Abstract
Morbidity and mortality from bovine respiratory disease (BRD) and associated losses in performance and carcass merit continue to plague the beef cattle industry. Several viral/bacterial agents are responsible for BRD, and interactions occur among the agents. Viral agents often predispose animals to bacterial infections, and Mannheimia haemolytica is the most frequently isolated organism in cattle with BRD. Laboratory tests are available to characterize organisms causing BRD using easily obtained nasal swab samples. Testing for persistent infection with bovine viral diarrhea virus can be done by a 2-stage technique using PCR and immunohistochemistry. Preconditioning programs that include preweaning viral vaccination programs along with castration could have a significant influence on decreasing BRD in the cattle feeding industry. Metaphylactic antibiotic programs continue to be effective; however, antibiotic resistance is a public concern, and additional management options (e.g., direct-fed microbials or other compounds with antimicrobial properties) deserve attention. Diets with an increased energy concentration achieved by decreasing the dietary roughage concentration may slightly increase the rate of BRD morbidity; however, these diets also increase ADG, DMI, and G:F compared with lower-energy, greater-roughage diets. The extent to which performance and BRD morbidity are affected by dietary protein concentration needs further study, but low and high protein concentrations should probably be avoided. Several trace minerals (e.g., Cu, Se, and Zn) affect immune function, but the effects of supplementation on performance and immune function in model challenge systems and in field studies are equivocal. Adding vitamin E to receiving diets at pharmacological levels (e.g., >1,000 IU·animal−1·day−1) seems beneficial for decreasing BRD morbidity, but it has little effect on performance. Given the limited ability to consistently modify immune function and BRD morbidity through dietary manipulations, we recommend that the diets for newly received cattle be formulated to adjust nutrient concentrations for low feed intake and to provide optimal performance during the receiving period.
Keywords: bovine respiratory disease, cattle, management, morbidity, nutrition
INTRODUCTION
Morbidity and mortality from bovine respiratory disease (BRD) in newly weaned/received cattle continue to be the most significant health problems facing the US beef cattle industry. In a recent survey of Kansas feedlots, there seemed to be a trend for increased death losses in feedlot cattle over the last decade (Babcock et al., 2006), and BRD was the leading cause of morbidity and mortality in a cross-sectional survey sent to 561 feedlots in 21 states (Woolums et al., 2005). Mortality from BRD and the expense of medicine and labor to treat BRD contribute to its negative economic and animal welfare costs, but feedlot performance and carcass merit also are affected negatively by BRD (Gardner et al., 1999), magnifying its economic consequences.
Montgomery et al. (1984) reported that BRD negatively affected marbling scores in 3 trials, and quality grade was significantly decreased in 2 of the 3 trials. Likewise, Roeber et al. (2001) reported lower HCW, marbling scores, and yield grades for cattle treated more than once for BRD compared with untreated cattle, and carcass grades were further decreased in cattle treated for BRD 2 or more times. Calves treated for BRD once returned $40.64 less, those receiving 2 medical treatments returned $58.35 less, and those receiving 3 or more treatments returned $291.93 less than calves that were not treated (Fulton et al., 2002).
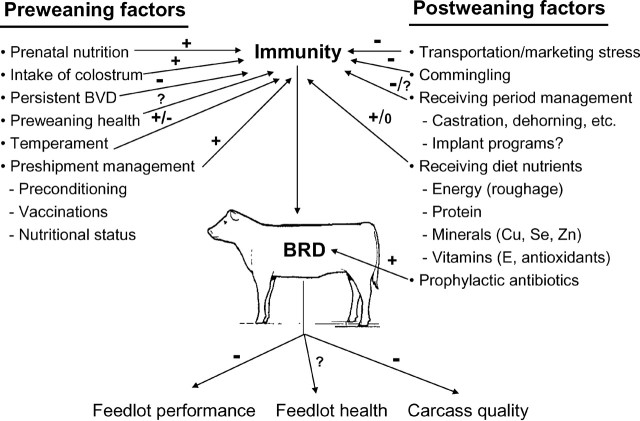
Although BRD is ultimately a viral/bacterial disease, it is a multifaceted problem with numerous potential exacerbating factors and outcomes (Figure 1). Stresses due to weaning, marketing, and transportation, previous plane of nutrition, genetics, and health history interact with exposure to viral and bacterial agents. Stress negatively affects the immune system (Blecha et al., 1984) at a time when the animal is more likely to be exposed to infectious agents as a result of commingling. Feed intake by stressed calves is low (Galyean and Hubbert, 1995; Cole, 1996), and low nutrient intake likely augments the negative effects of stress on immune function.
Figure 1.
Pre- and postweaning factors affecting bovine respiratory disease (BRD) in beef cattle and the resulting outcomes of the disease. + = decreased incidence or consequence; − = increased incidence or consequence; ? = effects not fully understood based on the available data. BVD = bovine viral diarrhea virus.
Along with our colleague Louis Perino, we previously reviewed the interaction of nutrition with beef cattle health and immunity (Galyean et al., 1999). Our objective in the present paper is to update topics covered in our 1999 review, to add additional information on strategies to prevent or treat BRD, and to provide suggestions for future research.
CAUSATIVE AGENTS FOR BRD
Although it is important that some discussion on the causes of BRD be included in this review, describing all of the potential causes and interactions associated with BRD is beyond the scope of this manuscript. Of the bacterial species, Pasteurella (Mannheimia) haemolytica, Pasteurella multocida, and Histophilus somni (formerly Haemophilus sommus) are of primary concern, with Mannheimia haemolytica serotype 1 being the organism most commonly associated with BRD (Pandher et al., 1998). In addition, viral agents, including infectious bovine rhinotraceitis (IBR), parainfluenza-3 (PI3), bovina viral diarrhea virus (BVDV), bovine respiratory syncytial virus (BRSV), and bovine enteric coronavirus have been associated with respiratory tract disease in feedlot calves (Plummer et al., 2004). Bovine adenovirus serotype 7 infections have been found in commingled calves and may be more common in calves with concurrent infections with other viruses (Fent et al., 2002). In Europe, Mycoplasma bovis is responsible for at least 25 to 33% of all pneumonia cases in calves suffering from BRD (Gevaert, 2006).
Much recent attention has focused on BVDV. These viruses are classified into 2 genotypes (type 1 and type 2; Ridpath et al., 1994) based on sequences from the 5′ untranslated region of the viral genome and are further characterized into subgenotypes (1a and 1b, Pellerin et al., 1994; and 2a and 2b, Flores et al., 2002). Within the 2 genotypes, a further division into cytopathic and noncytopathic strains is made based on the presence or absence of effects in vitro. Regardless of the biotype or genotype, significant losses can occur.
In cattle with a history of BRD, BVDV noncytophathic biotypes were isolated more often than cytopathic biotypes, and BVDV1 noncytopathic biotypes were isolated more frequently than BVDV2 genotypes (Fulton et al., 2000b). Moreover, BVDV1 genotypes were isolated more frequently than type 2 genotypes from necropsy of calves with fibrinous pneumonia. An almost equal distribution of BVDV1a and 1b isolates was noted from cattle with a history of BRD, but more BVDV1b than 1a was isolated in necropsy cases of cattle that died from pneumonia (Fulton et al., 2003). Of the US licensed and marketed BVDV vaccines, only one contains BVDV1b strains (Fulton et al., 2003), and although vaccines with BVDV1a and 2a are routinely administered to cattle entering feedlots, most vaccines may not provide adequate protection against BVDV1b (Fulton et al., 2006).
Thurmond (2005) described factors associated with the mode of transmission of BVDV. Transmission of BVD can be vertical (fetal infection) or horizontal (postnatal transmission). When an infection with a nonpathogenic strain occurs before d 42 to 125 in utero, calves can become persistently infected (PI; McClurkin et al., 1984). Persistent infections are lifelong, and because PI animals constantly shed the virus, this can be an important means of transmission.
Bovine viral diarrhea virus infections often occur in combination with infections by other viruses associated with BRD, particularly PI3 and BRSV (Fulton et al., 2000a). Early in the marketing process, highly susceptible calves are likely at risk to infections by IBR, BVDV types 1 and 2, and BRSV (Fulton et al., 2000a). Although much of the recent research conducted with viral vaccines has focused on BVD, bovine herpes virus 1 (BHV-1, commonly known as IBR) may predispose cattle to pneumonic pasteurellosis (Patel, 2005). Prevention of predisposing viral infections via preconditioning programs that include vaccination for viral and bacterial agents known to cause BRD should decrease the incidence of the disease.
DIAGNOSIS OF BOVINE RESPIRATORY DISEASE
Classical Methods
It is generally accepted that a variable but relatively high percentage of animals will succumb to BRD; thus, accurate diagnosis is critical in practical situations. Most animals are removed for examination and treatment for BRD on or before d 27 of the receiving period (Buhman et al., 2000). Traditional methods for detecting morbid cattle include visual appraisal once or twice daily, with animals displaying various signs including nasal or ocular discharge, depression, lethargy, emaciated body condition, labored breathing, or any combination of these, being removed from pens for further evaluation. Symptomatic animals with a rectal temperature ≥39.7°C are usually considered morbid and given therapeutic treatment.
Perino and Apley (1998) defined a clinical scoring system of: 0 = normal animal; 1 = noticeable depression without apparent signs of weakness; 2 = marked depression with moderate signs of weakness without a significantly altered gait; 3 = severe depression with signs of weakness such as a significantly altered gait; and 4 = moribund and unable to rise. According to their protocol, animals with a rectal temperature of ≥40°C and a clinical score of ≥1 should receive therapeutic treatment.
Given the subjective nature of such protocols, identification of animals with BRD is not always accurate. Pulmonary lesions indicative of BRD were present at slaughter in 68% of steers that were not treated for BRD, whereas lesions were present in 78% of treated steers (Wittum et al., 1996a). Similarly, in South African feedlots, Thompson et al. (2006) reported that 42.8% of all animals had lung lesions at slaughter, and of the animals with lung lesions at slaughter, 69.5% had never been treated for BRD. We previously suggested (Galyean et al., 1999) that a valuable tool for monitoring BRD diagnosis and treatment would be evaluation of lung lesions at slaughter. Bryant et al. (1999) provided a method for recording pulmonary lesions at slaughter; however, implementation in commercial settings is not common, and few published research studies have scored pulmonary lesions as an indicator of treatment or diagnosis of BRD. Failure to detect morbid animals using current protocols for diagnosis may be related to predator/prey behavior (Noffsinger and Locatelli, 2004), in that if the animals perceive the personnel handling the animals as predators, they will mask the signs of weakness (e.g., depression, illness, lameness, etc.). Therefore, development and implementation of quantitative measures to detect BRD is critical, and several possible candidates will be discussed in the subsequent sections.
Laboratory Tests for BRD and Body Temperature Measurements
Laboratory tests are often used to verify BRD; however, the optimal metabolite, compound, or organism to measure remains to be determined. Any laboratory procedure requires time to complete, which limits its value. Chute-side tests would be highly desirable; however, such tests are not widely available, are potentially cost-prohibitive, and little data are available to evaluate their efficacy.
Establishing the causative agents of BRD aids proper treatment. DeRosa et al. (2000) reported that nasal swab cultures contained the same bacterial species as transtracheal swab cultures 96% of the time, and nasal swab cultures were genetically identical with the organism causing disease within the lung for 70% of the calves tested. Furthermore, antibiotic susceptibility was generally similar between paired isolates for various antibiotics used to treat BRD. Thus, a nasal swab culture, which is easy to obtain and process, should be useful for identifying the species responsible for causing bacterial pneumonia and also should be indicative of antibiotic susceptibility (De-Rosa et al., 2000).
Immunohistochemistry (IHC) is frequently used to detect BVDV antigen in skin biopsies. Other methods include antigen-capture ELISA, virus neutralization, microplate virus isolation, and reverse-transcriptase PCR (RT-PCR) assays (Dubovi, 1996). Viral isolations from blood leukocytes or serum also can be used to identify PI BVDV animals. The time to complete these tests varies among laboratories, but the process can take approximately 10 d. In addition to assay costs, costs associated with sample collection, animal handling, sample shipment, etc. can be significant, and it is likely cost-prohibitive to sample all calves received in a given lot. The IHC test seems reliable, and calves recently vaccinated with modified live vaccines have not caused false positives (DuBois et al., 2000); however, skin biopsy samples cannot be pooled, thereby increasing costs. Larson et al. (2005) suggested using RT-PCR on pooled blood samples (30 animals), followed by an IHC test only on animals represented in the pooled samples that returned positive assay results. This 2-test strategy might be cost effective, but it would increase the lag time in detecting PI BVDV calves and increase the time they would be commingled with other cattle.
During the initial tissue insult of the disease, a set of reactions result in the release of soluble mediators termed the acute-phase response (Baumann and Gauldie, 1994). Several acute-phase proteins have been measured in cattle with BRD, including fibrinogen, haptoglobin, serum amyloid-A, α-1-acid glycoprotein, ceruloplasmin, α-2-macoglobulin, and C-reactive protein (Carter et al., 2002), and acute-phase proteins are altered by transportation in newly weaned calves (Arthington et al., 2003a). Initial reports suggested that haptoglobin concentration was unrelated to the severity of the case or the need for treatment in feedlot cattle (Wittum et al., 1996b), but subsequent results suggest that haptoglobin may have value for assessing morbidity (Carter et al., 2002). Berry et al. (2004a) reported that serum haptoglobin concentrations may be useful in predicting the number of treatments required by calves, and Wittum et al. (1996b) and Carter et al. (2002) suggested that haptoglobin was of value in assessing treatment efficacy. Dietary changes in energy and starch concentration had little effect on acute-phase protein response (Berry et al., 2004a); however, Cu status of the animal may be related to acute-phase proteins before and during an inflammatory challenge (Arthington et al., 2003b).
Technological approaches might allow for early detection of morbid animals. Schaefer et al. (2005) suggested that infrared thermography might be valuable for detection of BRD. Likewise, use of radio frequency implants containing temperature probes may allow for early detection of diseases that elevate body temperature (Reid and Dahl, 2005). Research on these 2 methods conducted in commercial applications is needed to verify the accuracy of the diagnosis. If these methods proved useful, they might be included with the national animal identification system being proposed by the USDA.
Feeding and Watering Behavior
Behavioral observations may have value in diagnosing BRD. Sowell et al. (1999), using a feeding behavior system with radio frequency technology (GrowSafe Systems Inc., Calgary, Alberta, Canada), suggested that the daily number of feeding bouts was a better predictor for steers that subsequently were identified as morbid than was watering behavior. Sowell et al. (1998) reported a 30% decrease in time at the feed bunk for morbid vs. healthy steers, and differences in feed intake seemed most pronounced during the first 4 d after arrival (Sowell et al., 1998, 1999). Buhman et al. (2000) reported that sick calves had a greater frequency and duration of drinking 4 to 5 d after arrival than animals that were not sick and further suggested that drinking behavior 4 to 5 d after arrival may be an indicator of BRD.
One widely accepted stressor of beef cattle is commingling. Loerch and Fluharty (2000) suggested that when calves from various sources are commingled in feedlot pens, the social hierarchy is destroyed, and additional stress is imposed. Calves with “trainer” cows in their pens had improved DMI during the first few days after arrival, and in some cases had improved gains and a decreased incidence of BRD (Loerch and Fluharty, 2000). In contrast, Gibb et al. (2000) reported that trainer cows did not improve calf performance, feeding behavior, or health, with calves actually avoiding cows at the bunk during the first few days in the pen. Perhaps surprisingly, Arthington et al. (2003a) reported a tendency for commingled calves to consume more daily DM over a 21-d period than noncommingled calves. Although the background of the commingled calves was unknown, previous exposure to feed bunks was offered as a possible explanation for the increased DMI.
FACTORS AFFECTING THE INCIDENCE OF BRD
Preconditioning
Most feedlot producers believe that preconditioning cattle is somewhat to extremely beneficial in decreasing morbidity and mortality in calves weighing less than 318 kg (USDA-APHIS, 2000a). Only 32.4% of all feedlots surveyed, however, received information about the previous history of the calves “always or most of the time” (USDA-APHIS, 2000b). Several formalized preconditioning programs exist, and many states have programs that certify preconditioned calves. One of the best-known preconditioning programs is the Texas A&M University Value Added Calf (Anonymous, 2005a) program. In general, preconditioning programs ensure that the animals have been weaned for a certain amount of time (usually 30 to 45 d), vaccinated (clostridial and viral vaccines), treated with anthelmintic, castrated, dehorned, and accustomed to feed bunks and water troughs.
Economics of Preconditioning
Dhuyvetter et al. (2005) suggested that based on a 45-d postweaning preconditioning program cow/calf producers can realize a $14.00 increase in returns compared with the sale of calves at weaning that are not preconditioned, and that feedlot producers also can benefit from such programs and can afford to pay premiums for preconditioned calves. Roeber et al. (2001) reported morbidity rates (cattle requiring at least 1 hospital visit) of 34.7, 36.7, and 77.3% and mortality of 1.1, 1.1, and 11.4% for cattle that had been subjected to 2 different preconditioning programs in Kentucky compared with auction-barn calves, respectively. Preconditioning calves on ryegrass pastures resulted in greater ADG and decreased feed costs compared with preconditioning in a drylot (St. Louis et al., 2003), but it was not determined whether the improved performance on grass was a result of deceased morbidity or other factors. For pasture-based preconditioning programs, an effective antibiotic regimen and vaccination program should be followed, along with pastures designed for low-stress handling of morbid animals (St. Louis et al., 2003). Preconditioning seems to be a highly effective means of decreasing BRD morbidity, but its application is not widespread. The ultimate value of preconditioning programs is the ancillary benefit of decreased morbidity in the feedlot, which may not be realized by cow/calf or stocker producers. In addition, perhaps improved information flow regarding the background of the cattle will result from the greater national emphasis on individual animal identification and trace-back, which might stimulate the demand for preconditioned calves.
Vaccination
Vaccination, including IBR, PI3, BVD, and BRSV vaccines, is an integral part of preconditioning programs. A sound working relationship between veterinarians, nutritionists, and farm/ranch managers is essential for an effective health management program. For the Value Added Calf program, vaccination is recommended 4 to 6 wk before weaning, followed by revaccination with a modified live virus at weaning (Anonymous, 2005b). If preweaning vaccination is not feasible, it is recommended that calves be vaccinated at weaning and revaccinated 14 to 21 d later. Nursing calves can be vaccinated with a product labeled for use in calves nursing pregnant cows.
Routine monitoring of the cow herd for potential viral or bacterial immunogens, or both, and administering annual boosters to the cows might result in transfer of greater levels of antibodies to the calves. Passive transfer of antibodies in colostrum is vital for the protection of the young calf against pathogens; however, high concentrations of maternally derived antibodies might interfere with the response to vaccination (Fulton et al., 2004). Zimmerman et al. (2006) reported that a single dose of a modified live vaccine containing BVDV administered at 4 to 5 wk of age stimulated a strong protective immune response to a challenge with virulent type 2 BVDV in calves in the presence of a high concentration of maternal antibodies against BVDV. Similarly, Patel (2005) evaluated a single intranasal vaccination with IBR and suggested the vaccine could provide significant protection in the face of maternally derived antibodies, and that this protection could be prolonged by a booster vaccination. Monitoring both cows and calves is necessary for effective vaccination protocols. Most vaccines used are inactivated (killed) because licensing issues prevent many modified live vaccines from being used on calves nursing pregnant cows. Current practices of vaccinating calves at branding, followed by boosters at or near weaning and at 2 to 4 wk after weaning with inactivated vaccines seem warranted (Fulton et al., 2004).
Little research has been conducted evaluating the efficacy and potential interactions of viral vaccines with nutrition or management. Lysine has been hypothesized as beneficial in treating herpes simplex virus in humans, and herpes simplex virus replication is inhibited by high intracellular concentrations of lysine and low arginine (Marcason, 2003). Maggs et al. (2003) reported that a daily oral dose of 400 mg of L-lysine to cats latently infected with feline herpes virus-1 resulted in decreased viral shedding after changes in housing and husbandry but not after administration of methylprednisolone to induce viral reactivation. Administration of ruminally inert lysine to increase serum lysine concentrations and the potential interaction with BHV-1 might deserve consideration.
Temperament
An excitable temperament in cattle negatively affects performance (Voisinet et al., 1997) and also may play a role in the response to vaccines (Oliphint, 2006; Oliphint et al., 2006). Brahman bull claves (6- to 7-mo-old) were divided into 2 groups (10 calves/temperament group) based on exit velocity from a squeeze chute and pen scores based on the response to confinement and human contact. Calves received clostridial vaccinations at the beginning of the 11-wk study and 42 d later. Both groups showed an initial antibody response by d 6 of the study, with a peak on d 13. Peak antibody response to the booster occurred on d 49 and 54 for calves classified as temperamental and calm, respectively; however, from d 49 to the end of the study, antibody response decreased 3-fold for temperamental calves, with no significant decrease for calm calves. At 11 wk, calm calves had a 1.6-fold greater antibody titer response than temperamental calves. As in previous research, calmer calves had 0.14 kg/d greater ADG than temperamental calves.
Persistent Infection with Bovine Viral Diarrhea Virus
The prevalence of PI calves may not be great, but the economic consequences could be. Wittum et al. (2001) screened 18,931 calves in 128 beef herds in 5 states for PI calves. On initial screening, a total of 56 BVDV-positive calves were found in 13 herds, and 61% of the initially positive calves remained BVDV positive at 6 mo of age. Out of 21,743 calves, Fulton et al. (2006) reported 86 PI calves, for a prevalence of 0.4%. Similarly, Loneragan et al. (2005) reported a prevalence of PI cattle was 0.3% at arrival at the feedlot; however, the prevalence of PI cattle was 2.5% in both chronically ill and dead cattle. Although the prevalence is small, PI calves affect transmission of the virus within groups of calves and associated health of cohorts. Including 1 PI calf positive for BVDV1b caused 68.4% (13 out of 19) of the calves exposed to seroconvert to BVDV1b (Fulton et al., 2005).
The role of exposure to PI calves on the health and performance of cohorts is less well established. O'Connor et al. (2005) reported that a PI calf in a feedlot pen was not associated with increased disease prevalence in commingled groups. Similarly, in a recent New Mexico study, exposure to PI calves had little effect on performance of the other calves (Elam, 2006a). Heifers (296) with a known background (vaccinated once at branding with a modified live IBR, PI3, BRSV, and a killed or modified live BVD) were processed on arrival, including vaccination with modified live IBR, PI3, BRSV, BVDV types 1 and 2, vaccination for clostridial organisms, metaphylactic treatment with a commercially available antimicrobial, and treatment for internal and external parasites. The PI-BVD calves were determined using the antigen capture ELISA test and confirmed with RT-PCR. Treatments were a negative control with no PI-BVD exposure, short-term exposure (60 h) followed by removal of the PI calf from the pen, and long-term exposure (for the duration of the study). In addition, spatially exposed groups included adjacent pens to the aforementioned treatments. No animals were treated for BRD, and no differences were observed in overall ADG, DMI, or G:F by heifers exposed to PI calves short- or long-term and directly or spatially.
Castration
Castration is often a major stress imposed on newly received intact bulls. In addition, it is common to castrate bulls shortly after arrival at the feedlot at the same time that other stresses are imposed (e.g., vaccinations, horn tipping, restraint, etc.). Daniels et al. (2000) reported that calves that were castrated on arrival had a 92% greater incidence of morbidity and 3.5 vs. 0% mortality compared with those castrated before entering the feedlot. Previously castrated calves gain 0.52 kg/d ADG during a 21-d receiving period vs. 0.21 kg/d for cattle castrated on arrival. Although daily feeding and watering behaviors were not affected by castration, calves castrated before arrival had more feeding and watering bouts per day than calves castrated on arrival (Daniels et al., 2000). Age of castration also is important. As the age of castration gets closer to birth, less weight is lost for the 30-d period after castration (Bretschneider, 2005). Cow/calf producers cite concerns about decreased weaning weight (Lents et al., 2006) as a reason for not castrating bulls; however, Lents et al. (2006) reported that intact bulls did not have an advantage in BW at 6 to 7 mo of age compared with bulls banded at birth or bulls banded at birth and implanted with 36 mg of zeranol. In addition, BW gain at weaning was decreased for at least 30 d when castration was delayed to later than 6 mo of age. Thus, producers concerned about decreased weaning weight because of castration could use estrogenic implants in suckling calves to maximize BW gain (Lents et al., 2006).
Prophylactic Medication
Preventative medication programs using prescription antibiotics are administered only under the supervision of licensed veterinarians. Economic considerations regarding the use of such programs should focus on decreasing morbidity and mortality (and associated labor issues) and improving performance. For “high risk” cattle, antibiotic therapy can be an effective means of controlling morbidity. Similarly, when morbidity and mortality are expected to be relatively low, including antibiotics in the feed of new cattle may be effective (Cole, 1993). Duff et al. (2000) evaluated the feeding of chlortetracycline at 10 mg/0.45 kg of BW for 5 d in newly received cattle. Cattle receiving chlortetracycline gained BW during the period that the antibiotic was fed, whereas the cattle not receiving the antibiotic lost weight during the same period. Including chlortetracycline in the feed near to the time of an outbreak of BRD might prove beneficial, but feed intake must be adequate to provide the proper dose of antibiotic. If feed intake of these cattle is an issue, antibiotics need to be injected to ensure the animal receives a sufficient quantity of the antibiotic.
Treatment of individual animals with antibiotics on a preventative or metaphylactic basis has been successful in decreasing the incidence of BRD. The classical work by Lofgreen (1983a), using a combination of long-acting oxytetracycline and sustained-release sulfadimethoxine, showed that morbidity was decreased from 63.3% in control calves to 7.1% in mass-medicated calves. Numerous studies have demonstrated that tilmicosin phosphate is effective for decreasing morbidity of newly received, stressed cattle (Galyean et al., 1995; Cusack, 2004; Guthrie et al., 2004), and preshipping medication programs do not seem to be more effective than arrival medication programs (Duff et al., 2000). Similary, Frank et al. (2002) reported that administration of florfenicol at arrival decreased the incidence of BRD and suggested that prophylactic use of antibiotics may be a means to decrease acute BRD for several days after arrival in the feedlot. More recently, tulathromycin was effective in decreasing the incidence of BRD when given before the onset of symptoms in high-risk cattle (Rooney et al., 2005).
The exact mode of action of preventative medication programs is unknown. One organism greatly affected by prophylactic medication programs is Mannheimia haemolytica. Frank and Duff (2000) and Frank et al. (2002) reported that tilmicosin phosphate and florfenicol inhibited colonization of Mannheimia haemolytica in the nasopharnynx of cattle. Frank et al. (2002) suggested that because of this effect, administration before shipment should decrease the incidence of acute respiratory tract disease during the first week after arrival, when the cattle are most susceptible to infection; however, as noted previously, no advantage was noted with such a protocol (Duff et al., 2000).
If injectable antibiotics negatively affect DMI, beneficial effects of the antibiotics might be offset by decreased performance. Although some injectable antibiotics might decrease feed intake, Daniels et al. (2000) reported that metaphylactic antibiotics (tilmicosin florfenicol given i.m. or s.c.) did not negatively alter feeding behavior, but they decreased morbidity and increased 21-d ADG compared with untreated controls. Neither danofloxacin nor tilmicosin affected neutrophil function or apoptosis (Fajt et al., 2003), suggesting that mass medication would not enhance or diminish any nutritional effects on neutrophil function; thus, interactions of mass medication with nutritional manipulations do not seem likely, but further research is needed.
Possible interactions of antibiotics with dietary nutrients deserve attention. Cole and Hutcheson (1987) reported a tendency for increased death loss of morbid calves with 4% added dietary fat. Because of the unique lipid solubility of certain antibiotics, either positive or negative associative effects might occur that would influence their efficacy. Added dietary fat increased serum concentrations of florfenicol at 6 (quadratic) and 24 (linear) h after injection compared with a diet that did not contain supplemental fat (Duff et al., 2003a). Further research should be conducted to evaluate the interaction of dietary fat with target tissue concentrations of antibiotics.
Post et al. (1991) analyzed 421 P. haemolytica and 158 P. multocida isolates, recovered from cattle with respiratory disease, for patterns of resistance to ampicillin, ceftiofur, erythromycin, gentamicin, penicillin, spectinomycin, sulfachlorpyridazine, sulfadimethoxine, tetracycline, and tylosin. All isolates tested were susceptible to ceftiofur and sulfachlorpyridazine. Pasteurella haemolytica isolates were resistant to ampicillin, penicillin, sulfadimethoxine, tetracycline, and tylosin. Pasteurella multocida isolates were resistant to sulfadimethoxine, tetracycline, and tylosin. A study conducted in the European Union examined the sensitivity of the major BRD bacteria [M. haemolytica, P. multocida, and H. somni (formerly H. somnus)] to commonly used antimicrobials (florfenicol, tilmicosin, and tulathromycin) using disc-diffusion methods (Montgomery, 2005). Bacterial samples were collected from cattle involved in field clinical studies of respiratory disease. The bacterial isolates included 367 M. haemolytica, 245 P. multocida, and 99 H. somni samples. The 711 different bacterial strains tested showed complete susceptibility to florfenicol, and a number of strains were resistant to or showed intermediate susceptibility to tilmicosin and tulathromycin. No mention of possible antimicrobial therapy of cattle used for sample collection was included (Montgomery, 2005).
Catry et al. (2005) measured antimicrobial resistance of Pasteurella and Mannheimia isolates from 57 calves in 13 dairy herds, 150 calves in 9 beef cattle herds, and 289 calves from 5 high-density veal calf herds. The overall resistance of the isolates to at least 1 antimicrobial was 17.6% for dairy, 21.9% for beef, and 71.9% for veal herds. They further reported that resistance to ceftiofur and florfenicol was not detected. Likewise, Rosenbusch et al. (2005) reported that florfenicol was found to be active in vitro against Mycoplasma bovis. Montgomery (2005) recommended frequent review of treatment protocols and that veterinarians evaluate case histories of any previous pneumonia outbreaks on a farm to determine the best intervention strategy. Given that the earlier work of Post et al. (1991) reported resistance to ceftiofur and the later work by Catry et al. (2005) revealed no resistance to ceftiofur, antibiotic resistance to ceftiofur is doubtful; however, more detailed information on previous antibiotic use in all cases needs to be reported before a definitive answer can be given. Because of the importance of antibiotics in therapeutic treatment of BRD, however, antimicrobial resistance needs to be monitored closely.
Nonantibiotic Alternatives
Given the potential for antibiotic resistance, evaluating alternatives to antibiotics is an important area of future research. In a pilot study using 13 weaned and transported calves, Schaefer et al. (2005) suggested that nitric oxide (administered via a nasal tube for 3 consecutive days at 160 or 200 ppm prophylactically or on early detection of BRD) may be an effective treatment. Rivera et al. (2003b) evaluated the effects of an intranasal lysozyme/carbopol preparation given at the time of arrival on health and performance of newly received calves. No differences were noted for ADG or G:F for the 28-d receiving period, but the preparation tended to decrease DMI and tended to increase morbidity during the receiving period. The authors speculated that the lysozyme preparation may have killed beneficial organisms in the nasopharynx, which could have facilitated colonization by M. haemolytica, P. multocida, or both. Research is needed to evaluate administration of lysozyme further down the respiratory tract or at different times after arrival.
Direct-fed microbials (DFM) might be useful to improve performance and decrease morbidity of newly received beef calves. From data collected by member feedlots reporting to the VetLife Benchmark Performance Program, McDonald et al. (2005) evaluated records on 73,870 lots containing 10,900,504 cattle. Feedyards using DFM had increased ADG of 1.9 and 1.4% for steers and heifers, respectively, along with 1.9 and 3.9% improvements in the efficiency of gain. Moreover, ancillary benefits of DFM might occur via improved health or response to antibiotic treatments. Performance advantages with DFM were much greater in cattle with greater (more than $20 per animal) processing and medical treatment charges (Mc-Donald et al., 2005).
Krehbiel et al. (2003) and Beauchemin et al. (2006) recently reviewed the use of DFM in ruminant diets. Krehbiel et al. (2003) cited a study conducted in their laboratory in which no difference in ADG was observed with DFM; however, calves receiving DFM as an oral gel during their first antimicrobial treatment were less likely to be treated again within 96 h, and fewer calves required a second treatment compared with those not given the gel. Krehbiel et al. (2003) suggested that DFM might be beneficial for newly received calves but also pointed out that Gill et al. (1987; as cited by Krehbiel et al., 2003) suggested that DFM might have limited value for extremely healthy or extremely sick calves. As with feed-grade antibiotics, attaining the desired intake of the product may pose problems for morbid calves.
The exact mode of action of DFM remains to be determined; however, Krehbiel et al. (2003) and McDonald et al. (2005) proposed alteration in intestinal microorganisms (including potential effects on ruminal fermentation) and thus competitive attachment of DFM vs. pathogens, as well as superior immune responses or greater gut permeability as possible factors responsible for improved health, performance, or both.
NUTRITIONAL STATUS EFFECTS
The nutritional status of cattle before a BRD challenge is likely critical to the outcome of the challenge. Effects of nutrition or stress during pregnancy on subsequent adult health (fetal programming) are topics of considerable interest in human nutrition (Moore, 1998; Godfrey and Barker, 2001; Owen et al., 2005). Fetal programming also has effects on livestock performance and health (Wu et al., 2006), but relationships between pre- or early postnatal nutrition and susceptibility to BRD in beef cattle have not been defined. As noted previously, effective passive transfer of immunoglobulins in colostrum is vital to calf health and immunity early (Perino, 1997) and later in life (Wittum and Perino, 1995). Perhaps somewhat surprisingly, little is known about the effects of plane of nutrition before a BRD challenge on the health and immunity of beef cattle. Indeed, the nutritional background of cattle used in practical experiments with BRD is typically unknown. Variation in nutritional status might explain the large variation in response to nutritional supplements, particularly protein, minerals, and vitamins, that is evident in the BRD literature.
For example, previous grazing of endophyte-infected fescue pastures might influence receiving period performance, and such cattle received during extreme hot or humid conditions, or both, may experience up to a 10% death loss unless they are cooled with water (personal communication, R. W. Sprowls, Texas A & M Veterinary Diagnostic Laboratory, Amarillo). The duration of carryover effects from fescue toxicosis have been reported to vary from 8 to 10 d (Aiken et al., 2006) to 14 d (Cole et al., 2001). Using cattle with a documented nutritional and management history might be preferable to using cattle from unknown backgrounds, but such cattle might be less likely to succumb to BRD (see the previous discussion on preconditioning, and Clark et al., 2006) and therefore less effective for modeling the disease. Applying nutrient depletion or repletion approaches to cattle for a known period of time before an immune system challenge might be a useful approach to evaluate the efficacy of nutritional or management changes. Although such experimental approaches would no doubt provide more precise estimates of the effects of nutritional or management modifications on health and immunity, well-designed experiments in “real world” settings will continue to be important for field application.
Dietary Energy Concentration
Energy restriction that does not result in malnutrition has increased the life span in rodent models, and it seems beneficial to immunity through increasing lymphocyte proliferation, attenuating age-related decreases in interleukin-2 production, and potentially modifying signal transduction in T-cell development and function (Pahlavani, 2000). Similarly, in periparturient, ruminally cannulated dairy cows with Johne's disease, the neutrophil responses to concanavalin A, phytohemagglutinin (PHA), or pokeweed mitogen were decreased in cows receiving additional feed through the cannula (Stabel et al., 2003); however, immunoglobulin secretion by peripheral blood mononuclear cells was increased in these cows, suggesting positive effects of added energy intake on some aspects of immune cell function.
In Holstein steers fed 210 or 60% of maintenance requirements, negative energy balance had little effect on the expression of adhesion molecules by leukocytes, and expression was increased by negative energy balance in some cases (Perkins et al., 2001). Nonetheless, Ritz and Gardner (2006) noted that aged, energy-restricted mice could not survive a primary influenza infection, and suggested that their innate immune function was diminished. Moreover, the low BW of energy-restricted mice contributed to the mortality because their body energy reserves were inadequate to withstand the infection. Because previously reported positive effects of energy restriction had been observed in challenges with influenza vaccine, Ritz and Gardner (2006) suggested that immunization should not serve as the sole response criterion for the immune response to viruses. Whether the effects on immunity noted with energy in aged rodent and primate models can be applied to lightweight, typically younger, beef cattle is open to question, but adequate energy intake and body energy stores should be important for all bodily functions, including immunity.
Because the energy concentration in beef cattle diets is typically altered by changing the dietary roughage concentration, few studies have evaluated changes in energy intake alone. Thus, effects of energy intake are often confounded with changes in dietary ingredients, particularly roughage. In summarizing classical research on dietary preferences of lightweight, stressed cattle, Lofgreen (1983b) noted such cattle have (1) an abnormally low feed intake relative to BW; and (2) a preference for and greater consumption of a high-concentrate than a high-roughage diet. When given a choice among feed mixtures varying in concentrate level during the first week after arrival at the feedlot, stressed calves selected diets with 72% concentrate (Lofgreen, 1983b). Thus, intake and performance by lightweight, newly received calves is typically optimized with greater compared with lower concentrate diets.
Calves started on a 75% concentrate diet, with or without long-stemmed alfalfa hay during the first week after arrival, gained more and ate more feed than those started on hay alone (Lofgreen, 1979). However, Lofgreen et al. (1981) reported that notwithstanding improved performance, calves fed a 75% concentrate diet tended to have more total sick days than those fed hay alone, although overall proportions of animals treated for BRD did not differ among diets. Fluharty and Loerch (1996) found that as dietary concentrate increased from 70 to 85% in newly received cattle, DMI, but not ADG, increased, and morbidity was not affected by the proportion of dietary concentrate.
Berry et al. (2004a, b) attempted to sort out the confounding effects of roughage and energy concentrations by feeding high and low starch concentrations within each of 2 dietary roughage concentrations. Energy concentration did not influence performance or overall morbidity, but morbid calves fed the greater-energy diets had less shedding of P. multocida and H. somnus than those fed the lower-energy diets. Dietary roughage concentration varied over a fairly narrow range of 35 to 45% in the Berry et al. (2004a, b) studies, and comparison with results of Lofgreen et al. (1981), where the variation in roughage/energy concentration was much greater, is not possible.
Whitney et al. (2006) fed Bermudagrass hay alone, hay with 0.175 or 0.35% of BW of supplemental soybean meal, or a 70% concentrate diet for an 84-d backgrounding phase in early weaned beef steers. Calves fed the concentrate diet had greater DMI and ADG than those fed the hay-based diets. After 84 d, all cattle were switched to the 70% concentrate diet and challenged with an intranasal dose of BHV-1. Serum IgG concentrations were greater before and after the challenge in cattle fed the hay-based diets during backgrounding. Although the differences were small, average rectal temperature on the day after the challenge was greater in calves that were fed the concentrate diet during the backgrounding period, suggesting these calves had a more intense febrile response than the calves fed the hay diets. Perhaps the increased sick days in calves fed greater concentrate diets noted by Lofgreen et al. (1981) reflects enhanced proinflammatory cytokine and febrile responses compared with their counterparts fed lower-energy roughage-based diets.
To evaluate the statistical relationships between BRD and dietary roughage concentration in lightweight, stressed cattle, Rivera et al. (2005) analyzed data collected by Glen Lofgreen at the New Mexico State University Clayton Livestock Research Center. Diets ranged from all-hay to 75% concentrate. Relationships between dietary roughage concentration (DM basis) and receiving-period morbidity, ADG, and DMI were evaluated with mixed-model regression methods. Morbidity (e.g., percentage of calves treated for BRD using visual observation and rectal temperature as a means of diagnosis) decreased slightly as dietary roughage concentration increased [morbidity, % = 49.59 − (0.0675 × roughage, %); P = 0.003]. The ADG and DMI were affected negatively (P < 0.001) by increasing the dietary roughage concentration, and economic analysis indicated that the slightly lesser morbidity noted with greater roughage concentrations would not offset the loss in profit resulting from decreased ADG. Rivera et al. (2005) concluded that greater concentrate, milled diets would likely provide the optimal receiving diet for lightweight, highly stressed, newly received cattle, with limited effects on BRD.
As noted previously, treating cattle for BRD has long-term consequences on feedlot performance and carcass characteristics. Similarly, decreasing energy intake during the receiving period seems to affect ADG, and calves fed low-quality, hay-based diets during receiving were unable to compensate for lost gain during subsequent finishing (Lofgreen 1983b, 1988). Perhaps caloric restriction during a time when the hypothalamic-pituitary-adrenal axis is activated, whether imposed by type of diet fed or as a result of negative health events, results in a permanent loss of performance. Using model systems to evaluate long-term performance effects of caloric restriction during stress would seem to be a good area for research.
Dietary Protein Concentration
In mice fed protein-free diets for 2 to 3 wk, bactericidal immune defense mechanisms were not affected, despite severe weight loss; however, protein deficiency resulted in a failure of the mice to eliminate influenza virus from the lungs, and viral infection suppressed bactericidal defenses (Jakab et al., 1981). Dai et al. (1998) reviewed the effects of nutrition on host responses to mycobacteria and concluded that with diseases like tuberculosis, protein malnutrition resulted in the loss of T-lymphocyte functions and cell-mediated resistance. Moreover, they suggested that generation, migration, or maturation of monocytes was negatively affected by protein deficiency, and that secretion of cytokines (interleukin-2 and interferon-γ) by these cells was impaired in protein-deficient animals. Conversely, transforming growth factor-β production by macrophages infected with Mycobacterium tuberculosis was increased in protein deficiency, which resulted in immunosuppressive effects. Thus, severe protein malnutrition can result in negative effects on immune function. Protein and energy deficiency are often confounded through negative effects of low-protein diets on energy intake, however, and attributing the effects on immunity noted in many experiments solely to protein deficiency is probably incorrect.
We previously concluded that although morbidity from BRD seemed to increase with increasing CP concentration in diets of newly received calves, ADG and DMI also increased as CP concentration increased (Galyean et al., 1999). This paradoxical response was attributed to 3 possible scenarios: (1) inaccurate diagnosis of BRD; (2) morbid calves fed greater-CP diets had greater performance than morbid calves fed lower-CP diets; or (3) performance by healthy calves within greater-CP diets was greater than by healthy calves fed the lower-CP diets. We further suggested that the effects of dietary CP concentration on immune function needed to be assessed; however, to our knowledge this has yet to be fully accomplished.
In neonatal calves, feeding a greater quantity of milk replacer with greater protein content had limited effects on the composition and functionality of peripheral blood mononuclear cell populations compared with calves fed a lower intake of a standard milk replacer (Foote et al., 2005). In early weaned calves fed Bermudagrass hay (6.7% CP, DM basis) alone or hay plus soybean meal at 0.175 or 0.35% of BW, ADG and DMI were increased during an 84-d backgrounding period by the supplemental protein, but no difference was found between the 2 supplemental protein levels (Whitney et al., 2006). When challenged with an intranasal dose of BHV-1, calves previously fed supplemental protein had greater average rectal temperatures for d 1, 2, 4, and 5 after the challenge than calves previously fed hay alone. Serum IgG concentrations after the virus challenge were not affected by protein supplementation. Considering the results of Whitney et al. (2006), and our previous observation (Galyean et al., 1999), calves fed greater CP diets might have an increased likelihood of being diagnosed with BRD as a result of elevated body temperature. Nonetheless, our suggestion that additional research is needed on the effects of dietary CP concentration on immune function in stressed cattle still seems to have merit.
Minerals
Because of low DMI, concentrations of most minerals need to be increased in receiving diets (NRC, 1996). In our earlier review of the potential effects of Cr, Cu, Se, and Zn supplementation on immune function, BRD morbidity, and performance by newly received calves, we noted that although some trials had shown immune function and health benefits from these minerals, many experiments had not (Galyean et al., 1999). Since that review, several reports have been published, particularly on Cu, Se, and Zn; however, little additional information beyond what we reported in 1999 seems to be available for Cr.
Copper.
Despite evidence that Cu is essential for immune function (Percival, 1998), the results of experiments that we reviewed previously did not provide compelling evidence for consistent effects on immunity and provided only limited evidence of responses to Cu supplementation in field studies with stressed calves (Galyean et al., 1999). Although it has been suggested that neutrophil function might be a valuable bio-marker of Cu status (Bonham et al., 2002), chemotaxis and adhesion molecule expression of neutrophils in cattle did not seem to be greatly affected by substantial changes in Cu status induced by Mo and S (Arthington et al., 1995, 1996).
Ward and Spears (1999) injected Angus bull calves with 90 mg of Cu glycinate 28 d before weaning and subsequently supplemented these calves with Cu from CuSO4 (7.5 and 5 mg/kg of DM during the receiving and growing phases, respectively). Control steers did not receive supplemental Cu during the study. During the growing phase, half of the steers in each Cu treatment group were supplemented with 5 mg of Mo/kg of DM, and at d 168 half of the steers were stressed by loading and transport. Supplemental Cu did not affect the skin-swelling response to PHA during the receiving period but increased the antibody response to ovalbumin during the growing phase. Moreover, supplemental Cu increased antibody titers to porcine red blood cells in calves that were subjected to loading and transport stress but decreased the titers in unstressed calves. The authors concluded that specific immunity of stressed cattle was not greatly affected by Cu deficiency and supplemental Mo.
Bailey et al. (2001) fed Angus × Hereford heifers a basal grass hay-based diet that contained 6 mg of Cu/ kg of DM or supplemented the basal diet with 49 mg/kg of DM as CuSO4; 22 mg/kg from CuSO4; 22 mg/kg from a 50:50 combination of CuSO4 and Cu-amino acid complex; or 22 mg of Cu/kg from a mixture of CuSO4, Cu-amino acid complex, and CuO (25:50:25). All diets contained added Mo, S, and Fe. Treatments did not affect ADG or cell-mediated immune function, but the combination of 22 mg/kg from CuSO4 and Cu-amino-acid complex was as effective at limiting liver Cu loss as feeding 49 mg of Cu/kg from CuSO4.
Source and level of supplemental Cu might have effects on other factors besides immunity and health that could affect performance. A bolus containing 12.5 g of CuO needles increased liver Cu concentration after 12 and 33 d in steers fed limpograss hay (Arthington, 2005), but steers in the CuO bolus group had lower NDF and CP digestibilities. In a second experiment, heifers fed stargrass hay were provided 0, 15, 60, or 120 mg of Cu/kg from CuSO4 in a molasses-cottonseed meal supplement. Feeding 15 mg of supplemental Cu/ kg of DM was more effective at maintaining liver Cu than greater concentrations. Heifers fed supplemental Cu tended to eat more forage than controls during the first 2 wk of a 12-wk period, but DM digestibility was not affected by Cu level. Measures of immune function were not reported.
Selenium.
If organic complexes of minerals are more bioavailable than inorganic ones, an organically complexed mineral might be beneficial in preventing or correcting deficiencies during stress and periods of low feed intake (Greene, 1995). With the development of Se-enriched yeast, research that has been published on Se since our previous review has largely focused on comparisons between Se-yeast and inorganic Se.
In cows and calves fed mineral supplements containing no supplemental Se or 26 mg of Se/kg of DM from sodium selenite or Se-yeast, Beck et al. (2005) evaluated lymphocyte blastogenesis and the skin-swelling response to PHA in the calves after weaning. Based on whole blood Se and glutathione peroxidase activity, control calves were deficient in Se. Lymphocyte proliferation response to mitogens was not affected by treatment, but macrophage phagocytosis was increased in calves supplemented with Se-yeast compared with control and sodium selenite calves. Skin swelling response after injection of PHA tended (P = 0.12) to be increased by Se supplementation, but it was not affected by Se source. Fry et al. (2005) fed Angus-crossbred calves fescue hay plus a corn-soybean meal supplement that provided no supplemental Se or 1.7 mg of supplemental Se/d from sodium selenite or Se-yeast. Both sources increased whole-blood Se, with the effects being more rapid in the Se-yeast group, but lymphocyte proliferation and phagocytosis did not differ among treatments. Hence, although Se-yeast might be more bioavailable in terms of the effects on whole-blood Se and other measures of Se status, additional research is needed to fully elucidate its effects on immune function.
Although Arthur et al. (2003) indicated that adequate dietary Se is considered vital for practically all components of the immune system, Suttle and Jones (1989) concluded that the evidence was not strong that Se affected resistance to infection in ruminants. We would not alter this conclusion substantially based on the data available since our previous review.
Zinc.
Beneficial effects of supplemental Zn for the prevention of pneumonia and diarrhea, or as an adjuvant to antimicrobial therapy for the treatment of pneumonia, are typically observed in Zn-deficient children (Hambidge, 2006). Likewise, beneficial effects of supplemental nutrients on immunity and the incidence of BRD in beef cattle would be most likely in animals with a marginal or deficient status of the nutrient. As noted previously, however, it is highly unusual to know the nutrient status of cattle used in most applied receiving studies.
Mineral source, particularly of Zn, was a major focus of our 1999 review, and the results of additional experiments have been published since then with Zn in various inorganic and organically complexed forms. Gunter et al. (2001) supplemented steers with 103 mg of Zn/d from ZnSO4, Zn-amino acid complex, or Zn-polysaccharide during a 116-d grazing period on Bermudagrass pastures, after which the steers were shipped (14 h) to a research feedlot, where they continued on the same Zn sources that were fed during grazing. Neither grazing nor feedlot performance or serum Zn concentrations were affected by Zn source, nor was the number of steers treated for BRD. Spears and Kegley (2002) fed Angus steers (246 kg of initial BW) growing (silage-based) and finishing (corn-based) diets with no added Zn or 25 mg of supplemental Zn/kg of DM from ZnO and 2 different zinc proteinates. All 3 sources of supplemental Zn increased ADG during the growing period, as well as carcass quality grade, whereas the 2 proteinates tended to increase finishing period ADG and improve G:F compared with ZnO. Lymphocyte blastogenesis and humoral antibody titers after IBR vaccination during the growing period did not differ among treatments.
Mineral Combinations.
In Angus and Simmental steers fed silage-based diets with 1,000 mg of supplemental Fe/kg for 149 d (Mullis et al., 2003), Cu and Zn supplemented in sulfate or proteinate forms to supply 5 mg of Cu and 25 mg of Zn/kg of DM did not affect performance or liver Cu and Zn concentrations. Liver Cu decreased with time on feed, and increasing the Cu to 10 mg/kg of DM did not prevent the decrease.
Salyer et al. (2004) evaluated health and performance responses of newly received heifers to dietary supplementation of Cu (10 mg of Cu/kg of dietary DM) and Zn (75 mg of Zn/kg of dietary DM) from sulfate and polysaccharide mineral complex sources. Effects of these same mineral sources on the humoral immune response to ovalbumin also were measured. Copper source × Zn source interactions were not detected for any variable. Neither Cu nor Zn source affected DMI, ADG, G:F, or BRD morbidity in the receiving study. Titers to ovalbumin were greater on d 14 (P = 0.02) and 21 (P = 0.06) after injection of ovalbumin in heifers that received the Zn-polysaccharide complex than in ZnSO4 heifers. In contrast, heifers receiving CuSO4 had greater titers to ovalbumin than those receiving the Cu-polysaccharide complex treatment on d 14 (P = 0.01) and 21 (P = 0.001). Thus, Cu and Zn sources affected the antibody response to ovalbumin, but source effects were not consistent for the 2 minerals.
Providing free-choice mineral supplements composed of all inorganic vs. half to two-thirds of the supplemental minerals from proteinate complexes of Cu, Mn, and Zn (10, 25, and 25 mg/kg of DM, respectively), with or without 0.15% supplemental P, to cows and calves before weaning and to calves after weaning had little effect on immune function measurements in newly weaned steers challenged with an intranasal inoculation of IBR virus (Engle et al., 1999). In a second experiment, in which the same treatments were repeated before and after weaning, receiving period performance was not affected, but plasma Cu was increased in calves fed the organically complexed mineral supplements (Engle et al., 1999).
Stanton et al. (2000) supplemented Angus cows and their calves with a low level of inorganic forms of Co, Cu, Mn, and Zn vs. high levels (2.1× for Cu, 1.44× for Mn and Zn, and 10× for Co vs. the low level) of inorganic or amino acid-complexed minerals and found no effect of treatments on the skin-swelling response to PHA in calves. Crossbred steer calves were arranged in a 2 × 2 factorial by Stanton et al. (2001) to evaluate the effects of high and low levels of inorganic or organically complexed trace minerals fed before and after weaning on performance and immune function. Iron, S, and Mo were added to all diets. Performance over 237 d was not affected by trace mineral source or level; titers to respiratory virus vaccines did not differ among treatments, and the skin-swelling response to PHA was not affected by trace mineral source or level.
Clark et al. (2006) treated low- and high-risk steer calves with a 5-mL (s.c.) injection containing Cu, Se, Mn, and Zn, and measured BRD morbidity (incidence of undifferentiated fever) and performance during a 28-d receiving period and subsequent finishing period. Low-risk calves were vaccinated against common diseases and weaned 45 d before the experiment, whereas high-risk calves were purchased via auction markets and were of unknown history. The incidence of undifferentiated fever was 64.4% in high-risk calves vs. 2% in low-risk calves, but it was unaffected by trace mineral injection. Trace mineral injection decreased ADG during the receiving period, but G:F was improved by the trace mineral injection for the finishing period.
In our earlier review (Galyean et al., 1999), we concluded that formulation of receiving diets should account for decreased DMI by highly stressed, newly received beef cattle and for any known nutrient deficiencies but that adding trace minerals to such diets beyond the concentrations needed to compensate for low feed intake is difficult to justify. Based on the additional data available since that review, we see no reason to substantially modify that conclusion. In addition, although there is some evidence that organically complexed mineral sources might occasionally have different effects on performance and immune function, the effects seem too variable to recommend feeding particular sources.
Vitamins
B Vitamins and Vitamin A.
Results with supplemental B vitamins in the diets of newly weaned/received cattle have been variable (Galyean et al., 1999), and there seems to be little justification for B-vitamin supplementation of nutritionally balanced receiving diets. Nonetheless, the practice of providing injections of B-vitamins is common in feedlots (31.4% of all feedlots surveyed; USDA-APHIS, 2001) as part of BRD treatment regimens, presumably as this is thought to stimulate immunity or perhaps feed intake. In a stress/ BHV-1 challenge model, Dubeski et al. (1996) noted that calves given an injection of B-vitamins and ascorbic acid had a nonsignificant increase in IgG titers to BHV-1 on d 14 and 28 after the challenge, but BW change and lymphocyte blastogenesis did not differ between treatments. Controlled studies to test the use of B-vitamin injections as an adjunct therapy for BRD treatment would be beneficial.
Vitamin A plays an important role in immune function, and vitamin A deficiency in humans and rats is associated with increased severity of infection (Twining et al., 1997). Chemotaxis and phagocytosis by neutophils was decreased in vitamin A-deficient rats, but these abilities were quickly restored by supplemental vitamin A. Semba (1999) reviewed the role of vitamin A in immunity and clinical outcomes of various human diseases. The efficacy of supplemental vitamin A in human trials has varied considerably, and trials with lower-tract respiratory infections have not shown marked clinical benefits (Semba, 1999). With respect to BRD, it would be important to provide vitamin A to calves that have a known deficiency or are potentially marginal in vitamin A status, with injection of vitamin A likely being the most rapid means of increasing body stores. In the absence of a frank deficiency, however, it would seem unlikely that supplemental vitamin A would have major effects on the incidence of BRD.
Vitamin E.
Carter et al. (2002) fed diets to provide 2,000 IU of vitamin E·animal−1·d−1 for 0, 7, 14, or 28 d after the arrival of 4 groups of lightweight heifers. Average daily gain was not affected by treatment, but the medical treatment cost was less for cattle fed supplemental vitamin E for 28 d. By d 28, serum amyloid-A concentrations were less in heifers that had been supplemented with vitamin E, as were α-1-acid glycoprotein concentrations, but fibrinogen and haptoglobin concentrations were not affected by vitamin E supplementation. Carter et al. (2005) evaluated the same vitamin E treatments in 7 truckloads of lightweight cattle during 42-d receiving studies. The duration of supplemental vitamin E feeding did not affect ADG and G:F. As in Carter et al. (2002), which seemed to include a subset of the animals used in Carter et al. (2005), medical costs were decreased for calves fed vitamin E for 28 d. Serum vitamin E concentrations were greatest on d 28 for calves fed vitamin E for the 28-d period and decreased as the length of supplementation decreased, leading Carter et al. (2005) to conclude that the effects of vitamin E on health were likely time-dependent.
Rivera et al. (2002) evaluated receiving diets containing 285, 570, or 1,140 IU of vitamin E·animal−1·d−1 in two 28-d receiving studies, 1 with steers and 1 with heifers. Vitamin E level did not affect ADG, DMI, or G:F during the receiving period; however, similar to the results of Carter et al. (2005), the greatest dose of supplemental vitamin E tended (P < 0.14) to decrease the BRD retreatment rate. In an experiment with beef steers, serum IgG titers to ovalbumin increased linearly with increasing dose of vitamin E. Rivera et al. (2003a) tested the effects of the same 3 doses of vitamin E used in their receiving studies on febrile and metabolic responses in individually fed steers after an intranasal challenge with IBR. Rectal temperature increased linearly in response to vitamin E dose on d 2 and 3 after the challenge, but vitamin E did not affect ADG or serum insulin, NEFA, and urea N concentrations. It was suggested that vitamin E supplementation increased the inflammatory response, which might explain the positive effects on humoral immunity reported by Rivera et al. (2002).
Elam (2006b) summarized data from Secrist et al. (1997), Rivera et al. (2003a), Carter et al. (2005), and Cusack et al. (2005) using mixed model statistical methods to evaluate the effects of vitamin E intake on performance and health of newly received cattle. Supplemental vitamin E intake ranged from 0 to 2,000 IU·animal−1·d−1. Average daily gain, DMI, and G:F were not significantly related to intake of vitamin E; however, BRD morbidity decreased (P = 0.08) by 0.35% for every 100 IU increase in daily vitamin E intake.
In our 1999 review (Galyean et al., 1999), we concluded that vitamin E at doses of greater than 400 IU·animal−1·d−1 seemed beneficial for increasing ADG and decreasing BRD morbidity. Results of the experiments reported since then are generally supportive of the positive effects of supplemental vitamin E on BRD morbidity but perhaps less indicative of the effects on ADG than we had previously concluded.
Other Dietary Considerations
Several other dietary factors might need special attention in receiving diets. Stressed calves are said to prefer dry diets over corn silage-based diets (NRC, 1996), and it is commonly recommended to avoid the use of silage in diets for lightweight, stressed cattle. Whether avoidance of wet ingredient diets is applicable to other feedstuffs (e.g., wet corn gluten feed, distiller's grains) or to only fermented feeds like silage is open to question, and direct comparisons between diets based on silage vs. hay-grain combinations are limited.
Limiting fat in receiving diets has been recommended (NRC, 1996). Added fat (4%) in receiving diets did not greatly affect performance, but it increased mortality compared with no added fat (Cole and Hutcheson, 1987). Fluharty and Loerch (1997), however, reported no effect of added fat (0 or 4% from an animal/ vegetable blend or from calcium soaps of fatty acids) on morbidity and mortality of newly received cattle, and the animal/vegetable blend improved G:F compared with no added fat and calcium soaps of fatty acids.
Based on a review of several articles, Cole (1996) recommended that urea concentrations in receiving diets be restricted to limit total urea intake to less than 0.5 to 0.75% of DM. Nonetheless, Duff et al. (2003b) reported that including urea at 1% of the dietary DM in a 70% concentrate diet for newly received calves did not affect performance or BRD morbidity during a 28-d receiving period.
Because ionophores can negatively affect DMI, their concentrations are often limited in receiving diets to avoid these negative effects. Duff et al. (1995) compared a no-ionophore control diet with diets containing lasalocid at 33 mg/kg, and monensin at 22 or 33 mg/ kg of the dietary DM. Ionophores decreased (P < 0.08) DMI compared with the control diet for the 28-d trial, but ADG and the efficiency of gain were not altered by treatments. Numerically, monensin at 33 mg/kg resulted in a lower DMI than lasalocid at the same concentration. All 3 ionophore treatments decreased the presence of coccidial oocysts. Thus, ionophores might have negative effects on DMI in newly received cattle, but these effects can be moderated by the choice of ionophore or, with monensin, by decreasing the dietary concentration.
SUMMARY AND CONCLUSIONS
Several viruses/bacteria interact to cause BRD, with M. haemolytica being the bacterial organism most often isolated. The effects of PI BVDV on transmission of virus and performance may be important in the development of BRD, but more research is needed. Our ability to diagnose BRD is less than optimal, and development of cost-effective, quantitative methods to more accurately detect animals afflicted with or likely to develop BRD would be valuable to the beef cattle industry. Preconditioning programs offer significant potential to decrease the incidence of BRD, and programs should include vaccination for viral agents and castration of bull calves. Metaphylactic antimicrobial programs continue to be an effective management option for high-risk beef calves, but close attention should be given to antimicrobial resistance, and alternative methods for prevention and/or treatment of calves for BRD deserve attention.
The nutrient status of animals purchased through commercial marketing channels and subsequently arriving at feedlots is rarely known, making it difficult to design diets to provide adequate nutrition and to predict the responses to supplemental nutrients. After decades of research, our ability to modify the incidence of BRD through nutritional manipulations seems limited. The same nutritional program applied to different groups of cattle under seemingly similar conditions may result in widely different rates of BRD morbidity. Based on our review, we recommend that the nutrient content of diets for newly received cattle be formulated to adjust for the low feed intake associated with stress. Adding greater concentrations of vitamin E may be beneficial for decreasing BRD, but research results with trace minerals are variable. Diets with increased energy density (e.g., increased concentrate feeds) that are formulated to provide adequate protein and other nutrients are likely to increase ADG and G:F compared with low-energy receiving diets, without substantially altering the occurrence of BRD.
LITERATURE CITED
- Aiken G. E., Looper M. L., Tabler S. F., Brauer D. K., Strickland J. R., and Schrick F. N.. 2006. Influence of stocking rate and steroidal implants on growth rate of steers grazing toxic tall fescue and subsequent physiological response. J. Anim. Sci. 84:1626–1632. [DOI] [PubMed] [Google Scholar]
- Anonymous. 2005a. Value added calf (VAC) – Management Program. Texas Coop. Ext. Service. http://animalscience.tamu.edu/ANSC/publications/beefpubs/vac_management.pdf Accessed June 15, 2005.
- Anonymous. 2005b. Value added calf (VAC) – Vaccination Programs. Texas Coop. Ext. Service. http://animalscience.tamu.edu/ANSC/publications/beefpubs/vac_vaccine.pdf Accessed June 15, 2005.
- Arthington J. D. 2005. Effects of copper oxide bolus administration or high-level copper supplementation on forage utilization and copper status in beef cattle. J. Anim. Sci. 83:2894–2900. [DOI] [PubMed] [Google Scholar]
- Arthington J. D., Corah L. R., Blecha F., and Hill D. A.. 1995. Effect of copper depletion and repletion on lymphocyte blastogenesis and neutrophil bactericidal function in beef heifers. J. Anim. Sci. 73:2079–2085. [DOI] [PubMed] [Google Scholar]
- Arthington J. D., Eicher S. D., Kunkle W. E., and Martin F. G.. 2003a. Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J. Anim. Sci. 81:1120–1125. [DOI] [PubMed] [Google Scholar]
- Arthington J. D., Martin F., and Blecha F.. 2003b. Effect of molybdenum and sulfur feeding on the acute phase protein response to inflammatory challenge in beef heifers. Prof. Anim. Sci. 19:221–226. [Google Scholar]
- Arthington J. D., Spell A. R., Corah L. R., and Blecha F.. 1996. Effect of molybdenum-induced copper deficiency on in vivo and in vitro measures of neutrophil chemotaxis both before and following an inflammatory stressor. J. Anim. Sci. 74:2759–2764. [DOI] [PubMed] [Google Scholar]
- Arthur J. R., McKenzie R. C., and Beckett G. J.. 2003. Selenium in the immune system. J. Nutr. 133:1457S–1459S. [DOI] [PubMed] [Google Scholar]
- Babcock A., Jones R., and Langemeier M.. 2006. Examining death loss in Kansas feedlots. Pages 46–52 in Beef Cattle Research–2006, Report of Prog. 959, Kansas State Univ., Manhattan: http://www.oznet.ksu.edu/library/lvstk2/srp959.pdf Accessed June 27, 2006. [Google Scholar]
- Bailey J. D., Ansotegui R. P., Paterson J. A., Swenson C. K., and Johnson A. B.. 2001. Effects of supplementing combinations of inorganic and complexed copper on performance and liver mineral status of beef heifers consuming antagonists. J. Anim. Sci. 79:2926–2934. [DOI] [PubMed] [Google Scholar]
- Baumann H., and Gauldie J.. 1994. The acute phase response. Immunol. Today 15:74–80. [DOI] [PubMed] [Google Scholar]
- Beauchemin K. A., Krehbiel C. R., and Newbold C. J.. 2006. Enzymes, bacterial direct-fed microbials and yeast: Principles for use in ruminant nutrition. Pages 251–284 in Biology of Nutrition in Growing Animals. Mosenthin R., Zentek J., and Zebrowska T. ed. Elsevier Limited, Amsterdam, the Netherlands. [Google Scholar]
- Beck P. A., Wistuba T. J., Dais M. E., and Gunter S. A.. 2005. Case study: Effects of feeding supplemental organic or inorganic selenium to cow-calf pairs on selenium status and immune responses of weaned beef calves. Prof. Anim. Sci. 21:114–120. [Google Scholar]
- Berry B. A., Confer A. W., Krehbiel C. R., Gill D. R., Smith R. A., and Montelongo M.. 2004a. Effects of dietary energy and starch concentrations for newly received feedlot calves: II. Acute-phase protein response. J. Anim. Sci. 82:845–850. [DOI] [PubMed] [Google Scholar]
- Berry B. A., Krehbiel C. R., Confer A. W., Gill D. R., Smith R. A., and Montelongo M.. 2004b. Effects of dietary energy and starch concentrations for newly received feedlot calves: I. Growth performance and health. J. Anim. Sci. 82:837–844. [DOI] [PubMed] [Google Scholar]
- Blecha F., Boyles S. L., and Riley J. G.. 1984. Shipping suppresses lymphocyte blastogenic responses in Angus and Brahman × Angus feeder calves. J. Anim. Sci. 59:576–583. [DOI] [PubMed] [Google Scholar]
- Bonham M., O'Connor J. M., Hannigan B. M., and Strain J. J.. 2002. The immune system as a physiological indicator of marginal copper status? Br. J. Nutr. 87:393–403. [DOI] [PubMed] [Google Scholar]
- Bretschneider G. 2005. Effects of age and method of castration on performance and stress response of beef male cattle. A review. Livest. Prod. Sci. 97:89–100. [Google Scholar]
- Bryant L. K., Perino L. J., Griffin D., Doster A. R., and Wittum T. E.. 1999. Method for recording pulmonary lesions of beef calves at slaughter, and the association of lesions with average daily gain. Bovine Pract. 33:163–173. [Google Scholar]
- Buhman M. J., Perino L. J., Galyean M. L., Wittum T. E., Montgomery T. H., and Swingle R. S.. 2000. Association between changes in eating and drinking behaviors and respiratory tract disease in newly arrived calves at the feedlot. Am. J. Vet. Res. 61:1163–1168. [DOI] [PubMed] [Google Scholar]
- Carter J. N., Gill D. R., Krehbiel C. R., Confer A. W., Smith R. A., Lalman D. L., Claypool P. L., and McDowell L. R.. 2005. Vitamin E supplementation of newly arrived feedlot calves. J. Anim. Sci. 83:1924–1932. [DOI] [PubMed] [Google Scholar]
- Carter J. N., Meredith G. L., Montelongo M., Gill D. R., Krehbiel C. R., Payton M. E., and Confer A. W.. 2002. Relationship of vitamin E supplementation and antimicrobial treatment with acute-phase protein responses in cattle affected by naturally acquired respiratory tract disease. Am. J. Vet. Res. 63:1111–1117. [DOI] [PubMed] [Google Scholar]
- Catry B., Haesebrouck F., De Vliegher S., Feyen B., Van Robaeys M., Opsomer G., Schwarz S., and De Kruif A.. 2005. Variability in acquired resistance of Pasteurella and Mannheimia isolates from the nasopharynx of calves, with particular reference to different herd types. Microb. Drug Resist. 11:387–394. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Olson K. C., Schmidt T. B., Larson R. L., Ellersieck M. R., Alkire D. O., Meyer D. L., Rentfrow G. K., and Carr C. C.. 2006. Effects of respiratory disease risk and bolus injection of trace minerals at receiving on growing and finishing performance by beef steers. Prof. Anim. Sci. 22:245–251. [Google Scholar]
- Cole N. A. 1993. Nutritional strategies for stressed feeder calves. Pages 1–9 in Proc. Southwest Nutr. Manage. Conf., University of Arizona, Tucson. [Google Scholar]
- Cole N. A. 1996. Review of bovine respiratory disease: Nutrition and disease interactions. Pages 57–74 in Review of Bovine Respiratory Disease—Schering-Plough Animal Health. Smith R. ed. Veterinary Learning Systems, Trenton, NJ. [Google Scholar]
- Cole N. A., and Hutcheson D. P.. 1987. Influence of receiving diet fat level on the health and performance of feeder calves. Nutr. Rep. Int. 36:965–970. [Google Scholar]
- Cole N. A., Stuedemann J. A., and Thompson F. N.. 2001. Influence of both endophyte infestation in fescue pastures and calf genotype on subsequent feedlot performance of steers. Prof. Anim. Sci. 17:174–182. [Google Scholar]
- Cusack P. M. V. 2004. Effect of mass medication with antibiotics at feedlot entry on the health and growth rate of cattle destined for the Australian domestic market. Aust. Vet. J. 82:154–156. [DOI] [PubMed] [Google Scholar]
- Cusack P. M. V., McMeniman N. P., and Lean I. J.. 2005. The physiological and production effects of increased dietary intake of vitamins E and C in feedlot cattle challenged with bovine herpesvirus 1. J. Anim. Sci. 83:2423–2433. [DOI] [PubMed] [Google Scholar]
- Dai G., Phalen S., and McMurray D. N.. 1998. Nutritional modulation of host responses to mycobacteria. Front. Biosci. 3:e110–e122. [DOI] [PubMed] [Google Scholar]
- Daniels T. K., Bowman J. G. P., Sowell B. F., Branine M. E., and Hubbert M. E.. 2000. Effects of metaphylactic antibiotics on behavior of feedlot calves. Prof. Anim. Sci. 16:247–253. [Google Scholar]
- DeRosa D. C., Mechor G. D., Staats J. J., Chengappa M. M., and Shryock T. R.. 2000. Comparison of Pasteurella spp. simultaneously isolated from nasal and transtracheal swabs from cattle with clinical signs of bovine respiratory disease. J. Clin. Microbiol. 38:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuyvetter K. C., Bryant A. M., and Blasi D. A.. 2005. Case study: Preconditioning beef calves: Are expected premiums sufficient to justify the practice? Prof. Anim. Sci. 21:502–514. [Google Scholar]
- Dubeski P. L., d'Offay J. M., Owens F. N., and Gill D. R.. 1996. Effects of B vitamin injection on bovine herpesvirus-1 infection and immunity in feed-restricted beef calves. J. Anim. Sci. 74:1367–1374. [DOI] [PubMed] [Google Scholar]
- DuBois W. R., Cooper V. L., Duffy J. C., Dean D. D., Ball R. L., and Starr, Jr B. D.. 2000. A preliminary evaluation of the effect of vaccination with modified live bovine viral diarrhea virus (BVDV) on detection of BVDV antigen in skin biopsies using immunohistochemical methods. Bovine Pract. 34:98–100. [Google Scholar]
- Dubovi E. J. 1996. Laboratory diagnosis of bovine viral diarrhea virus infections. Vet. Med. (Praha) 91:867–872. [DOI] [PubMed] [Google Scholar]
- Duff G. C., Hunsaker B. D., Anderson A. C., Roder J. D., and Walker D. A.. 2003a. Florfenicol concentration in serum of beef cattle fed 0, 3, or 6% added dietary fat in a 70% concentrate diet. J. Anim. Vet. Advan. 2:44–48. [Google Scholar]
- Duff G. C., Galyean M. L., Malcolm-Callis K. J., and Garcia D. R.. 1995. Effects of ionophore type and level in the receiving diet on performance by newly received beef calves. Clayton Livestock Res. Ctr. Prog. Rep. No. 96, New Mexico Agric. Exp. Stn., Las Cruces. [Google Scholar]
- Duff G. C., Malcolm-Callis K. J., Galyean M. L., and Walker D. A.. 2003b. Effects of dietary urea concentration on performance and health of receiving cattle and performance and carcass characteristics of finishing cattle. Can. J. Anim. Sci. 83:569–575. [Google Scholar]
- Duff G. C., Walker D. A., Malcolm-Callis K. J., Wiseman M. W., and Hallford D. M.. 2000. Effects of preshipping vs arrival medication with tilmicosin phosphate and feeding chlortetracycline on health and performance of newly received beef calves. J. Anim. Sci. 78:267–274. [DOI] [PubMed] [Google Scholar]
- Elam N. A. 2006a. Effects of long- or short-term exposure to a calf identified as persistently infected with Bovine Viral Diarrhea Virus on the performance of freshly weaned, stressed, receiving beef heifers. Clayton Livestock Res. Ctr. Prog. Rep. No. 109. New Mexico Agric. Exp. Stn., Las Cruces. [DOI] [PubMed] [Google Scholar]
- Elam N. A. 2006b. Impact of vitamin E supplementation on newly received calves. Proc. Colorado Nutr. Roundtable, Colorado State Univ., Ft. Collins. [Google Scholar]
- Engle T. E., Spears J. W., and Brown, Jr T. T.. 1999. Effects of dietary phosphorus and trace mineral source on immune function, mineral status, and performance of stressed steers. Prof. Anim. Sci. 15:238–244. [Google Scholar]
- Fajt V. R., Apley M. D., Roth J. A., Frank D. E., Brogden K. A., Skogerboe T. L., Shostrom V. K., and Chin Y.-L.. 2003. The effects of danofloxacin and tilmicosin on neutrophil function and lung consolidation in beef heifer calves with induced Pasteurella (Mannheimia) haemolytica pneumonia. J. Vet. Pharmacol. Therap. 26:173–179. [DOI] [PubMed] [Google Scholar]
- Fent G. M., Fulton R. W., Saliki J. T., Caseltine S. L., Lehmkuhl H. D., Confer A. W., Purdy C. W., Briggs R. E., Loan R. W., and Duff G. C.. 2002. Bovine adenovirus serotype 7 infections in postweaning calves. Am. J. Vet. Res. 63:976–978. [DOI] [PubMed] [Google Scholar]
- Flores E. F., Ridpath J. F., Weiblen R., Vogel F. S. F., and Gil L. H. V. G.. 2002. Phylogenetic analysis of Brazilian bovine viral diarrhea virus type 2 (BVDV-2) isolates: Evidence for a subgenotype within BVDV-2. Virus Res. 87:51–60. [DOI] [PubMed] [Google Scholar]
- Fluharty F. L., and Loerch S. C.. 1996. Effect of dietary energy source and level on performance of newly arrived feedlot cattle. J. Anim. Sci. 74:504–513. [DOI] [PubMed] [Google Scholar]
- Fluharty F. L., and Loerch S. C.. 1997. Effects of concentration and source of supplemental fat and protein on performance of newly arrived feedlot steers. J. Anim. Sci. 75:2308–2316. [DOI] [PubMed] [Google Scholar]
- Foote M. R., Nonnecke B. J., Waters W. R., Palmer M. V., Beitz D. C., Fowler M. A., Miller B. L., Johnson T. E., and Perry H. B.. 2005. Effects of increased dietary protein and energy on composition and functional capacities of blood mononuclear cells from vaccinated, neonatal calves. Int. J. Vitam. Nutr. Res. 75:357–368. [DOI] [PubMed] [Google Scholar]
- Frank G. H., Briggs R. E., Duff G. C., Loan R. W., and Purdy C. W.. 2002. Effects of vaccination before transit and administration of florfenicol at time of arrival in a feedlot on the health of transported calves and detection of Mannheimia haemolytica in nasal secretions. Am. J. Vet. Res. 63:251–256. [DOI] [PubMed] [Google Scholar]
- Frank G. H., and Duff G. C.. 2000. Effects of tilmicosin phosphate, administered before transport or at time of arrival, and feeding of chlortetracycline, after arrival in a feedlot, on Mannheimia haemolytica in nasal secretions of transported steers. Am. J. Vet. Res. 61:1479–1483. [DOI] [PubMed] [Google Scholar]
- Fry R. S., Kegley E. B., Davis M. E., Ratcliff M. D., Galloway D. L., and Dvorak R. A.. 2005. Level and source of supplement selenium for beef steers. Arkansas Anim. Sci. Dept. Rep., AAES Res. Ser. 535 http://www.uark.edu/depts/agripub/Publications/researchseries/535-26.pdf Accessed June 5, 2006. [Google Scholar]
- Fulton R. W., Briggs R. E., Payton M. E., Confer A. W., Saliki J. T., Ridpath J. F., Burge L. J., and Duff G. C.. 2004. Maternally-derived humoral immunity to bovine viral diarrhea virus (BVDV)1a, BVDV1b, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus, bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine 22:643–649. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Briggs R. E., Ridpath J. F., Saliki J. T., Confer A. W., Payton M. E., Duff G. C., Step D. L., and Walker D. A.. 2005. Transmission of bovine viral diarrhea virus 1 b to susceptible and vaccinated calves by exposure to persistently infected calves. Can. J. Vet. Res. 69:161–169. [PMC free article] [PubMed] [Google Scholar]
- Fulton R. W., Cook B. J., Step D. L., Confer A. W., Saliki J. T., Payton M. E., Burge L. J., Welsh R. D., and Blood K. S.. 2002. Evaluation of health status of calves and the impact on feedlot performance: Assessment of a retained ownership program for postweaning calves. Can. J. Vet. Res. 66:173–180. [PMC free article] [PubMed] [Google Scholar]
- Fulton R. W., Hessman B., Johnson B. J., Ridpath J. F., Saliki J. T., Burge L. J., Sjeklocha D., Confer A. W., Funk R. A., and Payton M. E.. 2006. Evaluation of diagnostic tests used for detection of bovine viral diarrhea virus and prevalence of subtypes 1a, 1b, and 2a in persistently infected cattle entering a feedlot. J. Am. Vet. Med. Assoc. 228:578–584. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Purdy C. W., Confer A. W., Saliki J. T., Loan R. W., Briggs R. E., and Burge L. J.. 2000a. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parinfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 64:151–159. [PMC free article] [PubMed] [Google Scholar]
- Fulton R. W., Ridpath J. F., Confer A. W., Saliki J. T., Burge L. J., and Payton M. E.. 2003. Bovine viral diarrhea virus antigenic diversity: Impact on disease and vaccination programmes. Biologicals 31:89–95. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Saliki J. T., Confer A. W., Burge L. J., d'Offay J. M., Helman R. G., Bolin S. R., Ridpath J. F., and Payton M. E.. 2000b. Bovine viral diarrhea virus cytopahtic and noncytopathic biotypes and type 1 and 2 geneotypes in diagnostic laboratory accessions: Clinical and necropsy samples from cattle. J. Vet. Diagn. Invest. 12:33–38. [DOI] [PubMed] [Google Scholar]
- Galyean M. L., Gunter S. A., and Malcolm-Callis K. J.. 1995. Effects of arrival medication with tilmicosin phosphate on health and performance of newly received beef cattle. J. Anim. Sci. 73:1219–1226. [DOI] [PubMed] [Google Scholar]
- Galyean M. L., and Hubbert M. E.. 1995. Effects of season, health, and management on feed intake by beef cattle. Pages 226–234 in Symposium: Intake by Feedlot Cattle. Owens F. N. ed. Oklahoma Agric. Exp. Stn., P-942. [Google Scholar]
- Galyean M. L., Perino L. J., and Duff G. C.. 1999. Interaction of cattle health/immunity and nutrition. J. Anim. Sci. 77:1120–1134. [DOI] [PubMed] [Google Scholar]
- Gardner B. A., Dolezal H. G., Bryant L. K., Owens F. N., and Smith R. A.. 1999. Health of finishing steers: Effects on performance, carcass traits, and meat tenderness. J. Anim. Sci. 77:3168–3175. [DOI] [PubMed] [Google Scholar]
- Gevaert D. 2006. The importance of Mycoplasma bovis in respiratory disease in calves. Tijdschr. Diergeneeskd. 131:124–126. [PubMed] [Google Scholar]
- Gibb D. J., Schwartzkopf-Genswein K. S., Stookey J. M., McKinnon J. J., Godson D. L., Wiedmeier R. D., and McAllister T. A.. 2000. Effect of a trainer cow on health, behavior, and performance of newly weaned beef calves. J. Anim. Sci. 78:1716–1725. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Smith R. A., and Ball R. L.. 1987. The effect of probiotic feeding on health and performance of newly-arrived stocker calves. Oklahoma Agric. Exp. Stn. MP-119:202–204.
- Godfrey K. M., and Barker D. J. P.. 2001. Fetal programming and adult health. Publ. Health Nutr. 4:611–624. [DOI] [PubMed] [Google Scholar]
- Greene L. W. 1995. The nutritional value of inorganic and organic mineral sources. Pages 23–31 in Proc. Plains Nutr. Counc. Symp. Texas Tech Univ., Lubbock. [Google Scholar]
- Gunter S. A., Malcolm-Callis K. J., Duff G. C., and Kegley E. B.. 2001. Performance of steers supplemented with zinc during grazing and receiving at the feedlot. Prof. Anim. Sci. 17:280–286. [Google Scholar]
- Guthrie C. A., Rogers K. C., Christmas R. A., Vogel G. J., Laudert S. B., and Mechor G. D.. 2004. Efficacy of metaphylactic tilmicosin for controlling bovine respiratory disease in high-risk northern feeder calves. Bovine Pract. 38:46–53. [Google Scholar]
- Hambidge K. M. 2006. Zinc and pneumonia. Am. J. Clin. Nutr. 83:991–992. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., and Astry C. L.. 1981. Alterations of pulmonary defense mechanisms by protein depletion diet. Infect. Immun. 34:610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel C. R., Rust S. R., Zhang G., and Gilliland S. E.. 2003. Bacterial direct-fed microbials in ruminant diets: Performance response and mode of action. J. Anim. Sci. 81(E. Suppl. 2): E120–E132. http://www.asas.org/symposia/03esupp2/jas2407.pdf Accessed June 7, 2006. [Google Scholar]
- Larson R. L., Miller R. B., Kleiboeker S. B., Miller M. A., and White B. J.. 2005. Economic costs associated with two testing strategies for screening feeder calves for persistent infection with bovine viral diarrhea virus. J. Am. Vet. Med. Assoc. 226:249–254. [DOI] [PubMed] [Google Scholar]
- Lents C. A., White F. J., Floyd L. N., Gay D. L., and Wettemann R. P.. 2006. Effects of method and timing of castration and the use of an estrogenic growth stimulant on weight gain of bull calves. Prof. Anim. Sci. 22:126–131. [Google Scholar]
- Loerch S. C., and Fluharty F. L.. 2000. Use of trainer animals to improve performance and health of newly arrived feedlot calves. J. Anim. Sci. 78:539–545. [DOI] [PubMed] [Google Scholar]
- Lofgreen G. P. 1979. A comparison of native and southern calves on three receiving rations. Clayton Livestock Res. Ctr. Prog. Rep. No. 10. New Mexico Agric. Exp. Stn., Las Cruces. [Google Scholar]
- Lofgreen G. P. 1983a. Mass medication in reducing shipping fever-bovine respiratory disease complex in highly-stressed calves. J. Anim. Sci. 56:529–536. [DOI] [PubMed] [Google Scholar]
- Lofgreen G. P. 1983b. Nutrition and management of stressed beef calves. Pages 87–101 in Veterinary Clinics of North America: Large Animal Practice. Vol. 5, No. 1, March, 1983. W. B. Saunders, Philadelphia, PA. [DOI] [PubMed] [Google Scholar]
- Lofgreen G. P. 1988. Nutrition and management of stressed beef calves. An update. Pages 509–522 in Veterinary Clinics of North America: Food Animal Practice. Vol. 4, No. 3, November 1988. W. B. Saunders, Philadelphia, PA. [DOI] [PubMed] [Google Scholar]
- Lofgreen G. P., El Tayeb A. E., and Kiesling H. E.. 1981. Millet and alfalfa hays alone and in combination with high-energy diets for receiving stressed calves. J. Anim. Sci. 52:959–968. [DOI] [PubMed] [Google Scholar]
- Loneragan G. H., Thomson D. U., Montgomery D. L., Mason G. L., and Larson R. L.. 2005. Prevalence, outcome, and health consequences associated with persistent infection with bovine viral diarrhea virus in feedlot cattle. J. Am. Vet. Med. Assoc. 226:595–601. [DOI] [PubMed] [Google Scholar]
- Maggs D. J., Nasisse M. P., and Kass P. H.. 2003. Efficacy of oral supplementation with L-lysine in cats latently infected with feline herpesvirus. Am. J. Vet. Res. 64:37–42. [DOI] [PubMed] [Google Scholar]
- Marcason W. 2003. Will taking the amino acid supplement lysine prevent or treat the herpes simplex virus? J. Am. Diet. Assoc. 103:351. [DOI] [PubMed] [Google Scholar]
- McClurkin A. W., Littledike E. T., Cutlip R. C., Frank G. H., and Coria M. F.. 1984. Production of cattle immunotolerant to bovine viral diarrhea virus. Can. J. Comp. Med. 48:156–161. [PMC free article] [PubMed] [Google Scholar]
- McDonald A., Andersen P., Defoor P., and Botts R.. 2005. DFMs improve health, performance of cattle. Feedstuffs 77:12–13. [Google Scholar]
- Montgomery A. 2005. Sensitivity of BRD pathogens to commonly-used antibiotics. Vet. Times 35:12. [Google Scholar]
- Montgomery T. H., Adams R., Cole N. A., Hutcheson D. P., and McLaren J. B.. 1984. Influence of feeder calf management and bovine respiratory disease on carcass traits of beef steers. Proc. West. Sec. Am. Soc. Anim. Sci. 35:319–322. [Google Scholar]
- Moore S. E. 1998. Nutrition, immunity and the fetal and infant origins of disease hypothesis in developing countries. Proc. Nutr. Soc. 57:241–247. [DOI] [PubMed] [Google Scholar]
- Mullis L. A., Spears J. W., and McCraw R. L.. 2003. Effects of breed (Angus vs Simmental) and copper and zinc source on mineral status of steers fed high dietary iron. J. Anim. Sci. 81:318–322. [DOI] [PubMed] [Google Scholar]
- Noffsinger T., and Locatelli L.. 2004. Low-stress cattle handling: An overlooked dimension of management. Pages 65–78 in Proc. Meet. Academy of Veterinary Consultants. Vol. XXXII, No. 2.
- NRC 1996. Nutrient Requirements of Beef Cattle. 7th ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- O'Connor A. M., Sorden S. D., and Apley M. D.. 2005. Association between the existence of calves persistently infected with bovine viral diarrhea virus and commingling on pen morbidity in feedlot cattle. Am. J. Vet. Res. 66:2130–2134. [DOI] [PubMed] [Google Scholar]
- Oliphint R. A. 2006. Evaluation of the inter-relationships of temperament, stress responsiveness and immune function in beef calves. M.S. Thesis. Texas A&M University, College Station. [Google Scholar]
- Oliphint R., Burdick N., Laurenz J., Curley K., Vann R., Randel R., and Welsh T.. 2006. Relationship of temperament with immunization response and lymphocyte proliferation in Brahman bulls. J. Anim. Sci. 84(Suppl. 2):32. (Abstr.)16361489 [Google Scholar]
- Owen D., Andrews M. H., and Matthews S. G.. 2005. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behavior. Neurosci. Biobehav. Rev. 29:209–226. [DOI] [PubMed] [Google Scholar]
- Pahlavani M. A. 2000. Caloric restriction and immunosenescence: A current perspective. Front. Biosci. 5:d580–d587. [DOI] [PubMed] [Google Scholar]
- Pandher K., Confer A. W., and Murphy G. L.. 1998. Genetic and immunologic analyses of PlpE, a lipoprotein important in complement-mediated killing of Pasteurella haemolytica serotype 1. Infect. Immun. 66:5613–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. R. 2005. Characteristics of live bovine herpesvirus-1 vaccines. Vet. J. 169:404–416. [DOI] [PubMed] [Google Scholar]
- Pellerin C., Van Den Hurk J., Lecomte J., and Tijssen P.. 1994. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology 203:260–268. [DOI] [PubMed] [Google Scholar]
- Percival S. S. 1998. Copper and immunity. Am. J. Clin. Nutr. 67(Suppl.):1064S–1068S. [DOI] [PubMed] [Google Scholar]
- Perino L. J. 1997. A guide to colostrum management in beef cows and calves. Vet. Med. (January):85–82. [Google Scholar]
- Perino L. J., and Apley M. D.. 1998. Clinical trial design in feedlots. Pages 343–365 in Veterinary Clinics of North America: Food Animal Practice. Hunt E. and Stokka G. L. ed. W. B. Saunders Co., Philadelphia, PA: Vol. 14. No. 2. [DOI] [PubMed] [Google Scholar]
- Perkins K. H., Vandhaar M. J., Tempelman R. J., and Burton J. L.. 2001. Negative energy balance does not decrease expression of leukocyte adhesion or antigen-presenting molecules in cattle. J. Dairy Sci. 84:421–428. [DOI] [PubMed] [Google Scholar]
- Plummer P. J., Rohrbach B. W., Daugherty R. A., Daugherty R. A., Thomas K. V., Wilkes R. P., Duggan F. E., and Kennedy M. A.. 2004. Effect of intranasal vaccination against bovine enteric cornonavirus on the occurrence of respiratory tract disease in a commercial backgrounding feedlot. J. Am. Vet. Med. Assoc. 225:726–731. [DOI] [PubMed] [Google Scholar]
- Post K. W., Cole N. A., and Raleigh R. H.. 1991. In vitro antimicrobial susceptibility of Pasteurella haemolytica and Pasteurella multocida recovered from cattle with bovine respiratory disease complex. J. Vet. Diagn. Invest. 3:124–126. [DOI] [PubMed] [Google Scholar]
- Reid E. D., and Dahl G. E.. 2005. Peripheral and core body temperature sensing using radio-frequency implants in steers challenged with lipopolysaccharide. J. Anim. Sci. 83(Suppl. 1):352. (Abstr.) [DOI] [PubMed] [Google Scholar]
- Ridpath J. F., Bolin S. R., and Dubovi E. J.. 1994. Segregation of bovine viral diarrhea virus into genotypes. Virology 205:66–74. [DOI] [PubMed] [Google Scholar]
- Rivera J. D., Duff G. C., Galyean M. L., Hallford D. M., and Ross T. T.. 2003a. Effects of graded levels of vitamin E on inflammatory response and evaluation of methods of delivering supplemental vitamin E on performance and health of beef steers. Prof. Anim. Sci. 19:171–177. [Google Scholar]
- Rivera J. D., Duff G. C., Galyean M. L., Walker D. A., and Nunnery G. A.. 2002. Effects of supplemental vitamin E on performance, health, and humoral immune response of beef cattle. J. Anim. Sci. 80:933–941. [DOI] [PubMed] [Google Scholar]
- Rivera J. D., Galyean M. L., and Nichols W. T.. 2005. Review: Dietary roughage concentration and health of newly received cattle. Prof. Anim. Sci. 21:345–351. [Google Scholar]
- Rivera J. D., Richeson J. T., Gleghorn J. F., Elam N. A., Galyean M. L., Hubbert M. E., and Bachman S. E.. 2003b. Effects of intranasal administration of a lysozyme/zinc/carbopol preparation on health and performance of newly received beef cattle. Proc. Western Sec. Am. Soc. Anim. Sci. 54:64–67. [Google Scholar]
- Ritz B. W., and Gardner E. M.. 2006. Malnutrition and energy restriction differentially affect viral immunity. J. Nutr. 136:1141–1144. [DOI] [PubMed] [Google Scholar]
- Roeber D. L., Speer N. C., Gentry J. G., Tatum J. D., Smith C. D., Whittier J. C., Jones G. F., Belk K. E., and Smith G. C.. 2001. Feeder cattle health management: Effects on morbidity rates, feedlot performance, carcass characteristics, and beef palatability. Prof. Anim. Sci. 17:39–44. [Google Scholar]
- Rooney K. A., Nutsch R. G., Skogerboe T. L., Weigel D. J., Gajewski K., and Kilgore W. R.. 2005. Efficacy of tulathromycin compared with tilmicosin and florfenicol for the control of respiratory disease in cattle at high risk of developing bovine respiratory disease. Vet. Therap. 6:154–166. [PubMed] [Google Scholar]
- Rosenbusch R. F., Kinyon J. M., Apley M., Funk N. D., Smith S., and Hoffman L. J.. 2005. In vitro antimicrobial inhibition profiles of Mycoplasma bovis isolates recovered from various regions of the United States from 2002 to 2003. J. Vet. Diagn. Invest. 17:436–441. [DOI] [PubMed] [Google Scholar]
- Salyer G. B., Galyean M. L., Defoor P. J., Nunnery G. A., Parsons C. H., and Rivera J. D.. 2004. Effects of copper and zinc source on performance and humoral immune response of newly received, lightweight beef heifers. J. Anim. Sci. 82:2467–2473. [DOI] [PubMed] [Google Scholar]
- Schaefer A. L., Perry B. J., Cook N. J., Church J. S., Miller C., and Stenzler A.. 2005. The early detection of bovine respiratory disease (BRD) with infrared thermography and treatment with nitric oxide. J. Anim. Sci. 83(Suppl. 1):350. (Abstr.)15644507 [Google Scholar]
- Secrist D. S., Owens F. N., and Gill D. R.. 1997. Effects of vitamin E on performance of feedlot cattle: A review. Prof. Anim. Sci. 13:47–54. [DOI] [PubMed] [Google Scholar]
- Semba R. D. 1999. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 58:719–727. [DOI] [PubMed] [Google Scholar]
- Sowell B. F., Branine M. E., Bowman J. G. P., Hubbert M. E., Sherwood H. E., and Quimby W.. 1999. Feeding and watering behavior of healthy and morbid steers in a commercial feedlot. J. Anim. Sci. 77:1105–1112. [DOI] [PubMed] [Google Scholar]
- Sowell B. F., Bowman J. G. P., Branine M. E., and Hubbert M. E.. 1998. Radio frequency technology to measure feeding behavior and health of feedlot steers. Appl. Anim. Behav. Sci. 59:277–284. [Google Scholar]
- Spears J. W., and Kegley E. B.. 2002. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J. Anim. Sci. 80:2747–2752. [DOI] [PubMed] [Google Scholar]
- Stabel J. R., Goff J. P., and Kimura K.. 2003. Effects of supplemental energy on metabolic and immune measurements in perparturient dairy cows with Johne's disease. J. Dairy Sci. 86:3527–3535. [DOI] [PubMed] [Google Scholar]
- Stanton T. L., Schutz D., and Swenson C.. 2001. Trace mineral supplementation in the presence of antagonists on growth performance, health, and carcass characteristics of transport-stressed calves. Prof. Anim. Sci. 17:101–108. [Google Scholar]
- Stanton T. L., Whittier J. C., Geary T. W., Kimberling C. V., and Johnson A. B.. 2000. Effects of trace mineral supplementation on cow-calf performance, reproduction and immune function. Prof. Anim. Sci. 16:121–127. [Google Scholar]
- St Louis D. G., Engelken T. J., Little R. D., and Edwards N. C.. 2003. Case study: Systems to reduce the cost of preconditioning calves. Prof. Anim. Sci. 19:357–361. [Google Scholar]
- Suttle N. F., and Jones D. G.. 1989. Recent developments in trace element metabolism and function: Trace elements, disease resistance and immune responsiveness in ruminants. J. Nutr. 119:1055–1061. [DOI] [PubMed] [Google Scholar]
- Thompson P. N., Stone A., and Schultheiss W. A.. 2006. Use of treatment records and lung lesion scoring to estimate the effect of respiratory disease on growth during early and late finishing periods in South African feedlot cattle. J. Anim. Sci. 84:488–498. [DOI] [PubMed] [Google Scholar]
- Thurmond M. C. 2005. Virus transmission. Pages 91–104 in Bovine Viral Diarrhea Virus: Diagnosis, Management and Control. Goyal S. M. and Ridpath J. F. ed. Blackwell Publishing, Ames, IA. [Google Scholar]
- Twining S. S., Schulte D. P., Wilson P. M., Fish B. L., and Moulder J. E.. 1997. Vitamin A deficiency alters rat neutrophil function. J. Nutr. 127:558–565. [DOI] [PubMed] [Google Scholar]
- USDA-APHIS 2000a. Attitudes Towards Pre-Arrival Processing in U.S. Feedlots. Report N340.1100. USDA-APHIS, Fort Collins, CO. [Google Scholar]
- USDA-APHIS 2000b. Part II: Baseline Reference of Feedlot Health Management, 1999. USDA:APHIS:VS, CEAH, National Animal Health Monitoring System; Fort Collins, CO. #N335.1000. [Google Scholar]
- USDA-APHIS 2001. National Animal Health Monitoring System. Info Sheet—Veterinary Services. Treatment of Respiratory Disease in U.S. Feedlots. Available: http://www.aphis.usda.gov/vs/ceah/ncahs/nahms/feedlot/feedlot99/FD99treatresp.pdf Accessed June 5, 2006.
- Voisinet B. D., Grandin T., Tatum T. D., O'Connor S. F., and Struthers J. J.. 1997. Feedlot cattle with calm temperaments have greater average daily gains than cattle with excitable temperaments. J. Anim. Sci. 75:892–896. [DOI] [PubMed] [Google Scholar]
- Ward J. D., and Spears J. W.. 1999. The effects of low-copper diets with or without supplemental molybdenum on specific immune response of stressed cattle. J. Anim. Sci. 77:230–237. [DOI] [PubMed] [Google Scholar]
- Whitney T. R., Duff G. C., Collins J. K., Schafer D. W., and Hallford D. M.. 2006. Effects of diet for early-weaned crossbred beef steers on metabolic profiles and febrile response to an infectious bovine herpesvirus-1 challenge. Livest. Sci. 101:1–9. [Google Scholar]
- Wittum T. E., Grotelueschen D. M., Brock K. V., Kvasnicka W. G., Floyd J. G., Kelling C. L., and Odde K. G.. 2001. Persistent bovine viral diarrhoea virus infection in US beef herds. Prev. Vet. Med. 49:83–94. [DOI] [PubMed] [Google Scholar]
- Wittum T. E., and Perino L. J.. 1995. Passive immune status at postpartum hour 24 and long-term health and performance of calves. Am. J. Vet. Res. 56:1149–1154. [PubMed] [Google Scholar]
- Wittum T. E., Woollen N. E., Perino L. J., and Littledike E. T.. 1996a. Relationship among treatment for respiratory disease, pulmonary lesions evident at slaughter, and rate of weight gain in feedlot cattle. J. Am. Vet. Med. Assoc. 209:814–818. [PubMed] [Google Scholar]
- Wittum T. E., Young C. R., Stanker L. H., Griffin D. D., Perino L. J., and Littledike E. T.. 1996b. Haptoglobin response to clinical respiratory tract disease in feedlot cattle. Am. J. Vet. Res. 57:646–649. [PubMed] [Google Scholar]
- Woolums A. R., Loneragan G. H., Hawkins L. L., and Williams S. M.. 2005. Baseline management practices and animal health data reported by US feedlots responding to a survey regarding acute interstitial pneumonia. Bovine Pract. 39:116–124. [Google Scholar]
- Wu G., Bazor F. W., Wallace J. M., and Spencer T. E.. 2006. BOARD-INVITED REVIEW: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 84:2316–2337. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. D., Boots R. E., Valli J. L., and Chase C. C. L.. 2006. Evaluation of protection against virulent bovine viral diarrhea virus type 2 in calves that had maternal antibodies and were vaccinated with a modified live vaccine. J. Am. Vet. Med. Assoc. 228:1757–1761. [DOI] [PubMed] [Google Scholar]