Abstract
Respiratory tract infections due to human metapneumovirus (hMPV) have been reported worldwide, with the exception of Africa. The prevalence of hMPV infection was studied among human immunodeficiency virus type 1 (HIV-1)–infected and HIV-1–uninfected African infants who were hospitalized for lower respiratory tract infections (LRTIs). Nasopharyngeal aspirate samples obtained from 81 HIV-1–infected and 110 HIV-1–uninfected infants who had tested negative for other respiratory viruses were selected for investigation. hMPV was detected in 10 HIV-1–uninfected infants (9.1%) and 3 HIV-1–infected infants (3.7%). Compared with the entire cohort of HIV-1–uninfected infants, hMPV was 4.6-fold less common than respiratory syncytial virus, but it was 3.2-fold more common than influenza virus and 2.1-fold more common than parainfluenza virus types 1–3. Genotyping of 7 of 14 isolates revealed the circulation of 2 major phylogenetic groups of the virus, which were similar to those described in North America and Europe.
Respiratory tract infections due to human metapneumovirus (hMPV), a recently described virus placed in the Paramyxoviridae family and Pneumovirinae subfamily, have been documented in Europe, North America, Australia, and Asia [1–8]. However, there are no data on the presence or importance of hMPV in Africa, where lower respiratory tract infections (LRTIs) are a leading cause of death, especially among infants (age, <12 months). Data from other continents implicate hMPV as the potential causative agent in 1.5%–10% of respiratory tract infections [2–4, 6, 7]. The clinical spectrum of illness appears to be similar to that caused by human respiratory syncytial virus (RSV) and ranges from mild rhinitis to severe bronchiolitis and pneumonitis requiring mechanical ventilation [1, 2, 8].
Although hMPV has been isolated among asymptomatic young adults who may well have had immunity due to prior infections during childhood [6], it was not isolated among 400 asymptomatic children in The Netherlands, compared with an isolation rate of ∼10% among children with respiratory tract infection in The Netherlands [1]. In developing sub-Saharan African countries, important risk factors for death due to LRTI include young age and HIV-1 infection [9]. The aim of this study was to retrospectively evaluate the prevalence of hMPV among HIV-1–infected and HIV-1–uninfected infants who had been hospitalized for severe LRTI during the autumn/winter/early spring seasons of 2000 (1 March 2000 through 30 September 2000).
Methods
The specimens analyzed in this study had been obtained from a cohort of infants who were participating in a phase 3 trial evaluating the efficacy of a nonavalent pneumococcal conjugate vaccine. Nasopharyngeal aspirate (NPA) samples were obtained from the infants at the time of hospitalization for LRTI. Full clinical details, which included obtainment of a clinical history and a physical examination, were performed by one of the study staff members. The method used for obtaining and processing the NPA samples has been described elsewhere [10]. All samples were evaluated for common respiratory viruses, including RSV, influenza viruses A and B, parainfluenza virus (PIV) types 1–3, and adenovirus, using a direct immunofluorescence assay (Respiratory Panel 1 Viral Screening and Identification Kit; Chemicon International). Of a total of 824 samples (326 obtained from HIV-1–infected infants, 486 obtained from HIV-1–uninfected infants, and 12 obtained from infants with unknown HIV-1 infection status), 196 samples were randomly selected and were stratified by HIV-1 infection status. These 824 samples were in addition to 454 samples (75, 367, and 12 obtained from HIV-1–infected infants, HIV-1–uninfected infants, and infants with unknown HIV-1 infection status, respectively) from which ⩾1 of the studied common respiratory viruses was identified. The HIV-1 infection status of the infants was determined using HIV-1 ELISAs (Axsym [Abbott] and Murex HIV 1+2 [Murex Diagnostics]) and confirmed using an HIV-1 PCR assay (HIV-1 DNA PCR, version 1.5; Roche Amplicor).
The NPA samples were aliquoted within 4 h after obtainment and were stored at -70°C until the time of processing for this study. We chose to limit the number of samples tested, as well as the period of time from which samples were selected for testing, because of the limited resources available to conduct this preliminary study, which aimed to document whether hMPV was an important pathogen among infants who are traditionally most at risk of being hospitalized for LRTI. Furthermore, we chose not to evaluate specimens that had tested positive for any of the other common respiratory viruses on the basis of the low frequency of dual-virus coinfections with hMPV and other viruses (0%–2%) observed in all but one of the other studies [1, 2, 4, 8]. The samples used were obtained during March through July, which is period when previous RSV epidemics had been described to occur in the community [11], as well as 2 months thereafter (August and September—i.e., early spring), to reflect the possibility that the seasonality of hMPV epidemics follows that of RSV epidemics.
The identification of hMPV was done by RT-PCR targeted against the fusion (F) protein of hMPV. Total RNA was extracted from stored NPAs using the High Pure RNA kit (Roche Molecular Biochemicals), as described elsewhere [11]. The hMPV was identified as described elsewhere [12]. The original RT-PCR assay was modified to a 1-step RT-PCR using the Titan One Tube RT-PCR system (Roche Molecular Biochemicals). In brief, 10 μL of total RNA was added to a 40-μL reaction mixture, in accordance with manufacturer's instructions. The reverse transcription procedure was performed at 50°C for 30 min, and PCR was performed as described elsewhere [12]. The resulting amplicons were purified from agarose gels using the Gene Clean kit (Bio 101). Both strands were sequenced using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems) with the ABI 310 automated sequencer (PE Applied Biosystems). The partial nucleotide sequences of the F gene were aligned using CLUSTAL × 1.81, with the published F nucleotide sequence obtained from The Netherlands (isolate HMPV001; GenBank accession no. AF371337). All samples were tested at the Respiratory and Meningeal Pathogens Research Unit in Johannesburg, South Africa.
Data were entered and analyzed using SAS (SAS Institute) and Epi Info, version 6.04d (Centers for Disease Control and Prevention), statistical software packages [13, 14]. Mean values were compared using Student's t test, and proportions were compared using the χ2 and Fisher's exact tests, as appropriate. ORs and 95% CIs were computed using Epi Info, version 6.04d.
Results
A total of 196 samples (81 samples obtained from HIV-1–infected infants, 110 obtained from HIV-1–uninfected infants, and 5 obtained from infants with unknown HIV-1 infection status) were analyzed. The mean age (°SD) of infants with hMPV-associated LRTI (6.0 ° 2.4 months) was similar to that of infants from whom hMPV was not isolated (5.2 ° 2.8 months; P = .29). Furthermore, there was no difference observed in the mean age of HIV-1–infected and HIV-1–uninfected infants from whom hMPV was isolated (5.9 ° 2.8 vs. 6.1 ° 0.2 months; P = .40).
Overall, hMPV was identified in 14 (7.1%) of the 196 infants, including 10 cases (9.1%) and 3 cases (3.7%) identified among HIV-1–uninfected and HIV-1–infected infants, respectively (P = .24). One additional case was detected among the 5 infants for whom the HIV-1 infection status had not been determined. There was a slight predominance of male sex (8 [57.1%] of 14) among infants with hMPV infection.
Because there were no clinical differences observed between HIV-1–infected and HIV-1–uninfected infants, possibly as a result of the limited number of infants from whom hMPV was isolated, the clinical features of all infants were analyzed together. The key clinical features of the individual infants are shown in table 1. The median duration of symptoms before hospitalization was 4.5 days (range, 1–30 days); the median oxygen saturation in room air, as measured by pulse oximetry, at the time of hospitalization was 92% (range, 84%–97%); and the mean axillary temperature (°SD) was 37.0°C ° 0.7°C. Seven (50%) of 14 infants had wheezing, and 8 (57.1%) of 14 infants had rales on auscultation of the chest. A clinical diagnosis of bronchiolitis was made for 7 (50%) of the 14 infants, and pneumonia was diagnosed for 9 infants, including 2 infants who also had bronchiolitis.
Table 1.
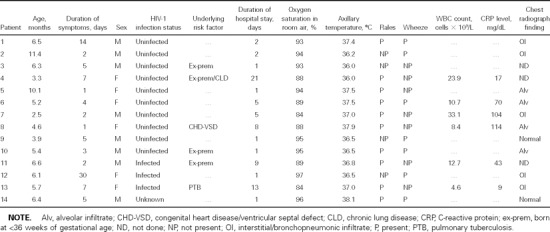
Clinical characteristics of African infants with human metapneumovirus–associated severe lower respiratory tract infection.
WBC counts and C-reactive protein levels at the time of hospitalization were determined for only 6 (42.9%) of 14 infants. The median C-reactive protein level was 56.5 mg/dL (range, 9.0–114 mg/dL; normal level, <3.0 mg/dL), and the median WBC count was 11.7 × 109 cells/L (range, 4.6–33.1 × 109 cells/L). The median duration of hospital stay was 2.0 days (range, 1–21 days). Although it was longer among HIV-1–infected infants (median, 9.0 days; range, 1–13 days), the duration of hospitalization did not differ significantly from that for HIV-1–uninfected infants (median, 2.0 days; range, 1–21 days; P = .38).
It is of interest that risk factors, such as premature birth, chronic lung disease, and congenital heart disease, that have been related to more-severe RSV illness among infants were commonly encountered among infants who had hMPV-associated LRTI (6 [42.9%] of 14 infants), including 2 infants who were HIV-1 infected and who had other underlying risk factors. The prevalence of these risk factors among infants who tested negative for hMPV was 20.3% (37 of 182 infants; P = .05). There was a trend toward a longer duration of hospitalization among infants with presumed underlying risk factors for severe disease (median duration, 8.5 days [range, 1–21 days]; median duration among infants with no underlying illness, 1.5 days [range, 1–5.0 days]; P = .11). For all infants, blood cultures were negative for bacteria; nevertheless, all infants were treated with antibiotics. Furthermore, 6 (42.9%) of 14 infants with hMPV-associated LRTI received supplemental oxygen therapy, and none of the infants required admission to the intensive care unit or died. Although Pneumocystis jiroveci was identified in 11 (44%) of 25 HIV-1–infected infants who were investigated for P. jiroveci pneumonia (PCP), none of the 3 HIV-1–infected infants in whom hMPV was identified had been evaluated for PCP.
Nucleotide comparison for the partial F gene sequences of 7 (50.%) of the 14 hMPV isolates revealed the isolation of 2 major phylogenetic groups, with 86%–88% similarity between the groups. The nucleotide homology in group 1 isolates was 95%–99%, and it was 94%–99% in group 2 isolates (figure 1).
Figure 1.
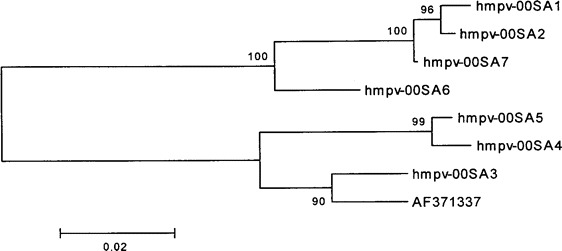
Phylogram obtained from analysis of the fusion gene open-reading frame (nucleotide 700–1088) of 7 human metapneumovirus (hmpv) isolates recovered from subjects in South Africa and The Netherlands (hmpv00-1; GenBank accession no. AF371337).
The frequency of detection of the other common respiratory viruses among the 401 HIV-1–infected and 853 HIV-1–uninfected infants who constituted the entire cohort of infants is shown in table 2. The extrapolated number of cases of hMPV for the entire cohort was based on the premise that concurrent infection involving hMPV and the other tested common respiratory viruses was uncommon. Consequently, it was estimated that hMPV could potentially have been identified in 15 (3.7%) of the 401 HIV-1–infected infants and in 78 (9.1%) of the 853 HIV-1–uninfected infants. Among HIV-1–uninfected infants, RSV was identified more often than was hMPV (31.8% vs. 9.1%; P < .0001); however, hMPV exceeded the other studied respiratory viruses as a potential cause of LRTI (table 2). Furthermore, among HIV-1–infected infants, RSV was also identified more often than was hMPV (11.2% vs. 3.7%; P < .0001); however, the rate of identification of hMPV was similar to that of influenza viruses A and B and PIV types 1–3 (table 2).
Table 2.
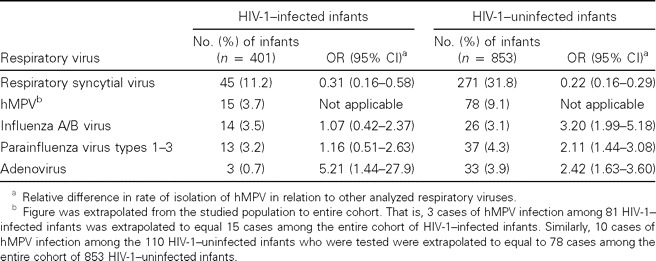
Relative importance of human metapneumovirus (hMPV), in relation to other common respiratory viruses, among infants hospitalized for lower respiratory tract infection.
Discussion
To our knowledge, this is the first study to document the existence of hMPV in Africa and among HIV-1–infected infants, and it confirms the global distribution of this virus. hMPV was identified as a possible cause of LRTI among 9.1% of HIV-1–uninfected and 3.7% of HIV-1–infected infants. The relatively lower prevalence of hMPV among HIV-1–infected infants, compared with HIV-1–uninfected infants, was most likely related to the greater importance of other respiratory pathogens among HIV-1–infected infants, including bacteria (⩾15% of cases) and P. jiroveci (∼45% of cases) [9, 15]. In a previous study, we showed that, despite RSV (5.3% vs. 18.1%), influenza virus (5.4% vs. 11.8%), and parainfluenza viruses (4.2% vs. 7.9%) being proportionally less commonly isolated among HIV-1–infected infants than among HIV-1–uninfected infants who had been hospitalized for severe LRTI, the relative risk of hospitalization for LRTI associated with these viruses was ∼2–8-fold greater among HIV-1–infected infants [10]. The design of this study was suboptimal to estimate the relative risk of hospitalization for hMPV-associated LRTI between HIV-1–infected and HIV-1–uninfected infants. Nevertheless, on the basis of the estimate that the prevalence of HIV-1 infection in the study cohort was ∼6% [16], it was extrapolated that, like other common respiratory viruses [10], hMPV was isolated from a lower proportion of HIV-1–infected infants hospitalized for LRTI. The population risk of hospitalization for hMPV-associated LRTI was, however, 2.9-fold (95% CI, 1.6–5.1-fold) greater among HIV-1–infected infants than among HIV-1–uninfected infants, as determined on the basis of calculation methods described elsewhere [10].
Although there were no significant clinical differences observed between HIV-1–infected and HIV-1–uninfected infants in our study, this observation may be biased because of the low number of infants studied. Comparison of the frequency of detection of hMPV in this study in relation to other published studies is confounded by differing spectrums of respiratory tract infections included in the various studies [1–8].
The frequency of detection of hMPV in this study was second only to that of RSV among HIV-1–infected and HIV-1–uninfected children. Among HIV-1–uninfected infants, hMPV was ∼4.6-fold less common than RSV, but it was 2–3-fold more common than influenza virus A, PIV types 1–3, or adenovirus during the studied period. In previous years, we documented that circulation of PIV types 1–3 and adenovirus is perennial and that circulation of influenza virus occurs during the period of the year we studied [10].
Other limitations of our study include the fact that only a fraction of samples from the study period were studied, although they were randomly selected, and that the method of diagnosing the other studied respiratory viruses (direct immunofluoresence testing) may have been less sensitive than the PCR technique used for detection of hMPV. Furthermore, none of the samples that had tested positive for other respiratory viruses were further tested for hMPV. This may have resulted in a lowering of the relative importance of hMPV in relation to the other viruses. Samples were also not evaluated for other potential respiratory viral pathogens, such as rhinovirus, enterovirus, and coronavirus. Concurrent viral infections have been reported among 2 (16.7%) of 12 children with hMPV-associated acute respiratory tract infections, as well as among 70% of children requiring mechanical ventilation for RSV-associated bronchiolitis [8, 17]. Furthermore, among HIV-1–infected children, P. jiroveci is often found, but it was not sought for the 3 HIV-1–infected children in whom hMPV was identified. A high rate (42%) of concurrent PCP and respiratory viral infection among children with LRTI was previously documented in this population [15]; this may explain the trend toward a longer duration of hospitalization among the HIV-1–infected infants in this study, compared with HIV-1–uninfected infants with hMPV infection.
Furthermore, the yield of hMPV-positive samples in our study may be low, because samples in our study were evaluated directly for hMPV by PCR without culture being performed [1, 2]. Therefore, we may have underestimated the importance of hMPV among African infants. A recent study using the same PCR technique as was used in our study, however, suggested that, on its own, RT-PCR may be adequate for identification of hMPV, even in the absence of viral culture [17]. Although an additional limitation of our study was the absence of a control group of healthy infants to exclude isolation of hMPV as coincidental in the studied infants, a more recent study [17] (in addition to the earliest report on hMPV [1]) has shown that hMPV infection/colonization is absent among infants without respiratory symptoms.
With regard to the clinical spectrum of disease observed among infants in this study, it is interesting that 4 (36.4%) of 11 infants had alveolar consolidation visible on their chest radiographs, as determined on the basis of the World Health Organization's standardized criteria for assessment of chest radiographs [18]. Furthermore, 4 (28.6%) of 14 infants had an axillary temperature of ⩾37.5°C at the time of presentation, and 4 (66.7%) of the 6 infants examined had C-reactive protein levels of ⩾40 mg/dL. In the absence of any bacterial growth on blood cultures, these findings suggest either that hMPV may be associated with a severe inflammatory response, including extensive lung parenchymal involvement, or that there may have been concurrent, unrecognized bacterial coinfections in these infants that may have contributed to the radiological and laboratory features that were observed. However, the findings in our study are in contrast with those in a study from Hong Kong, where the majority of children with hMPV infection were pyrexial (mean temperature, 39.2°C), and none had lobar consolidation visible on chest radiographs [5]. However, the children with hMPV infection in the Hong Kong study were much older (mean age, 31.7 months) than were those in our study [5]. The C-reactive protein levels and WBC counts observed among selected infants in our study also differed from those observed among Finnish children, in whom the mean C-reactive protein level was 9 mg/L and the mean WBC count was 9.5 × 109 cells/L [7]. This difference in C-reactive protein levels and WBC counts in our study may have been because these values were determined mainly in infants with severe disease, as evidenced by the longer duration of hospitalization among the infants for whom these measurements were made; in the Finland study, these tests were performed for children with clinically milder disease [7]. However, the duration of hospitalization in our study (median, 2.0 days) was similar to that observed among Finnish children (mean ° SD, 2.5 ° 1.6 days) [7].
Although our study did not aim to define risk factors for severe hMPV-associated LRTI, it is interesting to note that those risk factors, especially premature birth, were identified as frequently among hMPV-infected infants (28%) in this study as they were among infants with severe RSV disease from the same population [11]. hMPV has been widely postulated to behave clinically similarly to RSV [2]. Therefore, additional studies are warranted to clarify the importance of the identified risk factors as underlying conditions for severe RSV disease among infants. Although it was not significant, the finding of longer durations of hospital stay among infants with underlying medical conditions is a signal indicating greater severity of hMPV-associated LRTI amongst these infants.
Although genotyping was only performed for 7 hMPV isolates, the findings of our study corroborated those of other investigators in North America and Europe who described the simultaneous circulation of 2 major phylogenetic clusters of hMPV. Those findings were based on the nucleotide comparison of the F gene sequences and confirm the global existence of similar strains of hMPV-associated respiratory tract infections [1, 12, 17, 19, 20].
References
- 1.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin G, Abed Y, Pelletier G, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 3.Nissen MD, Siebert DJ, Mackay IM, Sloots TP, Withers SJ. Evidence of human metapneumovirus in Australian children. Med J Aust. 2002;176:188. doi: 10.5694/j.1326-5377.2002.tb04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freymouth F, Vabret A, Legrand L, et al. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr Infect Dis J. 2003;22:92–4. doi: 10.1097/00006454-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Peiris JSM, Tang W-H, Chan K-H, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong [serial online] Emerg Infect Dis. 2003;9:628–3. doi: 10.3201/eid0906.030009. Available at: http://www.cdc.gov/ncidod/EID/vol9no6/03-0009.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 7.Jartti T, van den Hoogen B, Garofalo RP, Osterhaus AD, Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002;360:1393–4. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greensill J, McNamara PS, Dove W, Flanagan B, Smyth RL, Hart CA. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–5. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000;31:170–6. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 10.Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 11.Madhi SA, Venter M, Madhi A, Petersen M-K, Klugman KP. Differing manifestations of respiratory syncytial virus-associated severe lower respiratory tract infections in human immunodeficiency virus type 1-infected and uninfected children. Pediatr Infect Dis J. 2001;20:164–70. doi: 10.1097/00006454-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Peret TC, Boivin G, Li Y, et al. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–3. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epi Info, version 6.04c statistical package. Atlanta, GA: Centers for Disease Control and Prevention; 1997. [Google Scholar]
- 14.SAS/STAT software: changes and enhancements through release 6.12. Cary, NC: SAS Institute; 1997. [Google Scholar]
- 15.Madhi SA, Cutland C, Ismail K, O'Reilly C, Mancha A, Klugman KP. Ineffectiveness of trimethoprim-sulfamethoxazole prophylaxis and the importance of bacterial and viral coinfections in African children with Pneumocystis carinii pneumonia. Clin Infect Dis. 2002;35:1120–6. doi: 10.1086/343049. [DOI] [PubMed] [Google Scholar]
- 16.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. Prevention of pneumonia and invasive pneumococcal disease by a 9-valent pneumococcal conjugate vaccine: efficacy in both HIV-infected and -uninfected children. Vaccine Trialists Group. N Eng J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 17.Boivin G, De Serres G, Côté S, et al. Human metapneumovirus infections in hospitalized children [serial online] Emerg Infect Dis. 2003;9:634–4. doi: 10.3201/eid0906.030017. Available at: http://www.cdc.gov/ncidod/EID/vol9no6/03-0017.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. Geneva: Pneumonia Vaccine Trial Investigators' Group, Department of Vaccines and Biologicals, World Health Organization; 2001. [Google Scholar]
- 19.van den Hoogen BG, Bestebroer TM, Osterhaus AD, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295:119–32. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- 20.Bastien N, Normand S, Taylor T, et al. Sequence analysis of the N. P. M and F genes of Canadian human metapneumovirus strains. Virus Res. 2003;93:51–62. doi: 10.1016/S0168-1702(03)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


