Abstract
Background. Severe acute respiratory syndrome (SARS) is a newly recognized infectious disease that has recently emerged in East Asia and North America. Although the clinical features of acute infection have been well described, mildly symptomatic or asymptomatic infections have not been well characterized.
Objective. To assess the spectrum of illness in health-care workers (HCWs).
Methods. A prospective seroepidemiologic cohort study was conducted on 372 HCWs in a large teaching hospital in Singapore who were both exposed and not exposed to patients with SARS. Participating HCWs completed a questionnaire and provided paired serum samples, which were analyzed by 2 different laboratories blinded to clinical data, by use of an enzyme-linked immunosorbent assay based on a protocol developed by the Centers for Disease Control and Prevention and a dot-blot immunoassay, with confirmation by a viral neutralization assay.
Results. A total of 21 patients with SARS were treated at our hospital. They were associated with transmission to 14 staff members, patients, and visitors in our hospital. Of the 372 HCWs participating in the present study, 8 were found to have positive antibodies to the SARS coronavirus in both samples by use of both test methods, and 6 had pneumonia and had been hospitalized for either probable or suspected SARS infection, whereas 2 had fever but did not have changes on chest radiographs. All seropositive HCWs had been exposed either directly or indirectly to patients with SARS. No asymptomatic, nonexposed staff members were found to be seropositive. There was a trend towards protection for HCWs who, while fully protected, had had contact with patients with SARS.
Conclusions. Although the majority of cases of SARS are associated with pneumonia, a small number of mildly symptomatic individuals do seroconvert. HCWs who are exposed to patients with SARS can be infected with SARS, regardless of the intensity of exposure. This has implications for surveillance and infection control planning, in the event that SARS returns next winter.
Severe acute respiratory syndrome (SARS) is a newly recognized coronavirus infection that recently emerged in East Asia, with subsequent global spread [1–3]. In Singapore, cases of SARS were diagnosed in early March 2003, and, on March 17, the Singapore Ministry of Health announced that all individuals with highly suspected or probable SARS infection were to be transferred to a designated SARS hospital, the Tan Tock Seng Hospital (TTSH), where the individual representing the index case and her contacts were treated [4]. TTSH was closed to individuals without SARS infection. Unfortunately, patients and visitors to TTSH during the preclosure period later presented to other hospitals in Singapore and triggered epidemics there [5]. Hospitals have been the major foci of infection, especially in Singapore, where >80% of cases have occurred in visitors, health-care workers (HCWs), and other patients who were in the same rooms as patients with unrecognized SARS [5]. The clinical features of typical SARS have been well described in large clinical studies [6–8]. Atypical presentations with no fever but with changes on chest radiographs have also been reported [9]. Asymptomatic or mild infections with no respiratory symptoms and no changes on chest radiographs have, however, not been previously reported to have occurred in contacts of patients with SARS, with the exception of 1 case report [10]. This has been documented in other emerging viral infections and has potential implications for the transmission and control of this emerging infection [11, 12].
The National University Hospital (NUH), Singapore, is a 900-bed teaching hospital that employs ∼3000 doctors, nurses, allied health professionals, and clerical staff members. Between 18 March and 29 April 2003, a total of 21 patients with SARS were treated in the hospital wards and emergency department before being transferred to the designated SARS hospital. An escalated policy on the use of more-complete personal protective equipment (PPE) and isolation of suspected patients was instituted, beginning with the required wearing of N-95 masks, gowns, and gloves for isolation-ward personnel only. Later, this was extended to personnel in all areas of the hospital, with regular audits of all HCWs. We conducted a seroepidemiologic study of HCWs in our hospital to assess the spectrum of illness seen in HCWs infected with the SARS virus, in particular to document whether asymptomatic or mild infections with no respiratory symptoms and no changes on chest radiographs occur in individuals with SARS.
Subjects, Materials, and Methods
After giving written, informed consent, unselected HCWs were recruited on a voluntary basis from all areas of the hospital, beginning with high-risk areas and extending to low-risk areas, including outpatient clinics and offices. They completed a simple questionnaire describing their workplaces, contact with patients with SARS, use of PPE, and symptoms experienced during the preceding 4 weeks. They also provided paired serum samples, which were collected initially at the peak of the outbreak and subsequently at a median interval of 31 days (range, 17–53 days) after initial collection. Samples were anonymized to ensure that the confidentiality of HCWs was preserved. The study was approved by the hospital institutional review board. All policies and procedures of the Ministry of Health, Singapore, Good Clinical Practice were followed in the conduct of this study.
Serum samples were stored at −80°C and subsequently were sent to 2 different external laboratories for serologic testing. The laboratory staff were unaware of the clinical details of the patients. The first laboratory used an ELISA based on a protocol and using antigens provided by Tom Ksiazek (Centers for Disease Control and Prevention, Atlanta, GA) [1]. Samples found to be positive for SARS by ELISA were confirmed by use of an indirect immunofluorescence assay. The second test was done at the National Environmental Agency, Singapore, and used a dot-blot immunoassay using antigens derived from virus culture supernatant. Positive samples, at a titer of ⩾1:100, were then subjected to a virus neutralization assay, in serial 2-fold dilution, starting at 1:10–1:320 and using amicroneutralization format described elsewhere for human enterovirus 71 [13]. A neutralizing antibody titer of ⩾10 was considered to be positive for SARS. Serum samples from volunteer patients with SARS and from nonexposed laboratory staff were included in all serologic assays as positive and negative controls, respectively. Thus, all the serum samples were tested by use of 2 screening tests (i.e., ELISA and dot-blot immunoassay), and the positive serum samples were confirmed by indirect immunofluorescence assay and virus neutralization assay, respectively. Results of the serologic testing from each laboratory were not revealed to the other laboratory until after completion of the study.
Definitions. A seropositive individual was defined as having provided a serum sample that received a positive confirmatory result by both the indirect immunofluorescence assay and the virus neutralization assay. An exposed HCW was defined as having worked in an area where a patient later confirmed to have SARS had been cared for. Direct contact was defined by use of the World Health Organization (WHO) definition of having cared for, having lived with, or having had direct contact with respiratory secretions and/or body fluids of an individual with SARS [14]. HCWs who worked in the same ward but did not have direct responsibility for patients with SARS or did not come into physical contact with respiratory secretions and/or body fluids of an individual with SARS would thus be defined as exposed-only HCWs.
Patients with SARS were defined by use of WHO criteria [14] for probable cases, which included fever (temperature >38°C), respiratory symptoms, and radiographic evidence of pneumonia or respiratory distress. Mildly symptomatic individuals were defined as those with fever significant enough to warrant evaluation at the staff clinic or emergency department but with no evidence of pneumonia on chest radiographs and prompt resolution of symptoms (within 48–72 h of symptomatic therapy).
Statistical analysis. Differences between groups were assessed by the χ2 test, Fisher's exact test, Mann-Whitney U test, Kruskal-Wallis test, or relative risk ratios with 95% confidence intervals, as appropriate. The results of these comparisons were reported as P values. All statistics were analyzed by use of the SPSS (version 11.5.1; SPSS) and STATA (version 7.0; StataCorp) software.
Results
Description of patients with SARS seen at NUH. The first patient with SARS seen in our hospital was a cardiology resident from TTSH who entered our emergency department on 18 March 2003. Since then, a total of 21 patients with SARS, including 5 HCWs from our hospital, have been seen in our wards and emergency department. All of these patients had positive antibodies to the SARS coronavirus. Six of the 7 tested also had SARS coronavirus isolated from stool samples, blood samples, and/or respiratory secretions. These 21 patients stayed in NUH a mean ± SD of 3.9 ± 4.8 days from admission or onset of symptoms to transfer to TTSH. There were 14 known nosocomial transmissions to staff members, visitors, and other patients, all of whom were eventually transferred to TTSH for treatment; 11 of these were linked to a single atypical case [15]. Initial policies on the use of PPE for HCWs, instituted on 17 March 2003, confined the mandatory use of gloves, gowns, and N95 masks to isolation wards only but, by 28 March, were extended to include intensive-care units and the emergency department. On 9 April, after the identification of an atypical case of SARS in an open general medical ward, full use of PPE was made mandatory for all staff in contact with patients.
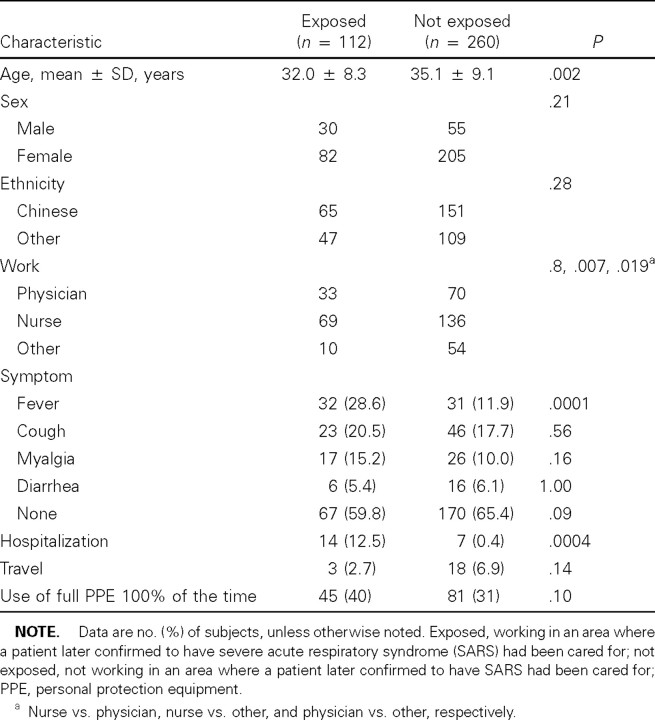
Demographic and clinical characteristics of participating HCWs. A total of 372 staff members participated, of whom paired serum samples were obtained from 303 (81.5%). Serum was drawn at 17–53 day intervals, beginning on 22 April and continuing to 5 June 2003—that is, beginning 4 weeks after the first case of SARS in NUH and ending 10 weeks after the last case was transferred to TTSH. Overall, mean ± SD age was 34.2 ± 9.0 years, and 287 (77.2%) were women, 103 (27.7%) were physicians, 205 (55.1%) were nurses, and the rest were allied health professionals and clerical staff members. One hundred twelve (30.1%) worked in areas where patients with SARS had been cared for (i.e., the exposed group), and the rest had no exposure at all to patients with SARS (i.e., the nonexposed group). The characteristics of the HCWs are listed in table 1. The exposed group was younger and included more nurses, probably because of a higher representation of emergency department and intensive-care unit staff members in this group. A large number of staff members reported a variety of symptoms during the study period, including fever (n = 63 [16.9%]), cough (n = 69 [18.5%]), and diarrhea (n = 22 [5.9%]). There was a significant difference in the frequency of fever between exposed (28.6%) and nonexposed (11.9%) HCWs (P = .0001). Twenty-one HCWs (5.6%) in our study cohort were hospitalized during this period, of whom 6 were classified by clinical criteria as probably infected with SARS. Twenty-one (5.6%) of the participants or their spouses had traveled to other SARS-affected areas during the study period.
Table 1.
Characteristics of health-care workers.
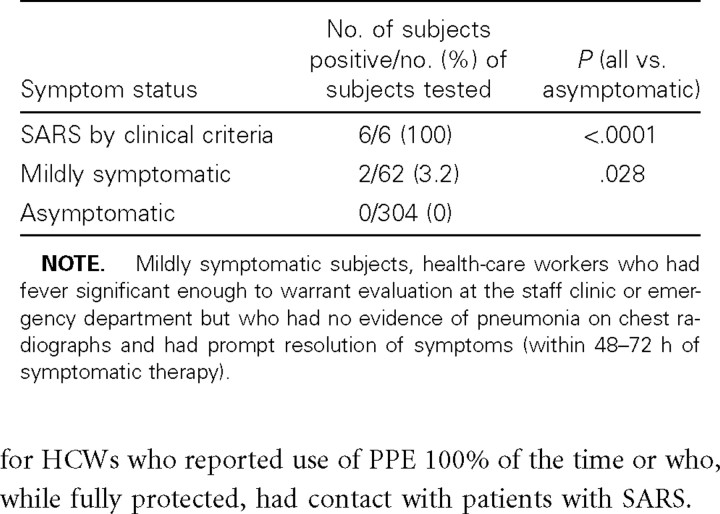
Serologic data on HCWs (table 2). Samples from 8 HCWs (2.2%) were found to be positive for SARS-associated coronavirus by ELISA, and these results were confirmed by indirect immunofluorescence assay using the CDC protocol, and samples from the same HCWs were found to be positive by use of the dot-blot method, and these results were confirmed by viral neutralization assay. Both samples tested for each HCW were found to be positive for all 8 HCWs, and no increase in titer could be demonstrated on their paired samples, since, from all 8 subjects, samples were obtained >4 weeks after the onset of symptoms. All 6 HCWs hospitalized at TTSH (including 5 who were first treated in our hospital) for probable SARS infection were found to be seropositive, and 2 additional individuals who had fever but did not meet SARS criteria (i.e., had symptoms <4 days in duration and had no changes on chest radiographs) were also seropositive. On the basis of data submitted anonymously for the study, as well as a review of hospital epidemiology and contact-tracing data, a profile of these 2 individuals could be constructed without compromising confidentiality: both worked in the emergency department at a time when patients with SARS were being treated in the department, wore full PPE, and did not have direct personal contact with any patients with SARS. One was admitted to an isolation room with fever, chills, and myalgias, which resolved completely within 3 days of symptomatic treatment; she had no changes on serial chest radiographs. The other was treated as an outpatient in the staff clinic; she presented with fever and upper respiratory symptoms. Again, chest radiographs were normal, and symptoms resolved within 3 days. No secondary cases resulted from any of the infected HCWs. No HCW who was completely asymptomatic was found by serologic testing to have SARS-associated coronaviral infection. It is interesting to note that, even after removing these 8 seropositve HCWs from the exposed and febrile groups in table 1, the difference between the exposed and nonexposed groups, in relation to being febrile, remains statistically significant (23.1% vs. 11.9%; P = .0095).
Table 2.
Seroprevalence of antibodies to severe acute respiratory syndrome (SARS) coronavirus.
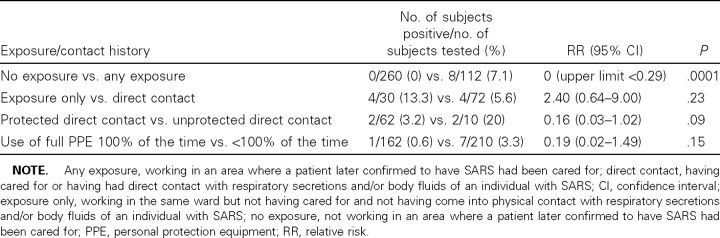
Relationship between serologic test results and exposure history. When analyzed by contact history, all of the seropositive HCWs worked in areas where patients with SARS had been cared for (table 3). Although only 4 of 8 had had direct contact by WHO definition, the remaining 4 worked in the same ward where the patients were located but did not have direct responsibility for these patients or come into physical contact with these patients' body fluids. Although the numbers of subjects in our study are small, there was a trend towards protection for HCWs who reported use of PPE 100% of the time or who, while fully protected, had contact with patients with SARS.
Table 3.
Seroprevalence of severe acute respiratory syndrome (SARS), by contact history.
Discussion
SARS is a novel coronavirus that has recently emerged in southern China and has caused widespread disruption to health-care services and international trade, especially in East Asia [16]. The vast majority of infections, with a few notable exceptions, have occurred in hospitals. As with all emerging infections, the clinical picture is only beginning to be described completely. The initial descriptions included atypical pneumonia that followed a prodrome with fever and myalgia [6–8] and that progressed almost universally to a severe respiratory illness, with a variety of changes on chest radiographs [17]. In approximately one-sixth of cases, this eventually progressed to acute respiratory distress syndrome and death [18]. Later reports highlighted gastrointestinal symptoms as a major element of a large community outbreak of SARS that was thought to be linked to environmental contamination [19]. Atypical presentations have also been reported, but all of these eventually led to the typical pattern of progressive respiratory distress with frank changes on radiographs [9].
The strengths of the present study include the prospective nature of the collection of serum samples and acquisition of clinical data, an adequate sample size with representative subjects from all areas of a large teaching hospital, use of paired serum samples, and application of 2 previously validated serologic tests, which were performed independently. The present study is the first to document SARS infection in HCWs with normal chest radiographs. Two of our seropositive HCWs had fever for <1 week and responded to symptomatic therapy for upper respiratory-tract infections. Their chest radiographs were repeatedly normal. Thus, they did not meet the clinical criteria for probable SARS infection. The possibility that our 2 individuals with mild illness were false positives is diminished by the fact that they were found to be positive on both assays, which were done by laboratory staff who were blinded to clinical data. That none of the nonexposed HCWs had evidence of infection and the unpublished reports that blood donors in a variety of settings had no serologic evidence of SARS support our hypothesis that these were indeed mild infections. Given our experience with other viral respiratory tract infections in which a spectrum of infections is the rule, the detection of mildly symptomatic infections is not surprising.
We also failed to detect seroconversion in totally asymptomatic individuals. This was not the experience of other investigators screening contacts of the Nipah virus or avian influenza, but those were retrospective serologic studies done some time after the outbreak, and mild clinical symptoms might not have been detected [11, 12]. Given the fact that the SARS coronavirus seems to be a completely novel pathogen with minimal genetic relatedness to other coronaviruses of humans and animals [20], it is perhaps not that surprising that it produced at least some clinical disease in all infected individuals with no preexisting cross-protective immunity. This is supported by our observation that, in our hospital, individuals who were exposed to patients with SARS had a significantly higher prevalence of fever.
The second important finding we observed is that individuals who did not have direct contact with patients with SARS also both developed clinical SARS and experienced seroconversion with milder illness. In such a setting, transmission is likely to occur on either environmental surfaces or the hands of other HCWs. However, our study strikingly shows the complete absence of transmission to individuals working in the same building but in different areas from locations where patients with SARS had been cared for. This also has implications, since it bears out our experience with the global epidemiology of SARS, in which, WHO global travel alerts notwithstanding, almost all transmissions in Hong Kong, Taiwan, Toronto, and Singapore and the majority in mainland China could be traced back to specific household or health-care settings. This should be taken into consideration in the event that SARS is detected again next winter, before widespread economic disruption is created by travel alerts and advisories.
Neither of the 2 individuals with mild cases of SARS was associated with any secondary transmission, despite not being isolated or quarantined for any significant period of time. Although the majority of cases of SARS in Singapore did not lead to any secondary infections [5], it is reassuring that mildly symptomatic cases are hopefully associated with lower virus loads [19] and less likely trigger epidemics. These cases might, however, allow for a low level of transmission of the virus, which might remain “below the radar” if they are not actively sought. This has been a concern with the reemergence of SARS in Toronto after a period during which transmission was believed to have been halted [21].
Data on SARS continue to emerge. We have shown that, as with most viral infections, there is a spectrum of illness associated with SARS, from mild febrile illness to severe respiratory distress. With data emerging about an animal reservoir [22] for SARS, it will be critical to detect periodic human infections, even if mild. Although SARS seems to have disappeared in the summer months, broad surveillance using more-sensitive assays will be critical in the event that SARS reappears next winter.
Acknowledgement
We are grateful to all the health-care workers who participated in this study. We would also like to thank the medical students Mo-Yee Chau, Hui-Yi Chia, Cherylin Foo, and Teck- Wei Tan for help with data entry.
References
- 1. Ksiazek TG, Erdman D, Goldsmith C, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003348:1953–66 [DOI] [PubMed] [Google Scholar]
- 2. Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003348:1967–76 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention Update: outbreak of severe acute respiratory syndrome—worldwide, 2003. MMWR Morb Mortal Wkly Rep. 200352:241–8 [PubMed] [Google Scholar]
- 4. Hsu LY, Lee CC, Green JA, et al. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis. 20039:713–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention Update: severe acute respiratory syndrome—Singapore, 2003. MMWR Morb Mortal Wkly Rep. 200352:405–11 [PubMed] [Google Scholar]
- 6. Lee N, Hui D, Alan W, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003348:1986–94 [DOI] [PubMed] [Google Scholar]
- 7. Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003348:1995–2005 [DOI] [PubMed] [Google Scholar]
- 8. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003289:2801–9 [DOI] [PubMed] [Google Scholar]
- 9. Fisher DA, Lim TK, Lim YT, Singh KS, Tambyah PA. Atypical presentations of SARS. Lancet. 2003361:1740. [DOI] [PubMed] [Google Scholar]
- 10. Li G, Zhao ZX, Chen LB, Zhou YH. Mild severe acute respiratory syndrome. Emerg Infect Dis. 20039:1182–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan KP, Rollin PE, Ksiazek TG, et al. A survey of Nipah virus infection among various risk groups in Singapore. Epidemiol Infect. 2002128:93–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bridges CB, Katz JM, Seto WH, et al. Risk of influenza A (H5N1) among healthcare workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000181:344–8 [DOI] [PubMed] [Google Scholar]
- 13. Ooi EE, Phoon MC, Ishak B, Chan SH. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 20028:995–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization Global surveillance for severe acute respiratory syndrome (SARS). Wkly Epidemiol Rec. 200378:100–19 [PubMed] [Google Scholar]
- 15. Fisher DA, Chew MH, Lim YT, Tambyah PA. Preventing local transmission of SARS: lessons from Singapore. Med J Aust. 2003178:555–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003361:1319–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muller NL, Ooi GC, Khong PL, Nicolau S. Severe acute respiratory syndrome: radiographic and CT findings. AJR Am J Roentgenol. 2003181:3–8 [DOI] [PubMed] [Google Scholar]
- 18. Lew TW, Kwek TK, Tai D, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003290:374–80 [DOI] [PubMed] [Google Scholar]
- 19. Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003361:1767–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruan YJ, Wei CL, Ling AE, et al. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet. 2003361:1779–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention Update: severe acute respiratory syndrome—Toronto, Canada, 2003. MMWR Morb Mortal Wkly Rep. 200352:547–550 [PubMed] [Google Scholar]
- 22. Guan Y, Zheng BJ, He YQ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003302:276–8 [DOI] [PubMed] [Google Scholar]