Abstract
This data article is related to a research paper entitled “Solvent- and metal-free hydroboration of alkynes under microwave irradiation” (Gioia et al. TETL-D-19-01698) [1]. Herein we present the spectral data acquired from the synthesis of (E)-alkenyl boronic acid pinacol esters. The data include the general information and the synthetic procedure affording the target derivatives, which were fully characterized by Nuclear Magnetic Resonance (1H and 13C NMR) and, for the most part, by Electrospray Ionization High Resolution Mass (ESI-MS). Proton and carbon NMR spectra and ESI-MS spectra were provided which will be useful for further organic chemists if they are interested in the synthesis of these building blocks.
Keywords: Hydroboration of alkynes, (E)-Alkenylboronic acid pinacol esters, Pinacolborane, Microwave irradiation
Specifications Table
| Subject | Chemistry |
| Specific subject area | Organic chemistry. Hydroboration of alkynes under microwave irradiation |
| Type of data | Figure 1H and13C NMR spectra ESI-MS spectra |
| How data were acquired | NMR (Bruker DRX400 Spectrometer, 400 MHz for 1H NMR and 100 MHz for13C NMR), NMR data processing (MestReNova software, version 11.0.2–18153, Mestrelab Research S.L. 2016), Electrospray Ionization High Resolution Mass (Bruker MicroTOF Q Spectrometer) |
| Data format | Raw and Analyzed |
| Parameters for data collection | All reagents and solvents were commercially available and used as received. The alkenylboronic acid pinacol esters were synthesized by the hydroboration of alkynes under microwave irradiation and all final compounds were purified by column chromatography. |
| Description of data collection | The isolated compounds were all characterized by NMR spectroscopy, and for the most part by HRMS. |
| Data source location | Université Claude Bernard Lyon 1, Lyon, France |
| Data accessibility | Data are available with the article |
| Related research article | Bruna Gioia, Alexandre Arnaud, Sylvie Radix, Nadia Walchshofer, Anne Doléans-Jordheim, Luc Rocheblave, Solvent- and metal-free hydroboration of alkynes under microwave irradiation, submitted to Tetrahedron Letters, reference number TETL-D-19-01698. |
Value of the Data
|
1. Data description
A series of eighteen (E)-alkenylboronic acid pinacol esters (2a-r) were synthesized from the hydroboration of aromatic or aliphatic alkynes in presence of pinacol borane according to a solvent- and metal-free procedure [1]. The synthetic scheme and the chemical structures of the target derivatives were described in Fig. 1. The final compounds 2a-r were all characterized by 1H and 13C NMR spectroscopy and HR mass spectra were recorded for the most part of synthesized boranes. All the spectra were provided in this data article (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17, Fig. 18, Fig. 19, Fig. 20, Fig. 21, Fig. 22, Fig. 23, Fig. 24, Fig. 25, Fig. 26, Fig. 27, Fig. 28, Fig. 29, Fig. 30, Fig. 31, Fig. 32, Fig. 33, Fig. 34, Fig. 35, Fig. 36, Fig. 37, Fig. 38, Fig. 39, Fig. 40, Fig. 41, Fig. 42, Fig. 43, Fig. 44, Fig. 45, Fig. 4). It is noteworthy that the 13C NMR signal for the alkenyl carbon next to the boron atom is identified in all carbon-13 spectra except for compound 2e.
Fig. 46.
ESI-MS spectra of compound 2r.
Fig. 1.
Synthesis and chemical structures of derivatives 2a-r.
Fig. 2.
1H NMR spectra of compound 2a (400 MHz) in DMSO‑d6.
Fig. 3.
13C NMR spectra of compound 2a (100 MHz) in DMSO‑d6.
Fig. 4.
1H NMR spectra of compound 2b (400 MHz) in CD3OD.
Fig. 5.
13C NMR spectra of compound 2b (100 MHz) in CD3OD.
Fig. 6.
1H NMR spectra of compound 2c (400 MHz) in CD3OD.
Fig. 7.
13C NMR spectra of compound 2c (100 MHz) in CD3OD.
Fig. 8.
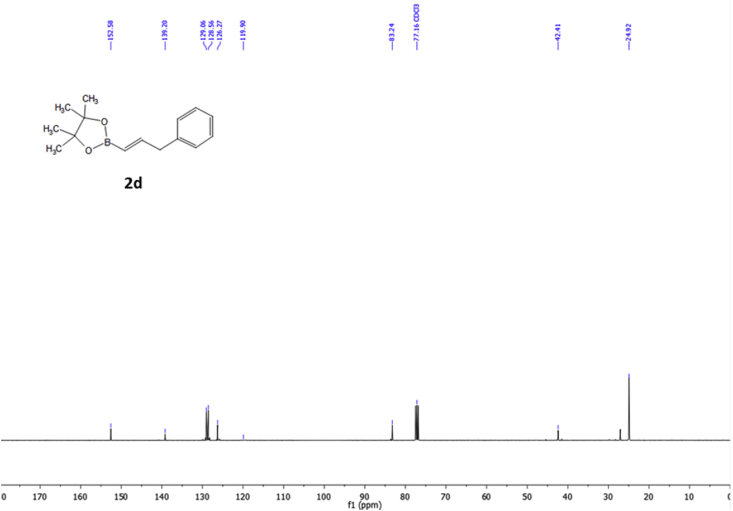
1H NMR spectra of compound 2d (400 MHz) in CDCl3.
Fig. 9.
13C NMR spectra of compound 2d (100 MHz) in CDCl3.
Fig. 10.
1H NMR spectra of compound 2e (400 MHz) in CDCl3.
Fig. 11.
13C NMR spectra of compound 2e (100 MHz) in CDCl3.
Fig. 12.
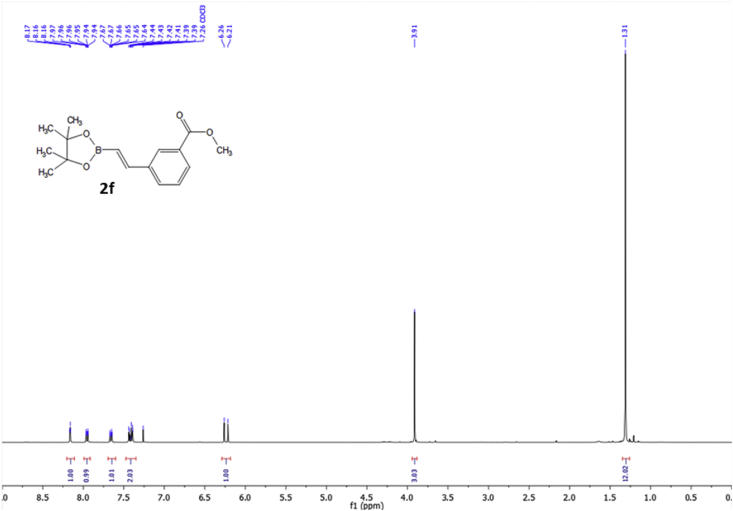
1H NMR spectra of compound 2f (400 MHz) in CDCl3.
Fig. 13.
13C NMR spectra of compound 2f (100 MHz) in CDCl3.
Fig. 14.
ESI-MS spectra of compound 2f.
Fig. 15.
1H NMR spectra of compound 2g (400 MHz) in CDCl3.
Fig. 16.
13C NMR spectra of compound 2g (100 MHz) in CDCl3.
Fig. 17.
1H NMR spectra of compound 2h (400 MHz) in CDCl3.
Fig. 18.
13C NMR spectra of compound 2h (100 MHz) in CDCl3.
Fig. 19.
1H NMR spectra of compound 2i (400 MHz) in CDCl3.
Fig. 20.
13C NMR spectra of compound 2i (100 MHz) in CDCl3.
Fig. 21.
1H NMR spectra of compound 2j (400 MHz) in CDCl3.
Fig. 22.
13C NMR spectra of compound 2j (100 MHz) in CDCl3.
Fig. 23.
1H NMR spectra of compound 2k (400 MHz) in CDCl3.
Fig. 24.
13C NMR spectra of compound 2k (100 MHz) in CDCl3.
Fig. 25.
ESI-MS spectra of compound 2k.
Fig. 26.
1H NMR spectra of compound 2l (400 MHz) in CDCl3.
Fig. 27.
13C NMR spectra of compound 2l (100 MHz) in CDCl3.
Fig. 28.
ESI-MS spectra of compound 2l.
Fig. 29.
1H NMR spectra of compound 2m (400 MHz) in CDCl3.
Fig. 30.
13C NMR spectra of compound 2m (100 MHz) in CDCl3.
Fig. 31.
ESI-MS spectra of compound 2m.
Fig. 32.
1H NMR spectra of compound 2n (400 MHz) in CDCl3.
Fig. 33.
13C NMR spectra of compound 2n (100 MHz) in CDCl3.
Fig. 34.
ESI-MS spectra of compound 2n.
Fig. 35.
1H NMR spectra of compound 2o (400 MHz) in CDCl3.
Fig. 36.
13C NMR spectra of compound 2o (100 MHz) in CDCl3.
Fig. 37.
ESI-MS spectra of compound 2o.
Fig. 38.
1H NMR spectra of compound 2p (400 MHz) in CDCl3.
Fig. 39.
13C NMR spectra of compound 2p (100 MHz) in CDCl3.
Fig. 40.
ESI-MS spectra of compound 2p.
Fig. 41.
1H NMR spectra of compound 2q (400 MHz) in CDCl3.
Fig. 42.
13C NMR spectra of compound 2q (100 MHz) in CDCl3.
Fig. 43.
ESI-MS spectra of compound 2q.
Fig. 44.
1H NMR spectra of compound 2r (400 MHz) in CDCl3.
Fig. 45.
13C NMR spectra of compound 2r (100 MHz) in CDCl3.
2. Experimental design, materials, and methods
2.1. General information
All reactions were performed under microwave irradiation using an Anton Paar Monowave 300 synthetizer. Pinacol borane (4,4,5,5-tetramethyl-1,3,2-dioxaborolane) was purchased from Sigma Aldrich. Alkynes and carboxylic acids were purchased from Sigma-Aldrich, Fisher Scientific or Fluorochem. Optima LC/MS grade acetonitrile was purchased from Fisher Scientific. 1,4-Dioxane was purchased from Carlo Erba, acetonitrile and dimethylformamide were purchased from Fisher Scientific and octane was purchased from Sigma-Aldrich. All purchased compounds or solvents were used as received. All reactions were monitored through thin-layer chromatography on GF254 plates purchased from Merck and spots were detected under a UV lamp (254 nm and 356 nm) or by spraying plates with 0.5% w/v aqueous KMnO4, followed by drying with heat gun. Chromatographic separations were performed on silica gel columns (Kieselgel 300–400 mesh) with eluent indicated for each compound. Organic solutions were concentrated under reduced pression on a rotary evaporator.
The samples were dissolved CDCl3, DMSO‑d6 or CD3OD to acquire the NMR spectra using a Bruker DRX400 Fourier transform NMR spectrometer. 1H and 13C NMR spectra were recorded respectively at 400 MHz and 100 MHz, using an internal deuterium lock. The chemical shift of the solvent residual signal was used as the reference. Data for 1H NMR are reported as follows: chemical shift (δ, ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, sext = sextuplet, dd = doublet of doublets, dt = doublet of triplets, m = multiplet), coupling constant (J, Hz) and integration. Data for 13C NMR are reported as a list of chemical shifts.
High-resolution mass spectroscopy (HRMS) measurements were performed by electrospray ionization (ESI-MS) using 2 mg/ml sample solutions in HPLC grade CH3CN or MeOH.
2.2. General procedure
Pinacol borane (1.4 mL, 9.44 mmol, 4 eq.), alkyne 1a-r (2.36 mmol, 1 eq) and 4-(dimethylamino)benzoic acid (5 mol%) were introduced in a 10 mL microwave sealed flask. Reactions conditions depended on alkyl or aromatic alkynes, time was set respectively at 30 and 15 min and temperature was set at 120 °C for both alkyne types. The reaction medium was directly purified by flash chromatography to obtain the final product 2a-r. Methyl tert-butyl ether (MTBE) was used for crystallization attempts.
2.3. Characterization data
2.3.1. (E)-2-Styrylboronic acid pinacol ester (2a) [2]

Purification by flash chromatography with cyclohexane: diethyl ether (97:3) to obtain a pale yellow oil (336 mg, 62%). 1H NMR (400MHz, DMSO‑d6) δ 7.58 (d, J 8.0 Hz, 2H), 7.40–7.28 (m, 4H), 6.14 (d, J 18.5 Hz, 1H), 1.24 (s, 12H); 13C NMR (100 MHz, DMSO‑d6) δ 149.21, 136.86, 129.14, 128.68, 127.02, 116.53, 83.02, 24.63.
2.3.2. (E)-2-(3-methoxystyryl)boronic acid pinacol ester (2b) [3]

Purification by flash chromatography with cyclohexane: diethyl ether (70:30) to obtain a colorless oil (374 mg, 60%). 1H NMR (400 MHz, CD3OD) δ 7.32 (d, J 18.4 Hz, 1H), 7.25 (m, 1H), 7.10–7.02 (m, 2H), 6.87 (m, 1H), 6.10 (d, J 18.4 Hz, 1H), 3.80 (s, 3H), 1.29 (s, 12H); 13C NMR (100 MHz, CD3OD) δ 161.44, 150.94, 149.38, 130.64, 120.69, 118.05, 115.83, 113.06, 84.62, 55.67, 25.13.
2.3.3. (E)-2-(4-methoxystyryl)boronic acid pinacol ester (2c) [2]

Purification by flash chromatography with cyclohexane: diethyl ether (70:30) to obtain a white solid (361.3 mg, 58%). 1H NMR (400 MHz, CD3OD) δ 7.44 (m, 2H), 7.30 (d, J 18.4 Hz, 1H), 6.89 (m, 2H), 5.94 (d, J 18.4 Hz, 1H), 3.80 (s, 3H), 1.29 (s, 12H); 13C NMR (100 MHz, CD3OD) δ 162.03, 150.72, 149.21, 131.60, 129.51, 115.04, 84.47, 55.75, 25.12.
2.3.4. (E)-2-(3-phenylprop-1-en-1-yl)boronic acid pinacol ester (2d) [4]

Purification by flash chromatography with cyclohexane: diethyl ether (90:10) to obtain a colorless oil (455.2 mg, 79%). 1H NMR (400 MHz, CDCl3) δ 7.28 (m, 2H), 7.23–7.09 (m, 3H), 6.77 (dt, J 17.8 Hz, 6.3 Hz, 1H), 5.46 (dt, J 17.8 Hz, 1.5 Hz, 1H), 3.49 (dd, J 6.3 Hz, 1.5 Hz, 2H), 1.26 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 152.58, 139.20, 129.05, 128.56, 126.27, 119.90, 83.24, 42.41, 24.92.
2.3.5. (Z)-2-(1,2-diphenylvinyl)boronic acid pinacol ester (2e) [5]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow solid (202.2 mg, 28%). 1H NMR (400 MHz, CDCl3) δ 7.36 (s, 1H), 7.28–7.24 (m, 2H), 7.21–7.18 (m, 1H), 7.17–7.14 (m, 2H), 7.11–7.09 (m, 3H), 7.05–7.03 (m, 2H), 1.20 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 143.29, 140.55, 137.11, 130.08, 128.98, 128.37, 127.97, 127.70, 126.39, 83.91, 24.93. Carbon signal next to boron atom was not observed.
2.3.6. Methyl (E)-3-(2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)vinyl)benzoate (2f)

Purification by flash chromatography with petroleum ether (40–60 °C): ethyl acetate (90:10) to obtain a pale yellow solid (446.0 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 8.16 (t, J 1.6 Hz, 1H), 7.95 (dt, J 7.8 Hz, 1.6 Hz, 1H), 7.66 (dt, J 7.8 Hz, 1.6 Hz, 1H), 7.47–7.36 (m, 2H), 6.24 (d, J 18.4 Hz, 1H), 3.91 (s, 3H), 1.31 (s, 12H); 13C NMR (100MHz, CDCl3) δ 166.99, 148.39, 137.95, 131.30, 130.69, 129.91, 128.80, 128.37, 118.28, 83.61, 52.31, 24.95; HRMS (ESI): calcd. for C16H22BO4: 289.1606; found [M+H]+: 289.1604.
2.3.7. Methyl (E)-4-(2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)vinyl)benzoate (2g) [2]

Purification by flash chromatography with petroleum ether (40–60 °C): ethyl acetate (90:10) to obtain a colourless solid (330.9 mg, 48%). 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J 8.3 Hz, 2H), 7.53 (d, J 8.3 Hz, 2H), 7.40 (d, J 18.4 Hz, 1H), 6.27 (d, J 18.4 Hz, 1H), 3.90 (s, 3H), 1.31 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 166.98, 148.25, 141.82, 130.25, 130.03, 127.02, 119.66, 83.69, 52.25, 24.94.
2.3.8. (E)-2-(3-methoxyprop-1-en-1-yl)boronic acid pinacol ester (2h) [6]

Purification by flash chromatography with petroleum ether (40–60 °C): ethyl acetate (50:50) to obtain a pale yellow oil (164.0 mg, 34%). 1H NMR (400 MHz, CDCl3) δ 6.62 (dt, J 18.2 Hz, 4.8 Hz, 1H), 5.68 (dt, J 18.2 Hz, 1.8 Hz, 1H), 3.99 (dd, J 4.8 Hz, 1.8 Hz, 2H), 3.34 (s, 3H), 1.26 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 149.17, 119.40, 83.38, 74.29, 58.42, 24.98.
2.3.9. (E)-2-(3-chlorostyryl)boronic acid pinacol ester (2i) [2]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (348.48 mg, 55%). 1H NMR (400 MHz, CDCl3) δ 7.46 (s, 1H), 7.37–7.25 (m, 4H), 6.17 (d, J 18.4 Hz, 1H), 1.32 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 147.65, 139.17, 134.39, 129.61, 128.57, 126.76, 125.01, 118.08, 83.32, 24.63.
2.3.10. (E)-2-(4-chlorostyryl)boronic acid pinacol ester (2j) [2]

Purification by flash chromatography with cyclohexane: ethyl acetate (60:40) to obtain a pale yellow solid (573.2 mg, 92%). 1H NMR (400 MHz, CDCl3) δ 7.41 (m, 2H), 7.37–7.24 (m, 3H), 6.13 (d, J 18.4 Hz, 1H), 1.31 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 148.14, 136.12, 134.74, 128.92, 128.36, 117.42, 83.59, 24.95.
2.3.11. (E)-2-(pent-1-en-1-yl)boronic acid pinacol ester (2k) [7]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (161.9 mg, 35%). 1H NMR (400 MHz, CDCl3) δ 6.62 (dt, J 18.0 Hz, 6.4 Hz, 1H), 5.42 (dt, J 18.0 Hz, 1.6 Hz, 1H), 2.11 (m, 2H), 1.43 (sext., J 7.4 Hz, 2H), 1.24 (s, 12H), 0.90 (t, J 7.4 Hz,3H); 13C NMR (100 MHz, CDCl3) δ 154.68, 118.63, 83.10, 38.06, 24.91, 21.55, 13.91; HRMS (ESI): calcd. for C11H22BO2: 197.1707; found [M+H]+: 197.1711.
2.3.12. (E)-2-(hex-1-en-1-yl)boronic acid pinacol ester (2l) [8]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (267.2 mg, 54%). 1H NMR (400 MHz, CDCl3) δ 6.63 (dt, J 17.9 Hz, 6.4 Hz, 1H), 5.42 (dt, J 17.9 Hz, 1.6 Hz, 1H), 2.14 (m, 2H), 1.39 (m, 2H), 1.24–1.28 (m, 14H), 0.88 (t, J 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 154.93, 118.42, 83.11, 35.65, 30.50, 24.92, 22.40, 14.06; HRMS (ESI): calcd. for C12H23BNaO2: 233.1683; found [M+Na]+: 233.1685.
2.3.13. (E)-2-(hept-1-en-1-yl)boronic acid pinacol ester (2m) [9]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (339.8 mg, 64%). 1H NMR (400 MHz, CDCl3) δ 6.63 (dt, J 18.0 Hz, 6.4 Hz, 1H), 5.41 (d, J 18.0 Hz, 1H), 2.13 (m, 2H), 1.41 (m, 2H), 1.26–1.29 (m, 16H), 0.88 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 154.97, 118.51, 83.10, 35.93, 31.55, 28.04, 24.92, 22.66, 14.14; HRMS (ESI): calcd. for C13H25BNaO2: 247.1840 found [M+Na]+: 247.1842.
2.3.14. (E)-2-(oct-1-en-1-yl)boronic acid pinacol ester (2n) [9]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (315.1 mg, 58%). 1H NMR (400 MHz, CDCl3) δ 6.63 (dt, J 17.9 Hz, 6.4 Hz, 1H), 5.41 (dt, J 17.9 Hz, 1.6 Hz, 1H), 2.14 (m, 2H), 1.40 (m, 2H), 1.26 (s, 18H), 0.86 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 154.98, 118.59, 83.10, 35.99, 31.87, 29.06, 28.34, 24.92, 22.74, 14.24; HRMS (ESI): calcd. for C14H28BO2: 239.2177; found [M+H]+: 239.2178.
2.3.15. (E)-2-(non-1-en-1-yl)boronic acid pinacol ester (2o) [9]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (282.4 mg, 50%). 1H NMR (400 MHz, CDCl3) δ 6.63 (dt, J 17.9 Hz, 6.4 Hz, 1H), 5.42 (dt, J 17.9 Hz, 1.6 Hz 1H), 2.13 (m, 2H), 1.40 (m, 2H), 1.26 (s, 20H), 0.86 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 155.00, 118.41, 83.11, 35.99, 31.95, 29.35, 29.31, 28.37, 24.92, 22.81, 14.25; HRMS (ESI): calcd. for C15H29BNaO2: 275.2153 found [M+Na]+: 275.2162.
2.3.16. (E)-2-(dec-1-en-1-yl)boronic acid pinacol ester (2p) [10]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (388.4 mg, 62%). 1H NMR (400 MHz, CDCl3) δ 6.63 (dt, J 18.0 Hz, 6.4 Hz, 1H), 5.42 (dd, J 18.0 Hz, 1.6 Hz, 1H), 2.14 (m, 2H), 1.40 (m, 2H), 1.26 (s, 22H), 0.86 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 155.00, 118.60, 83.11, 35.99, 32.03, 29.60, 29.39 (2xCH2), 28.37, 24.92, 22.82, 14.25; HRMS (ESI): calcd. for C16H31BNaO2: 289.2309 found [M+Na]+: 289.2311.
2.3.17. (E)-2-(undec-1-en-1-yl)boronic acid pinacol ester (2q) [11]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (405.4 mg, 61%). 1H NMR (400 MHz, CDCl3) δ 6.63 (dt, J 17.9 Hz, 6.4 Hz; 1H), 5.42 (dt, J 17.9 Hz, 1.6 Hz; 1H), 2.13 (m, 2H), 1.40 (m, 2H), 1.30–1.25 (m, 24H), 0.86 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 155.01, 118.58, 83.11, 35.99, 32.05, 29.69, 29.65, 29.48, 29.39, 28.38, 24.93, 22.83, 14.26; HRMS (ESI): calcd. for C17H33BNaO2: 303.2466; found [M+Na]+: 303.2469.
2.3.18. (E)-2-(dodec-1-en-1-yl)boronic acid pinacol ester (2r) [12]

Purification by flash chromatography with pentane: diethyl ether (97:3) to obtain a pale yellow oil (612.9 mg, 88%). 1H NMR (400 MHz, CDCl3) δ 6.63 (dt, J 17.9 Hz, 6.4 Hz, 1H), 5.42 (dt, J 17.9 Hz, 1.5 Hz, 1H), 2.13 (m, 2H), 1.40 (m, 2H), 1.30–1.25 (m, 26H), 0.87 (t, J 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 155.00, 118.53, 83.11, 35.99, 32.05, 29.77, 29.73, 26.65, 29.49, 29.39, 28.38, 24.93, 22.84, 14.27; HRMS (ESI): calcd. for C18H36BO2: 295.2803; found [M+H]+: 295.2804.
Acknowledgments
The authors would like to thank the Vaincre la Mucoviscidose (VLM) and Gregory Lemarchal associations for the financial support over these past two years. They thank the Ministère de l’Education Nationale de la Recherche et de Technologie (MENRT) and Ecole Doctorale Interdisciplinaire Sciences-Santé (EDISS) for the PhD fellowship of B. Gioia.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gioia B., Arnaud A., Radix S., Walchshofer N., Doléans-Jordheim A., Rocheblave L. Solvent- and metal-free hydroboration of alkynes under microwave irradiation. Tetrahedron Lett. 2020;61:151596. [Google Scholar]
- 2.Lu W., Shen Z. Direct synthesis of alkenylboronates from alkenes and pinacol diboron via copper catalysis. Org. Lett. 2019;21:142–146. doi: 10.1021/acs.orglett.8b03599. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z., Wei W., Xiong L., Feng Q., Shi Y., Wang N., Yu L. Selective and efficient synthesis of trans-arylvinylboronates and trans-hetarylvinylboronates using palladium catalyzed cross-coupling. New J. Chem. 2017;41:3172–3176. [Google Scholar]
- 4.Tanaka S., Saito Y., Yamamoto T., Hattori T. Electrophilic borylation of terminal alkenes with BBr3/2,6-disubstituted pyridines. Org. Lett. 2018;20:1828–1831. doi: 10.1021/acs.orglett.8b00335. [DOI] [PubMed] [Google Scholar]
- 5.Fleige M., Möbus J., vom Stein T., Glorius F., Stephan D.W. Lewis acid catalysis: catalytic hydroboration of alkynes initiated by Piers' borane. Chem. Commun. 2016;52:10830–10833. doi: 10.1039/c6cc05360b. [DOI] [PubMed] [Google Scholar]
- 6.Shirakawa K., Arase A., Hoshi M. Preparation of (E)-1-alkenylboronic acid Pinacol esters via transfer of Alkenyl Group from boron to boron. Synthesis. 2004:1814–1820. [Google Scholar]
- 7.Zhao J., Niu Z., Fu H., Li Y. Ligand-free hydroboration of alkynes catalyzed by heterogeneous copper powder with high efficiency. Chem. Commun. 2014;50:2058–2060. doi: 10.1039/c3cc48670b. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein E.K., Namirembe S., Morken J.P. Enantioselective conjunctive cros-coupling of bis-(alkenyl)borates: a general synthesis of chiral allylboron reagents. J. Am. Chem. Soc. 2017;139:5027–5030. doi: 10.1021/jacs.7b01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojha D.P., Prabhu K.R. Pd-catalyzed hydroborylation of alkynes: a ligand controlled regioselectivity switch for the synthesis of α- or β-vinylboronates. Org. Lett. 2016;18:432–435. doi: 10.1021/acs.orglett.5b03416. [DOI] [PubMed] [Google Scholar]
- 10.Mandal S., Verma P.K., Geetharani K. Lewis acid catalysis: regioselective hydroboration of alkynes and alkenes promoted by scandium triflate. Chem. Commun. 2018;54:13690–13693. doi: 10.1039/c8cc08361d. [DOI] [PubMed] [Google Scholar]
- 11.Yao Z.J., Hong S., Zhang W., Liu M., Deng W. Copper-catalyzed regioselective hydroboration of terminal alkynes in aqueous medium. Tetrahedron Lett. 2016;57:910–913. [Google Scholar]
- 12.Ho H.E., Asao N., Yamamoto Y., Jin T. Carboxylic acid-catalyzed highly efficient and selective hydroboration of alkynes with pinacolborane. Org. Lett. 2014;16:4670–4673. doi: 10.1021/ol502285s. [DOI] [PubMed] [Google Scholar]