Abstract
We compared severe graft-versus-host-disease (GvHD) free and relapse-free survival and other transplantation outcomes of acute myeloid leukemia (AML) patients given bone marrow (BM) without anti-thymocyte globulin (ATG) versus peripheral blood stem cells (PBSC) with ATG after myeloablative conditioning. In the cohort of patients receiving grafts from a human leukocyte antigen (HLA)-matched sibling donor, patients given PBSC with ATG (n=1,021) and those given BM without ATG (n=1,633) presented comparable severe GvHD-free relapse-free survival (GRSF)(hazard ratio [HR]=0.9, 95% confidence interval [CI]: 0.8-1.1, P=0.5) and overall survival (HR=1.0, 95% CI: 0.8-1.2, P=0.8). They had however, a lower incidence of chronic GvHD (cGvHD) (HR=0.7, 95% CI: 0.6-0.9, P=0.01). In the cohort of patients receiving grafts from HLA-matched unrelated donor , patients given PBSC with ATG (n=2,318) had better severe GvHD-free and relapse-free survival (GRFS) than those given BM without ATG (n=303) (HR=0.8, 95% CI: 0.6-0.9, P=0.001). They also had a lower incidence of cGvHD (HR=0.6, 95% CI: 0.5-0.8, P=0.0006) and better overall survival (HR=0.8, 95% CI: 0.6-1.0, P=0.04). In summary, these data suggest that PBSC with ATG results in comparable (in the case of sibling donor) or significantly better (in the case of unrelated donor) severe GRFS than BM without ATG in patients with AML in complete remission receiving grafts after myeloablative conditioning.
Introduction
A number of phase III trials have compared peripheral blood stem cells (PBSC) with bone marrow (BM) as the stem cell source for allogeneic hematopoietic cell transplantation in patients given grafts after myeloablative regimens.1,2 A meta-analysis of trials performed in patients given grafts from human leukocyte antigen (HLA)-matched sibling donors (MSD) revealed that the use PBSC of instead of BM was associated with a higher incidence of grade III-IV acute graft-versus-host disease (aGvHD) and (extensive) cGvHD, as well as a lower incidence of relapse (IR) and better overall survival (OS) in the subgroup of patients with late-stage disease.3 Further, the only phase III trial performed in patients given grafts from HLA-matched unrelated donors (MUD) showed that patients randomized to the BM group had a lower incidence of cGvHD, similar OS, and better patient-reported outcomes at 5 years after transplantation than those randomized to the PBSC arm.4,5 This might suggest that, at least in the MUD setting, BM may be preferred over PBSC as a stem cell source in patients receiving grafts after myeloablative regimens. In concordance with these findings, a recent European Group for Blood and Marrow Transplantation (EBMT) study observed a better GvHD-free relapse-free survival (GRFS) with BM than with peripheral blood (PB) as the stem cell source.6
In the last two decades, five phase III trials have assessed the impact of anti-thymocyte globulins (ATG) on transplantation outcomes in patients given grafts (mainly PBSC) from MSD (n=1)7 or MUD (n=4).8–11 These five trials demonstrated a lower incidence of cGvHD with ATG.12 Two of three trials assessing the impact of ATG on GRFS13 observed better GRFS in patients randomized to ATG,7,10 while the study by Soiffer et al. failed to demonstrate this effect.11 ATG was also found to improve GRFS in a registry study including data from patients given grafts from HLA-identical siblings after myeloablative fludarabine + busulfan regimens.13
These findings prompted us to compare transplantation outcomes in AML patients following BMT without ATG versus PBSC transplantation with ATG in the first or second complete remission (CR).
Methods
Ethics
The scientific board of the Acute Leukaemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) approved this research project. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines.
Inclusion/exclusion criteria and study endpoints
Inclusion criteria included adult patients, de novo or secondary AML, myeloablative conditioning, MSD or 10/10 HLA-matched unrelated donors (MUD), first or second CR, first allo-transplantation from 2000 to 2017 at an EBMT-affiliated center, and either BM without ATG, or PBSC with ATG. Exclusion criteria consisted of in vitro T-cell depletion of the graft, and prior administration of alemtuzumab. The primary endpoint was GRFS. Secondary endpoints included grade II-IV GvHD, RI, non-relapse mortality (NRM), leukemia-free survival (LFS) and OS.
Statistical analyses
Myeloablative conditioning was defined as use of ≥ 6 Gy TBI, busulfan > 8 mg/kg, or melphalan > 140 mg/m2 as previously reported.14 The cytogenetic AML risk was determined as previously reported.15,16
NRM was defined as death without prior or current disease recurrence. Events in the composite endpoint GRFS included grade III-IV aGvHD, extensive cGvHD, relapse or death as previously reported.17
OS, LFS and GRFS were estimated using the Kaplan-Meier estimates. Cumulative incidence functions (CIF) were used for RI and NRM in a competing risk setting, since death and relapse are competing together. In estimating the cumulative incidence of aGvHD and cGvHD, we considered relapse and death to be competing events. Univariate analyses were done using Gray’s test for CIF and the log-rank test for GRFS, OS and LFS. Since there were some imbalances between the groups we performed multivariate Cox models to further determine the impact of the graft type on transplantation outcome. These Cox models were adjusted for disease status at transplantation, female donor to male recipient or not, CMV serostatus, use or not of total body irradiation (TBI), year of transplantation, age at transplantation, primary versus secondary AML, and cytogenetic risk. In order to take into account the center effect, we introduced a frailty for each center into the model, as previously reported.18,19 Specifically, we introduced a random effect (also named frailty effect) in the Cox multivariate models. Then, the same random effect was shared by all patients within the same center. In order to assess whether there was a statistical interaction between the year of transplantation and the association between BMT without ATG versus PBSC with ATG and the incidence of relapse, we introduced the interaction term between cell source and year as a binary variable (with the median year by group as the cut-off) in the Cox multivariate model for relapse. Results are presented as hazard ratios (HR) and 95% confidence intervals (95% CI). The HR for year of transplantation corresponded to a 5-year increase and to a 10-year increase for the age at transplantation. As planned in the synopsis, the analyses were done separately for MSD and MUD. The interaction between graft type (i.e. BM without ATG or PBSC with ATG) and donor type (MSD versus MUD) was confirmed for several outcomes (data not shown).
Results
BM without ATG versus PBSC with ATG in MSD recipients
Patients
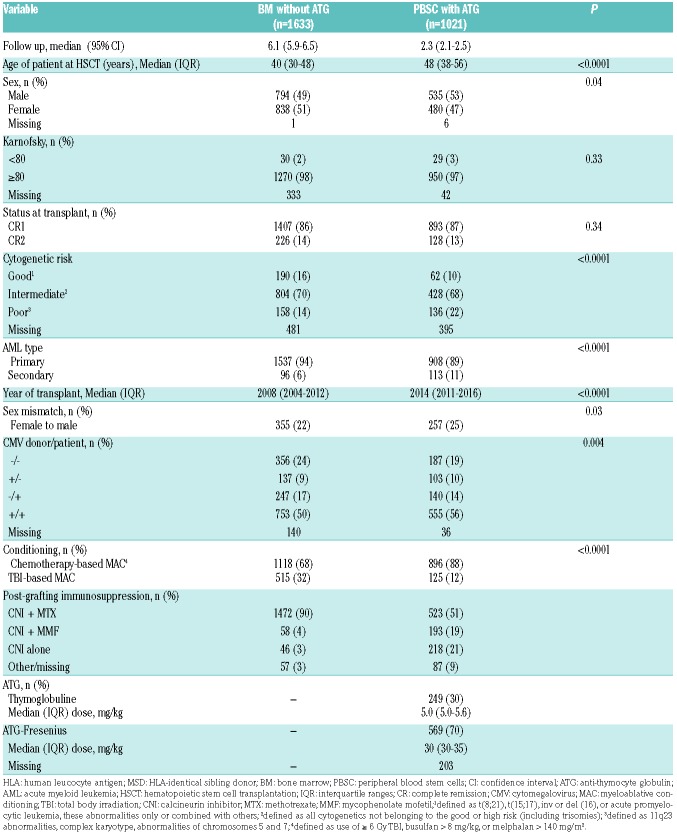
A total of 2,654 MSD recipients met the inclusion/exclusion criteria, comprising 1,633 BM without ATG patients and 1,021 PBSC with ATG patients (Table 1). In comparison to BM without ATG recipients, PBSC with ATG patients were older (median 48 years vs. 40 years old), were transplanted more recently (median year of transplantation 2014 vs. 2008), less likely to have a good cytogenetic risk (10% vs. 16%), more likely to have secondary AML (11% vs. 6%), less frequently received TBI in the conditioning regimen (12% vs. 32%), and were more frequently male patients receiving grafts from female donors (25% vs. 22%).
Table 1.
Patient characteristics among human leucocyte antigen-identical sibling donor (MSD) recipients
GvHD
The 100-day incidence of grade 2-4 aGvHD was 27% (95% CI: 25-29%) in BM without ATG recipients versus 18% (95% CI: 16-20%) in PBSC with ATG patients (P<0.001). For grades III-IV, the figures were 9% (95% CI: 8-11%) and 6% (95% CI: 4-7%), respectively (P=0.002). In multivariate analyses, the difference for grade II-IV aGvHD between PBSC with ATG versus BM without ATG was no longer statistically significant (HR=0.8, 95% CI: 0.7-1.1, P=0.12) (Table 2) while the use of TBI in the conditioning was associated with a higher incidence of grade II-IV aGvHD (HR=1.4, 95% C1: 1.1-1.8, P=0.004). In contrast, a more recent transplantation year was associated with a lower incidence of grade II-IV aGvHD (HR=0.8, 95% CI: 0.7-0.9, P=0.0004).
Table 2.
Outcomes (multivariate Cox models).
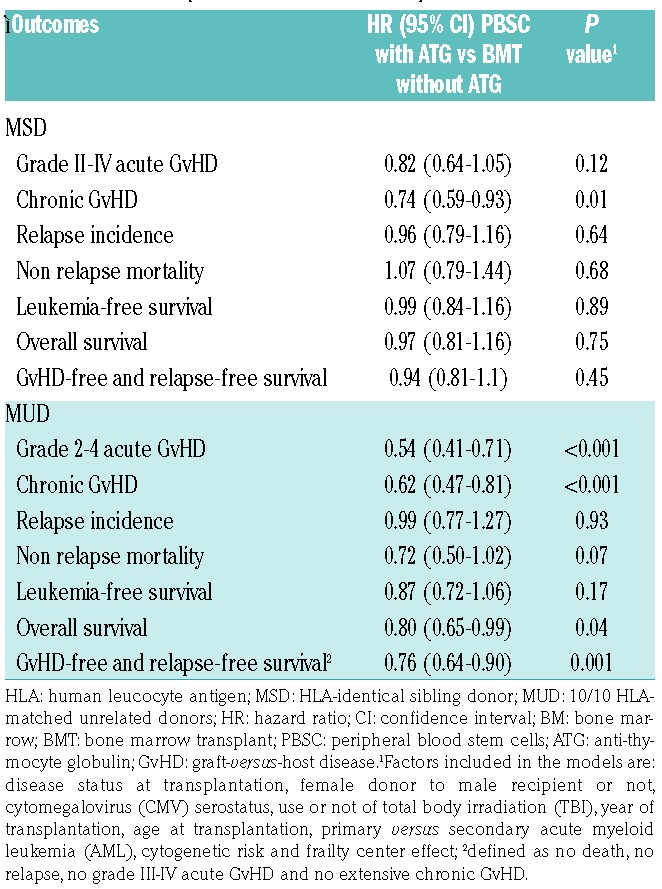
At 2 years the CI of cGvHD was 34% (95% CI: 31-36%) in BM without ATG recipients versus 30% (95% CI: 27-33%) in PBSC with ATG patients (P=0.02) (Figure 1E). This association remained statistically significant on multivariate analysis with a lower risk of cGvHD among PBSC with ATG recipients (HR=0.7, 95% CI: 0.6-0.9, P=0.01). In contrast, female donor to male recipient (HR=1.5, 95% C1: 1.3-1.7, P<0.001), and older age at transplantation (HR=1.07, 95% C1: 1.0-1.14, P=0.049) were associated with a higher incidence of cGvHD.
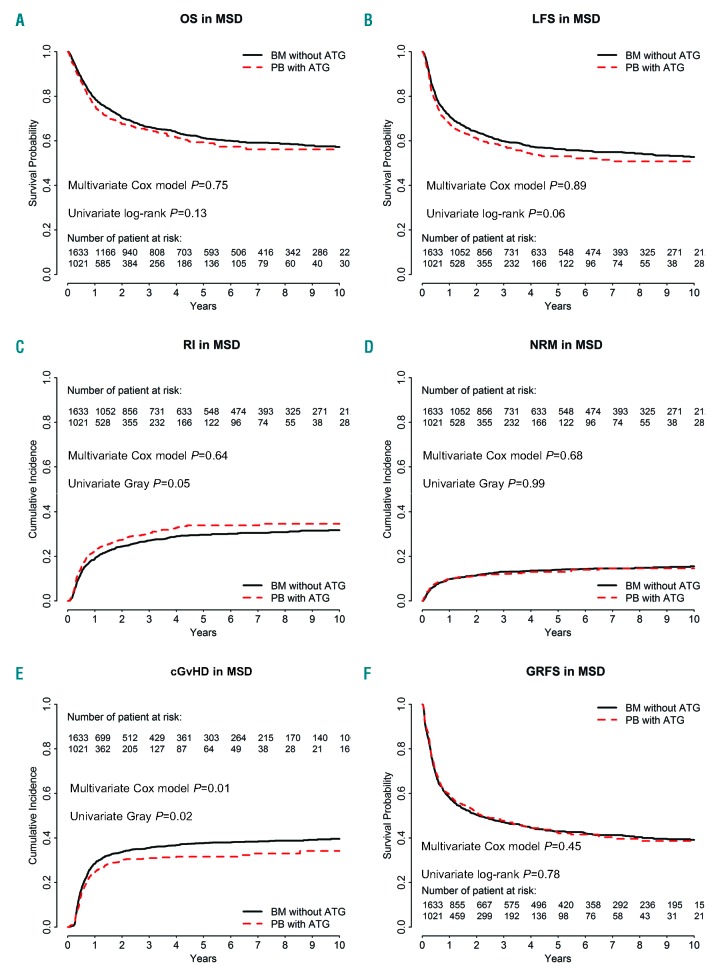
Figure 1.
Comparison of outcomes in bone marrow (BM) patients without anti-thymocyte globulin (ATG) and in those given peripheral blood (PB) stem cells with ATG (the dotted line shows the PBSC with ATG curve adjusted for relevant covariates) in the cohort of patients receiving grafts from HLA-identical sibling donor (MSD). (A) Overall survival (OS), P=0.13. (B) Leukemia-free survival (LFS), P=0.065. (C) Incidence of relapse (RI), P=0.0496. (D) Non-relapse mortality (NRM), P=0.989. (E) chronic graft-versus-host disease (cGvHD), P=0.0186. (F) GvHD-free and relapse-free survival (GRFS), P=0.782.
Relapse and non-relapse mortality
The 2-year CI of relapse was 25% (95% CI: 22-27%) in BM without ATG recipients versus 28% (95% CI: 25-31%) in PBSC with ATG patients (P=0.0496) (Figure 1C). After adjusting for potential confounding factors by multivariate analyses there was no significant impact of the graft type on relapse (HR=1.0, 95% CI: 0.8-1.2, P=0.6). In contrast, a more recent year of transplantation (HR=1.1, 95% C1: 1.0-1.2, P=0.02) was associated with a higher risk of relapse. There was no interaction between the association of graft type and the risk of relapse and year of transplantation (interaction test P=0.38). Unadjusted comparison of relapse incidence between the two groups per 6-year periods is shown in the Online Supplementary Table S2. We further investigated whether there was a statistical interaction between the use of TBI in the conditioning regimen and the impact of graft type on relapse risk. Among patients given graft from MSD, there was no such interaction (P-value for the interaction test = 0.97).
The 2-year CI of NRM was 11.5% in both groups (Figure 1D). In multivariate analyses, PBSC with ATG was associated with a similar risk of NRM to BM (HR=1.1, 95% CI: 0.8-1.4, P=0.7). In contrast, older age at transplantation (HR=1.5, 95% CI: 1.4-1.7, P<0.001) and secondary versus de novo AML (HR=1.5, 95% CI: 1.0-2.1, P=0.03) were associated with a higher NRM while a more recent year of transplantation was associated with a lower NRM (HR=0.7, 95% C1: 0.6-0.8, P<0.001).
OS and LFS
The 2-year OS and LFS were 70% (95% CI: 68-73%) and 64% (95% CI: 62-66%) in BMT without ATG patients versus 68% (95% CI: 64-71%, P=0.1) and 61% (95% CI: 58-64%, P=0.06) in PBSC with ATG patients, respectively (Figure 1A-B). After adjusting for potential confounding factors by multivariate analyses, the use of PBSC with ATG was no different to BM with respect to OS (HR=1.0, 95% CI: 0.8-1.2, P=0.8) and LFS (HR=1.0, 95% CI: 0.8-1.2, P=0.9). In contrast, second CR at transplantation (HR=1.3, 95% CI: 1.0-1.5, P=0.03), and older patient age (HR=1.2, 95% CI: 1.1-1.3, P<0.001) were associated with worse OS. The same factors were also significantly associated with worse LFS: second CR at transplantation (HR=1.2, 95% CI: 1.0-1.5; P=0.04), and older patient age (HR=1.2, 95% CI: 1.1-1.2, P<0.001).
GRFS
At 2 years, GRFS was 50% (95% CI: 48-53%) in BMT without ATG patients versus 52% (95% CI: 48-55%) in the PBSC with ATG group (P=0.8) (Figure 1F). This was confirmed on multivariate analysis including data from all patients (HR=0.9, 95% CI: 0.8-1.1, P=0.5) as well as in further sensitivity analysis restricted to patients given a combination of a calcineurin inhibitor and methotrexate as GvHD prophylaxis (HR=1.0, 95% CI: 0.8-1.2, P=0.9). In contrast, female donor to male recipient (HR=1.2, 95% CI: 1.0-1.3, P=0.01), and older patient age (HR=1.1, 95% CI: 1.1-1.1, P<0.001) were associated with a worse GRFS.
BM without ATG versus PBSC with ATG in MUD recipients
Patients
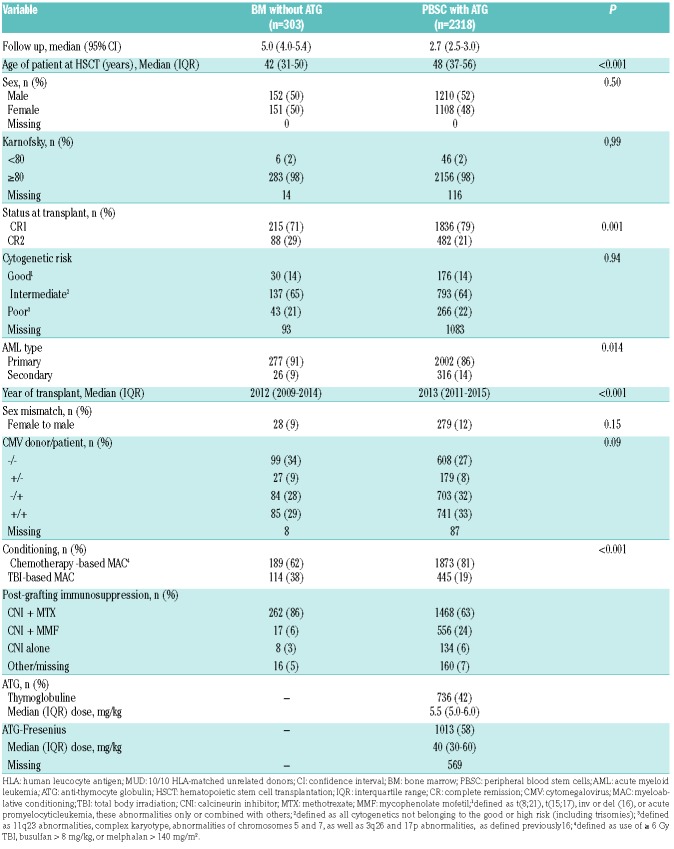
A total of 2,621 recipients met the inclusion/exclusion criteria. Comprising 303 patients in the BM group and 2,318 in the PBSC with ATG group (Table 3). In comparison to BM recipients, PBSC with ATG patients were older (median 48 years vs. 42 years old), were transplanted more recently (median year of transplantation 2013 versus 2012) and were more frequently transplanted in the first CR (79% vs. 71%).
Table 3.
Patient characteristics among 10/10 human leucocyte antigen-matched unrelated donor recipients.
GvHD
The 100-day incidence of grade II-IV aGvHD was 44% in BMT without ATG recipients vs. 24% in PBSC with ATG recipients (P<0.0001). For grade 3-4, the figures were 17% (95% CI: 13-21%) and 7% (95% CI: 6-8%), respectively (P<0.001). In multivariate analysis, PBSC with ATG patients had a significantly lower incidence of grade II-IV aGvHD than BM recipients (HR=0.5, 95% CI: 0.4-0.7; P<0.001). No other factor was significantly associated with grade II-IV aGvHD in multivariate analysis (Online Supplementary Table S4).
The 2-year CI of cGvHD was 43% (95% CI: 37-49%) in BM without ATG recipients versus 30% (95% CI: 28-32%) in PBSC with ATG patients (P<0.0001) (Figure 2E). On multivariate analysis, PBSC with ATG, in comparison to BM without ATG, was associated with a lower incidence of cGvHD (HR=0.6, 95% CI: 0.5-0.8, P=0.0006) (Table 2), but no other factor was significantly associated with cGvHD.
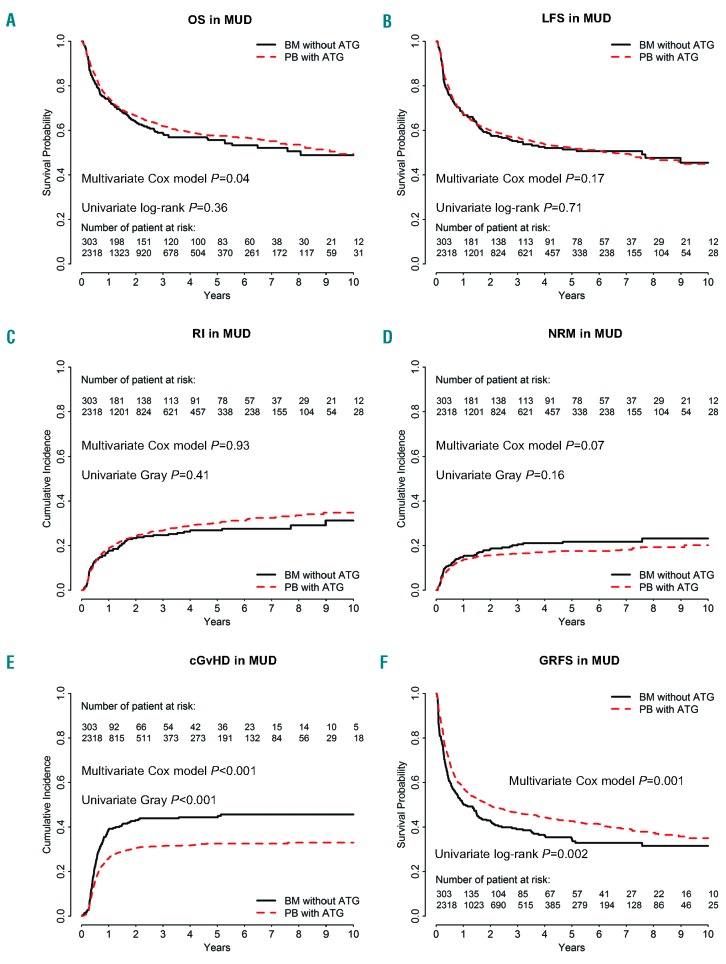
Figure 2.
Comparison of outcomes in bone marrow (BM) patients without anti-thymocyte globulin (ATG)and in those given peripheral blood (PB) stem cells with ATG (the dotted line shows the PBSC with ATG curve adjusted for relevant covariates) in the cohort of patients receiving grafts from 10/10 HLA-matched unrelated donors (MUD). (A) Overall survival (OS), P=0.36. (B) Leukemia-free survival (LFS), P=0.71. (C) Incidence of relapse (RI), P=0.41.(D) Non-relapse mortality (NRM), P=0.16. (E) Chronic graft-versus-host disease (cGvHD), P<0.001. (F) GvHD-free and relapse-free survival (GRFS), P=0.002.
Relapse and non-relapse mortality
The 2-year CI of relapse was 23% (95% CI: 19-29%) in BMT without ATG recipients versus 24% (95% CI: 23-26%) in PBSC with ATG patients (P=0.4) (Figure 2C). On multivariate analysis, as observed in the MSD cohort, there was no significant impact of the graft type on relapse (HR=1.0, 95% CI: 0.8-1.3, P=0.9), but a more recent transplantation was significantly associated with a higher risk of relapse (HR=1.2, 95% CI: 1.0-1.4, P=0.03) (Online Supplementary Table S1). There was no interaction between the association of graft type and the risk of relapse and year of transplantation (interaction test P=0.13). Unadjusted comparison of relapse incidence between the two groups per 6-year periods is shown in the Online Supplementalry Table S2. We further investigated whether there was a statistical interaction between the use of TBI in the conditioning regimen and the impact of the graft type on the relapse risk. Among patients given graft from MUD, there was such an interaction (P-value for the interaction test=0.05). Specifically, in the subgroup of patients given TBI-based conditioning we observed a lower risk of relapse in PBSC with ATG patients than in BMT without ATG patients (HR=0.56, 95% CI: 0.39-0.81, P=0.002) while this was not the case in patients given chemotherapy-based conditioning (HR=0.94, 95% CI: 0.70-1.26, P=0.68).
The 2-year CI of non-relapse mortality was 18% in BMT without ATG patients versus 16% in PBSC with ATG patients (P=0.16) (Figure 2D). On multivariate analysis, there was a non-significant trend for lower NRM in PBSC with ATG patients (HR=0.7, 95% CI: 0.7- 1.0, P=0.07). Factors associated with NRM on multivariate analysis included older age at transplantation (HR=1.4, 95% CI: 1.3-1.5, P<0.001) and secondary AML (HR=1.4, 95% CI: 1.1-1.9, P=0.01) while a more recent transplant year was associated with lower NRM (HR=0.8, 95% CI: 0.7-1.0, P=0.03).
OS and LFS
The 2-year OS and LFS were 64% (95% CI: 58-70%) and 58% (95% CI: 53-64%) in BMT without ATG patients versus 67% (95% CI: 64-69%, P=0.3) and 60% (95% CI: 58-62%, P=0.7) in PBSC with ATG patients, respectively (Figure 2A-B). After adjusting for potential confounding factors, the use of PBSC with ATG was associated with a better OS (HR=0.8, 95% CI: 0.6-1.0, P=0.04) than with BM, but there was no significant difference in LFS between the groups (HR=0.9, 95% CI: 0.7- 1.1, P=0.2). As well as being in the BMT group, older age was significantly associated with a poorer OS (HR=1.2, 95% CI: 1.1-1.3, P<0.0001) and a worse LFS (HR=1.1, 95% CI: 1.1-1.2, P<0.0001).
Causes of death were comparable in both groups expect for a higher frequency of infectious-related death in the PBSC with ATG group (23% vs. 11%) (Online Supplementary Table S3).
GRFS
The 2-year GRFS was 43% (95% CI: 37-49%) versus 50% (95% CI: 47-52%) in BMT without ATG and PBSC with ATG recipients, respectively (P=0.002) (Figure 2F). This was confirmed in multivariate analysis including data from all patients (HR=0.8, 95% CI: 0.6-0.9, P=0.001) as well as in further sensitivity analysis restricted to patients given a combination of a calcineurin inhibitor and methotrexate as GvHD prophylaxis (HR=0.8, 95% CI: 0.6-0.9, P=0.01). Finally, older patient age was also associated with worse GRFS (HR=1.1, 95% CI: 1.0-1.1, P=0.004) in the whole cohort of patients.
Discussion
Several studies have now established that the use of PBSC (without ATG) instead of BM is associated with a high incidence of (severe) cGvHD affecting the long-term well-being of the patients.2,5 In contrast, several phase III trials have demonstrated a beneficial impact of ATG on cGvHD.12 Here, we compare the outcomes of AML patients given BMT without ATG versus PBSC with ATG, in the setting of AML in first or second CR. We elected to restrict the analyses of patients given grafts after myeloablative conditioning since it was a inclusion criteria in most phase III ATG studies, although many AML patients are nowadays transplanted following reduced-toxicity regimens.20,21 Several observations can be made.
First, the use of PBSC with ATG was associated with a lower incidence of cGvHD both in the MSD and MUD datasets. This clearly demonstrates that ATG administration is able to counterbalance the high risk of cGvHD associated with the use of PBSC (instead of BMT without ATG).
Importantly, the incidence of relapse was not significantly different in BMT without ATG and in PBSC with ATG patients in both datasets. This may appear surprising given the tight association between cGvHD and graft-versus-leukemia effects, and is consistent with most phase III ATG trials showing,22–24 a reduction of cGvHD without an increase in the relapse risk.12 Similar findings were observed in the RIC setting in a large registry study.25
Given the data of the Soiffer et al. phase III study showing detrimental effects of ATG in patients given TBI-based conditioning regimen,11 we investigated whether, in our study, there was a statistical interaction between graft type and the risk of relapse and the use of TBI in the conditioning regimen. Interestingly, we observed a lower risk of relapse only in the subgroup of patients given TBI-based conditioning suggesting no adverse effects of ATG in patients given TBI-based conditioning. These data are in concordance with recent observations reported by our group.26
The main endpoint of our retrospective study was GRFS, a relatively new composite endpoint which aims at capturing the rate of cure without ongoing transplant-related morbidity.27 We observed a non-significant difference in GRFS with the two strategies in the cohort of patients given grafts from MSD, and a significantly better GRFS with PBSC with ATG in the cohort of patients given grafts from MUD. We thus advocate that our data might support the use of PBSC with ATG rather than BMT without ATG, in AML patients in CR at transplantation.
An interesting finding of our study was an increasing relapse incidence (but decreasing non relapse mortality incidence) with more recent transplantations. This might be due to a higher proportion at high risk of relapse (as example more patient with persistent minimal residual disease at transplantation) in more recent years of transplantation. Unfortunately, we do not have data on the MRD status at transplantation for many patients included in this survey.
There are some limitations of the study such as its design (it is however unlikely that a prospective random-ized phase III trial will address this question in the near future), the lack of data on mutational AML landscape and minimal residual disease, a high proportion of missing cytogenetic data, the lack of ATG dose data for several patients, and some imbalances between the groups. However these imbalances were adjusted for in the multivariate Cox models. The strengths of the study are the number of patients in each group and their relative uniformity (one single disease, all patients in first or second CR at transplantation, no HLA-mismatches, only myeloablative conditioning regimen).
In conclusion, our data suggest that PBSC transplantation with ATG results in comparable (in case of MSD) or significantly better (in case of MUD) GRFS than BMT without ATG in patients with AML in CR receiving grafts after myeloablative conditioning. These data might support the use of PBSC with ATG compared to BMT without ATG in patients receiving grafts from MSD or MUD after myeloablative conditioning as treatment for AML in CR.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/4/1138
References
- 1.Blaise D, Kuentz M, Fortanier C, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the SociÇtÇ Franáaise de Greffe de Moelle. J Clin Oncol. 2000;18(3):537–571. [DOI] [PubMed] [Google Scholar]
- 2.Flowers MED, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002; 100(2):415–419. [DOI] [PubMed] [Google Scholar]
- 3.Stem Cell Trialists’ Collaborative G Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23(22):5074–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SJ, Logan B, Westervelt P, et al. Comparison of patient-reported outcomes in 5-Year survivors who received bone marrow vs peripheral blood unrelated donor transplantation: long-term follow-up of a randomized clinical trial. JAMA Oncol. 2016;2(12):1583–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battipaglia G, Ruggeri A, Labopin M, et al. Refined graft-versus-host disease/relapse-free survival in transplant from HLA-identical related or unrelated donors in acute myeloid leukemia. Bone Marrow Transplant. 2018;53(10):1295–1303. [DOI] [PubMed] [Google Scholar]
- 7.Kroger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43–53. [DOI] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12(5):560–565. [DOI] [PubMed] [Google Scholar]
- 9.Walker I, Schultz KR, Toze CL, et al. Thymoglobulin decreases the need for immunosuppression at 12 months after myeloablative and nonmyeloablative unrelated donor transplantation: CBMTG 0801, a randomized, controlled trial. Blood. 2014; 124(21):38. [Google Scholar]
- 10.Finke J, Schmoor C, Bethge WA, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017; 4(6):e293–e301. [DOI] [PubMed] [Google Scholar]
- 11.Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, randomized, double-blind, Phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35(36):4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron F, Mohty M, Blaise D, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102(2):224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio MT, D’Aveni-Piney M, Labopin M, et al. Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine iv-busulfan myeloablative regimen: a report from the EBMT Acute Leukemia Working Party. J Hematol Oncol. 2017;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron F, Ruggeri A, Beohou E, et al. Occurrence of graft-versus-host disease increases mortality after umbilical cord blood transplantation for acute myeloid leukemia: a report from Eurocord and the ALWP of the EBMT. J Intern Med. 2018; 283(2):178–189. [DOI] [PubMed] [Google Scholar]
- 15.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 16.Baron F, Ruggeri A, Beohou E, et al. RIC versus MAC UCBT in adults with AML: A report from Eurocord, the ALWP and the CTIWP of the EBMT. Oncotarget. 2016; 7(28):43027–43038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51(4):610–611. [DOI] [PubMed] [Google Scholar]
- 18.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999; 18(12):1489–1500. [DOI] [PubMed] [Google Scholar]
- 19.Baron F, Labopin M, Ruggeri A, et al. Impact of donor type in patients with AML given allogeneic hematopoietic cell transplantation after low-dose TBI-based regimen. Clin Cancer Res. 2018; 24(12):2794–2803. [DOI] [PubMed] [Google Scholar]
- 20.Potdar RR, Gupta S, Giebel S, et al. Current status and Perspectives of irradiation-based conditioning regimens for patients with acute leukemia undergoing hematopoietic stem cell transplantation. Clin Hematol International. 2019;1(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron F, Labopin M, Peniket A, et al. Reduced-intensity conditioning with fludarabine and busulfan versus fludarabine and melphalan for patients with acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2015;121(7):1048–1055. [DOI] [PubMed] [Google Scholar]
- 22.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED, the Seattle Marrow Transplant T Antileukemic effect of chronic graft-versus-host disease. Contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981; 304(25):1529–1533. [DOI] [PubMed] [Google Scholar]
- 23.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23(9):1993–2003. [DOI] [PubMed] [Google Scholar]
- 24.Baron F, Labopin M, Niederwieser D, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26(12):2462–2468. [DOI] [PubMed] [Google Scholar]
- 25.Baron F, Labopin M, Blaise D, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(3):389–396. [DOI] [PubMed] [Google Scholar]
- 26.Nagler A, Labopin M, Dholaria B, et al. Impact of antithymocyte globulin on outcomes of allogeneic hematopoietic cell transplantation with TBI. Blood Adv. 2019; 3(13):1950–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]