Abstract
Little is known about the impact of human rhinovirus (HRV) and coronavirus infections in hematopoietic stem cell transplant (HSCT) recipients. We tested bronchoalveolar lavage (BAL) samples obtained from HSCT recipients with acute pulmonary infiltrates for HRV (n = 122) and coronavirus (n = 46) by reverse-transcriptase polymerase chain reaction. HRV RNA was detected in 6 (8%) of 77 patients, and coronavirus RNA was detected in 0 of 46 of BAL samples from HSCT recipients. The fatality rate in HRV-infected patients was high (83%), but all patients had significant coinfections, and the overall mortality rate was not different from that of patients who were negative for HRV in BAL samples. These results suggest that HRV may be a cause of lower respiratory tract infections in HSCT recipients and that its detection in BAL samples is associated with frequent copathogens. Whether the poor prognosis is due to HRV or the copathogen is not clear.
Human rhinovirus (HRV) and coronavirus are the agents most commonly associated with the common cold and are generally considered to replicate principally within the upper respiratory tract (URT) [1]. Most patients with common colds present with a self-limited syndrome characterized by nasal obstruction, coryza, sneezing, sore throat, and cough; fever is very uncommon in immunocompetent adults with rhinoviral colds [2]. In patients with predisposing conditions, such as underlying lung disease and compromised immunity, HRV has been associated with lower respiratory tract disease, including reports of possible viral pneumonia in infants, hospitalized elderly persons, and transplant recipients [3–7]. Rhinoviruses have recently been demonstrated to replicate effectively at core temperature (37°C) [8]. In addition, several studies have recovered HRV from the lower airways present in the lower tracheobronchial tree [9–17], and replicative-strand rhinoviral RNA has been detected in situ from bronchial biopsy samples from patients who had been inoculated experimentally with rhinovirus [18].
The spectrum of disease and outcomes of infection with HRV and human coronavirus infections in hematopoietic stem cell transplant (HSCT) recipients have received limited study. Bowden [4] detected HRV infection by viral culture in 25% of 127 HSCT recipients with acute respiratory illnesses; all had URT infections, and only 1 had virus recovered from the lower respiratory tract. In contrast, Ghosh et al. [19] recently described the clinical course of 22 myelosuppressed adults, who represented ∼1% of HSCT recipients during the period of the study, with HRV infection identified by viral culture. Of these patients, 7 (32%) developed fatal pneumonia that was attributed to HRV infection of the lower airways. HRV was cultured antemortem from lower respiratory tract samples in 6 patients (86%). The mean time between the onset of acute respiratory symptoms and respiratory failure in these patients was 12 days (range, 3–21 days), and the duration of viral shedding for these patients was 8 days (range, 1–18 days). One case report has described the occurrence of human coronavirus pneumonia in a transplant recipient [20]. This patient developed transient respiratory distress, which required intubation, but recovered from the infection.
To better clarify the prevalence of HRV and human coronavirus lower respiratory tract involvement in HSCT recipients, we tested 122 consecutively collected bronchoalveolar lavage (BAL) samples from 77 patients with acute pulmonary infiltrates for the presence of HRV and, in a limited subgroup, human coronavirus RNA by RT-PCR, the most sensitive and appropriate detection technique currently available for these pathogens [2].
Materials and Methods
Consecutive BAL samples, obtained from HSCT recipients at the Fred Hutchinson Cancer Research Center (FHCRC; Seattle) with acute pulmonary infiltrates during one respiratory virus season, were used for the study. The initial evaluation of the samples included routine viral, bacterial, fungal, and mycobacterial cultures; shell-vial cultures for cytomegalovirus (CMV) and respiratory syncytial virus (RSV); direct fluorescent antibody staining for herpesviruses, respiratory viruses, and Legionella species; and cytologic testing with use of methods discussed elsewhere [21, 22]. In brief, BAL samples were inoculated into human foreskin fibroblast (HFF), A-549, and rhesus monkey kidney (RMK) cell cultures and maintained for 10 (A-549 and RMK) or 28 days (HFF), all at 35°C–37°C. Shell-vial centrifugation cultures were performed using CMV- and RSV-specific antibodies, according to standard techniques described elsewhere [21]. Bacterial, fungal, and mycobacterial cultures used standard methodology in the Clinical Microbiology Laboratory at FHCRC [22]. Direct fluorescent antibody staining was performed for CMV, adenovirus, parainfluenza viruses 1–4, influenza viruses A and B, RSV, and Legionella species with use of pathogen-specific monoclonal antibodies (Bartels) according to the manufacturer's instructions. An aliquot of BAL sample was kept frozen at -70°C and subsequently tested under blinded conditions at the University of Virginia for HRV and human coronavirus, using RT-PCR by techniques described elsewhere [23, 24]. Human coronavirus RT-PCR was only done on the first 46 samples. The detection of amplification products was done by microplate hybridization according to published methods, with minor modifications [25]. Patient characteristics and treatment records were obtained from the electronic database and from patient charts maintained at FHCRC.
Results
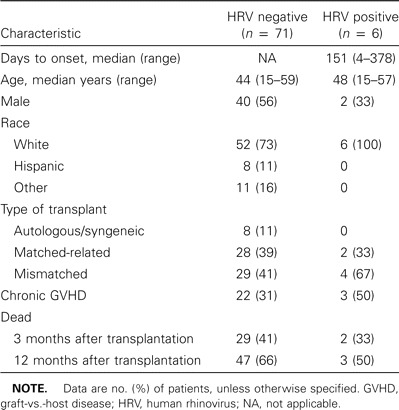
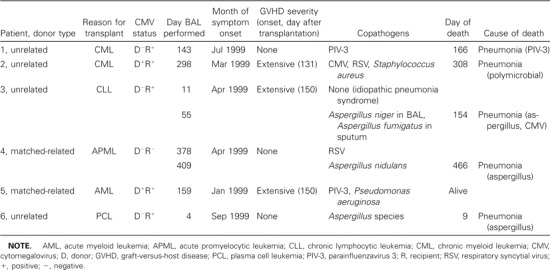
Seventy-seven patients with newly diagnosed pulmonary infiltrates by chest radiograph underwent bronchoscopy with BAL (a total of 122 samples) during the study period (table 1). No human coronavirus RNA was detected in 46 samples tested. Six patients (8%) had HRV RNA detected from 8 (6%) of 122 BAL samples. None of the patients had cultures positive for HRV, although the culture method was not optimized to grow HRV. Two patients had 2 samples positive for HRV; the 2 BAL samples were collected 31 and 44 days apart, respectively. One patient (3; table 2) was first diagnosed with idiopathic pneumonia syndrome by biopsy and BAL and improved with steroid therapy; the patient then developed a lingual lobe infiltrate that prompted the second BAL. The other patient (4; table 2) had persistent pulmonary nodules that did not improve after treatment for RSV; the second BAL revealed Aspergillus nidulans in addition to HRV. Patients 1 and 3 complained of upper respiratory symptoms prior to the onset of respiratory distress, and patient 4 developed an expiratory wheeze concomitant with the identification of RSV in her BAL fluid.
Table 1.
Patient characteristics and virological results.
Table 2.
Characteristics of patients with ⩾1 bronchoalveolar lavage (BAL) sample positive for human rhinovirus (HRV).
Among those positive for HRV RNA, the onset of acute pulmonary infiltrate averaged 151 days after transplantation (range, 4–378 days). The onset of HRV infection occurred during the first 100 days in only 2 (33%) of the 6 HRV-positive patients. All of the patients were the recipients of allogeneic HSCT (4 unrelated mismatched donors and 2 matched-related). Fifty percent had chronic graft-versus-host disease at the time of illness onset. None of the HRV-positive BAL specimens grew HRV on routine cell culture. None of the 6 patients had rhinovirus detected by culture in upper respiratory secretions.
All of the patients had copathogens detected along with HRV in their BAL samples (table 2); 1 patient had an initial BAL specimen that grew only HRV and a subsequent BAL 44 days later that grew both HRV and Aspergillus niger. The most frequent copathogens were Aspergillus species from 3 patients, parainfluenza virus 3 from 3 patients, and RSV from 2 patients. One patient each had coinfection with CMV, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae.
Death occurred in all but 1 patient; 3 patients died within 1 month of onset of respiratory illness, and 4 patients died within 3 months of the onset of illness. The mean time between illness onset and death was 54 days (median, 23 days; range, 5–143 days). No autopsy data were available from any of these fatal cases. No relapse of the underlying disease was diagnosed in any of the patients who died.
Discussion
Our findings indicate that the detection of HRV in the BAL fluid of HSCT recipients with pneumonia is associated with a high mortality rate and frequent coinfection. This suggests that HRV could cause lower respiratory tract disease in highly immunosuppressed HSCT recipients. In addition, the present study demonstrates the importance of using RT-PCR—the most sensitive available technique for diagnosing HRV infections—in this patient population, because cell culture results may be insensitive unless specific efforts are taken to isolate rhinoviruses [2, 26].
Respiratory viruses have been increasingly recognized to be associated with severe respiratory complications in immunosuppressed HSCT recipients [27]. The present study provides additional evidence that HRV can be associated with life-threatening lower respiratory tract disease in such patients. HRV RNA was detected in 8% of patients who had a bronchoscopy with BAL for an acute pulmonary infiltrate. Half of the HRV-positive patients died during the first month after infection, and, ultimately, 5 of 6 died. However, all of the patients with HRV had coinfection with at least 1 other pathogen that is likewise associated with increased mortality in this population. It is unclear from the available information which agent, the HRV or the copathogen, was the primary cause of death in these patients. It is also not clear from these data whether rhinovirus by itself can cause lower respiratory tract disease. It is of interest that 2 of 6 patients identified appeared to show persistent HRV infection. These patients may have had more-compromised immunity that predisposed them to polymicrobial infections and a higher risk of infectious mortality. The frequency of coinfections suggests that HRV may predispose patients to additional infections, possibly through an immunomodulatory mechanism, as has been demonstrated with RSV and parainfluenza virus [21, 28] and indicated in vitro for HRV [29]. More data are needed to confirm this hypothesis.
We found that most patients presented with HRV infection after the first 100 days after transplantation, which is consistent with a community acquisition of infection. In contrast, Ghosh et al. [19] reported that all their patients presented with infection within the first 10 days after transplantation, which is indicative of nosocomial acquisition. Strict infection-control practices—including the screening of symptomatic patients, instituting early isolation and cohorting, the universal use of gloves and masks for all contact with symptomatic patients, strict implementation of hand washing, and restricting staff and visitors with upper respiratory symptoms and children aged <12 years from visiting patients—have been in place for the past 10 years at FHCRC and may have contributed to reduced risk of nosocomial HRV infections, particularly early after transplantation. In addition, our study took place after the institution of a policy that prohibited staff with uncontrolled respiratory secretions from having patient contact, which could have further limited the spread of HRV and coronavirus. It is also possible that earlier symptoms, restricted to the URT, may have been missed. Most studies, however, have suggested a rapid progression to lower airway involvement, if it occurs. As a result, it is unlikely that these patients were infected for a long period prior to their BALs. Future studies should do prospective viral cultures of the upper airways to get a better sense of the epidemiology of respiratory viruses in this population.
There are several limitations to the present study. It was conducted during a single season at a single center and thus may not be representative of HRV infections in different areas of the country or with different viruses that may not have circulated during our study period. All the samples were detected in patients who had been concurrently diagnosed with pneumonia by chest radiograph. As a result, the study focused on patients with severe disease and does not provide data on the overall incidence of HRV infections in HSCT recipients. The selection of patients with pneumonia probably overestimates the risk of mortality secondary to HRV infection and gives no indication of the frequency of progression from upper airway disease to lower airway disease. A prior report by Bowden [4] in HSCT recipients suggested that this risk is low.
There is the possibility that HRV may have been introduced into the lower respiratory tract during the bronchoscopy procedure in patients who had URT infection. Unfortunately, we could not test this possibility by PCR, because no upper respiratory samples were saved. In this context, earlier studies performed mainly with CMV may be instructive. Those studies demonstrated that patients with documented CMV shedding in the throat often have negative BAL for CMV [30, 31]. Thus, viral shedding in the throat is not necessarily associated with lower respiratory tract contamination of the virus when optimized bronchoscopy techniques are used.
Intervention studies are also needed to identify the optimal way to mange these infections. Capsid-binding (pleconaril) and 3C protease (ruprintrivir) HRV inhibitors are currently under investigation for the treatment and prevention of HRV infections in immunocompetent patients, and these agents could be tested to see whether they favorably affect the clinical course of complicated HRV infections in HSCT recipients. Pleconaril is currently available via a compassionate-use program for the management of HRV in immunocompromised hosts, and there has been one report [32] of a bone marrow transplant recipient with a possible rhinovirus pneumonia who was successfully treated, with pleconaril, as part of this program.
In conclusion, the present study suggests that HRV infection may sometimes be associated with severe lower respiratory tract disease and pneumonia in HSCT recipients. Further studies are needed to determine whether HRV causes lytic infection or acts primarily via an indirect immunosuppressive effect, resulting in an increased predisposition to superinfections. In addition, studies are needed to better clarify the epidemiology of HRV infection in HSCT recipients and to better understand the risk factors for progression of HRV URT infections into the lower respiratory tract. A prospective surveillance study in which all HSCT recipients with upper or lower respiratory symptoms have URT and, as possible, lower respiratory tract samples collected to assess the presence of HRV and human coronavirus by PCR would be needed to delineate the true epidemiology of these respiratory viral diseases in this population. Such a prospective study is ongoing.
Acknowledgments
We thank Gary Schoch for data retrieval, Tom Novicki and the Clinical Microbiology Laboratory staff for sample preparation and storage, and Gina McBrayer for chart review.
Footnotes
Financial support: National Institutes of Health (grants CA-18029 and CA-15704).
References
- 1.Gwaltney JM, Jr, Heinz BA. Rhinovirus. In: Hayden FG, editor. Clinical virology. Washington, DC: American Society for Microbiology Press; 2002. pp. 995–1018. [Google Scholar]
- 2.Arruda E, Pitkaranta A, Witek TJ, Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–8. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez Y, Martino R, Badell I, et al. Pulmonary enterovirus infections in stem cell transplant recipients. Bone Marrow Transplant. 1999;23:511–3. doi: 10.1038/sj.bmt.1701605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. 42–3. [DOI] [PubMed] [Google Scholar]
- 5.Crooks BN, Taylor CE, Turner AJ, et al. Respiratory viral infections in primary immune deficiencies: significance and relevance to clinical outcome in a single BMT unit. Bone Marrow Transplant. 2000;26:1097–102. doi: 10.1038/sj.bmt.1702656. [DOI] [PubMed] [Google Scholar]
- 6.Monto AS, Fendrick AM, Sarnes MW. Respiratory illness caused by picornavirus infection: a review of clinical outcomes. Clin Ther. 2001;23:1615–27. doi: 10.1016/S0149-2918(01)80133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabella N, Rodriguez P, Labeaga R, et al. Conventional respiratory viruses recovered from immunocompromised patients: clinical considerations. Clin Infect Dis. 1999;28:1043–8. doi: 10.1086/514738. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos NG, Sanderson G, Hunter J, Johnston SL. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999;58:100–4. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–61. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 10.Connolly MG, Jr, Baughman RP, Dohn MN, Linnemann CC., Jr Recovery of viruses other than cytomegalovirus from bronchoalveolar lavage fluid. Chest. 1994;105:1775–81. doi: 10.1378/chest.105.6.1775. [DOI] [PubMed] [Google Scholar]
- 11.Halperin SA, Eggleston PA, Hendley JO, Suratt PM, Groschel DH, Gwaltney JM., Jr Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am Rev Respir Dis. 1983;128:806–10. doi: 10.1164/arrd.1983.128.5.806. [DOI] [PubMed] [Google Scholar]
- 12.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 13.Mosser AG, Brockman-Schneider R, Amineva S, et al. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–43. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 14.Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–41. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malcolm E, Arruda E, Hayden FG, Kaiser L. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J Clin Virol. 2001;21:9–16. doi: 10.1016/s1386-6532(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 16.El-Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imakita M, Shiraki K, Yutani C, Ishibashi-Ueda H. Pneumonia caused by rhinovirus. Clin Infect Dis. 2000;30:611–2. doi: 10.1086/313723. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, Champlin R, Couch R, et al. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:528–32. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 20.Folz RJ, Elkordy MA. Coronavirus pneumonia following autologous bone marrow transplantation for breast cancer. Chest. 1999;115:901–5. doi: 10.1378/chest.115.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–8. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 22.Crippa F, Holmberg L, Carter RA, et al. Infectious complications after autologous CD34-selected peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:281–9. doi: 10.1053/bbmt.2002.v8.pm12064366. [DOI] [PubMed] [Google Scholar]
- 23.Arruda E, Hayden FG. Detection of human rhinovirus RNA in nasal washings by PCR. Mol Cell Probes. 1993;7:373–9. doi: 10.1006/mcpr.1993.1055. [DOI] [PubMed] [Google Scholar]
- 24.Pitkaranta A, Arruda E, Malmberg H, Hayden FG. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–3. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitkaranta A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102:291–5. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 26.Arruda E, Crump CE, Rollins BS, Ohlin A, Hayden FG. Comparative susceptibilities of human embryonic fibroblasts and HeLa cells for isolation of human rhinoviruses. J Clin Microbiol. 1996;34:1277–9. doi: 10.1128/jcm.34.5.1277-1279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ison MG, Hayden FG. Viral infections in immunocompromised patients: what's new with respiratory viruses? Curr Opin Infect Dis. 2002;15:355–67. doi: 10.1097/00001432-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–66. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 29.Gern JE, Joseph B, Galagan DM, Borcherding WR, Dick EC. Rhinovirus inhibits antigen-specific T cell proliferation through an intercellular adhesion molecule-1-dependent mechanism. J Infect Dis. 1996;174:1143–50. doi: 10.1093/infdis/174.6.1143. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt GM, Horak DA, Niland JC, Duncan SR, Forman SJ, Zaia JA. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants: the City of Hope-Stanford-Syntex CMV Study Group. N Engl J Med. 1991;324:1005–11. doi: 10.1056/NEJM199104113241501. [DOI] [PubMed] [Google Scholar]
- 31.Slavin MA, Gooley TA, Bowden RA. Prediction of cytomegalovirus pneumonia after marrow transplantation from cellular characteristics and cytomegalovirus culture of bronchoalveolar lavage fluid. Transplantation. 1994;58:915–9. doi: 10.1097/00007890-199410270-00010. [DOI] [PubMed] [Google Scholar]
- 32.Rotbart HA, Webster AD, The Pleconaril Treatment Registry Group Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis. 2001;32:228–35. doi: 10.1086/318452. [DOI] [PubMed] [Google Scholar]


