Abstract
The treatment or prevention of bleeding in patients with hemophilia A relies on replacement therapy with different factor VIII (FVIII)-containing products or on the use of by-passing agents, i.e., activated prothrombin complex concentrates or recombinant activated factor VII. Emerging approaches include the use of bispecific anti-factor IXa/factor X antibodies, anti-tissue factor pathway inhibitor antibodies, interfering RNA to antithrombin, and activated protein C-specific serpins or gene therapy. The latter strategies are, however, hampered by the short clinical experience and potential adverse effects including the absence of tight temporal and spatial control of coagulation and the risk of uncontrolled insertional mutagenesis. Systemic delivery of mRNA allows endogenous production of the corresponding encoded protein. Thus, injection of erythropoietin-encoding mRNA in a lipid nanoparticle formulation resulted in increased erythropoiesis in mice and macaques. Here, we demonstrate that a single injection of in vitro transcribed B domain-deleted FVIII-encoding mRNA to FVIII-deficient mice enables endogenous production of pro-coagulant FVIII. Circulating FVIII:C levels above 5% of normal levels were maintained for up to 72 h, with an estimated half-life of FVIII production of 17.9 h, and corrected the bleeding phenotype in a tail clipping assay. The endogenously produced FVIII did however exhibit low specific activity and induced a potent neutralizing IgG response upon repeated administration of the mRNA. Our results suggest that the administration of mRNA is a plausible strategy for the endogenous production of proteins characterized by poor translational efficacy. The use of alternative mRNA delivery systems and improved FVIII-encoding mRNA should foster the production of functional molecules and reduce their immunogenicity.
Introduction
Hemophilia A is a rare X-linked hemorrhagic disorder that results from insufficient plasma levels of pro-coagulant factor VIII (FVIII).1 Replacement therapy using exogenous FVIII is to date the most efficient strategy to treat or prevent bleeds. It is however extremely expensive because of the elevated production costs, the short half-life of therapeutic FVIII and the need for life-long treatment. Several alternative strategies to correct bleeding include the use of FVIII by-passing agents, such as activated prothrombin complex concentrates, recombinant factor VIIa or monoclonal FVIII-mimicking bispecific antibodies,2 the injection of anti-tissue factor pathway inhibitor,3 of interfering RNA to antithrombin (AT)4 or of activated protein C-specific serpins,5 and gene therapy.6 Each of these promising therapies does, however, have intrinsic challenges that may limit broad application.
In vivo production of proteins following the administration of mRNA was demonstrated in the early 1990s in the case of luciferase and β-galactosidase,7 leading to the first clinical trial with mRNA a decade later.8 Concomitantly, both double- and single-stranded RNA were found to trigger innate immunity upon ligation of TLR3, 7 and 8, and RIG-1.9–12 The replacement of uridines by 1-methylpseudouridines and the removal of double-stranded RNA by high performance liquid chromatography was demonstrated to abrogate the activation of innate immune cells,13,14 and allowed the in vivo production of different proteins, including erythropoietin, factor IX and anti-human immunodeficiency virus antibodies without the induction of overt neutralizing immune responses.15–19 Conversely, the administration of synthetic mRNA was also used in vaccination strategies either by direct injection20 or upon adoptive transfer of ex vivo-transfected dendritic cells.21,22
Because of its monogenic nature and the requirements for low amounts of FVIII to correct the bleeding phenotype of affected patients, hemophilia A is a particularly suitable disease for treatment with mRNA. Furthermore, transfection with mRNA is by essence not integrative, and thus avoids risks of uncontrolled insertional mutagenesis that may occur with DNA-based gene therapy approaches. In addition, mRNA is translated only transiently and is degraded by physiological pathways, thus ensuring its safety and facilitating the control of the bioavailability of the encoded protein. Here, we investigated whether the intravenous administration of FVIII-encoding mRNA enables the production of therapeutic levels of pro-coagulant FVIII in FVIII-deficient mice.
Methods
Cloning of factor VIII
The cDNA encoding human B domain-deleted (BDD) FVIII (FVIIIHSQ), containing the 14-amino acid segment SFSQNPPVLKRHQR in place of the B domain, cloned in the ReNeo mammalian expression plasmid with geneticin resistance, has been described previously.23 Codon optimization of the DNA sequence encoding human BDD-FVIII was adapted to the bias of Homo sapiens using in-house proprietary software (GeneOptimizer) from GeneArt (Thermo Fisher, Darmstadt, Germany). The GeneOptimizer software also calculates removal of cis-acting sequence motifs, including internal TATA-boxes, chi-sites and ribosomal entry sites, AT- or GC-rich sequence stretches, AU-rich elements, inhibitory and cis-acting repressor sequence elements, repeat sequences, RNA secondary structures, and all cryptic splice sites. The codon-optimized BDD-FVIII-encoding cDNA was also cloned in the ReNeo vector.
In vitro transcription of mRNA
mRNA were transcribed as previously described15 using the linearized plasmids encoding BDD-FVIII (FVIIIHSQ), the codon-optimized BDD-FVIII (CoFVIIIHSQ) and firefly luciferase (Luciferase). The Megascript T7 RNA polymerase kit (Thermo Fisher) was used for transcription, and UTP was replaced with 1-methylpseudouridine triphosphate (m1ΨTP; TriLink, San Diego, CA, USA) to generate m1Ψ-containing mRNA. All mRNA were transcribed to contain 100-nucleotide long poly(A) tails. To obtain cap1, RNA was incubated with guanylyltransferase and 2′-O-methyltransferase (Vaccinia capping system; New England Biolabs, Frankfurt, Germany). All mRNA were purified and stored at −20°C.
In vitro transfection
For transient in vitro production of FVIII, baby hamster kidney (BHK) cells (0.5×106 cells in 48-well plates) were transfected with FVIIIHSQ or CoFVIIIHSQ cloned in the ReNeo vector (0.1 μg) using lipofectamine (Invitrogen, Carlsbad, CA, USA). For in vitro transfection using mRNA, mRNA (0.4 μg) was mixed with TransIT®-mRNA reagent (0.45 μL, Mirus Bio, Madison, WI, USA) and Boost reagent (0.29 μL) in a final volume of 50 μL of Dulbecco modified Eagle medium (DMEM) for 2 min at room temperature. HEK293 cells (50,000 cells/130 μL) were incubated with the formulated mRNA overnight in DMEM-F12 (Thermo Fisher). FVIII was measured in the supernatant after 24 h. Supernatant was kept frozen at −80°C until use.
Treatment of mice
Mice were 8- to 12-week old F8 exon 16 knockout C57BL/6 mice (a kind gift from Prof H.H. Kazazian, Department of Genetics, University of Pennsylvania School of Medicine, Philadelphia, PA, USA). Mice were injected intravenously with recombinant BDD-FVIII (rFVIII, Refacto®, Pfizer, 150 IU/kg), or with mRNA (1 to 5 μg) formulated in TransIT® (100 to 350 μL final volume). Blood was collected from the retro-orbital sinus 6, 24, 48, 72 or 120 h following the injection of mRNA. Plasma was kept frozen at −80°C until use. Animals were handled in agreement with local ethical authorities (approval by Charles Darwin ethics committee, authorization #3335 2015121718044892). FVIII:Ag, FVIII:C, anti-FVIII IgG and FVIII inhibitors were measured as described in the Online Supplementary Methods.
Results
Codon-optimization of cDNA encoding factor VIIIHSQ improves in vitro factor VIII production
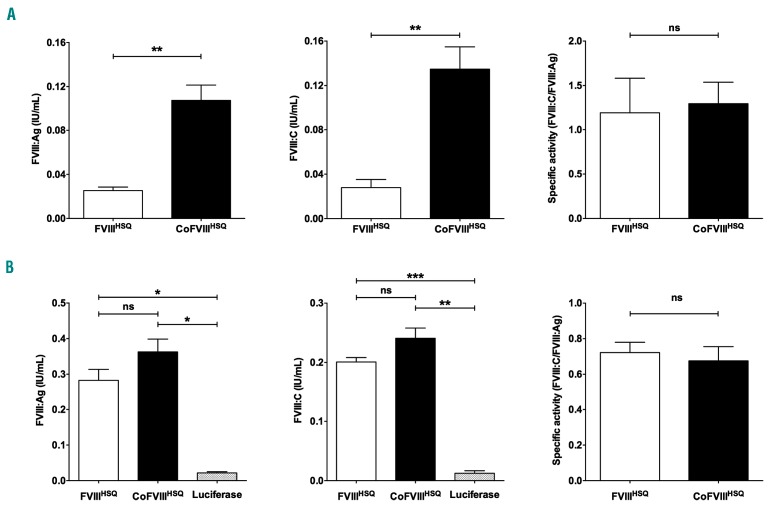
We first investigated whether codon-optimization of FVIIIHSQ improves the production of FVIII by BHK transfected cells. To this end, CoFVIIIHSQ was synthesized and inserted in the Reneo vector. BHK cells were transiently transfected with 0.1 μg FVIIIHSQ or CoFVIIIHSQ-encoding cDNA (Figure 1A). FVIII:Ag and FVIII:C were measured in the supernatant 24 h later by enzyme-linked immunosorbent assay (ELISA) and chromogenic assay. Transfection with CoFVIIIHSQ-encoding cDNA produced 4.2-fold more FVIII:Ag (mean ± standard error of mean: 0.11±0.01 IU/mL vs. 0.03±0.00 IU/mL, respectively; P<0.01) and 4.8-fold more FVIII:C (0.14±0.02 IU/mL vs. 0.03±0.01 IU/mL, respectively; P<0.01), than transfection with the non-optimized FVIIIHSQ-encoding cDNA. Our data confirm previous findings obtained upon gene therapy in preclinical models of hemophilia A,24,25 on the capacity of codon optimization to increase the yields of FVIII production.
Figure 1.
In vitro production of wildtype and codon-optimized factor VIII. (A, B) DNA in lipofectamine (0.1 μg) (A) or mRNA formulated in TransIT® (0.4 μg) (B) encoding B domain-deleted (BDD) factor VIII (FVIIIHSQ) and codon-optimized BDD-FVIII (CoFVIIIHSQ) were used to transfect BHK (A) or HEK293 (B) cells. mRNA encoding luciferase was used as a control (B). FVIII:Ag (left panels) and FVIII:C (middle panels) were measured in cell supernatant 24 h after transfection. The right panels show the specific activities as calculated by dividing the FVIII:C values by the FVIII:Ag values. Statistical differences were assessed using a two-tailed t test (ns: non-significant, *P<0.05, **P<0.01, ***P<0.001). Results are presented as the mean ± standard error of mean of three independent experiments.
Transfection with factor VIII-encoding mRNA leads to factor VIII production in vitro
We then validated the capacity of mRNA transcribed in vitro, using the FVIIIHSQ and CoFVIIIHSQ-encoding cDNA as templates, to promote FVIII production. As the codon-optimization is for Homo sapiens, mRNA encoding FVIIIHSQ or CoFVIIIHSQ formulated in TransIT® was used to transfect human HEK293 cells (Figure 1B). As a negative control, HEK293 cells were transfected with luciferase-encoding mRNA. Transfection with the two FVIII-encoding mRNA led to the in vitro production of similar amounts of FVIII (FVIII:Ag: 0.28±0.03 IU/mL vs. 0.36±0.04 IU/mL for FVIIIHSQ and CoFVIIIHSQ, respectively) and similar activities (FVIII:C: 0.20±0.01 IU/mL vs. 0.24±0.02 IU/mL, respectively). Accordingly, the specific activity of FVIII produced using both mRNA did not differ (0.72±0.06 and 0.68±0.08, respectively), suggesting that codon optimization has no beneficial effect on the production of FVIII when cells are transfected with mRNA, in our experimental system.
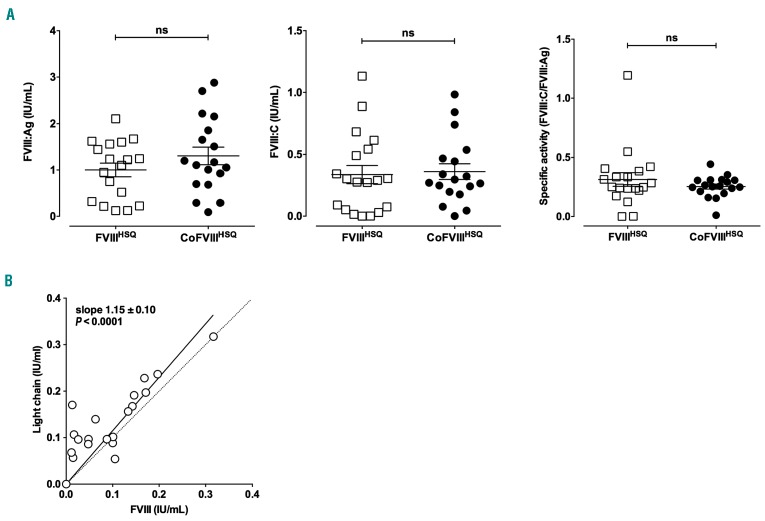
Systemic delivery of factor VIII-encoding mRNA to factor VIII-deficient mice leads to the endogenous production of factor VIII
FVIII-deficient mice were injected intravenously with 1 μg FVIIIHSQ- or CoFVIIIHSQ-encoding mRNA formulated in TransIT® (Figure 2A). Plasma levels of FVIII:Ag and FVIII:C, measured after 24 h, were 1.00±0.15 IU/mL and 0.34±0.07 IU/mL in the case of FVIIIHSQ-encoding mRNA, and 1.30±0.19 IU/mL and 0.36±0.06 IU/mL in the case of CoFVIIIHSQ-encoding mRNA. Likewise, the specific activities of FVIIIHSQ and CoFVIIIHSQ (defined as the ratios of FVIII:C over FVIII:Ag) did not differ significantly: 0.31±0.06 and 0.25±0.02, respectively, but were more than 2-fold lower than specific activities measured in vitro (Figure 1B). To investigate for the presence of non-functional FVIII molecules, we measured plasma levels of FVIII light chain using a dedicated ELISA. Levels of FVIII:Ag and light chain were perfectly correlated (slope=1.15±0.10; P<0.0001) (Figure 2B). However, 14 of the 20 tested samples showed >10% more FVIII light chain than total FVIII, indicating that some FVIII in the plasma samples lacked its A2 domain (A2 domain dissociation is a feature of FVIII inactivation26).
Figure 2.
Delivery of factor VIII-encoding mRNA allows endogenous production of factor VIII. mRNA encoding B domain-deleted (BDD) factor VIII (FVIIIHSQ) or codon-optimized BDD-FVIII (CoFVIIIHSQ) was formulated in TransIT®. FVIII-deficient mice were then injected intravenously with 1 µg FVIII-encoding mRNA. (A) FVIII:Ag and FVIII:C were measured in plasma after 24 h. Individual symbols on the graphs represent individual mice; horizontal bars represent means ± standard error of mean. Statistical differences were assessed using a two-tailed t test (ns: non-significant). (B) FVIII:Ag and FVIII light chain were measured in plasma by enzyme-linked immunosorbent assay. Individual symbols on the graphs represent individual mice. The full line curve represents the linear regression of the experimental data. The dotted line represents the theoretic correlation with a slope of 1.
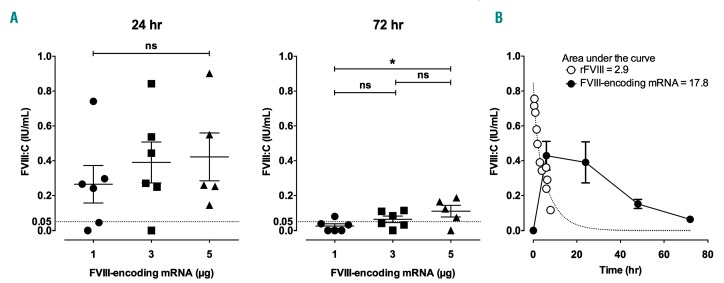
The endogenous production of FVIII was then followed over 72 h after the injection of 1, 3 or 5 µg of CoFVIIIHSQ-encoding mRNA. At 24 h, the levels of FVIII:C reached in the circulation ranged from 0.27±0.11 to 0.42±0.14 IU/mL (Figure 3A, left panel). The effect of the dose of injected mRNA on the level of endogenously produced FVIII was statistically significant at 72 h (Figure 3A, right panel). Interestingly, the mean residual FVIII activity 72 h after the injection of 3 and 5 μg of mRNA, was above 5% of the theoretical FVIII:C in normal plasma (0.06±0.02 IU/mL and 0.11±0.03 IU/mL, respectively). We then estimated the total amount of FVIII:C produced over 72 h following injection of FVIII-encoding mRNA. To this end, mice were injected intravenously with either 3 IU of human rFVIII or 3 μg of CoFVIIIHSQ-encoding mRNA formulated in TransIT® (Figure 3B). The areas under the curves depicting the changes in FVIII:C plasma levels over time were 2.9 and 17.8 IU/mL x h, respectively, showing that the injection of 3 μg of mRNA allows the endogenous production over a period of 72 h of amounts of FVIII:C 6-fold greater than the amount of rFVIII injected at once. The half-life of rFVIII in the circulation was fitted using a two-phase decay equation: short and long half-lives of 0.9 and 4.7 h were calculated. An apparent half-life for the production of FVIII of 17.9 h was calculated by fitting the FVIII:C levels measured at 24, 48 and 72 h with a one-phase decay equation.
Figure 3.
Time-dependent production of endogenous factor VIII after a single injection of factor VIII-encoding mRNA. mRNA encoding codon-optimized B domain-deleted (BDD) factor VIII (CoFVIIIHSQ) was formulated in TransIT®. (A) FVIII-deficient mice were then injected intravenously with 1, 3 or 5 μg FVIII-encoding mRNA. FVIII:C was measured in plasma after 24 and 72 h. Individual symbols on the graphs represent individual mice; horizontal bars represent means ± standard error of mean. The dotted line indicates the critical FVIII level (i.e., 5%) required to drastically reduce joint bleeds. Statistical differences were assessed using a two-tailed t test (ns: non-significant, *P<0.05). (B) Mice were injected with 3 μg CoFVIIIHSQ-encoding mRNA formulated in TransIT®, or with 100 μL of 10 nM recombinant BDD-FVIII (3 IU rFVIII). FVIII:C in plasma was measured after 30, 60, 90, 120, 180, 240, 360, 390 and 480 min in the case of rFVIII, in the case of mRNA. The dotted line depicts the non-linear fit (two-phase exponential decay: y=0.62*e-0.15x+0.2211*e-0.80x) of the experimental data obtained with rFVIII. The full circles and full line curve represent means ± standard error of mean of six mice treated with mRNA (representative of two independent experiments; fitted with a one-phase exponential decay for the values obtained at 24, 48 and 72 h: y=0.99*e-0.03874x). Areas under the curves were calculated using Prism GraphPad (version 6).
Correction of the bleeding phenotype of factor VIII-deficient mice by the endogenously produced factor VIII
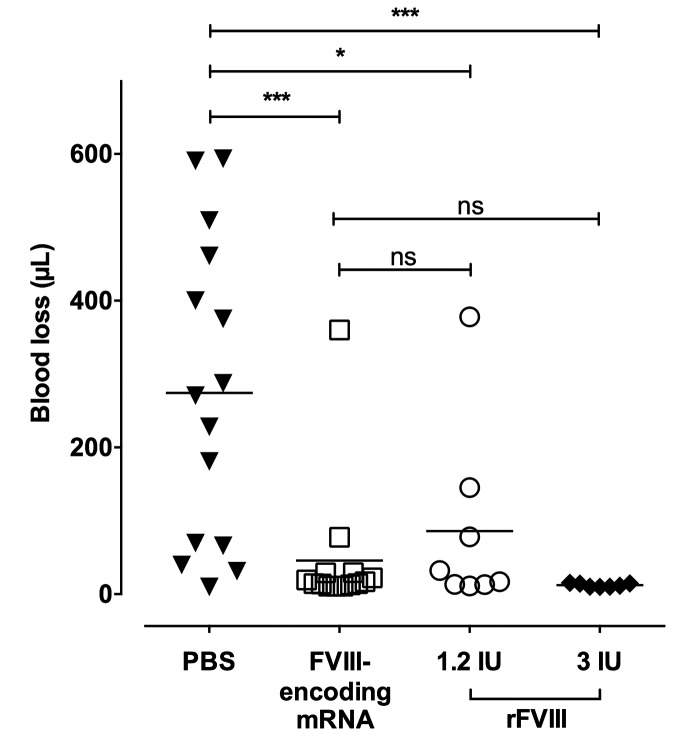
We then investigated the effect of a single injection of FVIII-encoding mRNA on the bleeding phenotype of FVIII-deficient mice. FVIII-deficient mice were injected with phosphate-buffered saline (PBS), with FVIII-encoding mRNA or with 1.2 or 3 IU rFVIII (Figure 4). The tip of the mouse tail was clipped after 30 min in the case of rFVIII or after 24 h in the case of PBS or mRNA, which correspond to the activity peak of each individual treatment. Blood loss was followed over 20 min. While the PBS-injected mice lost 274.3±53.4 μL of blood, injection of rFVIII protected the mice from major bleeding with blood loss of 86.0±44.9 μL and 12.2±0.9 μL for 1.2 IU (P<0.05) and 3 IU (P<0.001) rFVIII, respectively. Interestingly, mice injected with FVIII-encoding mRNA lost 45.8±24.6 μL of blood (P<0.001 as compared to PBS-treated mice), showing correction of the bleeding phenotype.
Figure 4.
Injection of factor VIII-encoding mRNA corrects acute bleeding in factor VIII-deficient mice. Factor VIII (FVIII)-deficient mice were injected intravenously with 3 μg of FVIII-encoding mRNA (empty squares) formulated in TransIT®, with 1.2 IU (empty circles) or 3 IU (full diamonds) recombinant B domain-deleted-FVIII (rFVIII) or with phosphate-buffered saline (PBS) (full triangles). Mice tails were clipped 3 mm from the tip, 24 h after mRNA and PBS injection, or 30 min after rFVIII injection. Bleeding intensity was measured as the volume of blood lost during 20 min. Horizontal bars represent means and individual dots represent individual mice. Statistical differences were assessed using the double-sided Mann-Whitney test (ns: non-significant; *P<0.05; ***P<0.001).
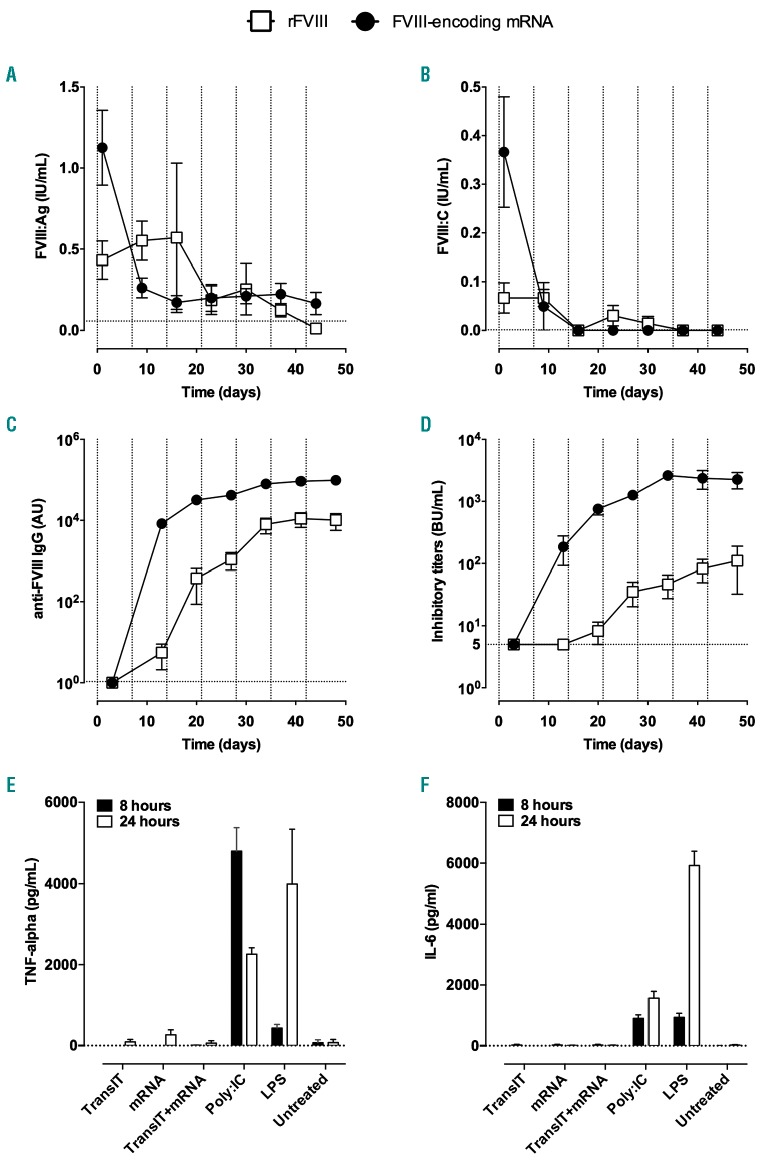
Multiple injections of factor VIII-encoding mRNA trigger an anti-factor VIII immune response
The repeated administration of rFVIII is known to trigger the production of inhibitory anti-FVIII IgG in mice after 3-5 injections.27 We thus assessed the effect of multiple injections of FVIII-encoding mRNA on the onset of an anti-FVIII immune response. We treated FVIII-deficient mice with 1 μg FVIII-encoding mRNA once a week for 7 weeks. As a control, FVIII-deficient mice were injected once a week with 5 IU rFVIII. First, we measured residual plasma FVIII levels 24 h after rFVIII or mRNA injection (Figure 5A, B). Consistent with the short half-life of FVIII in mice, very low FVIII:Ag and FVIII:C were measured in plasma 24 h following injection of rFVIII during the first 3 weeks of treatment. In the case of mRNA-treated mice, a sharp decrease in both FVIII:Ag and FVIII:C was observed between the first (1.13±0.23 IU/mL and 0.37±0.11 IU/mL, respectively) and second (0.26±0.06 IU/mL and 0.05±0.05 IU/mL, respectively) injections. From the third week of treatment onwards, FVIII:C levels were below the level of detection.
Figure 5.
Induction of an anti-factor VIII immune response after repeated injections of factor VIII-encoding mRNA. (A-D) Factor VIII (FVIII)-deficient mice were injected intravenously with 1 μg of coFVIIIHSQ-encoding mRNA formulated in TransIT® (full circles), or with 5 IU recombinant BDD-FVIII (empty squares), once a week for 7 weeks. FVIII:Ag (A) and FVIII:C (B) were measured in plasma 24 h after each injection. Anti-FVIII IgG (C) and inhibitory titers (D) were measured in plasma 72 h after each injection. Results are depicted as the mean ± standard error of mean (SEM) for four to seven mice per group. The vertical dotted lines represent the days of FVIII/mRNA administration. The horizontal dotted lines represent the background levels (calculated as “mean + 1 x standard deviation”) measured in six or seven FVIII-deficient mice (A: 0.045+0.013 IU/mL; B: 0.001±0.000 IU/mL; C: 0.84±0.23 μg/mL); or the threshold for detection (D: 5 BU/mL). (E and F) Five-day old immature monocyte-derived dendritic cells were incubated alone (Untreated) or in the presence of Poly:IC, lipopolysaccharide (LPS), TransIT® alone, 1 μg FVIII-encoding mRNA (mRNA) alone or 1 μg FVIII-encoding mRNA formulated in TransIT®. The panels show the levels of tumor necrosis factor-α (E) and interleukin-6 (F) measured in the culture supernatant 8 h (full bars) and 24 h (empty bars) later. Data are presented as the mean ± standard error of mean from two independent experiments. Differences between TransIT, mRNA, TransIT+mRNA and Untreated were not statistically significant (two-sided Mann-Whitney test).
We then investigated the presence of inhibitory anti-FVIII IgG 5 days after each administration of mRNA or rFVIII to the mice (Figure 5C, D). As described previously, the intravenous administration of rFVIII induced a progressive increase in levels of anti-FVIII IgG which reached a plateau after five injections (10168±4501 AU after 7 injections) (Figure 5C). This was mirrored by a gradual increase in levels of FVIII inhibitors, which crossed 10 BU/mL 5 days after the fourth injection to plateau at 111±79 BU/mL (Figure 5D). In the case of FVIII-encoding mRNA, levels of anti-FVIII IgG as high as those obtained after five injections of rFVIII were detected as early as 5 days following the second treatment. The plateau after 7 weeks of treatment (98060±8251 AU) was 10-fold higher in the case of mRNA than in the case of rFVIII. Likewise, while no FVIII inhibitory activity was detected 5 days after the first injection of mRNA, levels of 383±196 BU/mL were generated 5 days after the second injection, and gradually increased to reach a plateau at 2258±669 BU/mL after 7 weeks of treatment. The plateau of FVIII inhibitors was thus 20-fold higher in mice treated with mRNA than in mice treated with rFVIII. Of note, the incubation of FVIII-encoding mRNA, TransIT® or TransIT®-formulated mRNA with immature human monocyte-derived dendritic cells failed to induce the production of tumor necrosis factor-α and interleukin-6 (Figure 5E, F), indicating that mRNA and TransIT® do not activate innate immune cells and are not responsible for triggering the anti-FVIII immune response. In additional experiments, we investigated the site of production of mRNA-encoded proteins following formulation in TransIT®. To this end, Balb/c mice were injected with luciferase-encoding mRNA formulated in TransIT®. The luminescence measured 24 h later was at least 2-fold greater in the spleen than in the liver (Online Supplementary Figure S1), suggesting that the liver and hepatocytes are not the main target for TransIT®-formulated mRNA. Attempts to detect FVIII in FVIII-encoding mRNA-treated FVIII-deficient mice led to occasional FVIII signals in the marginal zone of the spleen and constant absence of FVIII detection in the liver (data not shown).
Discussion
The present work documents the sustained endogenous production of pro-coagulant FVIII following the injection of FVIII-encoding mRNA into FVIII-deficient mice, and is of potential relevance for the improvement of treatment for patients with hemophilia A. FVIII levels achieved 24 h after the injection of mRNA were about 40% of the levels in normal plasma. The levels of FVIII expression are notoriously low because of poor transcriptional and translation efficacies27–29 as well as retention of the protein in the endoplasmic reticulum.30,31 Attempts to improve the levels of expression of FVIII include partial removal of the B domain with conservation of essential N-glycosylation sites,24,31 mutation of an immunoglobulin binding-protein (BiP) binding site32 to ensure improved transfer from the endoplasmic reticulum to the Golgi apparatus,33 and codon optimization. Here, mRNA was generated using wildtype23 or codon-optimized cDNA encoding human BDD-FVIII. While codon optimization improved FVIII production following transfection of eukaryotic cells with plasmid DNA in vitro, it did not increase the levels of FVIII:C or FVIII:Ag following in vitro mRNA transfection of cells or in vivo transfection of FVIII-deficient mice. Codon optimization aims at improving translation rates by using codons for which the cognate tRNA levels are not limiting. Thus, codon optimization of FVIII-encoding cDNA cloned in lentiviral vectors or adenoviral-associated vectors led to more than 10-fold increased FVIII levels after in vitro transfection of cell lines and after injection into wildtype or FVIII-deficient mice.24,25 The lack of improvement in protein production associated with the administration of codon-optimized mRNA encoding FVIII suggests that codon optimization of FVIII preferentially targets transcriptional rather than translational events, as previously shown.34,35 Alternatively, levels of mRNA introduced into each cell upon in vivo transfection with TransIT® may be much lower than that transcribed endogenously following transfection using DNA, and insufficient to exhaust non-abundant tRNA. Increasing the amount of mRNA injected in vivo did not, however, have drastic effects on the FVIII levels reached in the circulation.
Frequent spontaneous joint and muscle bleeds in patients with severe hemophilia A eventually lead to the development of arthropathy and functional joint impairment. An association between joint bleeds and baseline FVIII activity levels was demonstrated,36 with FVIII levels above 5% of the normal values drastically reducing the occurrence of joint bleeds. Because the half-life of human FVIII in patients with hemophilia A is between 12 and 18 h, prophylactic replacement therapy is the gold standard to maintain healthy joint function.37 In FVIII-deficient mice, the half-life of human FVIII is between 4 and 6 h. Interestingly, delivery of FVIII-encoding mRNA yielded FVIII levels that corrected acute bleeding in a tail clipping assay, and were maintained above 5% for up to 72 h. The longer residence time of FVIII produced after mRNA delivery as compared to that of rFVIII results from the cumulative lifespans of the transfected mRNA in the cells and of the FVIII released in the circulation. mRNA-based therapy can be improved by engineering the encoded protein to extend its half-life.38 For instance, a FVIII molecule with an increased half-life has been developed by fusion of FVIII to the Fc fragment of human IgG1.48 We anticipate that the use of mRNA encoding long-lasting FVIII glycoproteins will allow a further increase in the duration of FVIII detection in vivo. Because peak expression levels of FVIII were reached in the mice between 6 and 24 h after the administration of mRNA, mRNA-based therapy may represent a surrogate for prophylactic treatment rather than for the on-demand treatment of sudden acute hemorrhagic events.
The specific activity of the rFVIII produced by eukaryotic cells in vitro was about 1.2 following transfection using cDNA and about 0.7 following transfection with mRNA. In contrast, the specific activity of the FVIII produced endogenously following injection of mRNA to the mice was about 0.3, suggesting the absence of pro-coagulant activity for a substantial proportion of the endogenously produced FVIII molecules. In parallel, we compared the levels of intact FVIII measured using a standard ELISA, wherein FVIII is captured with an anti-light chain antibody and detected with anti-heavy chain antibody, with that of FVIII light chain measured using a sandwich light chain-specific ELISA. A perfect correlation was obtained although with an overall tendency for 2-fold more light chains than intact molecules. Importantly, the levels of light chain may be under-estimated because of the short in vivo half-life of the light chain alone. Future experiments will indicate whether the use of mRNA encoding FVIII lacking the furin cleavage site39,40 between the heavy and light chains, or FVIII mutants with increased FVIII A2 subunit stability41 yields FVIII with improved specific activity. An alternative and non-exclusive explanation for the poor specific activity of the endogenously produced FVIII is the fact that the TransIT® used to formulate the mRNA does not target particular cell types. In fact, we show here that luciferase is produced by the spleen, rather than by the liver, following injection of mice with luciferase-encoding mRNA formulated with TransIT®. Accordingly, immuno-fluorescence experiments on mice treated with FVIII-encoding mRNA occasionally detected faint amounts of FVIII in the marginal zone of the spleen but never in the liver (not shown). Hence, TransIT® delivers FVIII mRNA to cells that are not dedicated to the production of FVIII,42,43 thus potentially generating FVIII molecules that are improperly folded or have undergone incorrect post-translational modifications.
In patients with severe hemophilia A, inhibitory anti-FVIII IgG (FVIII inhibitors) generally develop within the first 20 cumulated days of exposure to therapeutic FVIII.44 In the present work, and in agreement with previous studies in FVIII-deficient mice,45 FVIII-binding IgG and FVIII inhibitors were detected after two and three intravenous injections of rFVIII, respectively. The levels of anti-FVIII IgG increased to reach a plateau at 10- to 100-fold higher levels after two to three additional injections of rFVIII. In the case of mRNA administration, however, close to maximal levels of FVIII-binding IgG and inhibitory antibodies were reached after only two injections of FVIII-encoding mRNA. Accordingly, endogenous FVIII:C was undetectable as early as after the second administration of mRNA. Different explanations for such a brisk and intense immune response to the endogenously produced FVIII may be envisaged. Because the mRNA used in our study was engineered to contain 1-methylpseudouridines and was purified by high performance liquid chromatography to remove double-stranded RNA, thus abrogating mRNA recognition by TLR3, TLR7, TLR8 and RIG-I,9–12 it is not probable that the very mRNA plays an adjuvant role in the onset of the anti-FVIII immune response. Indeed, FVIII-encoding mRNA, alone or formulated in TransIT®, failed to activate human immature monocyte-derived dendritic cells in vitro. Interestingly, previous work on in vivo mRNA transfection without apparent induction of neutralizing immune responses used TransIT®15 or other types of nanoparticles.16,18 Most studies were however performed in immunocompromised animals46 or in animals expressing the corresponding endogenous protein (e.g., the absence of induction of an anti-erythropoietin immune response in animals receiving erythropoietin-encoding mRNA15,17 probably relates to the presence of the endogenous erythropoieitin molecule and associated ongoing active immune tolerance).
In fact, the very formulation of mRNA in TransIT® may be a reason for induction of a strong anti-FVIII immune response. TransIT® was initially conceived for in vitro and not in vivo gene transfection. As explained above, protein production following injection of TransIT®-formulated mRNA is not targeted to the liver or endothelial cells, which produce FVIII under physiological conditions. This is reminiscent of the early works on gene therapy for hemophilia wherein the use of promoters with poor specificity for hepatocytes was associated with the induction of neutralizing anti-FVIII or anti-factor IX antibodies.47
Several lines of evidence suggest that the FVIII produced may itself be responsible for the sharp anti-FVIII immune response. As explained above, FVIII is a particularly immunogenic glycoprotein: e.g., a fusion protein between the light chain of FVIII and the first domain of hemagglutinin 1 (HA1) demonstrated greater anti-HA1 immunogenicity upon intravenous injection to FVIII-deficient mice than the HA1 molecule alone.48 In contrast, the production of factor IX was induced in mice following administration of mRNA without report of a neutralizing immune response.18 Of note, a relationship between the dose of FVIII injected and the kinetics of detection of the anti-FVIII IgG response was reported in mice45 and in patients.49 This is particularly relevant in view of the fact that the amount of active FVIII produced over 72 h after one injection of FVIII-encoding mRNA was equivalent to 6-fold the amount of rFVIII administered in a single injection. Furthermore, based on the poor specific activity of the endogenously produced FVIII, the total amount of FVIII molecules (active and inactive) probably exceeds 18-fold that of injected rFVIII. Further work will indicate whether targeting mRNA delivery to hepatocytes or to endothelial cells using improved lipid nanoparticle-based formulating agents,50 using mRNA encoding single chain or A2 variant stable FVIII39,40,41 and including miRNA target sequences to prevent off-target expression in hematopoietic cells51 are plausible strategies to improve the specific activity and reduce the immunogenicity of the endogenously produced molecule.
Acknowledgment
We thank Dr Katalin Karikó (BioNTech RNA Pharmaceuticals, Mainz, Germany) for her constant support regarding this project and for providing the IVT mRNA. This study was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Sorbonne Université, and by grants from CSL-Behring (Paris, France), ANR (Exfiltrin project: ANR-18-CE17-0010-02) and CEFIPRA (IFC/7126/ Hemophilia/1353). JR was the recipient of a fellowship from Ministère de l’Enseignement Supérieur et de la Recherche. We also thank the staff from the Center of Histology, Cell Imaging and Flow Cytometry (CHIC) and Centre d’Expérimentation Fonctionnelle for assistance (Centre de Recherche des Cordeliers, Paris).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/4/1129
References
- 1.Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–1779. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818. [DOI] [PubMed] [Google Scholar]
- 3.Chowdary P, Lethagen S, Friedrich U, et al. Safety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trial. J Thromb Haemost. 2015;13(5):743–754. [DOI] [PubMed] [Google Scholar]
- 4.Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med. 2017;377(9):819–828. [DOI] [PubMed] [Google Scholar]
- 5.Polderdijk SG, Adams TE, Ivanciu L, et al. Design and characterization of an APC-specific serpin for the treatment of hemophilia. Blood. 2017;129(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathwani AC, Davidoff AM, Tuddenham EGD. Advances in gene therapy for hemophilia. Hum Gene Ther. 2017;28(11): 1004–1012. [DOI] [PubMed] [Google Scholar]
- 7.Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. [DOI] [PubMed] [Google Scholar]
- 8.Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. [DOI] [PubMed] [Google Scholar]
- 10.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. [DOI] [PubMed] [Google Scholar]
- 11.Hornung V, Ellegast J, Kim S, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–997. [DOI] [PubMed] [Google Scholar]
- 12.Schlee M, Roth A, Hornung V, et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. [DOI] [PubMed] [Google Scholar]
- 14.Kariko K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39(21):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kariko K, Muramatsu H, Keller JM, Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol Ther. 2012;20(5): 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Luo X, Deng B, et al. An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Lett. 2015;15(12):8099–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thess A, Grund S, Mui BL, et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol Ther. 2015;23(9):1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramaswamy S, Tonnu N, Tachikawa K, et al. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc Natl Acad Sci U S A. 2017;114(10):E1941–E1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richner JM, Himansu S, Dowd KA, et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168(6):1114–1125e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63(9):2127–2133. [PubMed] [Google Scholar]
- 22.Kyte JA, Kvalheim G, Lislerud K, et al. T cell responses in melanoma patients after vaccination with tumor-mRNA transfected dendritic cells. Cancer Immunol Immunother. 2007;56(5):659–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healey JF, Barrow RT, Tamim HM, et al. Residues Glu2181-Val2243 contain a major determinant of the inhibitory epitope in the C2 domain of human factor VIII. Blood. 1998;92(10):3701–3709. [PubMed] [Google Scholar]
- 24.McIntosh J, Lenting PJ, Rosales C, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121(17):3335–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward NJ, Buckley SM, Waddington SN, et al. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117(3):798–807. [DOI] [PubMed] [Google Scholar]
- 26.Pipe SW, Eickhorst AN, McKinley SH, Saenko EL, Kaufman RJ. Mild hemophilia A caused by increased rate of factor VIII A2 subunit dissociation: evidence for nonproteolytic inactivation of factor VIIIa in vivo. Blood. 1999;93(1):176–183. [PubMed] [Google Scholar]
- 27.Kaufman RJ, Wasley LC, Davies MV, et al. Effect of von Willebrand factor coexpression on the synthesis and secretion of factor VIII in Chinese hamster ovary cells. Mol Cell Biol. 1989;9(3):1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch CM, Israel DI, Kaufman RJ, Miller AD. Sequences in the coding region of clotting factor VIII act as dominant inhibitors of RNA accumulation and protein production. Hum Gene Ther. 1993;4(3):259–272. [DOI] [PubMed] [Google Scholar]
- 29.Hoeben RC, Fallaux FJ, Cramer SJ, et al. Expression of the blood-clotting factor-VIII cDNA is repressed by a transcriptional silencer located in its coding region. Blood. 1995;85(9):2447–2454. [PubMed] [Google Scholar]
- 30.Dorner AJ, Bole DG, Kaufman RJ. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987;105(6 Pt 1):2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao HZ, Sirachainan N, Palmer L, et al. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103(9): 3412–3419. [DOI] [PubMed] [Google Scholar]
- 32.Swaroop M, Moussalli M, Pipe S, Kaufman R. Mutagenesis of a potential immunoglobulin-binding protein-binding site enhances secretion of coagulation factor VIII. J Biol Chem. 1997;272:24121–24124. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, McGee B, Yamaoka JS, et al. Combined deficiency of factor V and factor VIII is due to mutations in either LMAN1 or MCFD2. Blood. 2006;107(5):1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, Dang Y, Zhou M, et al. Codon usage is an important determinant of gene expression levels largely through its effects on transcription. Proc Natl Acad Sci U S A. 2016;113(41):E6117–E6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudla G, Lipinski L, Caffin F, Helwak A, Zylicz M. High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 2006;4(6):e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.den Uijl IE, Fischer K, Van Der Bom JG, et al. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44. [DOI] [PubMed] [Google Scholar]
- 37.Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125(13):2038–2044. [DOI] [PubMed] [Google Scholar]
- 38.Farelli JD, Asrani KH, Isaacs C, et al. Leveraging rational protein engineering to improve mRNA therapeutics. Nucleic Acid Ther. 2018;28(2):74–85. [DOI] [PubMed] [Google Scholar]
- 39.Siner JI, Samelson-Jones BJ, Crudele JM, et al. Circumventing furin enhances factor VIII biological activity and ameliorates bleeding phenotypes in hemophilia models. JCI Insight. 2016;1(16):e89371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen GN, George LA, Siner JI, et al. Novel factor VIII variants with a modified furin cleavage site improve the efficacy of gene therapy for hemophilia A. J Thromb Haemost. 2017;15(1):110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monaghan M, Wakabayashi H, Griffiths A, Wintermute J, Fay PJ. Enhanced factor VIIIa stability of A2 domain interface variants results from an increased apparent affinity for the A2 subunit. Results from an increased apparent affinity for the A2 subunit. Thromb Haemost. 2014;112(3):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahani T, Lavend’homme R, Luttun A, et al. Activation of human endothelial cells from specific vascular beds induces the release of a FVIII storage pool. Blood. 2010;115(23):4902–4909. [DOI] [PubMed] [Google Scholar]
- 43.Pan J, Dinh TT, Rajaraman A, et al. Patterns of expression of factor VIII and von Willebrand factor by endothelial cell subsets in vivo. Blood. 2016;128(1):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046–4055. [DOI] [PubMed] [Google Scholar]
- 45.Reipert BM, Ahmad RU, Turecek PL, et al. Characterization of antibodies induced by human factor VIII in a murine knockout model of hemophilia A. Thromb Haemost. 2000;84(5):826–832. [PubMed] [Google Scholar]
- 46.Pardi N, Secreto AJ, Shan X, et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun. 2017;8:14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VandenDriessche T, Collen D, Chuah MK. Gene therapy for the hemophilias. J Thromb Haemost. 2003;1(7):1550–1558. [DOI] [PubMed] [Google Scholar]
- 48.Ing M, Gupta N, Teyssandier M, et al. Immunogenicity of long-lasting recombinant factor VIII products. Cell Immunol. 2016;301:40–48. [DOI] [PubMed] [Google Scholar]
- 49.Gouw SC, van den Berg HM, le Cessie S, van der Bom JG. Treatment characteristics and the risk of inhibitor development: a multicenter cohort study among previously untreated patients with severe hemophilia A. J Thromb Haemost. 2007;5(7):1383–1390. [DOI] [PubMed] [Google Scholar]
- 50.Dahlman JE, Barnes C, Khan O, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9(8):648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui H, Hegadorn C, Ozelo M, et al. A microRNA-regulated and GP64-pseudotyped lentiviral vector mediates stable expression of FVIII in a murine model of hemophilia A. Mol Ther. 2011;19(4):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]