A recently discovered mechanism showed that in mice, anti-glycoprotein (GP)Ib/IX platelet antibodies interfere with hepatocyte thrombopoietin (TPO) production. This mechanism may potentially also contribute to the relatively low TPO levels observed in patients suffering from immune thrombocytopenia (ITP), an autoimmune bleeding disorder in which anti-platelet autoantibodies are able to target platelets. To investigate this, we reviewed a large cohort of thrombocytopenic patients which we assessed for anti-platelet autoantibodies and TPO levels (n=3,490). We show for the first time that anti-GPIb/IX antibodies, occurring alone or together with other anti-platelet autoantibodies such as anti-GPV and/or with anti-GPIIb/IIIa antibodies, do not influence circulating TPO levels in ITP patients. This suggests that anti-GPIb/IX autoantibodies do not interfere directly with TPO production in humans.
Platelet production is regulated mainly by TPO, a hematopoietic growth factor that interacts with the myeloproliferative leukemia protein receptor (Mpl; CD110) on megakaryocytes and circulating platelets.1,2 The primary site of TPO synthesis is the liver, and to a lesser extent kidney, spleen and bone marrow cells.3 Interestingly, it was suggested that TPO production is induced by the binding of desialylated aged platelets interacting with the hepatocyte asialoglycoprotein receptor (ASGPR), also known as the hepatic Ashwell-Morrell receptor (AMR).4 Furthermore, circulating TPO levels are influenced by binding of TPO to platelet- and megakaryocyte-Mpl.5,6
Immune thrombocytopenia is an autoimmune bleeding disorder with a complex pathophysiology.7 Many ITP patients show autoantibodies to platelet GPIIb/IIIa, GPIb/IX and GPV. In ITP patients, there appears to be an ongoing platelet destruction, but with normal or mildly elevated TPO levels.8,9 Recently, a novel mechanism of TPO production was described, in which platelet GPIb, in an AMR-independent manner, induces hepatocytic TPO production, and was independent of platelet desialylation.10 In this mouse study, monoclonal antibodies to GPIb were shown to inhibit hepatic TPO production.10 This mechanism might play an additional role in the relatively low TPO levels observed in ITP patients. However, it has not been investigated if anti-GPIb antibodies are indeed able to interfere with circulating TPO levels in ITP patients.
To address this unresolved question, we evaluated TPO levels in ITP patients with anti-platelet autoantibodies, including a subgroup with only anti-GPIb IgG antibodies, using a large cohort of thrombocytopenic patients evaluated in our national reference laboratory (Sanquin Diagnostic Services, Amsterdam, The Netherlands) for antigen-specific platelet autoantibodies (years 2011-2019; 3490 patients and 201 healthy controls). Data were handled under National Responsible Use policies (Code of Conduct for Use of Data in Health Research; https://www.federa.org/codes-conduct). All of these thrombocytopenic samples were tested for platelet autoantibodies against GPIbIX, GPV and GPIIb/IIIa using a modified monoclonal antibody-immobilization of platelet antigens (MAIPA) assay.11 In addition, circulating TPO levels were measured in fresh EDTA plasma by an in-house ELISA, as previously described.12,13 Control samples were obtained from non-thrombocytopenic healthy blood donors. Unfortunately, platelet counts at the time of analysis were not available in our laboratory information system. A two-sided alpha value of 0.05 was used as cut-off for statistical significance. Children below one year of age were excluded. The total cohort which was analyzed was made up of 3,490 individual thrombocytopenic patients with 2,979 and 2,239 samples for direct and indirect tests, respectively, and 201 healthy controls. Platelet-associated IgG autoantibodies (direct test) and/or circulating anti-platelet IgG (indirect test) were assessed using MAIPA. Although not all ITP patients have detectable autoantibodies by MAIPA, we have previously reported that a direct antibody test has 98% specificity for clinically diagnosed ITP.11
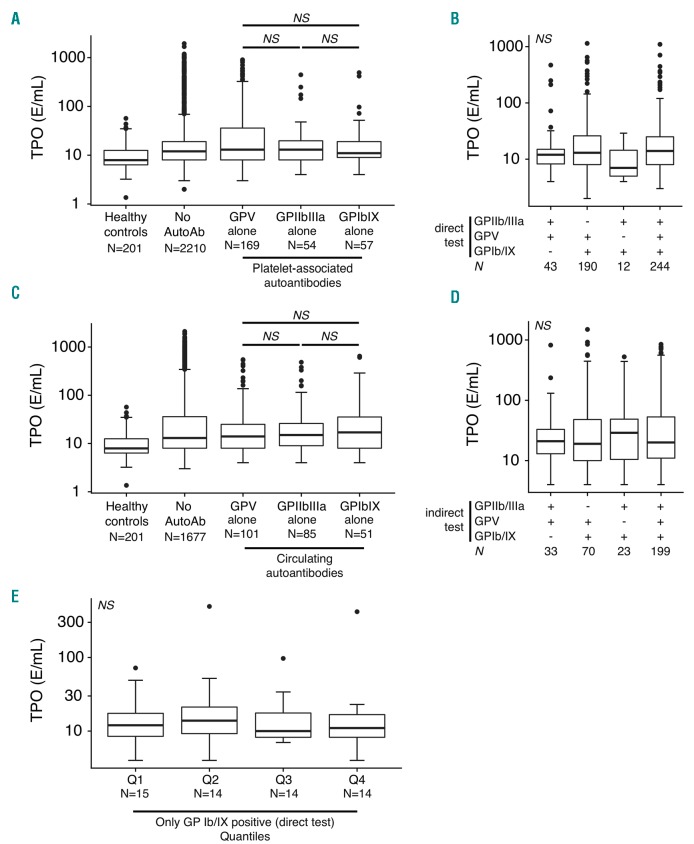
In the current study, we found that, in agreement with previous studies,8,9 TPO levels in ITP patients were significantly increased compared to healthy controls (P<3.5×10−3 vs. healthy controls) (Figure 1). However, all patients with detectable antibodies to GPIIb/IIIa, GPV or GPIb/IX, as determined with a direct test, showed similar TPO levels (Figure 1A). Among the majority of ITP patients with multiple anti-platelet glycoprotein antibodies, presence of anti-GPIb antibodies did not affect TPO levels (Figure 1B). Identically to the direct test, also using the indirect test, patients with circulating antibodies against GPIb alone displayed no differences in TPO levels compared to patients with anti-GPIIb/IIIa or anti-GPV antibodies (Figure 1C). In addition, we did not observe any differences in TPO levels when anti-GPIb/IX antibodies co-occurred with antibodies against GPV and/or GPIIb/IIIa (Figure 1D). It is conceivable that a certain antibody level is required to achieve sufficient opsonization to block GPIb-hepatocyte interactions. However, in agreement with the results above, we did not observe any significant differences between patients with low or high anti-GPIb IgG antibody levels and TPO (Figure 1E).
Figure 1.
Anti-GPIbIX autoantibodies do not correlate with circulating thrombopoietin (TPO) levels in patients with immune thrombocytopenia. (A) TPO levels, dependent on platelet-associated antibodies (direct test) for the indicated platelet antigens. Only single-positive antibody results are displayed. Exact P-values are (post-hoc Nemenyi test): anti-GPV vs. anti-GPIb/IX, P=0.84; anti-GPIIb/IIIa vs. anti-GPIb/IX, P=0.99; anti-GPV vs. anti-GPIIb/IIIa, P=0.76. (B) TPO levels for multiple specificities of anti-platelet autoantibodies in a direct test. Kruskal-Wallis test, P=0.19. (C) TPO levels for single-positive circulating autoantibodies (indirect test). Exact P-values are (post-hoc Nemenyi test): anti-GPV vs. anti-GPIb/IX, P=0.95; anti-GPIIb/IIIa vs. anti-GPIb/IX, P=0.99; anti-GPV vs. anti-GPIIb/IIIa, P=0.95. (D) TPO levels by multiple specificities of anti-platelet autoantibodies in an indirect test. Kruskal-Wallis test, P=0.93. (E) No dose-dependent effect of anti-GPIbIX autoantibodies (direct test) on TPO levels in immune thrombocytopenia. Patients were categorized in 25% quantiles based on observed antibody levels. Kruskal-Wallis test, P=0.80. NS: not significant; N: number.
Our findings in human ITP samples are not in agreement with the recently proposed mechanism stating that anti-GPIb autoantibodies impair TPO production in mice.10 Alternatively, it may be possible that platelet activation, complement activation or a mechanical feature induced by anti-GPIb antibodies14 determines the ability to induce Fc-independent platelet clearance, which, just like anti-GPIIb/IIIa Fc-mediated platelet clearance, may not induce increased hepatic TPO generation. A limitation of our study is that no additional clinical information, such as co-morbidities and platelet counts, were available. Key strengths of our study are the large number of clinical patient samples available for analysis, the ability to distinguish antibodies against multiple platelet antigens, and the standardized analysis of anti-platelet antibodies in our laboratory. To our knowledge, this is the first time such a large-scale analysis is performed investigating the association between anti-platelet GPIb, GPV, and GPIIb/IIIa antibodies versus circulating TPO levels in thrombocytopenic patients. Our results further support the notion that the majority of ITP patients clearly demonstrate the simultaneous presence of antibodies to multiple platelet-glycoproteins, including anti-GPV antibodies which were found in large quantities, as also previously reported in ITP.11,15
In conclusion, our data show that, in ITP patients, anti-GPIb/IX antibodies, alone or co-occurring with anti-GPV and/or with anti-GPIIb/IIIa antibodies, do not influence circulating TPO levels. It therefore appears that, in humans, blocking of GPIb by anti-platelet GPIb antibodies does not directly account for the reduced TPO levels observed in ITP. More research is required to understand the mechanisms to account for the slightly elevated TPO levels in ITP patients.
Acknowledgments
We are thankful for the contributions of the staff of the Platelet and Leukocyte Serology Laboratory, Sanquin Research, in particular Gonda Oldert and Elly Huiskes.
Footnotes
Funding: this work was supported by a research grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR) and a doctoral stipend to D.E.S. by the Studienstiftung des Deutschen Volkes.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushansky K. Thrombopoietin. N Engl J Med. 1998;339(11):746–754. [DOI] [PubMed] [Google Scholar]
- 3.Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89(1):101–107. [PubMed] [Google Scholar]
- 4.Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015;21(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folman CC, de Jong SM, de Haas M, Borne von dem AE. Analysis of the kinetics of TPO uptake during platelet transfusion. Transfusion. 2001;41(4):517–521. [DOI] [PubMed] [Google Scholar]
- 6.Fielder PJ, Gurney AL, Stefanich E, et al. Regulation of thrombopoietin levels by c-mpl-mediated binding to platelets. Blood. 1996;87(6):2154–2161. [PubMed] [Google Scholar]
- 7.Zufferey A, Kapur R, Semple JW. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porcelijn L, Folman CC, Bossers B, et al. The diagnostic value of thrombopoietin level measurements in thrombocytopenia. Thromb. Haemost. 1998;79(6):1101–1105. [PubMed] [Google Scholar]
- 9.Kosugi S, Kurata Y, Tomiyama Y, et al. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br J Haematol. 1996;93(3):704–706. [DOI] [PubMed] [Google Scholar]
- 10.Xu M, Li J, Neves MAD, et al. GPIbα is required for platelet-mediated hepatic thrombopoietin generation. Blood. 2018;132(6):622–634. [DOI] [PubMed] [Google Scholar]
- 11.Porcelijn L, Huiskes E, Oldert G, et al. Detection of platelet autoantibodies to identify immune thrombocytopenia: state of the art. Br J Haematol. 2018;39(Suppl. 1):195. [DOI] [PubMed] [Google Scholar]
- 12.Folman CC, Borne von dem AE, Rensink IH, et al. Sensitive measurement of thrombopoietin by a monoclonal antibody based sandwich enzyme-linked immunosorbent assay. Thromb Haemost. 1997; 78(4):1262–1267. [PubMed] [Google Scholar]
- 13.Porcelijn L, Folman CC, de Haas M, et al. Fetal and neonatal thrombopoietin levels in alloimmune thrombocytopenia. Pediatr Res. 2002;52(1):105–108. [DOI] [PubMed] [Google Scholar]
- 14.Quach ME, Dragovich MA, Chen W, et al. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets. Blood. 2018;131(7):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vollenberg R, Jouni R, Norris PAA, et al. Glycoprotein V is a relevant immune target in patients with immune thrombocytopenia. Haematologica. 2019;104(6):1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]