenotype consistent with both mo-DC and DC3 have been reported in colorectal, breast, and lung tumors (Laoui et al., 2016, Lavin et al., 2017, Michea et al., 2018). DC3 are increased in the blood of patients with melanoma (Bakdash et al., 2016) and systemic lupus erythematosus (Dutertre et al., 2019), whereas blood DC3 infiltrate the lung alveolar space upon LPS-induced acute inflammation (Jardine et al., 2019). The respective role of these two DC subtypes in cancer or inflammation remains elusive. Our findings will pave the way for a better characterization of DC3 and mo-DC in pathological settings, ultimately allowing their manipulation for therapeutic strategies.
Limitations of the Study
In this work, we have identified one antibody (VHH D4) that is specific for human mo-DC present in ascites or derived in vitro from monocytes using a combination of M-CSF, IL-4, and TNFα. Further work is needed to address whether VHH D4 also stains the mo-DC that have been described in other human tissues, such as skin, lung, or gut. In addition, complementary markers, such as defined in the Discussion, should be used for the accurate identification of myeloid cells in human tissues.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by Inserm, CNRS, Agence Nationale de la Recherche (ANR-10-LABX-0043, ANR-CHIN-0002, ANR-10-IDEX-0001-02 PSL), Institut Curie (CIC IGR-Curie 1428), the European Research Council (2013-AdG N° 340046 DCBIOX), and Cancéropôle Île-de-France (2017-1-EMERG-56-ICR-1).
The authors wish to thank A. Coillard for technical assistance, the Flow Cytometry Platform of Institut Curie for cell sorting and the TAb-IP platform of Institut Curie for the production of the VHH.
Author Contributions
S.M., S.A., F.P., and E.S. designed experiments. S.M., A.B., A.S., B.L., and E.S. performed experiments. S.M., A.B., D.L., and E.S. analyzed the data. E.S. prepared the figures and wrote the manuscript, with input from all authors.
Declaration of Interests
S.M., S.A., F.P., and E.S. are co-inventors of a patent entitled "New anti-LSP1 antibody" (PCT/EP2017/0761). S.M. and F.P. are co-inventors of a patent that covers the commercial use of the library (WO/2015/063331). The authors declare no other competing interest. The authors adhere to Cell Press policy on sharing materials.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100987.
Data and Code Availability
Proteomics data are available via ProteomeXchange (identifier PXD015647).
Supplemental Information
References
- Alcantara-Hernandez M., Leylek R., Wagar L.E., Engleman E.G., Keler T., Marinkovich M.P., Davis M.M., Nolan G.P., Idoyaga J. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity. 2017;47:1037–1050.e6. doi: 10.1016/j.immuni.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakdash G., Buschow S.I., Gorris M.A., Halilovic A., Hato S.V., Skold A.E., Schreibelt G., Sittig S.P., Torensma R., Duiveman-De Boer T. Expansion of A Bdca1+Cd14+ myeloid cell population in melanoma patients may attenuate the efficacy of dendritic cell vaccines. Cancer Res. 2016;76:4332–4346. doi: 10.1158/0008-5472.CAN-15-1695. [DOI] [PubMed] [Google Scholar]
- Coillard A., Segura E. In vivo differentiation of human monocytes. Front. Immunol. 2019;10:1907. doi: 10.3389/fimmu.2019.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cytlak U., Resteu A., Pagan S., Green K., Milne P., Maisuria S., Mcdonald D., Hulme G., Filby A., Carpenter B. SSRN; 2019. Differential Irf8 Requirement Defines Two Pathways of Dendritic Cell Development in Humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand M., Walter T., Pirnay T., Naessens T., Gueguen P., Goudot C., Lameiras S., Chang Q., Talaei N., Ornatsky O. Human lymphoid organ Cdc2 and macrophages play complementary roles in T follicular helper responses. J. Exp. Med. 2019;216:1561–1581. doi: 10.1084/jem.20181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre C.A., Becht E., Irac S.E., Khalilnezhad A., Narang V., Khalilnezhad S., Ng P.Y., Van Den Hoogen L.L., Leong J.Y., Lee B. Single-cell analysis of human mononuclear phagocytes reveals subset-defining markers and identifies circulating inflammatory dendritic cells. Immunity. 2019;51:573–589.e8. doi: 10.1016/j.immuni.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Goudot C., Coillard A., Villani A.C., Gueguen P., Cros A., Sarkizova S., Tang-Huau T.L., Bohec M., Baulande S., Hacohen N. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity. 2017;47:582–596 E6. doi: 10.1016/j.immuni.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Guilliams M., Ginhoux F., Jakubzick C., Naik S.H., Onai N., Schraml B.U., Segura E., Tussiwand R., Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C.V., Randolph G.J., Henson P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017;17:349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- Jardine L., Wiscombe S., Reynolds G., Mcdonald D., Fuller A., Green K., Filby A., Forrest I., Ruchaud-Sparagano M.H., Scott J. Lipopolysaccharide inhalation recruits monocytes and dendritic cell subsets to the alveolar airspace. Nat. Commun. 2019;10:1999. doi: 10.1038/s41467-019-09913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D.P., Jongstra-Bilen J., Ogryzlo K., Chong R., Jongstra J. Lymphocyte-specific Ca2+-binding protein Lsp1 is associated with the cytoplasmic face of the plasma membrane. Mol. Cell Biol. 1989;9:3043–3048. doi: 10.1128/mcb.9.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoui D., Keirsse J., Morias Y., Van Overmeire E., Geeraerts X., Elkrim Y., Kiss M., Bolli E., Lahmar Q., Sichien D. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat. Commun. 2016;7:13720. doi: 10.1038/ncomms13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y., Kobayashi S., Leader A., Amir E.D., Elefant N., Bigenwald C., Remark R., Sweeney R., Becker C.D., Levine J.H. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–765.e17. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michea P., Noel F., Zakine E., Czerwinska U., Sirven P., Abouzid O., Goudot C., Scholer-Dahirel A., Vincent-Salomon A., Reyal F. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat. Immunol. 2018;19:885–897. doi: 10.1038/s41590-018-0145-8. [DOI] [PubMed] [Google Scholar]
- Moutel S., Bery N., Bernard V., Keller L., Lemesre E., De Marco A., Ligat L., Rain J.C., Favre G., Olichon A., Perez F. Nali-H1: a universal synthetic library of humanized nanobodies providing highly functional antibodies and intrabodies. Elife. 2016;5:e16228. doi: 10.7554/eLife.16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K., Jones M., Banham A.H., Haralambieva E., Mason D.Y. Lymphocyte-specific protein 1: a specific marker of human leucocytes. Immunology. 1999;96:262–271. doi: 10.1046/j.1365-2567.1999.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E., Touzot M., Bohineust A., Cappuccio A., Chiocchia G., Hosmalin A., Dalod M., Soumelis V., Amigorena S. Human inflammatory dendritic cells induce Th17 Cell differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Tang-Huau T.L., Gueguen P., Goudot C., Durand M., Bohec M., Baulande S., Pasquier B., Amigorena S., Segura E. Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway. Nat. Commun. 2018;9:2570. doi: 10.1038/s41467-018-04985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A.C., Satija R., Reynolds G., Sarkizova S., Shekhar K., Fletcher J., Griesbeck M., Butler A., Zheng S., Lazo S. Single-cell RNA-Seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356:eaah4573. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Proteomics data are available via ProteomeXchange (identifier PXD015647).