Abstract
Cross-reactivity between antibodies to different human coronaviruses (HCoVs) has not been systematically studied. By use of Western blot analysis, indirect immunofluorescence assay (IFA), and enzyme-linked immunosorbent assay (ELISA), antigenic cross-reactivity between severe acute respiratory syndrome (SARS)—associated coronavirus (SARS-CoV) and 2 HCoVs (229E and OC43) was demonstrated in immunized animals and human serum. In 5 of 11 and 10 of 11 patients with SARS, paired serum samples showed a ⩾4-fold increase in antibody titers against HCoV-229E and HCoV-OC43, respectively, by IFA. Overall, serum samples from convalescent patients who had SARS had a 1-way cross-reactivity with the 2 known HCoVs. Antigens of SARS-CoV and HCoV-OC43 were more cross-reactive than were those of SARS-CoV and HCoV-229E.
Severe acute respiratory syndrome (SARS) is an emerging infectious disease caused by a novel coronavirus (CoV) designated “SARS-associated CoV” (SARS-CoV) [1]. Three known antigenic groups of CoVs are associated with diseases in animals and humans [2]. The known human CoVs (HCoVs)—HCoV-229E, in CoV group 1, and HCoV-OC43, in CoV group 2—are generally recognized to cause mild upper respiratory tract diseases and, rarely, lower respiratory tract diseases [2]. Phylogenetic analyses show that SARS-CoV is not closely related to any of the previously characterized CoVs [3]. However, some investigators, using SARS-CoV—infected Vero cells in immunohistochemical antibody tests, have observed cross-reactions between SARS-CoV and group I CoVs, but seroepidemiological studies revealed that there were no cross-reactions with SARS-CoV—infected Vero cells in 13 and 14 paired serum samples from patients with HCoV-OC43 and HCoV-229E, respectively [1]. Serum samples from group I CoV-infected animals also cross-reacted with the recombinant nucleocapsid protein of SARS-CoV [4]. Results of the recombinant nucleocapsid protein—based ELISA were positive in 1.04% of serum samples from healthy blood donors [5]. The nature of this antigenic cross-reactivity is still unknown. In the present study, we cloned the nucleocapsid genes of SARS-CoV, HCoV-229E, and HCoV-OC43 and produced specific animal antisera to determine if the nucleocapsid protein is responsible for the observed antigenic cross-reactivity. In addition, the antigenic relationships among SARS-CoV, HCoV-229E, and HCoV-OC43 were further studied using serum samples from healthy donors and patients with SARS.
Subjects, materials, and methods. HCoV strains 229E (ATCC VR740) and OC43 (ATCC VR759) were maintained in normal human fetal lung fibroblast cells (MRC-5; ATCC CCL-171) and African green monkey kidney cells (BSC-1; ATCC CCL-26) in MEM (Gibco BRL) supplemented with 10% fetal bovine serum (Gibco BRL). A SARS-CoV (HKU-39849) strain isolated from a patient with SARS in Hong Kong was inoculated into Vero E6 cells as described elsewhere [6, 7]. All experiments with live viruses were performed in a biosafety level 3 laboratory.
Murine monoclonal antibodies specific for the nucleocapsid proteins of HCoV-229E and HCoV-OC43 were obtained from a commercial source (Chemicon International).Amurinemonoclonal antibody specific for the nucleocapsid protein of SARSCoV was produced in our laboratory [8]. Antiserum to whole virus was prepared in female New Zealand White rabbits as described elsewhere [9].
The cDNA fragments of the nucleocapsid protein of the 3 CoVs were cloned into the prokaryotic expression vector pQE30 (Qiagen) in frame and upstream of the 6 histidine (His6) residue series, and the His6-tagged nucleocapsid proteins were expressed and purified using an Ni-NTA affinity column (Qiagen) in accordance with the manufacturer's instructions. The expressed recombinant nucleocapsid proteins were identified by Western blot analysis as described elsewhere [8].
SARS-CoV—specific IgG was identified using a commercially available indirect immunofluorescence assay (IFA) kit (Euroimmun) in accordance with the manufacturer's instructions. HCoV-229E— and HCoV-OC43—specific IgG was identified by an inhouse IFA, as described elsewhere [7], that was modified to use HCoV-229E—infected MRC-5 cells and HCoV-OC43—infected BSC-1 cells, respectively.
IgM and IgG antibodies to SARS-CoV were identified using an ELISA test kit (Huada GBI Biotechnology) in accordance with the manufacturer's instructions. The nucleocapsid protein—based ELISA was performed as described elsewhere [6]. To ensure biosafety, experiments using serum samples from patients were performed in a biosafety level 2 laboratory.
One hundred serum samples were collected randomly from healthy adult donors in October 2003. Serum samples were collected from 34 patients with SARS 8–81 days after the onset of symptoms. Paired serum samples were obtained from 11 of these patients who exhibited seroconversion (table 1), from whom the acute- and convalescent-phase serum samples were collected on days 1–7 and days 12–159 after the onset of symptoms. SARS was diagnosed in accordance with the World Health Organization's criteria and was confirmed by assessment of seroconversion or a ⩾4-fold increase in antibody titers against SARS-CoV by IFA.
Table 1.
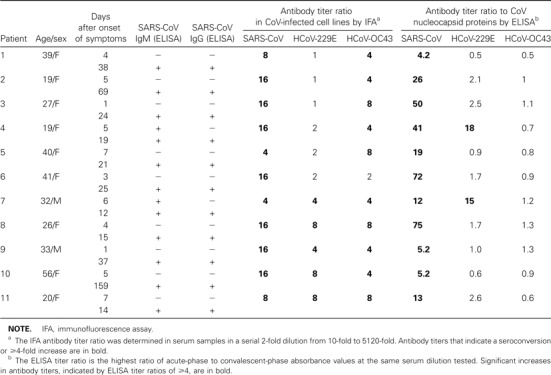
Antibody responses to human coronavirus (HCoV)—229E, HCoV-OC43, and severe acute respiratory syndrome (SARS)—associated CoV (SARS-CoV) in 11 paired serum samples from patients with SARS
Results. The full lengths of 3 nucleocapsid genes from HCoV-229E, HCoV-OC43, and SARS-CoV were amplified with their corresponding primer pairs. The gene sizes were 1182, 1359, and 1281 bp for HCoV-229E, HCoV-OC43, and SARSCoV, respectively. The recombinant plasmids were sequenced, and they were all in frame and had sequences matching those of the nucleocapsid genes of the 3 CoVs. The expressed recombinant His6-tagged N-terminal nucleocapsid proteins were identified by Western blot analysis using anti-His monoclonal antibodies. The immunoreactive protein bands with expected sizes are shown in figure 1A. The nucleocapsid proteins of HCoV-229E, HCoV-OC43, and SARS-CoV reacted strongly and specifically with the rabbit serum immune to the corresponding nucleocapsid protein as immunoreactive protein bands on the Western blot (figure 1B). No cross-reactivity was demonstrated among the nucleocapsid proteins of HCoV-229E, HCoV-OC43, and SARS-CoV. These results indicate that no substantial antigenic cross-reactivity occurred among the nucleocapsid proteins of HCoV-229E, HCoV-OC43, and SARS-CoV when immune rabbit serum was used.
Figure 1.
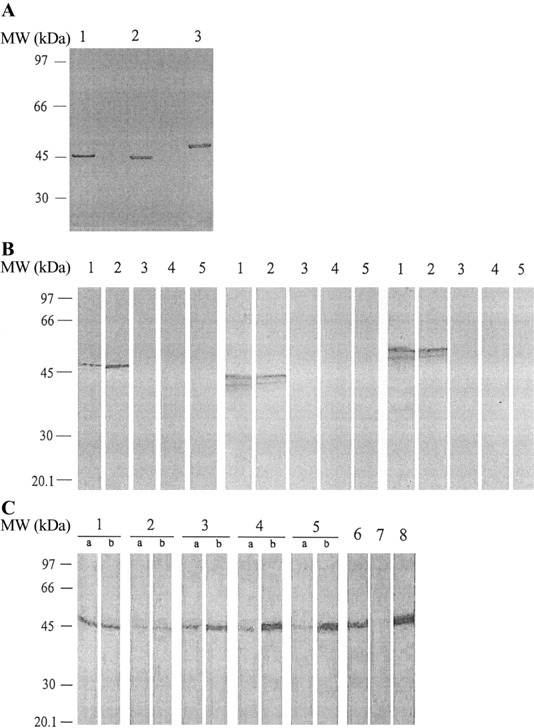
A, Western blot analysis of the expressed recombinant nucleocapsid proteins with anti-His monoclonal antibody. Each protein had a 6 histidine tag at the N-terminal end. Western blot results showed clear and specific bands at molecular weight (MW) positions 47, 44, and 50 kDa, corresponding to the recombinant nucleocapsid proteins of severe acute respiratory syndrome (SARS)—Cassociated coronavirus (CoV) (lane 1), human CoV (HCoV)—229E (lane 2), and HCoV-OC43 (lane 3). B, Western blot analysis of the antigenicities of recombinant nucleocapsid proteins. Left, Nucleocapsid protein of SARS-CoV with SARS-CoV—immune rabbit serum (lane 1), monoclonal antibody to the nucleocapsid protein of SARS-CoV (lane 2), HCoV-229E—immune rabbit serum (lane 3), HCoV-OC43—immune rabbit serum (lane 4), and nonimmune rabbit serum (lane 5). Center, Nucleocapsid protein of HCoV-229E with HCoV-229E—immune rabbit serum (lane 1), monoclonal antibody to the nucleocapsid protein of HCoV-229E (lane 2), SARS-CoV—immune rabbit serum (lane 3), HCoV-OC43—immune rabbit serum (lane 4), and nonimmune rabbit serum (lane 5). Right, Nucleocapsid protein of HCoV-OC43 with HCoV-OC43—immune rabbit serum (lane 1), monoclonal antibody to the nucleocapsid protein of HCoV-OC43 (lane 2), SARS-CoV—immune rabbit serum (lane 3), HCoV-229E—immune rabbit serum (lane 4), and nonimmune rabbit serum (lane 5). All bands appear at the proper MW position. C, Western blot analysis of the HCoV-229E culture filtrates with paired serum samples from patients with SARS (lanes 1—5), HCoV-229E—immune rabbit serum (lane 6), HCoV-OC43—immune rabbit serum (lane 7), and monoclonal antibody to the nucleocapsid protein of HCoV-229E (lane 8). Lanes 4b (patient 4) and 5b (patient 7), which show results of convalescent-phase serum samples from 2 patients with SARS who had high titers in the HCoV-229E nucleocapsid protein—based ELISA, exhibit prominent immunoreactive bands at ∼44 kDa. Reactions with specific monoclonal antibody to the nucleocapsid protein of HCoV-229E are visible at the same MW. Three pairs of acute- and convalescent-phase serum samples had the same reactive band at ∼44 kDa (lane 1, patient 10; lane 2, patient 8; lane 3, patient 3). All bands appear at the proper MW position.
SARS-CoV–immune rabbit serum reacted very strongly with SARS-CoV—infected cells, moderately with HCoV-229E—infected cells, and weakly with HCoV-OC43—infected cells by IFA. Conversely, HCoV-229E—immune rabbit serum reacted very strongly with HCoV-229E—infected cells but did not react with either SARS-CoV- or HCoV-OC43—infected cells. HCoV-OC43—immune rabbit serum reacted very strongly with HCoV-OC43—infected cells and strongly with HCoV-229E—infected cells but did not react with SARS-CoV—infected cells. Furthermore, SARS-CoV— and HCoV-OC43—immune rabbit serum showed weak fluorescent signals from uninfected MRC-5 and BSC-1 cells, compared with the response in nonimmune rabbit serum.
To determine the serological response to nucleocapsid proteins of the 3 CoVs, 100 serum samples collected from healthy donors and 34 serum samples collected from patients with SARS were tested with recombinant nucleocapsid proteins of HCoV-229E, HCoV-OC43, and SARS-CoV using a Western blot analysis. The serum samples from healthy donors showed strong reactivity to the nucleocapsid proteins of HCoV-229E and HCoV-OC43, with positive results in 97% and 99% of the samples, respectively. Only 2 samples (2%) reacted with the SARS-CoV nucleocapsid protein. In contrast, the serum samples from patients with SARS obtained 8–81 days after the onset of symptoms showed strong immunoreactivity to the nucleocapsid proteins of HCoV-229E, HCoV-OC43, and SARS-CoV, with positive results in 97%, 100%, and 100% of the samples, respectively.
When CoV-infected cells were used, results were positive for IgG antibodies by IFA in 98% (HCoV-229E), 100% (HCoV-OC43), and 1% (SARS-CoV) of the serum samples from healthy donors. Two samples from healthy donors had no antibody response to HCoV-229E by IFA and no antibody response to the nucleocapsid protein of HCoV-229E by Western blot analysis. One sample collected in October 2003 had an antibody response to SARS-CoV by IFA and to the nucleocapsid protein of SARS-CoV by Western blot analysis. However, 100% of the samples from 34 patients with SARS had antibody responses to HCoV-229E, HCoV-OC43, and SARS-CoV. In healthy donors, the results of the IFA showed the presence of antibodies in response to the nucleocapsid proteins of HCoV-229E and HCoV-OC43 in association with the presence of IgG antibodies to both HCoVs, but antibodies to SARS-CoV were absent in all samples except 1, which had a low antibody titer of 1:10, compared with the usual antibody titer of at least 1:100 in most patients with SARS. The serum samples from patients with SARS had antibody responses to SARS-CoV as well as to HCoV-229E and HCoV-OC43 when nucleocapsid proteins were used in the Western blot analysis and when CoV—infected cells were used in the IFA.
Paired serum samples from 11 patients with SARS were used to determine antigenic relationships among the 3 CoVs by IFA and ELISA. The antibody titers to HCoV-229E, HCoV-OC43, and SARS-CoV are shown in table 1. To determine which viral antigen was responsible for the cross-reactions, the culture filtrate from cells infected with HCoV-229E and HCoV-OC43 was immunoblotted with the paired serum samples. A strong band at ∼44 kDa, the same molecular weight at which there was a reaction with a specific monoclonal antibody to the nucleocapsid protein of HCoV-229E, was observed in convalescent- phase samples from 2 patients with SARS (figure 1C). These serum samples also displayed higher antibody titers in the HCoV-229E nucleocapsid protein—based ELISA. The paired serum samples from the 9 other patients also had a weak or moderate reactive band at ∼44 kDa when they were immunoblotted with HCoV-229E. Acute-phase or convalescent-phase serum from 11 paired serum samples had a weak reactive band at ∼50 kDa, the same molecular weight at which there was a reaction with a specific monoclonal antibody to the nucleocapsid protein of HCoV-OC43 when it was immunoblotted with HCoV-OC43 (data not shown).
Discussion. Our results indicate that no nucleocapsid protein antigenic cross-reactivity was found between SARS-CoV and rabbit serum immune to either HCoV-229E or HCoV-OC43. In our previous studies, neither specific monoclonal nor polyclonal antibodies to the nucleocapsid protein of SARS-CoV crossreacted with HCoV-229E or HCoV-OC43 [8, 10]. However, a previous study has described cross-reactivity between the nucleocapsid proteins of SARS-CoV and those of group I CoVs [4]. This cross-reactivity may depend on the type of serum used. Another investigator has demonstrated that HCoV-229E—immune animal serum cross-reacted with SARS-CoV [1]. In the present study, when we used immunofluorescent staining of CoV-infected cells, HCoV-229E— and HCoV-OC43—immune rabbit serum did not cross-react with SARS-CoV—infected cells, whereas SARS-CoV—immune rabbit serum had moderate cross-reactivity with HCoV-229E—infected cells and weak cross-reactivity with HCoV-OC43—infected cells. Although SARS-CoV—immune rabbit serum that had high antibody titers for SARS-CoV was slightly contaminated with antibodies to host cell components, it is apparent that the serum had cross-reactivity with HCoV-229E and HCoV-OC43. In addition, the IFA showed that HCoV-OC43—immune rabbit serum had strong cross-reactivity with HCoV-229E. This cross-reactivity has been observed by some investigators [11, 12] but not by others [13, 14]. It is possible that some antibodies in the rabbit serum reacted against the host cells or cross-reacted with HCoV-229E.
Because the 2 known HCoVs are responsible for ∼30% of all common colds [2], it is not unexpected that 97% and 99% of serum samples from healthy donors had antibodies to HCoV-229E and HCoV-OC43, respectively. Therefore, it is expected that the antibodies to HCoV-229E and HCoV-OC43 found in the serum samples from patients with SARS either preexisted or were cross-reacting antibodies to HCoV-229E and HCoV-OC43. Further studies of this issue are warranted. The IFA showed that paired serum samples exhibited a ⩾4-fold increase in antibody titers against HCoV-229E and HCoV-OC43 in 5 of 11 and 10 of 11 patients with SARS, respectively. Such a high antibody titer response to the known HCoVs in patients with SARS may represent an anamnestic reaction to previous infections with the 2 known HCoVs or other CoVs or a crossreaction between SARS-CoV and HCoV-229E or HCoV-OC43. However, the nucleocapsid protein—based ELISA detected increases in antibody titers in only 2 of 11 paired serum samples from patients with SARS when the nucleocapsid protein of HCoV-229E was used as an antigen, and they had a consistently increased signal reaction to the nucleocapsid protein from HCoV-229E—infected cell culture filtrate by Western blot analysis (figure 1C). Paired serum samples from patients with SARS showed no consistent increase in antibody titers in the HCoV-OC43 nucleocapsid protein—based ELISA and no increase in signal reaction to the nucleocapsid protein from HCoV-OC43—infected cell culture filtrate by Western blot analysis. These results confirm the suggestion that the major antigenic crossreactivity with HCoV-OC43 in the convalescent-phase serum samples of patients with SARS is due not to nucleocapsid proteins but to other viral components. Although the paired serum samples from patients with SARS showed a partial cross-reaction to HCoV-229E, there was no significant close correlation between the IFA titer ratios, which were determined in response to whole virus—infected cells, and the ELISA titer ratios, which were determined in response to nucleocapsid proteins. Furthermore, antibodies to SARS-CoV could be detected in only 1 serum sample from a healthy donor by either IFA or nucleocapsid protein–based Western blot analysis, even though patients with SARS had antibodies to HCoV-229E and HCoV-OC43. Therefore, there is no serological cross-reactivity with SARS-CoV in healthy donors even though they have a high reactivity to HCoVs [15].
In summary, SARS-CoV had apparent antigenic 1-way crossreactivity to the 2 known HCoVs. Although it is not clear which antigenic determinants were involved, the overall results suggest that SARS-CoV and HCoV-OC43 are more closely antigenically related than are SARS-CoV and HCoV-229E. Studies using a number of purified recombinant viral components from CoVs as antigens to identify the antibodies produced during infection and to determine the antigenic relationships among CoVs are in progress.
Acknowledgments
We thank Biao Di (Centers for Disease Control and Prevention of Guangzhou, People's Republic of China), for providing the serological data of patients with severe acute respiratory syndrome for analysis, and San Francisco Edit, for assistance in editing the manuscript.
Footnotes
Financial support: Ministry of Science and Technology of the People's Republic of China Special Programs of SARS; Research Project of Guangdong Province for SARS Prevention and Treatment.
References
- 1.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 2.Myint SH. Human coronavirus infections. In: Siddell SG, editor. The Coronaviridae. New York: Plenum Press; 1995. pp. 389–401. [Google Scholar]
- 3.Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 4.Sun ZF, Meng XJ. Antigenic cross-reactivity between the nucleocapsid protein of severe acute respiratory syndrome (SARS) coronavirus and polyclonal antisera of antigenic group I animal coronaviruses: implication for SARS diagnosis. J Clin Microbiol. 2004;42:2351–2. doi: 10.1128/JCM.42.5.2351-2352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang LR, Chiu CM, Yeh SH, et al. Evaluation of antibody responses against SARS coronaviral nucleocapsid or spike proteins by immunoblotting or ELISA. J Med Virol. 2004;73:338–46. doi: 10.1002/jmv.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo PC, Lau SK, Tsoi HW, et al. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Lancet. 2004;363:841–5. doi: 10.1016/S0140-6736(04)15729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KH, Poon LL, Cheng VC, et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294–9. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Che XY, Qiu LW, Pan YX, et al. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. 2004;42:2629–35. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogue BG, King B, Brian DA. Antigenic relationships among proteins of bovine coronavirus, human respiratory coronavirus OC43, and mouse hepatitis coronavirus A59. J Virol. 1984;51:384–8. doi: 10.1128/jvi.51.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau SK, Woo PC, Wong BH, et al. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in SARS patients by enzyme-linked immunosorbent assay. J Clin Microbiol. 2004;42:2884–9. doi: 10.1128/JCM.42.7.2884-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradburne AF. Antigenic relationships amongst coronaviruses. Arch Gesamte Virusforsch. 1970;31:352–64. doi: 10.1007/BF01253769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntosh K, Kapikian AZ, Hardison KA, Hartley JW, Chanock RM. Antigenic relationships among the coronaviruses of man and between human and animal coronaviruses. J Immunol. 1969;102:1109–18. [PubMed] [Google Scholar]
- 13.Pedersen NC, Ward J, Mengeling WL. Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Arch Virol. 1978;58:45–53. doi: 10.1007/BF01315534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt OW, Kenny GE. Immunogenicity and antigenicity of human coronaviruses 229E and OC43. Infect Immun. 1981;32:1000–6. doi: 10.1128/iai.32.3.1000-1006.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


