Abstract
Objectives
The aim of this study was to assess the effect of commercial aurintricarboxylic acid (ATA) against Cryptosporidium parvum.
Methods
The anticryptosporidial effect of ATA was evaluated in vitro using cell culture and double fluorogenic staining, and in vivo in experimentally infected neonatal C57BL/6 mice. Mice were orally treated for 9 consecutive days starting on the day of infection with daily ATA doses of 50 and 100 µmol/kg. Paromomycin (100 mg/kg) was used as a positive control.
Results
In both in vitro models, ATA at concentrations of 100 and 10 µmol/L completely inhibited sporozoites within 10 and 60 min, respectively. Viability of oocysts exposed to 100 µmol/L and assessed by flow cytometry and in cell culture was reduced by 65% and 61%, respectively. The treatment of neonatal mice with a daily ATA dose of 100 µmol/kg led to 97–99% inhibition of infection without any observable negative effects on the animals. In comparison, the mean reduction of infection for paromomycin was 79–84%.
Conclusions
ATA exerted high anticryptosporidial activity and should be considered for further study.
Keywords: flow cytometry, cell culture, chemotherapy, experimental infection, neonatal mice
Introduction
Cryptosporidiosis is responsible for several intestinal disorders in a wide range of hosts. The infection is typically found in AIDS patients and in other immunocompromised persons. Cryptosporidium species are also common and dangerous waterborne pathogens.1Cryptosporidium parvum is one of the most frequent causes of enteritis and diarrhoea in postnatal calves, lambs, goat kids and in other agriculturally important animals. The economical, hygienic and zoohygienic impact of cryptosporidiosis is significant.1,2
Control of the infection in human and veterinary practice is complicated by a lack of effective therapy. Although immunotherapy, mainly administration of hyperimmune colostrum, may improve the clinical status of patients, chemotherapy is the main therapeutic approach.3 However, none of the known anticryptosporidial drugs, such as paromomycin, azithromycin and nitazoxanide used mostly in humans, or halofuginone-lactate approved for anticryptosporidial therapy of calves, is absolutely effective.4,5 Better anticryptosporidial drugs with minimal residuality and toxicity are still needed in human and veterinary clinical practice.
One of the drugs with potential anticryptosporidial use is aurintricarboxylic acid (ATA). It has been shown that ATA is minimally toxic6 and that it is biologically highly active. For example, ATA is a potent inhibitor of endonuclease activity and other protein–nucleic acid interactions.7,8 ATA also inhibits angiogenesis9 or von Willebrand factor binding to platelets, and thus prevents blood coagulation.10 Antimicrobial properties of ATA have also been described, as this compound suppresses Yersinia pestis pathogenicity.11 Interestingly, it has been found that ATA also strongly inhibits the replication of the coronavirus causing severe acute respiratory syndrome (SARS).12 In contrast, no data on the antiprotozoal activity of this compound are available.
The aim of this study was to assess efficacy of ATA against C. parvum using both in vitro and in vivo laboratory models.
Materials and methods
In vitro tests
Commercially available ATA (A1895; Sigma-Aldrich) was freshly dissolved in dimethylsulfoxide to prepare a 0.1 M stock solution. Working solutions of 0.1, 10 and 100 µmol/L were subsequently prepared by diluting the stock solution in DMEM and PBS for cell cultivation and flow cytometry, respectively. Fresh oocysts of C. parvum were obtained from a human AIDS patient; their viability was assessed before use by staining with DAPI (4′-6-diamidino-2-phenylindole) and propidium iodide and using an excystation assay.13 Both intact oocysts and free sporozoites were examined separately.
The in vitro anticryptosporidial activity of ATA was evaluated using flow cytometry and a cell culture model as described in detail previously.13 Concisely, in the flow cytometry assay, oocysts and sporozoites were exposed to the three different concentrations of drug for 0–180 min. Then the parasites were stained by fluorescein diacetate and propidium iodide and analysed by flow cytometry. This analysis demonstrated the presence of four populations of organisms: (i) green-fluorescent organisms (viable cells); (ii) red-fluorescent organisms (non-viable organisms); (iii) organisms that showed both red and green fluorescence (injured or dying cells); and (iv) particles that showed no fluorescence (cellular debris or cystic forms that are poorly permeable to fluorescein diacetate).
In the in vitro cell culture test, fresh confluent monolayers of A-549 cells were experimentally infected with intact oocysts or sporozoites that had also been previously exposed to the three test concentrations of drug for 0–180 min. After infection, the cells were cultivated with the parasite for 48 h under standard conditions. The intensity of the infection was then assessed in 100 random fields using Nomarski interference contrast optics. Only meronts and gamonts were enumerated to avoid counting non-viable, but adherent, sporozoites or merozoites.
In vivo test
Three-day-old, SPF C57BL/6 baby mice (Velaz, Czech Republic) were randomly assigned to groups of nine. To avoid rejection of pups, randomized baby mice were wetted with urine of their adoptive mother and then placed into the respective nest. The experiment was initiated 1 day later, after ensuring that all pups were healthy. The baby mice were maintained together with their (adoptive) mother in sterile microisolators equipped with filter tops (Tecniplast, Italy). The cages were opened only in a biosafety cabinet. Sterilized water and standardized food pellets were administered ad libitum.
Mouse litters were assigned to one of five groups as follows: group A, infected and treated with ATA at a daily dose of 100 µmol/kg; group B, infected and receiving ATA at a daily dose of 50 µmol/kg; group C, uninfected and receiving ATA at a daily dose of 100 µmol/kg; group D, infected and treated with paromomycin (100 mg/kg/day; positive control); and group E, infected and receiving PBS (control).
Oocysts of C. parvum isolate VUZV-N were propagated in a neonatal Holstein calf 5–6 weeks before the experiment. Oocysts were purified on a secondary sucrose gradient,14 surface-sterilized in 1% sodium hypochlorite and washed three times in sterile PBS. Oocysts were stored in sterile PBS at 4°C until they were used.
The in vivo model15 was modified as follows: 4-day-old mice were orally infected with 105 oocysts and the treatment was initiated at the same time; the daily dose of ATA was divided into two equal portions and orally administered at 12 h intervals. Paromomycin (Duchefa Biochemie, The Netherlands) was dissolved in deionized water and orally administered at 24 h intervals. The same ATA as used in in vitro tests was freshly prepared before each use by complete dissociation in aqueous NaOH at a 1:3 (ATA/NaOH) molar ratio and filter-sterilized with a sterile 0.22 µm pore size syringe filter. The treatment was continued for 9 days. During this period, mice were weighed daily to adjust the drug dosage and monitor growth.
Mice were euthanized by cervical dislocation 24 h after completing the treatment. Their intestinal tract (from duodenum to rectum) was extracted, weighed, homogenized and resuspended in 25 mL of cold PBS. The homogenate was then vigorously shaken with the same volume of cold petroleum ether and centrifuged (10 min, 3000 g, 2°C). Extracted fat, mucus and petroleum ether were discarded and the pellet resuspended in PBS. The suspension was then treated with 1% sodium hypochlorite, washed three times in PBS, the volume adjusted to 2.5-fold of the initial weight of the extracted intestine, and the oocysts enumerated in a haemocytometer. Intensity of infection was expressed as the number of oocysts extracted from 1 mg of the intestinal tissue. To correctly diagnose the infection, aliquots of the intestinal extract were concentrated four times by centrifugation and also examined by the acid-fast staining technique. One-third of the PBS volume in the concentrated samples was replaced with bovine blood plasma before the samples were smeared onto a slide. The slide was then dried, fixed and stained with carbol-fuchsin as described previously.16
The in vivo experiment was repeated a second time using the same protocol. Results were statistically evaluated with non-parametric ANOVA (statistical package QC Expert, version 2.5; TriloByte, Czech Republic).
The in vivo experiments were approved by The Ethics Committee of the Institute of Animal Science in Praha (Project 14/2006) and by the Central Commission for Animal Welfare at The Ministry of Agriculture of the Czech Republic (Project MZe 812).
Results and discussion
Flow cytometry demonstrated that the percentage of viable sporozoites decreased to an undetectable level after 10 and 60 min of exposure to 100 and 10 µmol/L ATA, respectively (Figure 1). ATA at 100 µmol/L reduced oocyst viability by 65% (Figure 2).
Figure 1.
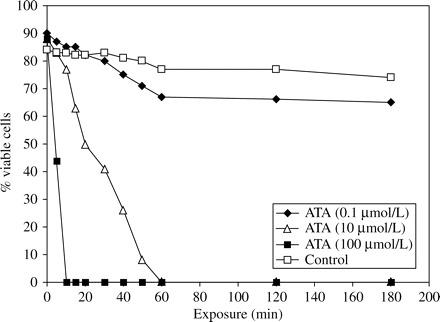
Flow cytometric analysis of sporozoite susceptibility to aurintricarboxylic acid (ATA).
Figure 2.
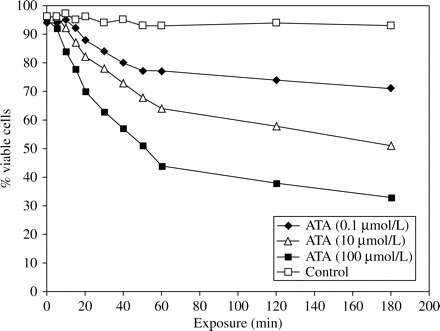
Flow cytometric analysis of oocysts exposed to aurintricarboxylic acid (ATA).
Similarly, in cell culture, complete inhibition of parasite growth was observed after 10 and 60 min of exposure of sporozoites to 10 and 100 µmol/L ATA, respectively (Figure 3). In the oocyst series, ATA exposure resulted in a reduction of 61% at 100 µmol/L (Figure 4). Both, flow cytometry and cell culture assay showed a high activity of ATA against free sporozoites, but surprisingly also against oocysts. Data obtained from the repeated in vitro tests produced analogous results (data not shown).
Figure 3.
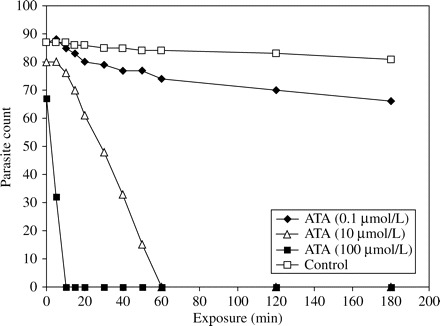
Assessment in cell culture of sporozoite susceptibility to aurintricarboxylic acid (ATA).
Figure 4.
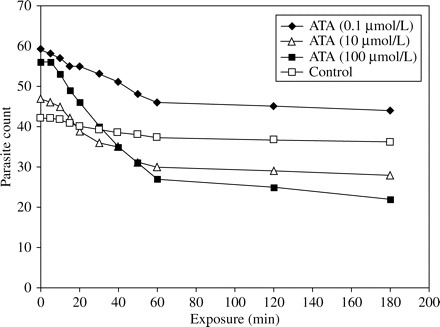
Assessment in cell culture of oocyst susceptibility to aurintricarboxylic acid (ATA).
In mice, a dose-dependent inhibition of the infection by ATA was observed (Figure 5). Whereas the mean inhibition in animals treated with 100 µmol/kg was 97% and 99% (P < 0.001) in Experiments I and II, respectively, a dose of 50 µmol/kg reduced the infection by 72% and 76% (P < 0.001). In addition, some animals treated with the higher drug dose, particularly in Experiment II, were found to be C. parvum-negative at the end of the treatment.
Figure 5.
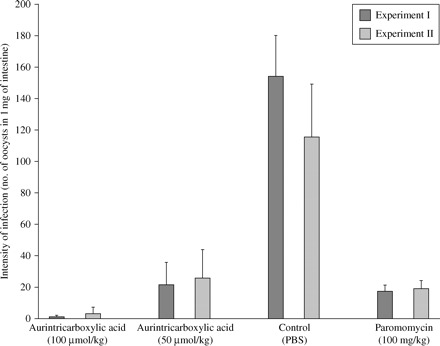
Effect of 9 day aurintricarboxylic acid treatment on C. parvum infection in C57BL/6 neonatal mice.
Treatment with paromomycin (100 mg/kg) led to a mean inhibition of infection of 79% and 84% (P < 0.001; Figure 5). Nevertheless, paromomycin exerted slight toxicity and caused moderate depression of growth, as the average daily weight gain of mice in this group, between age 4 and 14 days, was 0.39 g. In contrast, the average daily gain in the other groups (A–C and E) during the same period was 0.43 g, indicating that ATA had no observable negative effect on the animals.
Available data on toxicity of ATA are unclear. Commercially available ATA preparations contain usually ∼15% of impurities, and it is possible that the toxicity of ATAs from different sources may vary. Moreover, many biological studies of this compound did not distinguish between the ammonium salt of ATA, known also as aluminon, and ATA itself. Further toxicological studies on ATA are therefore necessary. Results of this in vivo experiment are nevertheless consistent with the results of Balzarini et al.6 who found that the LD50 of ATA in mice is as high as 0.34 g/kg.
The mechanism by which ATA inhibits the parasite, and the compound’s therapeutical target, also remain unknown. Indeed, commercial ATA used in most biological studies, including the present, is a chemical compound whose chemical structure is still being discussed. ATA was originally thought to be a triphenylmethane dye, but the dye’s structure is still incorrectly used in much of the ATA-related literature. Commercially available ATA is actually a heterogeneous mixture of polymers of the phenol formaldehyde type.17 In addition, it is known that ATA readily polymerizes and that its biological activity varies with the molecular weight.8 A study on the effect of ATA fractions on nuclear swelling and on in vitro RNA and DNA synthesis even found that significant biological activity can be attributed to formaurindicarboxylic acid, an impurity found in commercial preparations of ATA, whereas pure ATA was found to be almost inactive.18
A complete understanding of the relationship between the chemical structure and the biological properties of ATA is essential before ATA is considered for clinical use, including as an antiprotozoal drug. Our data indicate that commercial ATA preparations have strong anticryptosporidial activity and that the treatment was well tolerated in mice. In light of the limited therapeutic options for treating cryptosporidiosis, further evaluation of ATA is clearly justified.
Funding
The study was supported by the Ministry of Agriculture of The Czech Republic (project MZe-0002701403).
Transparency declarations
None to declare.
References
- 1.O’Donoughe PJ. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–95. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 2.de Graaf DC, Vanopdenbosch E, Ortega-Mora LM, et al. A review of the importance of cryptosporidiosis in farm animals. Int J Parasitol. 1999;29:1269–87. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins MC. Present and future control of cryptosporidiosis in humans and animals. Expert Rev Vaccines. 2004;3:669–71. doi: 10.1586/14760584.3.6.669. [DOI] [PubMed] [Google Scholar]
- 4.Giacometti A, Burzacchini F, Cirioni O, et al. Efficacy of treatment with paromomycin, azithromycin, and nitazoxanide in a patient with disseminated cryptosporidiosis. Eur J Clin Microbiol Infect Dis. 1999;18:885–9. doi: 10.1007/s100960050424. [DOI] [PubMed] [Google Scholar]
- 5.Klein P. Preventive and therapeutic efficacy of halofuginone-lactate against Cryptosporidium parvum in spontaneously infected calves: a centralised, randomised, double-blind, placebo-controlled study. Vet J. 2008;177:429–31. doi: 10.1016/j.tvjl.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balzarini J, Mitsuya H, De Clercq E, et al. Aurintricarboxylic acid and Evans Blue represent two different classes of anionic compounds which selectively inhibit the cytopathogenicity of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. Biochem Biophys Res Commun. 1986;136:64–71. doi: 10.1016/0006-291x(86)90877-6. [DOI] [PubMed] [Google Scholar]
- 7.Hallick RB, Chelm BK, Gray PW, et al. Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977;4:3055–64. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González RG, Blackburn BJ, Schleich T. Fractionation and structural elucidation of the active components of aurintricarboxylic acid, a potent inhibitor of protein nucleic acid interactions. Biochim Biophys Acta. 1979;562:534–45. doi: 10.1016/0005-2787(79)90116-3. [DOI] [PubMed] [Google Scholar]
- 9.Gagliardi AR, Collins DC. Inhibition of angiogenesis by aurintricarboxylic acid. Anticancer Res. 1994;14:475–9. [PubMed] [Google Scholar]
- 10.Owens MR, Holme S. Aurin tricarboxylic acid inhibits adhesion of platelets to subendothelium. Thromb Res. 1996;81:177–85. doi: 10.1016/0049-3848(95)00234-0. [DOI] [PubMed] [Google Scholar]
- 11.Liang F, Huang Z, Lee SY, et al. Aurintricarboxylic acid blocks in vitro and in vivo activity of YopH, an essential virulent factor of Yersinia pestis, the agent of plague. J Biol Chem. 2003;278:41734–41. doi: 10.1074/jbc.M307152200. [DOI] [PubMed] [Google Scholar]
- 12.He R, Adonov A, Traykova-Adonova M, et al. Potent and selective inhibition of SARS coronavirus replication by aurintricarboxylic acid. Biochem Biophys Res Commun. 2004;320:1199–203. doi: 10.1016/j.bbrc.2004.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacometti A, Cirioni O, Del Prete MS, et al. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J Antimicrob Chemother. 2003;51:843–7. doi: 10.1093/jac/dkg149. [DOI] [PubMed] [Google Scholar]
- 14.Arrowood MJ, Sterling CR. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–9. [PubMed] [Google Scholar]
- 15.Tzipori S. Cryptosporidiosis: laboratory investigations and chemotherapy. Adv Parasitol. 1998;40:187–221. doi: 10.1016/s0065-308x(08)60121-9. [DOI] [PubMed] [Google Scholar]
- 16.Casemore DP, Armstrong M, Sands RL. Laboratory diagnosis of cryptosporidiosis. J Clin Pathol. 1985;38:1337–41. doi: 10.1136/jcp.38.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushman M, Kanamathareddy S, De Clercq E, et al. Synthesis and anti-HIV activities of low molecular weight aurintricarboxylic acid fragments and related compounds. J Med Chem. 1991;34:337–42. doi: 10.1021/jm00105a053. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui K, Seki S, Tsutsui K, et al. Fractionation of aurintricarboxylic acid and effects of its components on nuclear swelling and nucleic acid synthesis. Biochim Biophys Acta. 1978;517:14–23. doi: 10.1016/0005-2787(78)90029-1. [DOI] [PubMed] [Google Scholar]


