Abstract
For the establishment of a diagnostic tool for mycobacterial species, a part of the dnaA gene was amplified and sequenced from clinically relevant 27 mycobacterial species as well as 49 clinical isolates. Sequence variability in the amplified segment of the dnaA gene allowed the differentiation of all species except for Mycobacterium tuberculosis, Mycobacterium africanum and Mycobacterium microti, which had identical sequences. Partial sequences of dnaA from clinical isolates belonging to three frequently isolated species revealed a very high intraspecies similarity, with a range of 96.0–100%. Based on the dnaA sequences, a species-specific primer set for Mycobacterium kansasii and Mycobacterium gastri was successfully designed for a simple loop-mediated isothermal amplification method. These results demonstrate that the variable sequences in the dnaA gene were species specific and were sufficient for the development of an accurate and rapid diagnosis of Mycobacterium species.
Keywords: Mycobacterium spp., dnaA gene, differential diagnosis, LAMP assay
Introduction
Increasing reports of opportunistic infection by nontuberculous mycobacteria (NTM) in immunocompromised patients such as AIDS patients and elderly people are a matter of serious concern to public health (Horsburg, 1991; Montessori , 1996; Primm , 2004). The routine diagnosis of mycobacteriosis relies primarily on the detection of acid-fast-stained bacilli in the samples by microscopic observation, and the infecting mycobacterial species can be identified with conventional tests including observation of colony morphology and pigmentation, growth rate, and biochemical characteristics (Cernoch , 1994; Metchock , 1999). Disadvantages of this approach include the time taken to provide clinically relevant information. The clinician must initiate therapy for Mycobacterium tuberculosis against NTM infection several weeks before species identification (Montessori , 1996), which may increase health care costs, and may reduce the social activity of the patients. Therefore rapid detection and identification of the species level of mycobacteria is required, both to decide whether measures are needed to prevent the spread of the disease and for adequate therapy (American Thoracic Society, 1997).
The mycobacterium species often implicated in NTM infection are Mycobacterium avium–Mycobacterium intracellulare complex (MAC), Mycobacterium kansasii, Mycobacterium chelonae, Mycobacterium abscessus, and Mycobacterium xenopi (Wayne & Sramek, 1992; Metchock , 1999; Primm , 2004). Mycobacterium gordonae, Mycobacterium gastri, or most of the rapidly growing species are rarely pathogenic, but are often encountered as contaminant in clinical samples. Therefore, the discrimination of these species from pathogenic ones is an important diagnostic issue (Primm , 2004).
Several studies have been conducted to develop rapid methods based on molecular technique for identifying mycobacterial species in recent years. The DNA sequences reported for such usage are those of 16S rRNA gene (Kirschner , 1993; De Beenhouwer , 1995; Cloud , 2002), recA (Blackwood , 2000), rpoB (Kim , 1999), gyrB (Kasai , 2000), hsp65 (Plikaytis , 1992; Brunello , 2001), or 16S–23S internal transcribed spacer (ITS) (De Smet , 1995; Roth , 1998). The 16S rRNA gene and ITS-based methods are currently widely accepted as rapid and accurate for identifying mycobacteria (Plikaytis , 1992; De Smet , 1995; Park , 2000; Turenne , 2001). However, some species have the same sequence or a very high similarity (Kim , 1999; Kasai , 2000). This fact indicates the need to develop more reliable and user-friendly molecule-based diagnostic tools.
Recently, Notomi (2000) have reported a novel nucleic acid amplification method, termed loop-mediated isothermal amplification (LAMP), that amplifies DNA with high specificity, efficacy, and rapidity under isothermal conditions. The LAMP reaction requires a Bst DNA polymerase with strand displacement activity and a set of four specially designed primers that recognize six distinct sequences on the target DNA, the specificity of which should be extremely high. The amplification products are stem-loop DNA structures with several inverted repeats of the target. The advantage of the LAMP method is that the reaction is performed under isothermal conditions of between 60 and 65°C. As a result, it requires only simple and cost-effective reaction equipment. The LAMP method has emerged as a powerful tool to facilitate genetic testing for various infectious diseases (Enosawa , 2003; Iwamoto , 2003; Kuboki , 2003; Ihira , 2004; Parida , 2004; Thai , 2004).
The purpose of our work is to identify a species-specific region of Mycobacterium sp., and to develop a LAMP assay that can differentiate clinically relevant species.
Materials and methods
Bacterial strains and preparation of genomic DNA
The bacteria used in this study comprised 27 strains and 49 clinical isolates as shown in Table 1. All strains except for Mycobacterium leprae were cultured on 1% Ogawa medium (Nissui, Tokyo, Japan) at 37°C. Mycobacterium leprae was prepared from infected nude mouse food pad (Shepard, 1960). Genomic DNA was extracted from mycobacterial strains as follows. Mycobacterial cells were resuspended in 1.8 mL of sterile phosphate-buffered saline (PBS) containing 0.1 mm diameter zirconia/silica beads (BioSpec Products Inc., Bartlesville, OK). The mixture was beaded for 20 s with a Beads Homogenizer Model BC-20 (Central Scientific Commerce, Tokyo, Japan), transferred to a 1.5 mL microcentrifuge tube, and the genomic DNA was purified with proteinase K treatment and phenol/chloroform extraction followed by ethanol precipitation, then suspended in 100 μL distilled water.
1.
Mycobacterium species and strains used in this study and results of the loop-mediated isothermal amplification assay
| Species | Strains | Accession number | Primer set | |
| Kan32 | Gas583 | |||
| Mycobacterium abscessus | JATA 63-01 (ATCC 19977) | AB087684 | − | − |
| Mycobacterium africanum | KK 13-02 (ATCC 25420) | AB087685 | − | − |
| Mycobacterium avium | JATA 51-01 (ATCC 25291) | AB087686 | − | − |
| Clinical isolate 22 strains | ||||
| Mycobacterium bovis | JATA 12-01 (ATCC 19210) | AB087687 | − | − |
| Mycobacterium chelonae | JATA 62-01 (ATCC 35752) | AB087688 | − | − |
| Mycobacterium fortuitum | JATA 61-01 (ATCC 6841) | AB087689 | − | − |
| Mycobacterium gastri | KK 44-02 (ATCC 15754) | AB087690 | − | + |
| Mycobacterium gordonae | JATA 33-01 (ATCC 14470) | AB087691 | − | − |
| Mycobacterium intracellulare | JATA 52-01 (ATCC 13950) | AB087692 | − | − |
| Clinical isolate 17 strains | ||||
| Mycobacterium kansasii | KK 21-01 (ATCC 12478) | AB087693 | + | − |
| Clinical isolate 10 strains | + | − | ||
| Mycobacterium leprae | Thai-53 | AB087694 | − | − |
| Mycobacterium malmoense | JATA 47-01 (ATCC 29571) | AB087695 | − | − |
| Mycobacterium marinum | JATA 22-01 (ATCC 927) | AB087696 | − | − |
| Mycobacterium microti | KK 14-01 (ATCC 19422) | AB087697 | − | − |
| Mycobacterium nonchromogenicum | JATA 45-01 (ATCC 19530) | AB087698 | − | − |
| Mycobacterium parafortuitum | ATCC 25807 | AB087699 | − | − |
| Mycobacterium phlei | ATCC 19249 | AB087700 | − | − |
| Mycobacterium scrofulaceum | JATA 31-01 (ATCC 19981) | AB087701 | − | − |
| Mycobacterium simiae | KK 23-08 (ATCC 25275) | AB087702 | − | − |
| Mycobacterium smegmatis | JATA 64-01 | AB087703 | − | − |
| Mycobacterium szulgai | JATA 32-01 | AB087704 | − | − |
| Mycobacterium terrae | KK 46-01 (ATCC 15755) | AB087705 | − | − |
| Mycobacterium triviale | KK 50-02 (ATCC 23292) | AB087706 | − | − |
| Mycobacterium tuberculosis | JATA 11-01 (H37Rv) | AB087707 | − | − |
| Mycobacterium ulcerans | KK 43-01 | AB087708 | − | − |
| Mycobacterium vaccae | KK 66-01 | AB087709 | − | − |
| Mycobacterium xenopi | KK 42-01 (ATCC 19250) | AB087710 | − | − |
All strains were kindly donated by Dr Kashiwabara, NIID.
Clinical isolates were identified by Amplicore Mycobacterium kit (Roche Pharma, Basel, Switzerland) or conventional biochemical test (Jamal , 2000).
Amplification of the region within dnaA gene
Highly polymorphic regions flanked by conserved regions were identified by aligning the Mycobacterium spp. dnaA sequences, which were available in GenBank at the time this study was initiated. These regions were used to design a pair of degenerate primers, U1F 5′-GTS CAR AAC GAR ATC GAR CG-3′ and U1R 5′-CCB GAY TCR CCC CAG ATG AA-3′. A schematic representation of the primer design is shown in Fig. 1a. PCR was performed in a TAKARA Thermal Cycler MP (TAKARA Biomedical, Otsu, Japan) with a reaction mixture consisting of 1 μL of genomic DNA, each deoxynucleoside triphosphate at a concentration of 200 μM, each primer at a concentration of 0.4 μM, 1 × PCR buffer with 1.5 mM MgCl2 (TAKARA Biomedical), and 1.25 U of ExTaq (TAKARA Biomedical), with 10 μL PCRX Enhancer System solution (Gibco BRL, Rockville, MD) in a total volume of 50 μL. The PCR thermocycles were 3 min at 94°C, followed by 30 cycles of 94°C for 10 s, 50°C for 20 s, and 72°C for 45 s, with a final extension step at 72°C for 7 min. PCR products were visualized by UV illumination of an ethidium bromide-stained 1.5% agarose gel and cut out to purify with EASYTRAP Ver.2 (TAKARA Biomedical) according to the manufacturer's instruction.
1.
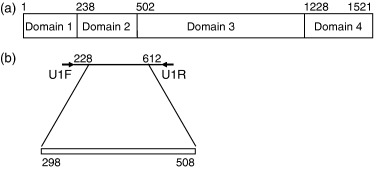
Schematic representation of the DnaA protein and primer design for the amplification of the partial mycobacterial dnaA gene. Number indicates the nucleotide position of Mycobacterium tuberculosis, GenBank accession number AL021427. (a) The DnaA protein from M. tuberculosis contains four domains. Domain 1 is involved in interaction with DnaB. Domain 2 constitutes a flexible loop. DNA unwinding required Domain 3. Domain 4 is sufficient for specific binding to DNA. Primers U1F and U1R were used to generate about 400 bp fragment from dnaA of 27 mycobacterial spp. (b) Analysis and comparison region used in this study are indicated by a bar (298–508 bp).
DNA sequencing and sequencing analysis
The ABI Prism BigDye Terminator v3.1 Cycle Sequencing Kit (PE Biosystems, Foster City, CA) was used for the sequencing of the PCR products. The same primers for amplification were used for sequencing. The sequencing reaction was performed in accordance with the instruction of the manufacturer. Sequencing products were purified with a Centrisep column (Princeton Separations, Adelphia, NJ).
The sequencing output was analyzed by using the DNA Sequence Analyzer computer software (PE Biosystems). The partial dnaA sequences were aligned using the Clustal W algorithm (Thompson , 1994) of the software DNASpace ver. 3.5 (Hitachi Software Engineering, Yokohama, Japan), and the alignment was manually corrected. A phylogenetic tree was generated by DNASpace ver. 3.5 (Hitachi Software Engineering) with a total of 1000 bootstraps. Pairwise similarity of the partial dnaA sequences was determined by using DNASIS package (Hitachi Software Engineering).
Species-specific LAMP assay for Mycobacterium kansasii and Mycobacterium gastri
A set of four primers comprising two inner primers and two outer primers that recognized six distinct regions on the target sequence were designed with PrimerExplorer Ver.3 (Fujitu, Tokyo, Japan). The detailed sequences of the primers are shown in Fig. 3. The two inner primers are called the forward inner primer (FIP) and the backward inner primer (BIP), and each contains two distinct sequences corresponding to the sense and antisense sequences of the target DNA, one for priming in the first stage and the other for self-priming in late stages. FIP contains the sequence complementary F1 (F1c) and F2. BIP contains the complementary B1 (B1c) and B2. The two outer primers consist of F3 and B3.
3.

Location of oligonucleotide primer sets Kan 32 and Gas 583, used for the loop-mediated isothermal amplification method. For Mycobacterium kansasii partial dnaA gene (GenBank accession number AB087693) and for Mycobacterium gastri partial dnaA gene (GenBank accession number AB087690). A right arrow indicates the sense sequence which is used as the primer. A left arrow indicates that a complementary sequence is used as the primer. The unique restriction enzyme recognition sites in the amplified product are shown with a bold bar. (b) List of each primer sequence.
The LAMP reaction was carried out in 25 μL of reaction mixture by using the Loopamp DNA amplification kit (Eiken Chemical Co. Ltd., Tochigi, Japan) containing 2.4 μM (each) FIP and BIP, 0.2 μM (each) of the outer primers, F3 and B3, 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 8 mM MgSO4, 10 mM (NH4)2SO4, 0.1% Tween 20, 0.8 M betaine, 1.4 mM (each) of dNTP, 8 U of Bst DNA polymerase (New England BioLabs, Beverly, MA), and the template DNA. Amplification was undertaken in 0.5 μL microtubes in a heatblock under isothermal conditions of 63°C for 60 min, followed by 80°C for 2 min to terminate the reaction. Positive and negative controls were included in each run, and precautions to prevent cross-contamination were observed. Two microliter aliquots of LAMP products were subjected to electrophoresis on a 4% agarose gel in Tris-borate-EDTA buffer followed by staining with ethidium bromide and were visualized on a UV transilluminator at 302 nm. The specificity of the LAMP-amplified products were further validated by restriction enzyme digestion with NaeI and HaeII for M. kansasii and M. gastri, respectively. The diluted genomic DNA was used for determining the sensitivity of the species-specific LAMP assay.
Results
Comparison of partial dnaA sequence to identify the Mycobacterium species
For the species identification of mycobacterial species, we analyzed some possible variable regions of mycobacterial sequences deposited in the GenBank, and found the 5′ part of the dnaA gene as a candidate target for PCR amplification. The PCR products with U1F and U1R, from 27 mycobacterial species, showed the ragged pattern around 400 bp in size (data not shown). Therefore, we determined nucleotide sequences, corresponding to position 228–612 bp of Mycobacterium tuberculosis, of all 27 species (Fig. 1a). The alignment of the sequence shows that the region (298–508 bp) in the amplified products had the highest species-specific variability (Fig. 1b). The size of the variable fragment in dnaA ranged from 154 bp in M. triviale to 232 bp in M. kansasii. The variable region exhibits a reasonable number of nucleotide substitution and insertion or deletion sites, which is important for the development of a differential diagnostic tool. The lowest interspecies similarity was 28.2% in M. leprae versus M. vaccae. The similarity between M. avium and M. intracellulare was 78.3% and that between M. marinum and M. ulcerans was 97.7%. Pathogenic M. kansasii were easily differentiated from nonpathogenic M. gastri (83.6%). The sequences of M. tuberculosis, M. microti, M. africanum, and M. bovis were found to be identical, except for one nucleotide substitution that occurred in M. bovis. When clinical isolates from clinically relevant mycobacterial strains were analyzed, the following minor variation was found among each species: 97.7–100% (M. avium) and 96.0–100% (M. intracellulare). We did not find any intraspecies variation in 10 clinical isolates and the standard strain of M. kansasii. Because other reports using different systems revealed the existence of more than one sequevar (Yang , 1993; Alcaide , 1997), we may need to examine a bigger number of clinical isolates.
The unrooted phylogenetic tree showed that the 27 mycobacterial species were resolved by the variable region in the dnaA sequence (Fig. 2). All rapidly growing species, M. abscessus, M. chelonae, M. fortuitum, M. parafortuitum, M. phlei, M. vaccae, and M. smegmatis, made a cluster that was clearly separated from those of the other species so far examined. On the other hand, M. kansasii, M. gastri, M. avium, and M. intracellulare are clinically relevant species; however, the branch of the former two species was obviously segregated from one of the later two species, which was supported by high bootstrap values. The results indicated that the partial dnaA sequence could be useful for the differentiation of NTM (Fig. 2).
2.
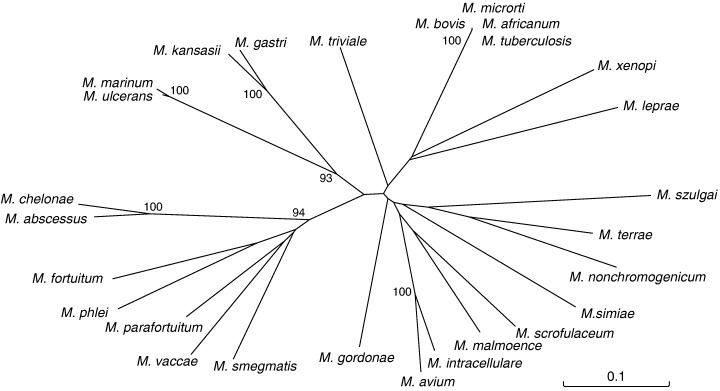
Phylogenetic relationship of 27 Mycobacterium species. Unrooted tree based on the dnaA sequences. The tree was generated from DNASpace (Hitachi Software Engineering) with the Clustal W algorithm. The numbers on the dendrogram indicate the percentages of occurrence in 1000 bootstrapped trees; only values of >90% are shown.
Identification of mycobacteria by dnaA sequence-targeted species-specific LAMP assay
Several sets of primers designed from the dnaA sequence were evaluated for their specificity and sensitivity by the LAMP method. One set of primers named Kan-32 for M. kansasii and Gas-583 for M. gastri was selected (Fig. 3), and by using these primer sets, a successful LAMP product appeared as a ladder of multiple bands (Fig. 3a).
The species specificity and intraspecies stability of each primer set were examined with purified DNA from 27 mycobacterial species and 10 clinical isolates of M. kansasii. We subjected each sample to amplification using Kan-32 or Gas-583 primer set. The results obtained by electrophoretic examination are summarized in Table 1. Although 200 pg of nontargeted species DNA were not amplified, significant amplification of targeted respective isolates was observed after a 60 min incubation at 63°C. To confirm that the amplification products had corresponding DNA structures, the amplified products were digested with restriction enzymes and the size of the fragments was analyzed by electrophoresis. NaeI cuts between F1 and B1c for the M. kansasii amplicon; HaeII was used for the M. gastri amplicons. The sizes of the fragments generated after digestion were in good agreement with sizes predicted theoretically from the expected DNA structure: 100 and 93 bp by NaeI digestion, and 123 and 98 bp by HaeII digestion (Fig. 4a). Thus, we concluded that each primer set was species specific.
4.
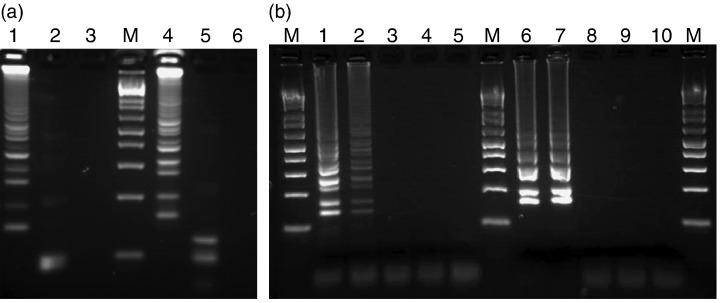
(a) Four percent agarose gel electrophoresis and restriction enzyme analysis of loop-mediated isothermal amplification (LAMP) products of partial dnaA gene of Mycobacterium kansasii and Mycobacterium gastri. Lanes: M, 100 bp DNA ladder; lanes 1–3, LAMP carried out with M. kansasii primer, Kan 32, in the presence of genomic DNA from M. kansasii (lanes 1 and 2) and M. gastri (lane 3); lane 2, LAMP product from lane 1 after digestion with Nae I; lanes 4–6, LAMP carried out with M. gastri primer, Gas 583, in the presence of genomic DNA from M. gastri (lanes 4 and 5) and M. kansasii (lane 6). lane 5, LAMP product from lane 4 after digestion with Hae II. (b) Serial dilution of purified M. kansasii or M. gastri genomic DNA was amplified to determine the sensitivities by LAMP. Lanes: M, 100 bp DNA ladder; lanes 1–5 LAMP carried out with Kan 32 primer set in the presence of genomic DNA of M. kansasii, lane 1, 1000 copies; lane 2, 500 copies; lane 3, 100 copies; lane 4, 10 copy; lane 5, distilled water. lanes 6–10 LAMP carried out with gas 583 primer set in the presence of genomic DNA of M. gastri, lane 6, 1000 copies; lane 7, 300 copies; lane 8, 100 copies; lanes 9, 10 copy; lane 10, distilled water.
We next assessed the sensitivity of the assay. Serially diluted M. kansasii or M. gastri genomic DNA was used. The results of a typical experiment are shown in Fig. 4b. Amplified DNA was readily visible when 500 copies of genomic DNA were present in a 60 min incubation assay. The detection limit did not change with a longer incubation period (data not shown).
Discussion and conclusions
For the identification of species, a target gene must be conserved among strains and species. As the DnaA protein is generally conserved among microbial organisms (Mizrahi , 2000), this coding region could be used for the target analysis. Four functional domains of the DnaA protein have been defined (Messer , 1998). Domain 1 is involved in oligomerization and interaction with DnaB, Domain 2 constitutes a flexible loop, Domain 3 has ATPase function, and Domain 4 is sufficient for specific binding to DNA. The variable region that we identified in the dnaA sequence was equivalent to the Domain 2 coding nucleotide sequence (Fig. 1). This domain is the least conserved region in the dna A gene with respect to sequence and length among M. smegmatis, M. tuberculosis, and M. leprae (Fsihi , 1996). However, comparative studies of this region using 27 mycobacteria have not been reported and, as far as we know, this is the first report indicating the usefulness of the dnaA Domain 2 sequence as a differential diagnostic tool.
An accurate and rapid bacterial identification greatly contributes to this field of medication. Several methods based on molecular biological techniques have been reported. The sequences that have been reported include hsp65, 16S rRNA gene, and ITS (Plikaytis , 1992; De Smet , 1995; Springer , 1996; Messer & Weigel, 1997; Roth , 1998; Brunello , 2001). Each gene has several advantages and disadvantages. An excessive degree of variability is found in the hsp65 gene (Telenti , 1993), which may hinder the development of reliable probes. While 16s rRNA gene sequence is identical in M. kansasii and M. gastri and shows narrow divergenicity within species (Taylor , 1997), ITS sequence can be used to distinguish between M. kansasii and M. gastri (Roth , 1998). While M. kansasii is a representative pathogenic mycobacteria, M. gastri does not induce an apparent disease. The discrimination between these mycobacteria provides useful information to select the appropriate therapy. The percent similarity of ITS between two species was 93% (Roth , 1998), and that of the dnaA variable region was found to be 83.6%. These observations may indicate the usefulness of the dnaA gene for discrimination of these species, at least in complement with ITS.
The recent trend in genetic testing is to make systems fully automatic with high-throughput analysis. Although this may be an ideal approach, it requires expensive equipment as well as a well-trained person in diagnostic laboratories. The LAMP method could be conducted under isothermal conditions ranging from 60 to 65°C by a single enzyme. The only equipment needed for LAMP reaction is a regular laboratory water bath or a heat block that furnishes a constant temperature around 63°C. LAMP does not require a thermal cycling step, and an isothermal reaction for a short time (60 min) is enough to amplify the target DNA to a detectable level. As PCR and other molecular biological techniques are conducted in well-equipped laboratories, these methodologies are often impracticable under a field diagnosis.
In this paper, we demonstrated that the dnaA region could be an effective new nucleotide region for the diagnosis of NTM infection and that the LAMP method could be applied for a dnaA gene-based differential diagnostic tool.
Acknowledgements
We are grateful to Dr Y. Kashiwabara for the mycobacterial strains. This work was supported in part by a Health Science Research Grants-Research on Emerging and Re-emerging Infectious Diseases, Ministry of Health, Labour and Welfare, Japan.
References
- Alcaide F, Richter I, Bernasconi C, Springer B, Hagenau C, Schulze-Robbecke R, Tortoli E, Martin R, Bottger EC, Telenti A. (1997) Heterogeneity and clonality among isolates of Mycobacterium kansasii: implications for epidemiological and pathogenicity studies. J Clin Microbiol 35: 1959–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society (1997) Diagnosis and treatment of diseases caused by nontuberculous mycobacteria. Am J Respir Crit Care Med 156: S1–S25. [DOI] [PubMed] [Google Scholar]
- De Beenhouwer H, Liang Z, De Rijk P, Van Eekeren C, Portaels F. (1995) Detection and identification of mycobacteria by DNA amplification and oligonucleotide-specific capture plate hybridization. J Clin Microbiol 33: 2994–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood KS, He C, Gunton J, Turenne CY, Wolfe J, Kabani AM. (2000) Evaluation of recA sequences for identification of Mycobacterium species. J Clin Microbiol 38: 2846–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunello F, Ligozzi M, Cristelli E, Bnora S, Tortoli E, Fontana R. (2001) Identification of 54 mycobacterial species by PCR-restriction fragment length polymorphism analysis of the hsp65 gene. J Clin Microbiol 39: 2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernoch PL, Enns RK, Saubolle MA, Wallace RA., Jr (1994) Laboratory diagnosis of the Mycobacterioses. Cumitech 16A (Weissfield AS, Coordinating ed.), American Society for Microbiology, Washington, DC. [Google Scholar]
- Cloud JL, Neal H, Rosenberry R, Turenne CY, Jama M, Hillyard DR, Carroll1 KC. (2002) Identification of Mycobacterium spp. by using a commercial 16S ribosomal DNA sequencing kit and additional sequencing libraries. J Clin Microbiol 40: 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet KA, Brown IN, Yates M, Ivanyi J. (1995) Ribosomal internal transcribed spacer sequences are identical among Mycobacterium avium–intracellulare complex isolates from AIDS patients, but vary among isolates from elderly pulmonary disease patients. Microbiology 141: 2739–2747. [DOI] [PubMed] [Google Scholar]
- Enosawa M, Kageyama S, Sawai K, Watanabe K, Notomi T, Onoe S, Mori Y, Yokomizo Y. (2003) Use of loop-mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis. J Clin Microbiol 41: 4359–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fsihi H, De Rossi E, Salazar L, Cantoni R, Labo M, Riccardi G, Takiff HE, Eiglmeier K, Bergh S, Cole ST. (1996) Gene arrangement and organization in approximately 76 kb fragment encompassing the oriC region of the chromosome of Mycobacterium leprae. Microbiology 142: 3147–3161. [DOI] [PubMed] [Google Scholar]
- Horsburg CR., Jr (1991) Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med 324: 1332–1338. [DOI] [PubMed] [Google Scholar]
- Ihira M, Yoshikawa T, Enomoto Y, et al (2004) Rapid diagnosis of human herpesvirus 6 infection by a novel DNA amplification method, loop-mediated isothermal amplification. J Clin Microbiol 42: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Sonobe T, Hayashi K. (2003) Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J Clin Microbiol 41: 2616–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal MA, Maeda S, Nakata N, Kai M, Fukuchi K, Kashiwabara Y. (2000) Molecular basis of clarithromycin-resistance in Mycobacterium avium intracellulare complex. Tuberc Lung Dis 80: 1–4. [DOI] [PubMed] [Google Scholar]
- Kasai H, Ezaki T, Harayama S. (2000) Differentiation of phylogenetically related slowly growing mycobacteria by their gyrB sequences. J Clin Microbiol 38: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, Chae GT, Kim EC, Cha CY, Kook YH. (1999) Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J Clin Microbiol 37: 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner P, Springer B, Vogel U, Meier A, Wrede A, Kiekenbeck M, Bange FC, Bottger EC. (1993) Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol 31: 2882–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I. (2003) Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol 41: 5517–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W, Blaesing F, Majka J, et al (1998) Functional domains of Dna A proteins. Biochimie 81: 819–825. [DOI] [PubMed] [Google Scholar]
- Messer W, Weigel C. (1997) Dna A initiator – also a transcription factor. Mol Microbiol 24: 1–6. [DOI] [PubMed] [Google Scholar]
- Metchock BG, Nolte FS, Wallace RJ., Jr (1999) Mycobacterium. Manual of Clinical Microbiology, 7th edn (Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, eds), pp.399–437. American Society for Microbiology, Washington, DC. [Google Scholar]
- Mizrahi VS, Dawes S, Rubin H. (2000) DNA replication. Molecular Genetics of Mycobacteria (Hatful GF, Jacobs WR, Jr, eds), pp.159–172. American Society for Microbiology, Washington, DC. [Google Scholar]
- Montessori V, Phillips P, Montaner J, Haley L, Craib K, Bessuille E, Black W. (1996) Species distribution in human immunodeficiency virus-related mycobacterial infections: implications for selection of initial treatment. Clin Infect Dis 22: 989–992. [DOI] [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28: E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida M, Posadas G, Inoue S, Hasebe F, Morita K. (2004) Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol 42: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Jang H, Kim C, Chung B, Chang CL, Park SK, Song S. (2000) Detection and identification of mycobacteria by amplification of the internal transcribed spacer regions with genus- and species-specific PCR primers. J Clin Microbiol 38: 4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikaytis BB, Plikaytis BD, Yakrus MA, Butler WR, Woodley CL, Silcox VA, Shinnick TM. (1992) Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol 30: 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primm TP, Lucero CA, Falkinham JO., III (2004) Health impacts of environmental mycobacteria. Clin Microbiol Rev 17: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Fischer M, Hamid ME, Michalke S, Ludwig W, Mauch H. (1998) Differentiation of phylogenetically related slowly growing mycobacteria based on 16S–23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol 36: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CC. (1960) The experimental diseases that follows the injection of human leprosy bacilli into foot-pads of mice. J Exp Med 112: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B, Stockman L, Teschner K, Roberts GD, Bottger EC. (1996) Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J Clin Microbiol 34: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TB, Patterson C, Hale Y, Safranek WW. (1997) Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol 35: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. (1993) Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol 31: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai HTC, Le MQ, Vuong CD, Parida M, Minekawa H, Notomi T, Hasebe F, Morita K. (2004) Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J Clin Microbiol 42: 1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turenne CY, Tschetter L, Wolfe J, Kabani A. (2001) Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J Clin Microbiol 39: 3637–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne LG, Sramek HA. (1992) Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev 5: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ross BC, Dwyer B. (1993) Isolation of a DNA probe for identification of Mycobacterium kansasii, including the genetic subgroup. J Clin Microbiol 31: 2769–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]


