Induced pluripotent stem cells (iPSC) could differentiate into different cells including hematopoietic stem and progenitor cells (HSPC).1,2 However, in vitro generation of transplantable HSPC from iPSC is still challenging.2,3 Recent studies reported that engraftable HSPC could be isolated from teratomas formed from mouse and human iPSC.4–6 Therefore, it would be worth exploring whether in vivo generated HSPC are functional for HSPC-based gene therapy.
Anti-Factor VIII (FVIII) neutralizing antibody (inhibitors) development is a serious problem in the replacement therapy of hemophilia A (HA).7,8 Platelet-targeted gene therapy is a promising approach for HA with inhibitors. FVIII-releasing platelets generated from FVIII-modified HSPC could ameliorate the hemorrhage diathesis in the presence of inhibitors.9–11 CRISPR/Cas9 provides a convenient method for targeted integration of a therapeutic gene to cure genetic diseases.12–14 Meanwhile, genome editing of iPSC is easier and more efficient than that of HSPC, due to the fact that iPSC can be easily proliferated and screened.15 In this study, we aim to explore the possibility of using in vivo-generated HSPC from genome-edited iPSC in platelet-targeted gene therapy of HA.
Firstly, we codon-optimized a FVIII cassette (named opF8) and confirmed the high expression efficiency of opF8 in HEK293T cells and HA mice (Online Supplementary Figure S1A-E). Then, we put opF8 under control of the megakaryocyte/platelet specific αIIb promoter (2bopF8) and verified the specificity of the cassette in Dami and HepG2 cells (Online Supplementary Figure S1F). On the other hand, we utilized an optimized reprogramming method to generate HA mouse iPSC (HA-iPSC) (Online Supplementary Figure S2A and B). Pluripotency of the cells was confirmed (Online Supplementary Table S1 and Online Supplementary Figure S2C-G).
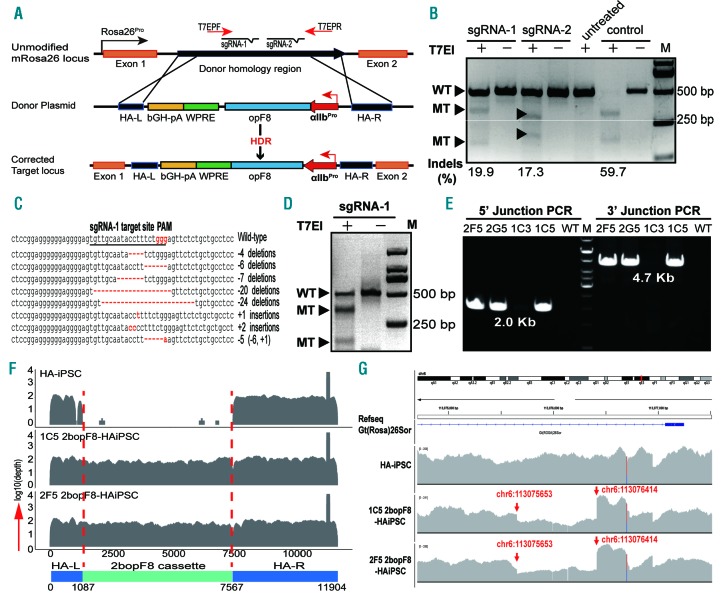
We designed two sgRNA targeting intron one of mouse Rosa26 locus and chose Cas9/sgRNA-1 for its higher efficiency (Figure 1A-D, Online Supplementary Table S2 and Online Supplementary Figure S3A and B). We acquired 2bopF8 target-integrated HA-iPSC clones (2bopF8-HAiPSC) by co-transfection of Cas9/sgRNA-1 plasmid and 2bopF8 donor plasmid into HA-iPSC. The integration site of 2bopF8 was confirmed (Figure 1E and Online Supplementary Figure S3C) and the copy number of 2bopF8 is one per cell (Online Supplementary Figure S3D).
Figure 1.
CRISPR/Cas9 mediated targeted integration of the 2bopF8 cassette in mouse Rosa26 locus of 3T3 cells and hemophilia A induced pluripotent stem cells (HA-iPSC). (A) Schematic of CRISPR/Cas9-mediated target integration of 2bopF8 to the intron one of mouse Rosa26 locus. The sgRNA-1 and sgRNA-2 were designed targeting intron one of Rosa26. HA-L and HA-R represent left and right homologous arm, respectively. HDR: homology-directed repair; T7EPF/R: primers used in T7EI assay. (B) T7EI assay to evaluate the efficiency of sgRNA in Cas9/sgRNA-transfected 3T3 cells. Rate of indels is higher in Cas9/sgRNA-1 transfected cells. Untreated: non-transfection; Control: positive targeting control; WT: wild-type band; MT: mutated band. Indel rates are indicated at the bottom of the corresponding lanes. (C) Sanger sequencing results of the cloned polymerase chain reaction (PCR) products from Cas9/sgRNA-1 transfected cells (see B) confirmed the indels generated at the predicted targeted site. (D) Cleavage efficiency of the Cas9/sgRNA-1 in HA-iPSC confirmed by T7EI assay. The indel rate is 47.3%. (E) Junction PCR analysis of the integration of 2bopF8 in the Rosa26 locus of HA-iPSC clones after Cas9/sgRNA-1 and 2bopF8 donor plasmid transfection. The HA-iPSC clones (2F5, 2G5 and 1C5) are positive for both 5’ junction and 3’ junction. Clone 1C3: genome edited HA-iPSC mono-clone; WT: non-edited HA-iPSC control. (F) Whole genome sequencing (WGS) reads coverage of the Rosa26 target integration site before and after genome editing. The integrated region is shown between two red dotted lines. There is integration of 2bopF8 in clones 1C5 and 2F5, but no 2bopF8 in HA-iPSC. (G) WGS blast between mouse mm10 reference genome and iPSCs genome via IGV tool. The red arrows show the breakpoints due to 2bopF8 cassette integration.
The off-target mutation in 2bopF8-HAiPSC was verified by off-target prediction and whole genome sequencing (WGS). Most of the predicted potential sites were on the non-functional regions (Online Supplementary Table S3). No indel was found in the top ten potential off-target sites except OT-6 (Online Supplementary Figure S4A), which was also found in HA-iPSC, suggesting that the indel was not triggered by Cas9/sgRNA-1 (data not shown). Through WGS, we found only two small indels and one single nucleotide variation (SNV) in the inter-genic or intronic regions of clone 1C5 and one indel and two SNV in clone 2F5 (Online Supplementary Tables S4 and S5). These mutations do not locate in the predicted off-target regions (Online Supplementary Figure S4B). The WGS data also confirmed the integration of the 2bopF8 cassette in the Rosa26 target site and no other insertion sites were found (Figure 1F and G and Online Supplementary Figure S4C and D). These results demonstrated the successful integration of 2bopF8 and no obvious off-target mutations in 2bopF8-HAiPSC.
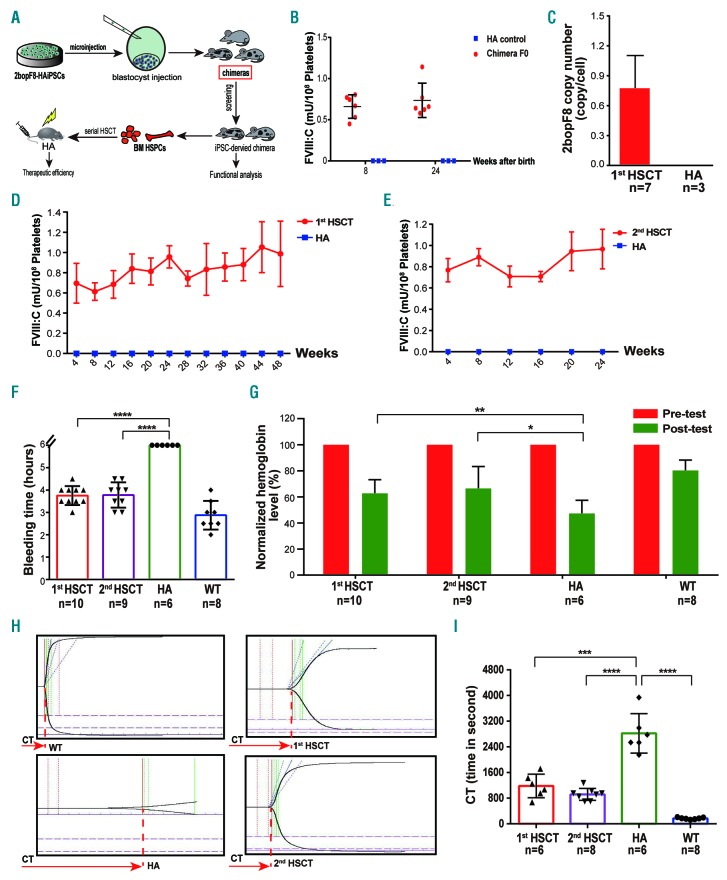
To determine if the pluripotency of 2bopF8-HAiPSC was affected by the genome editing, we selected three 2bopF8-HAiPSC clones to generate chimeric mice by blastocyst injection (Figure 2A). We analyzed six chimeric mice generated from clone 1C5 (Online Supplementary Table S6 and Online Supplementary Figure S5A). All six chimeric mice were 2bopF8 positive, and the integration site of 2bopF8 was demonstrated in peripheral blood cell (PBC) DNA (Online Supplementary Figure S5B and C), suggesting that pluripotency of 2bopF8-HAiPSC was maintained. FVIII:C was detected in the platelets and lasted for 24 weeks (Figure 2B), demonstrating that there are platelets containing FVIII that originated from 2bopF8-HAiPSC. Furthermore, we isolated Sca-1+ cells from bone marrow (BM) of the chimeric mice and transplanted the cells into lethally irradiated HA mice. All recipients except one were 2bopF8 positive in the PBC DNA, and 2bopF8 integration was confirmed (Figure 2C and Online Supplementary Figure S6A and B). The FVIII:C in platelets remained between 0.5-1.2 mU/108 platelets for 48 weeks until the end of the study (Figure 2D). The platelet-stored FVIII could be released after activation of platelets (Online Supplementary Figure S7A). No FVIII or inhibitor in plasma and no FVIII in mononuclear cells (MNC) of hematopoietic stem cell transplantation (HSCT) recipients were detected (Online Supplementary Figures S8A and S9A and B). The PBC counts returned to the normal range from week 8 (Online Supplementary Figure S10A); hence, hematopoiesis was successfully established and sustained in the recipients. We further performed a secondary HSCT. FVIII:C in the platelets of recipients was detected and maintained between 0.6-1.2 mU/108 platelets (Figure 2E). FVIII could also be released after platelet activation (Online Supplementary Figure S7B). The PBC counts recovered to the normal range after eight weeks (Online Supplementary Figure S10B). These data demonstrate the existence of 2bopF8-HAiPSC-derived HSPC in the chimeric mice, and that the 2bopF8-HAiPSC-derived HSPC have hematopoietic reconstitution capacity and long-term engraftment ability. Phenotypic correction of the recipients was assessed by tail bleeding time (BT) and relative blood loss. The BT of the first and secondary HSCT recipients is significantly shorter than that of HA mice (Figure 2F). The remaining hemoglobin in the recipients is significantly higher than that in the HA group (Figure 2G). We also used thrombelastograph (TEG) to analyze whole blood clotting time (WBCT). The WBCT of first and secondary recipients is significantly shorter than that of HA mice (Figure 2H and I). We then tried to evaluate if functional HSPC could be generated from genome-edited iPSC by means of teratoma. To promote the differentiation of 2bopF8-HAiPSC towards HSPC, we introduced hHoxB4/GFP into 2bopF8-HAiPSC (Online Supplementary Figure S11A). We injected hHoxB4-modified 2bopF8-HAiPSC together with OP9 stroma cells and cytokines intramuscularly into NOD-SCID mice to generate teratoma in the mice. Histological analysis of teratoma sections clearly demonstrates the presence of BM-like structures (Online Supplementary Figure S11B). We screened GFP+ cells from the teratoma and identified CD45+ cells and Lin– Sca1+ c-Kit+ (LSK) cells in GFP+ cells, indicating that these cells originated from the hHoxB4-modified 2bopF8-HAiPSC (Figure 3A and Online Supplementary Figure S11C). 2bopF8 and hHoxB4 transgene were detected in BM and PB cells of the teratoma-bearing mice (Figure 3B). GFP+ CD45+ cells were identified in BM and PB, and GFP+ CD150+ CD48– LSK cells (LT-HSC) in BM (Figure 3C and Online Supplementary Figure S11D). The results suggested that some iPSC-derived HSPC migrated and homed to the BM. Colony forming cell assay showed different colony-forming units of hematopoietic lineages, and flow cytometry analysis of colony forming cells revealed terminally hematopoietic lineage differentiation (Online Supplementary Figure S11E and F).
Figure 2.
Characterization of 2bopF8-hemophilia A induced pluripotent stem cell (HA-iPSC)-derived hematopoietic stem and progenitor cells (HSPC) from chimeric mice. (A) Flow chart of generating 2bopF8-HAiPSC-derived chimeras via blastocyst injection and subsequent serial hematopoietic stem cell transplantation (HSCT). (B) FVIII:C in platelets of F0 chimeras were measured at week 8 and week 24. (C) The average copy number of the 2bopF8 cassette was 0.77±0.30 copies per white blood cell (WBC) in the first HSCT recipients (n=7). WBC genomic DNA from HA mice was used as controls. (D) FVIII:C in the platelets of the first HSCT recipients, the level was maintained throughout the study period (n=8). (E) FVIII:C levels in the platelets of the secondary HSCT recipients, the level was maintained during the entire study period and is comparable to that of first recipients (n=9). (F) Tail bleeding time assessment of the mice. The tail bleeding time of both first (n=10) and secondary (n=9) recipients were significantly lower than that of HA mice, which continued bleeding after six hours. (G) Blood loss assessment of the mice. Remaining hemoglobin (Hb) after the tail bleeding test (post-test) was normalized to the Hb before the test (pre-test). The remaining Hb of first and secondary recipients was significantly higher than that of HA mice. (H) Thrombelastograph (TEG) analysis was performed between 8 and 12 weeks after HSCT. Representative TEG traces of WT, HA, first and secondary HSCT recipients are shown. (I) Whole blood clotting time (WBCT) was measured through TEG analysis. WBCT of the first and secondary recipients was significantly shorter than that of HA mice. Data are represented as mean±standard deviation. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Figure 3.
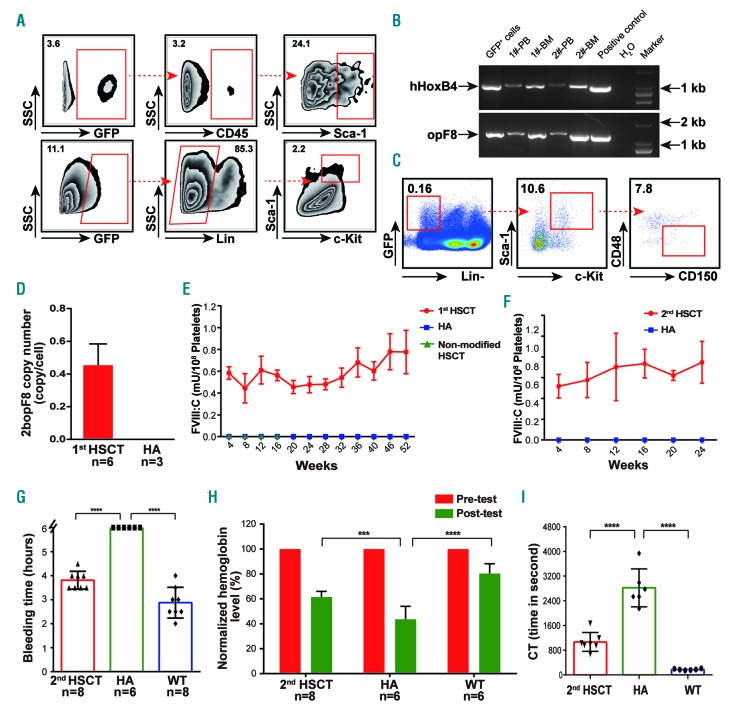
Characterization and functional assessment of teratoma-derived hematopoietic stem and progenitor cells (HSPC). (A) Flow cytometry analysis of GFP+ cells from the teratomas showed typical GFP+ HSPC generated from induced pluripotent stem cells (iPSC)-derived teratoma cells. (B) Transgene specific PCR analysis of peripheral blood (PB) and bone marrow (BM) cells from two teratoma bearing NOD-SCID mice. hHoxB4 and opF8 were positive in the mouse PB and BM cells, indicating that some teratoma-derived HSPC homed to the mouse BM. (C) Flow cytometry analysis identified GFP+CD150+CD48–LSK cells from the BM of teratoma-bearing mice. (D) Copy number of 2bopF8 in the first hematopoietic stem cell transplantation (HSCT) recipients (n=6). Hemophilia A (HA) mice were used as controls. (E) FVIII:C level in the platelets of first HSCT recipients (n=6). The level was stable during the entire study period, and no FVIII:C was detected in the platelets of recipients transplanted with HA-iPSC-derived HSPC. (F) FVIII:C level was stable in the secondary HSCT recipients (n=7). (G) Bleeding time of the mice in the tail-bleeding test. BT of the secondary HSCT recipients was significantly lower than that of HA mice. (H) Relative blood loss of the mice in the tail-bleeding test. Blood loss of the secondary HSCT recipients was significantly lower than that of HA mice. (I) Whole blood clotting time (WBCT) was measured by thrombelastograph (TEG) analysis at week 12 after HSCT. The WBCT of the secondary recipients was significantly lower than that of HA mice. Data are represented as mean±standard deviation. ***P<0.001; ****P<0.0001.
Next, we performed HSCT with Sca-1+ cells purified from the teratomas. GFP mRNA was detected in BM and PB cells of the recipients, and 2bopF8 in the PBC DNA (Figure 3D and Online Supplementary Figure S6C and D). Approximately 45.4% of PB cells were from iPSC-derived HSPC in the transplanted recipient mice. PBC counts returned to normal eight weeks after transplantation (Online Supplementary Figure S10C). The FVIII:C in platelets fluctuated between 0.5-1.0 mU/108 platelets throughout the entire study period (Figure 3E). FVIII release on activation of platelets was demonstrated (Online Supplementary Figure S7C). No FVIII was found in the plasma and MNC, and no FVIII inhibitor in the plasma of first recipients (Online Supplementary Figures S8B and S9C and D). We then performed secondary HSCT. The FVIII:C in platelets of the secondary recipients was maintained between 0.5-1.0 mU/108 platelets (Figure 3F). Releasing of FVIII from activated platelets was also demonstrated (Online Supplementary Figure S7D). PBC counts reached normal range after eight weeks (Online Supplementary Figure S10D). The results demonstrate the long-term engraftment capacity of teratoma-derived HSPC. Phenotypic correction evaluation was performed on the secondary recipients. BT is significantly lower for the recipients than HA mice (Figure 3G) while the remaining hemoglobin is significantly higher in the recipients than the HA controls (Figure 3H). WBCT of the recipients is significantly shorter than that of HA controls (Figure 3I). The results demonstrate that the bleeding tendency was ameliorated in the HSCT recipients. During the whole study period, we did not find any difference between the recipients and HA mice in terms of the morphology of lymph nodes, spleen, liver, and other organs, as well as the portions of hematopoietic lineages and LSK in BM (Online Supplementary Figure S12). The data demonstrate that long-term engraftable HSPC could come from 2bopF8-HAiPSC by way of teratoma formation, and that teratoma-derived HSPC is functional after HSCT and could serve as a reliable cell source for platelet-targeted gene therapy of HA.
In summary, we edited a HA mouse iPS cell line with a platelet-targeted FVIII expression cassette via CRISPR/Cas9. We obtained HSPC from the genome-edited iPSC through in vivo differentiation approaches. We prove that the iPSC-derived HSPC possess long-term engraftment and hematopoiesis reconstitution capacity. Hemorrhage diathesis of HA mice could be rescued by generating FVIII-releasing platelets from the iPSC-derived HSPC, providing a new potential route for platelet-targeted gene therapy of HA.
The generation of HSC with in vivo engraftability and multilineage potential from iPSC has been a long-sought goal in hematology research. Currently, HSPC derived from teratoma are not acceptable for clinical application. However, this technique still provides several advantages, such as the technical simplicity, low cost and scalability,4,5 and, most of all, it could provide a stable in vivo environment for HSPC generation without introducing any artificial interference to the HSPC. Our proof of concept work provides evidence that teratoma-derived HSPC are functional and safe in HSCT-based gene therapy. Further investigation of the teratoma-derived HSPC might provide new insights for producing clinical applicable HSPC.
Acknowledgments
The authors would like to thank Prof. Mitsujiro Osawa (Chiba University, Japan) and David A. Wilcox (Medical college of Wisconsin, USA) for providing the hHoxB4 plasmid and GPαIIb promoter, respectively. We are grateful to Yan Shen in our hospital for helping with animal procedures; to Fan Tan and Shumin Xiong for providing technical assistance in histology and morphology analysis; to Dr. Ruilin Sun for his assistance in Blastocyst injection; and to the CloudHealth (Shanghai, China) for WGS.
Footnotes
Funding: this work was supported by 111 Project (B17029), Shanghai Collaborative Innovation Program on Regenerative Medicine and Stem Cell Research (2019CXJQ01), the Chinese National Key Basic Research Project (2013CB966804), The National Natural Science Fund of China (81170531), Zhejiang Provincial Natural Science Foundation of China (LY17H080004), Novo Nordisk Hemophilia Research Fund in China (NN-SGTMRF-2013 and 2019), and National Science and Technology Major Project (2018ZX09101001).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Blau HM, Daley GQ. Stem cells in the treatment of Disease. N Engl J Med. 2019;380(18):1748–1760. [DOI] [PubMed] [Google Scholar]
- 2.Sugimura R, Jha DK, Han A, et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017; 545(7655):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan YT, Ye L, Xie F, et al. Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc Natl Acad Sci USA. 2018;115(9):2180–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amabile G, Welner RS, Nombela-Arrieta C, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121(8):1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki N, Yamazaki S, Yamaguchi T, et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013;21(7):1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukada M, Ota Y, Wilkinson AC, et al. In Vivo Generation of Engraftable Murine Hematopoietic Stem Cells by Gfi1b, c-Fos, and Gata2 Overexpression within Teratoma. Stem Cell Reports. 2017;9(4):1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouw SC, van der Bom JG, Marijke van den Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007; 109(11):4648–4654. [DOI] [PubMed] [Google Scholar]
- 8.Ingerslev J, Sorensen B. Parallel use of by-passing agents in haemophilia with inhibitors: a critical review. Br J Haematol. 2011; 155(2):256–262. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox DA, Olsen JC, Ishizawa L, Griffith M, White GC., 2nd Integrin alphaIIb promoter-targeted expression of gene products in megakaryocytes derived from retrovirus-transduced human hematopoietic cells. Proc Natl Acad Sci USA. 1999;96(17):9654–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarovoi HV, Kufrin D, Eslin DE, et al. Factor VIII ectopically expressed in platelets: efficacy in hemophilia A treatment. Blood. 2003;102(12):4006–4013. [DOI] [PubMed] [Google Scholar]
- 11.Shi Q, Wilcox DA, Fahs SA, et al. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116(7):1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345(6201):1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CW, Lai YS, Westin E, et al. Modeling human severe combined immunodeficiency and correction by CRISPR/Cas9-enhanced gene targeting. Cell Rep. 2015;12(10):1668–1677. [DOI] [PubMed] [Google Scholar]
- 15.Hockemeyer D, Jaenisch R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell. 2016;18(5):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]