Abstract
Background. Rhinoviruses frequently cause the common cold but have not been considered important causes of acute respiratory hospitalizations in children.
Methods. A population-based surveillance study was performed among children <5 years of age who were hospitalized with respiratory symptoms or fever and who resided within counties encompassing Nashville, Tennessee, or Rochester, New York, from October 2000 through September 2001. Data collected included questionnaires, nasal and throat swabs for viral culture and polymerase chain reaction testing, and chart review. Rates of rhinovirus-associated hospitalizations were calculated.
Results. Of 592 children enrolled, 156 (26%) were rhinovirus positive, representing 4.8 (95% confidence interval [CI], 4.3–5.2) rhinovirus-associated hospitalizations/1000 children. Age-specific rates per 1000 children were 17.6 (95% CI, 14.9–20.6) for 0–5-month-olds, 6.0 (95% CI, 5.0–7.0) for 6–23-month-olds, and 2.0 (95% CI, 1.6, 2.4) for 24–59-month-olds (P<.01) Children with a history of wheezing/asthma had significantly more rhinovirusassociated hospitalizations than those without a history (25.3/1000 children [95% CI, 21.6–29.5/1000 children] vs. 3.1/1000 children [95% CI, 2.7–3.5/1000 children]).
Conclusions. Rhinoviruses were associated with nearly 5 hospitalizations/1000 children <5 years of age and were highest in children with a history of wheezing/asthma.
Viral acute respiratory infections (ARIs) cause significant morbidity in children [1–5]. Although earlier reports have tried to assess this burden, most, to our knowledge, were not prospective, population-based studies and were conducted before the advent of sensitive viral detection methods such as polymerase chain reaction (PCR), which markedly enhances the identification of pathogens, including rhinoviruses [1–8]. Rhinoviruses, members of the family Picornaviridae, were first isolated in 1956, and, currently, >100 serotypes have been identified [9, 10]. Although once thought to cause only the common cold, the role of rhinoviruses in lower respiratory infections and asthma exacerbations in adults [11–15] and children [16–29] has been recently appreciated. However, the actual population-based rate of rhinovirus-associated hospitalizations in infants and children has not been described.
The New Vaccine Surveillance Network (NVSN), established by the Centers for Disease Control and Prevention (CDC) in 1999, conducts active, population-based surveillance in children <5 years of age hospitalized with ARI or fever in Davidson County, Tennessee (Nashville), and Monroe County, New York (Rochester) [3, 30]. Using data from the NVSN, we evaluated the prevalence of rhinovirus infection in hospitalized children <5 years of age and determined the population-based rates of hospitalization. Demographic and clinical characteristics of children with confirmed rhinovirus were compared with those with other viral illnesses.
Subjects and Methods
Study design. Prospective surveillance was conducted among children <5 years of age hospitalized with acute respiratory symptoms or fever from 1 October 2000 through 30 September 2001 in hospitals that provided care for >95% of hospitalized children in Davidson County, Tennessee, and Monroe County, New York. Institutional review boards of the 2 study sites, the 5 participating surveillance hospitals, and the CDC approved the study.
Four days each week, study nurses identified children who were hospitalized at each surveillance hospital. Written, informed consent was obtained from a parent or guardian. Both nasal and throat swabs were obtained from all enrolled children and combined into a single tube of transport medium. Samples were tested by cell culture isolation using Rh-MK and HEp2 cell lines; those with cytopathic effects were tested by immunofluorescence staining for respiratory syncytial virus (RSV), influenza virus types A and B, parainfluenza viruses (PIV) types 1–3, and picornavirus (including both rhinovirus and enterovirus), as described elsewhere [7, 8]. Aliquots of samples were also tested at the CDC by reverse-transcription (RT) PCR for RSV, influenza virus types A and B, PIV-1–3, human metapneumovirus (HMPV), and picornavirus (inclusive of both enterovirus and rhinovirus) [7,31,32]. A sample was considered to be positive for a given virus if the culture was positive or both an initial and confirmatory RT-PCR were both positive.
To further discriminate between enterovirus and rhinovirus, all samples positive for picornavirus by PCR were further subjected to another round of PCR using the same forward primer from the primary reaction, PICO-F3 (+) 5'-GGCCCCTGAATGYGGCTAA-3', and an internal reverse primer, PICO-R4 (−)5'-CAAAGTAGTTGGTCCCATCC-3'. Amplifications were performed by adding 0.5 µL of the primary amplification product to 49.5 µL of the reaction mixture containing 1 × reaction buffer with Mg++, 0.2 mmol/L each deoxynucleotide triphosphate, 0.2 U of Taq DNA polymerase (Roche Diagnostics), and 0.2 µmol/L of each primer. Thermocycling was performed on a MicroAmp 9600 (Applied Biosystems) instrument set for 94°C denaturation for 2 min; 30 cycles of amplification at 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min; and a final amplicon extension at 72°C for 5 min. Amplicons were analyzed by electrophoresis on 2% agarose gels with ethidium bromide staining, with a subset sequenced to confirm rhinovirus specificity. Standardized viral RNA extracts and nuclease-free water were always included as positive and negative controls, respectively.
Demographic and clinical information. Demographic and clinical information from each parent or guardian was collected on a standardized questionnaire [30]. Subgroup comparisons were identified a priori. History of underlying medical conditions, health insurance status, symptom duration, admission diagnoses, microbiology laboratory results, laboratory and chest radiograph results, hospital course, and discharge diagnoses were obtained from the medical record. Prematurity was defined as birth at <36 weeks gestational age. A maximum of 10 ICD-9 discharge diagnosis codes were recorded for each hospitalization by nonstudy hospital coding staff. Children were classified as having asthma/wheezing if their parent or guardian reported or the medical record documented that a physician had diagnosed asthma or had noted prior wheezing.
Population-based hospitalization rates. Rates of rhinovirus-associated hospitalization per 1000 children were calculated as the weighted number of hospitalizations for rhinovirus ARI or fever divided by the number of children in the county population as determined by the 2000 US Census, multiplied by 1000. Rates were calculated using Stata (version 8.2; StataCorp) and SAS software (version 9.1; SAS Institute) procedures by weighting the observed number of enrolled hospitalizations to account for sampling 4 days/week and eligible patients who were not enrolled; age and study site were the sampling strata. Rates were calculated overall and by viral etiology and demographic subsets, with 95% confidence intervals (CIs) calculated using the Confidence Interval Analysis program (version 2.0.0; British Medical Journal). This program assumes events to have a Poisson distribution, and population is constant with no sampling variation. Rates were compared between the age groups using conditional and normal theory test [33]. Children admitted multiple times were regarded as independent hospitalizations; no records were dropped in analysis.
Because we did not determine the overall proportion of all children with underlying high-risk medical conditions, we assumed that 10% of children <5 years old had high-risk medical conditions based on national and local Rochester, New York, data (P. G. Szilagyi, University of Rochester Medical Center, Rochester, NY, personal communication). Asthma prevalence was obtained from the National Center for Health Statistics, which reports results from multiple data sets including the National Health Interview Survey (NHIS). NHIS asks parents if a doctor or other health professional has ever told them that their child had asthma or has had an asthma attack during the last 12 months. Using the projected asthma prevalence from this survey of 7.5% in children <5 years of age in the United States, rates of rhinovirus-associated hospitalization among children with asthma were also calculated [34]. A x2 test was used for categorical variables. Multivariable analysis was performed using logistic regression to determine odds ratios.
Results
Study population. Of 812 eligible children admitted to surveillance hospitals, 592 (73%) children were enrolled. Of 220 children not enrolled, 127 (58%) parents or guardians refused participation; 44 (20%) parents or guardians were not available; 40 (18%) were discharged before enrollment; and 9 (4%) were secondary to missed admissions, interpreter unavailability, or physician refusal. Twenty children had multiple hospitalizations; 18 had 2 admissions; and 1 each had 3 and 4 admissions. Sex, race/ethnicity, and age were not significantly different between children with multiple and 1 hospitalizations. Chart audits revealed that numbers of hospital admissions and diagnoses during the 3 nonsurveillance days each week were similar to those seen during the 4 surveillance days. Enrolled children were comparable to nonenrolled children for sex, race/ethnicity, and county of residence [30].
Viral etiology. Viruses were detected by culture or PCR in 352 (61%) of 592 samples obtained. Overall, rhinovirus was detected in 26%, RSV in 20%, influenza in 3%, PIV in 7%, HMPV in 3%, and enteroviruses in 2%. Only 20 (13%) of the rhinovirus-positive children were coinfected with another virus—11 with RSV, 5 with PIV, and 4 with HMPV. All 9 children who had rhinoviruses detected by viral culture were also positive for rhinovirus by PCR. Of children with multiple hospitalizations, 12 (60%) had at least 1 hospitalization with rhinovirus, and 2 had rhinovirus detected on separate hospitalizations (1 separated by 5.5 months and the other by 1.5 months).
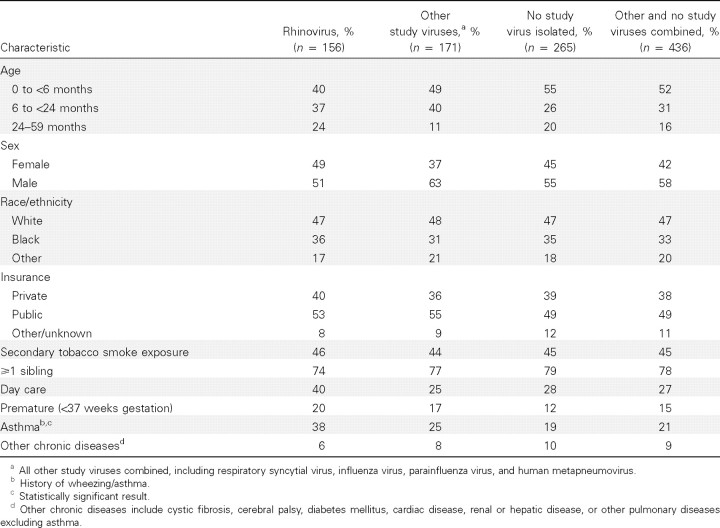
Demographic and clinical characteristics. Among the 592 study children with ARI or fever, demographic and clinical characteristics were similar among those with confirmed rhinovirus infections, those with other confirmed viral infections, and those with no virus detected (Table 1). Approximately three-quarters of enrolled children were 0–23 months of age, half were male, half had public insurance (Medicaid), and one-third were black. Approximately 46% of enrolled children lived with a smoker, nearly 75% had siblings, and 20% were born prematurely; proportions did not differ among the 3 groups. More rhinovirus-positive children attended day care, but these differences were not statistically significant.
Table 1.
Demographic and clinical characteristics of 592 children hospitalized with acute respiratory illness or fever, by rhinovirus vs. other study viruses or no study virus isolated.
The only statistically significant difference in the characteristics of the 3 groups was that 38% of children with rhinovirus had asthma/wheezing, compared with 25% of those with another virus and 19% of those with no virus isolated (P=.001). Fewer than 10% of all enrolled children had another chronic disease, excluding asthma, and this finding did not vary among groups.
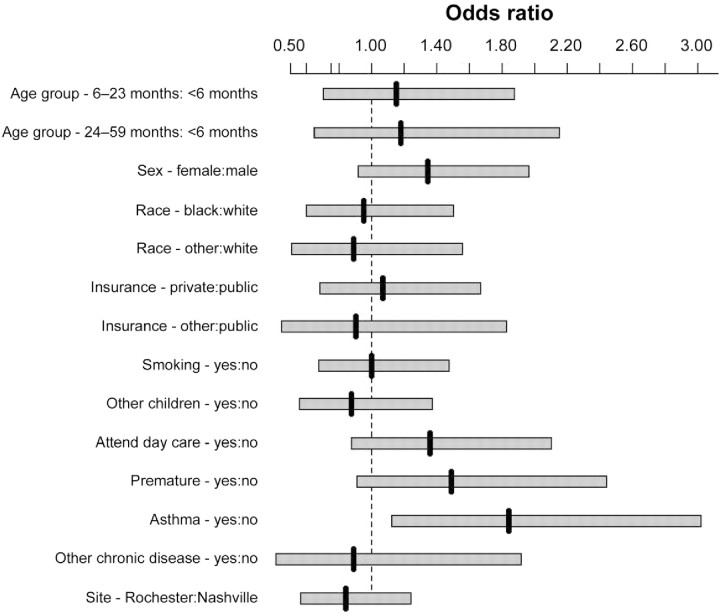
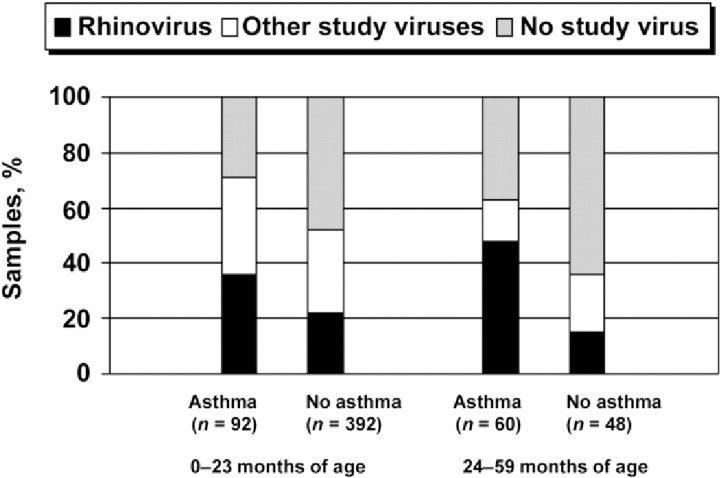
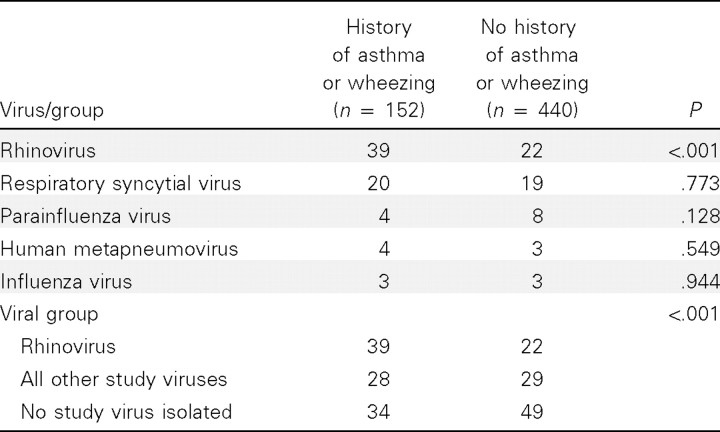
As shown in figure 1, when all variables were included in the multivariable analyses, the only factor independently associated with rhinovirus detection was a history of wheezing/ asthma (P=.02). Of the 152 hospitalized children with this history, 114 (75%) were actually given a discharge diagnosis of asthma or wheezing. Of rhinovirus-positive children 24–59 months of age, 76% had a history of asthma. As shown in figure 2, among children 24–59 months of age with this history, the proportion with rhinovirus (47%) was significantly higher than with other viruses (15%) (P=.01). Of rhinovirus-positive patients 0–23 months of age, 26% had a history of asthma or wheezing. Rhinovirus was the only study virus significantly associated with a history of asthma or wheezing (P<.001 ) (Table 2).
Figure 1.
Odds of rhinovirus-associated hospitalization, compared with hospitalization for acute respiratory infection/fever in which rhinovirus was not detected, with 95% confidence intervals calculated by multivariable logistic regression. “Asthma” indicates history of asthma or wheezing.
Figure 2.
Percent distribution of specific virus-associated hospitalizations, by age and history of asthma or wheezing.
Table 2.
History of asthma or wheezing, by study virus.
Clinical manifestations. In children with confirmed rhinovirus infection, the median length of stay was 3 days (range, 1–22 days) and was the same for those with other study viruses (range, 1–71 days) or no virus (range, 1–32 days). Differences in clinical manifestations among those with rhinovirus, those with other viruses, or those with no isolated viruses were not statistically significant. Supplemental oxygen was required in 36% of children with rhinovirus, compared with 51% and 23% of those with other study virus or no study virus. Four children (3%) with rhinovirus required mechanical ventilation, compared with 3% and 1% of those with other study virus or no study virus. Blood cultures were obtained in 52% of children with rhinovirus, 51% of those with other viruses, and 70% of those with no virus. Blood cultures obtained in children with rhinovirus were predominantly in younger children (60% of children 0–6 months of age; 27% of children 6–23 months of age). Twenty-six percent of those with rhinovirus had cerebrospinal fluid (CSF) obtained, compared with 18% and 37% of those with other study viruses or no study virus. In children with rhinovirus who had CSF obtained, 93% were 0–6 months of age. Twenty-four percent of children with rhinovirus had urine cultures, compared with 31% and 45% of those with other study viruses or no study virus. Of rhinovirus-positive children who had urine cultures obtained, 79% were 0–6 months of age.
In 62 infants 0–6 months of age with rhinovirus, admission diagnoses included asthma (n=2), pneumonia (n=5), sepsis (n=20), bronchiolitis (n=11), febrile neonate (n=15), RSV (n=9), otitis media (n=2), and apnea (n=7). Of the 15 febrile neonates with rhinovirus, 5 were coinfected with RSV and 1 with PIV; 3 were diagnosed with sepsis and 1 with pneumonia; and 11 had cough or nasal symptoms, 10 anorexia, 9 vomiting, 8 dyspnea, and 7 diarrhea. In 94 children 6–59 months of age with rhinovirus, admission diagnoses included asthma (n=43), pneumonia (n=20), sepsis (n=1), bronchiolitis (n=6), febrile syndromes (n=11), RSV (n=2), croup (n=9), respiratory distress (n=9), otitis media (n=5), upper respiratory illness (n=5), febrile seizures (n=4), pharyngitis (n=1), and group A streptococcal pharyngitis (n=1).
There were no significant differences n clinical manifestations between those with rhinovirus alone and those with dual infection. Of the 25 rhinovirus-positive children with pneumonia, 4 were coinfected with another virus (2 RSV, 1 PIV, 1 HMPV), and 5 were infants <6 months of age. These were not confirmed as bacterial pneumonia nor associated with bacteremia. In addition, 21 of 25 children with pneumonias and 23 of 26 children with febrile illness had no additional viruses isolated. Three rhinovirus-positive children had bacterial coinfections; one child had bacteremia with group B Streptococcus, another had meningitis with group B Streptococcus, and one child had a urinary tract infection with Klebsiella pneumoniae.
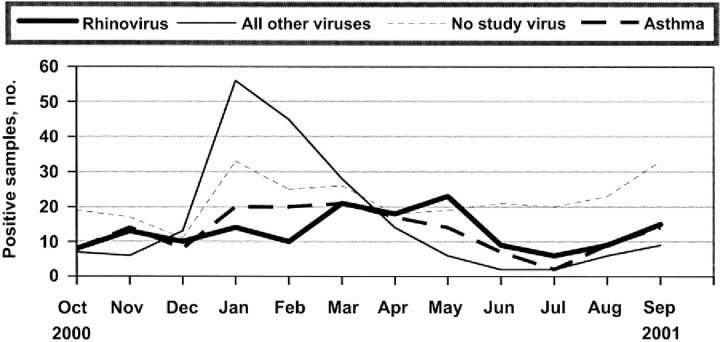
Seasonality. The distinct seasonal patterns associated with various study viruses are shown in figure 3. Of rhinoviruses detected, 40% were identified in children hospitalized in the spring, 15% in the summer, 23% in the fall, and 22% in the winter. In contrast, 25% of the nonrhinovirus isolates were detected in the spring, 5% in the summer, 11% in the fall, and 59% in the winter (P<.01 ). The rhinovirus-associated hospitalizations peaked in May, whereas hospitalizations associated with the other respiratory viruses peaked in January and February.
Figure 3.
Seasonal distribution of hospitalizations for acute respiratory infection (ARI) or fever, by virus, identified for all children; and seasonal distribution of all hospitalizations for ARI and fever for children with a history of asthma or wheezing.
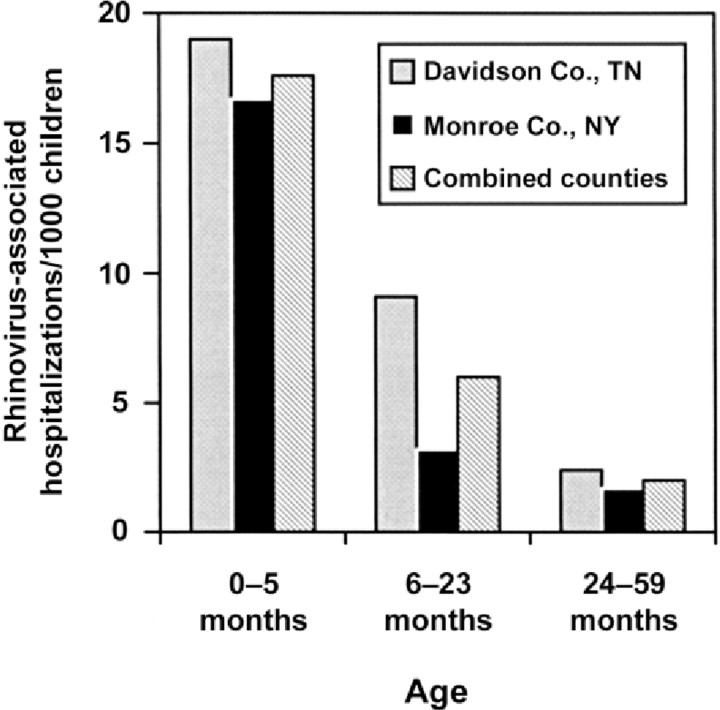
Rates of rhinovirus-associated hospitalization. In 2000, a total of 84,790 children <5 years of age resided in the 2 surveillance areas. This denominator was used to determine the rhinovirus-associated hospitalization rates by age group in figure 4. Although the rates in Davidson County were slightly higher than those in Monroe County, these differences were not statistically significant. The overall rate of hospitalized children with rhinovirus detected was 4.8/1000 children 0–59 months of age/year (95% CI, 4.3–5.2). Age-specific hospitalization rates for children with a rhinovirus isolate were 17.6/ 1000 children 0–5 months of age (95% CI, 14.9–20.6), 6.0/1000 children 6–23 months of age (95% CI, 5.0–7.0), and 2.0/1000 children 24–59 months of age (95% CI, 1.6–2.4) (P<.001, for any pair of rate comparisons between 2 age groups). Among children with and without a history of asthma or wheezing, the estimated rates of rhinovirus-associated hospitalizations were 25.3/1000 children (95% CI, 21.6–29.5) and 3.1/1000 children (95% CI, 2.7–3.5), respectively (P<.001 ).
Figure 4.
Rates of hospitalizations for rhinovirus-associated acute respiratory infection or fever in individual and combined counties, by age group.
Excluding rhinovirus coinfections, the overall rate of hospitalizations for children with rhinovirus detected was 4.2/1000 children 0–59 months of age/year (95% CI, 3.8–4.7); for each age group, the rates were as follows: 14.9/1000 children 0–5 months of age (95% CI, 12.4–17.7), 5.0/1000 children 6–23 months of age (95% CI, 4.2–6.0), and 2.0/1000 children 24–59 months of age (95% CI, 1.6–2.4) (P<.001, for any pair of rate comparisons between 2 age groups), which were similar to rates in rhinovirus-coinfected children.
Discussion
Historically, RSV has been regarded as the predominant virus associated with hospitalizations for ARI in young children [35, 36]. We detected more rhinoviruses (26%) than RSV (20%) among children <5 years of age hospitalized with ARI or fever. Furthermore, children with a history of asthma or wheezing had the highest rate of rhinovirus-associated hospitalizations at 25–28/1000 children/year. In determining rates of rhinovirus-associated hospitalization in children with wheezing or asthma, we used the background prevalence of 7.5% derived from data obtained from the National Center for Health Statistics [34]. Although this estimate of asthma prevalence is subject to variations based on socioeconomic status, geographic location, sex, and race/ethnicity, our data suggest that rhinoviruses are important contributors to many of these hospitalizations. The rhinovirus-positive children had distinct clinical and epidemiologic features, particularly the predilection for children with a history of wheezing or asthma. To our knowledge, our estimates for age-specific rates of hospitalizations for children with rhinovirus infections are the first population-based rates for pediatric hospitalizations associated with rhinovirus.
Although rhinovirus was detected year-round in this 1-year study, 40% of all cases occurred in the spring. This supports prior observations that rhinovirus is predominant in the spring and fall [37–39]. Interestingly, the seasonal trend for asthma hospitalizations mirrored the seasonal trend for rhinovirus, with the exception of an additional peak during the winter when RSV and influenza virus circulated, as has been reported elsewhere [23, 40–42]. Many study children (45%) hospitalized with ARI or fever lived in homes with smokers, significantly greater than the 21% national prevalence of smoking (P<.001) [43]. Although most children with rhinovirus infections were hospitalized for <3 days, many children required supplemental oxygen, and a few required mechanical ventilation. Approximately half of the children hospitalized with rhinovirus infection had blood, urine, or CSF cultures obtained, and most were obtained in children 0–6 months of age. The majority of bacterial cultures were negative, but, as mentioned above, one child had bacteremia with group B Streptococcus, another had meningitis with group B Streptococcus, and one child had a urinary tract infection with Klebsiella pneumoniae. Of note, 10% of rhinovirus-positive children <24 months of age presented with a primary diagnosis of fever alone. Although many of the febrile neonates with rhinovirus had the “usual” nasal symptoms and cough that have been classically associated with rhinoviruses, several were reported to have emesis, diarrhea, or poor appetite, and 25% had no respiratory symptoms at all. Pneumonia was diagnosed in 16% of patients with rhinovirus, none of which was confirmed as bacterial. In 84% of cases of pneumonia and 88% of cases of febrile illness in rhinovirus-positive children, no additional viral pathogens were isolated.
In multivariable analysis, asthma/wheezing was the only statistically significant factor that distinguished children hospitalized with rhinoviruses from those with either other viruses or no viruses. Overall, rhinovirus was found in 48% of children 24–59 months of age and in 36% of those 0–23 months of age who were hospitalized with a history of asthma or wheezing. This finding is consistent with previous reports that have associated rhinovirus infection with hospitalizations in children with asthma [24–26] and in adults [11, 14, 15, 22].
It is unclear whether children who are predisposed to asthma are more likely to be infected with rhinovirus or whether they are more likely to have severe illness when infected with rhinovirus. Blomqvist et al. found that 22% of all children had at least 1 rhinovirus infection by 6 months of age [44]. Although we do not have incidence data on infection, our hospitalization data suggest that the children hospitalized with rhinovirus may not be at greater risk of infection but instead may be more likely to have a worse outcome. In the few studies investigating rhinovirus infection in asymptomatic children who have underlying asthma, the proportion with rhinovirus tends to be lower (10%–12%) [12, 16] than in other studies of asymptomatic children without asthma (18%–20%) [27, 45]. A recently published study evaluating the role of viruses in outpatient respiratory illnesses in a birth cohort of children 0–1 year of age found that 69% of ARIs were viral, and, of those, 48.5% were attributable to rhinoviruses. These investigators also found that 11.5% of asymptomatic children in the birth cohort were infected with rhinoviruses and were reported to be clinically well within the 4-week period surrounding viral detection [46]. We found a similar distribution among hospitalized children, with a 44% detection of rhinoviruses in the viral-positive specimens.
Study limitations. Despite the strength of our surveillance system, there are several limitations. First, we did not test concurrent healthy controls to determine the prevalence of asymptomatic rhinovirus shedding. However, we did perform viral surveillance in all enrolled children and did not detect additional viruses in the majority of the children with rhinovirus. Although it is possible that rhinovirus infection was an incidental finding in children with other viral or bacterial pathogens, few of the children had viral coinfections detected. In addition, the rate of asymptomatic rhinovirus infection from previous studies is less than the rate of infection seen in the present study [12, 27, 42, 45–48]. Another limitation is that we only evaluated rhinovirus during a single year, and the seasonality may not reflect the rates in other years or other geographic areas. Although surveillance hospitals included at least 95% of county children admitted with fever or respiratory illness and we adjusted for nonenrolled children and nonsurveillance days, enrolled children may not have been representative of the entire county populations. Finally, although the present study utilized sensitive PCR methods coupled with culture, we may have failed to detect some viruses due to RNA instability, low titers, or as-yet unidentified viruses. Although some studies have suggested higher detection rates with nasal aspirates/washes rather than nasal/throat swabs, other studies have not confirmed these findings [8, 49]. Swabs are more acceptable to families.
Conclusion. In conclusion, this unique population-based surveillance study revealed a high proportion of hospitalizations for pediatric ARI or fever associated with rhinoviruses. Asthma/ wheezing was the only risk factor significantly associated with rhinovirus hospitalizations. Further studies of hospitalized children and healthy controls are needed to better understand the proportion of disease caused by infection and the clinical and epidemiologic features of these infections. The frequency of rhinovirus detection in young children suggests that it has an underappreciated role in ARI hospitalizations.
New Vaccine Surveillance Network (NVSN)
NVSN acute respiratory infection inpatient study collaborators are as follows. University of Rochester: Linda Anderson, Gerry Lofthus, Andrea Marino, Anne Mowrer, Kenneth Schnabel, Laura Shone, and Peter Szilagyi; Vanderbilt University: Kathy Holland, Diane Kent, Ayesha Khan, Yi-Wei Tang, Nayleen Whitehead, Sandra Yoder, and Yuwei Zhu; and Centers for Disease Control and Prevention: Barbara Anderson, John Copeland, Paul Gangarosa, James A. Mullins, Ben Schwartz, David Shay, and Fran Walker.
Acknowledgments
We thank all the parents and children who participated in this study and all the members of the New Vaccine Surveillance Network.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: VIII International Symposium for Respiratory Viral Infection (poster), Kohala Coast, Hawaii, 16–19 March 2006; Pediatric Academic Societies (poster), Washington, D.C., 13–15 May 2005.
Financial support: Centers for Disease Control and Prevention (CDC; cooperative agreement 1 U01 IP000022); Agency for Healthcare Quality and Research (grant T32 HS 13833-02); CDC National Immunization Program (cooperative agreement U38/CCU217969 with the University of Rochester; cooperative agreement U38/ CCU417958 with Vanderbilt University); National Vaccine Program Office; Robert Wood Johnson Generalist Physician Faculty Scholars Program (grant K23 AI065805 to K.A.P.); Agency for Healthcare Quality and Research (grant T32 HS 13833-02 to E.K.M.); National Institutes of Health (grants RO1 AI 50884 and NIH MO1 000095 to T.V.H.); Thrasher Research Grant Fund (to T.V.H.).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
References
- 1.West JV. Acute upper airway infections. Br Med Bull. 2002;61:215–30. doi: 10.1093/bmb/61.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acute respiratory infections: the forgotten pandemic. Int J Tuberc Lung Dis; Communique from the International Conference on Acute Respiratory Infections; 7–10 July 1997; Canberra, Australia. 1998. pp. 2–4. [PubMed] [Google Scholar]
- 3.Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J. 2004;23(Suppl 11):S188–92. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 4.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–94. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 5.Bertino JS. Cost burden of viral respiratory infections: issues for formulary decision makers. Am J Med. 2002;112(Suppl 6A):42–9. doi: 10.1016/s0002-9343(01)01063-4. [DOI] [PubMed] [Google Scholar]
- 6.Jennings LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of acute respiratory tract infections in children presenting to hospital: role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23:1003–7. doi: 10.1097/01.inf.0000143648.04673.6c. [DOI] [PubMed] [Google Scholar]
- 7.Erdman DD, Weinberg GA, Edwards KM, et al. GeneScan reverse transcription-PCR assay for detection of six common respiratory viruses in young children hospitalized with acute respiratory illness. J Clin Microbiol. 2003;41:4298–303. doi: 10.1128/JCM.41.9.4298-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg GA, Erdman DD, Edwards KM, et al. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–10. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 9.Pelon W, Mogabgab WJ, Phillips IA, Pierce WE. A cytopathogenic agent isolated from naval recruits with mild respiratory illnesses. Proc Soc Exp Biol Med. 1957;94:262–7. doi: 10.3181/00379727-94-22915. [DOI] [PubMed] [Google Scholar]
- 10.El-Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atmar RL, Guy E, Guntupalli KK, et al. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–9. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambrano JC, Carper HT, Rakes GP, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–16. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 14.Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest. 1997;112:591–6. doi: 10.1378/chest.112.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira A, Williams Z, Donninger H, van Schalkwyk EM, Bardin PG. Rhinovirus is associated with severe asthma exacerbations and raised nasal interleukin-12. Respiration. 2002;69:136–42. doi: 10.1159/000056316. [DOI] [PubMed] [Google Scholar]
- 16.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14:17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulos NG, Johnston SL. Rhinoviruses as pathogens of the lower respiratory tract. Can Respir J. 2000;7:409–14. doi: 10.1155/2000/169814. [DOI] [PubMed] [Google Scholar]
- 20.Gern JE. Rhinovirus respiratory infections and asthma. Am J Med. 2002;112(Suppl 6A):19–27. doi: 10.1016/s0002-9343(01)01060-9. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos NG, Papi A, Psarras S, Johnston SL. Mechanisms of rhinovirus-induced asthma. Paediatr Respir Rev. 2004;5:255–60. doi: 10.1016/j.prrv.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11:21–6. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 23.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 24.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy—the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thumerelle C, Deschildre A, Bouquillon C, et al. Role of viruses and atypical bacteria in exacerbations of asthma in hospitalized children: a prospective study in the Nord-Pas de Calais region (France) Pediatr Pulmonol. 2003;35:75–82. doi: 10.1002/ppul.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–90. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 28.Krilov L, Pierik L, Keller E, et al. The association of rhinoviruses with lower respiratory tract disease in hospitalized patients. J Med Virol. 1986;19:345–52. doi: 10.1002/jmv.1890190407. [DOI] [PubMed] [Google Scholar]
- 29.Juven T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–8. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–64. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 31.Mullins JA, Erdman DD, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–5. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roghmann M, Ball K, Erdman D, Lovchik J, Anderson LJ, Edelman R. Active surveillance for respiratory virus infections in adults who have undergone bone marrow and peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;32:1085–8. doi: 10.1038/sj.bmt.1704257. [DOI] [PubMed] [Google Scholar]
- 33.5th ed. Duxbury, CA: Duxbury Press; 2005. Bernard Rosner fundamentals of biostatistics. [Google Scholar]
- 34.Percentage of children aged 18 years who have ever had asthma diagnosed, by age group—United States 2003. MMWR Morb Mortal Wkly Rep. 2005;54:412. [Google Scholar]
- 35.Levy BT, Graber MA. Respiratory syncytial virus infection in infants and young children. J Fam Pract. 1997;45:473–81. [PubMed] [Google Scholar]
- 36.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 37.Monto AS. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther. 2002;24:1987–97. doi: 10.1016/S0149-2918(02)80093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monto AS. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J. 2004;23(Suppl 1):S58–64. doi: 10.1097/01.inf.0000108193.91607.34. [DOI] [PubMed] [Google Scholar]
- 39.Arruda E, Pitkaranta A, Witek TJ, Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–8. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntosh K, Ellis EF, Hoffman LS, Lybass TG, Eller JJ, Fulginiti VA. Association of viral and bacterial respiratory infection with exacerbations of wheezing in young asthmatic children. Chest. 1973;63(Suppl):S43. doi: 10.1378/chest.63.4_Supplement.43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnston NW, Johnston SL, Duncan JM, et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115:132–8. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.State-specific prevalence of cigarette smoking and quitting among adults—United States, 2004. MMWR Morb Mortal Wkly Rep. 2005;54:1124–7. [PubMed] [Google Scholar]
- 44.Blomqvist S, Roivainen M, Puhakka T, et al. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol. 2002;66:263–8. doi: 10.1002/jmv.2140. [DOI] [PubMed] [Google Scholar]
- 45.Nokso-Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkaranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66:417–20. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusel M, de Klerk N, Holt P, et al. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life. Pediatr Infect Dis J. 2006;25:680–6. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 47.van Benten I, Koopman L, Niesters B, et al. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003;14:363–70. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–9. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 49.Frayha H, Castriciano S, Mahony J, Chernesky M. Nasopharyngeal swabs and nasopharyngeal aspirates equally effective for the diagnosis of viral respiratory disease in hospitalized children. J Clin Microbiol. 1989;27:1387–9. doi: 10.1128/jcm.27.6.1387-1389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]