Abstract
Severe acute respiratory syndrome (SARS) coronavirus has been known to damage multiple organs; however, little is known about its impact on the reproductive system. In the present study, we analyzed the pathological changes of testes from six patients who died of SARS. Results suggested that SARS caused orchitis. All SARS testes displayed widespread germ cell destruction, few or no spermatozoon in the seminiferous tubule, thickened basement membrane, and leukocyte infiltration. The numbers of CD3+ T lymphocytes and CD68+ macrophages increased significantly in the interstitial tissue compared with the control group (P < 0.05). SARS viral genomic sequences were not detected in the testes by in situ hybridization. Immunohistochemistry demonstrated abundant IgG precipitation in the seminiferous epithelium of SARS testes, indicating possible immune response as the cause for the damage. Our findings indicated that orchitis is a complication of SARS. It further suggests that the reproductive functions should be followed and evaluated in recovered male SARS patients.
Keywords: immunohistochemistry, in situ hybridization, orchitis, SARS, spermatogenesis, testis
Introduction
Since the first appearance in Guangdong province, China, in November 2002, the global outbreak of severe acute respiratory syndrome (SARS) had spread to more than 28 countries in three continents, and resulted in more than 8000 infections and close to 800 deaths within the following 9 mo [1]. For what appeared initially as a mere infection of the respiratory tract like a common cold, the death toll was alarming, and many lives of health-care workers were claimed. With vigilant public health controls and strict preventive measures on further spread within the hospital environment, the epidemic was brought under control. The concern over this global outbreak brought together scientists from all over the world, and with their joint effort, the SARS virus, a novel coronavirus, was isolated, and subsequently the full genomic sequences of the SARS virus were determined [2–4]. Although a lot had been learned about the epidemiology, mode of spread, and certain aspects of the pathogenesis, a number of SARS-related complications are still waiting to be studied.
Pathological studies revealed that the lungs of SARS patients had the most dramatic changes, with severe degeneration of the epithelium, hyaline membrane formation, exudation of fibrin fluid, and vasculitis, with many alveoli collapsed [5, 6]. In addition, other organs were also infected and damaged by the SARS virus. The targets included the lymphocytes, the epithelium of the distal tubules of the kidney, and the submucosal lymphoid complex of the gut, the spleen, and the lymph nodes [7, 8]. However, only sporadic information is available regarding the involvement of reproductive organs in SARS patients [9, 10]. Because it is known that viruses such as HIV, HBV, and mumps can enter the testis and cause viral orchitis and, in some instances, result in male infertility and testicular tumor [11], we investigated the possible damage of the testis in SARS patients and the effects of SARS on spermatogenesis.
Materials and Methods
Materials
Autopsy specimens of testis were obtained from six patients (cases S01, S03, S05, S08, S11, and S15) who died of SARS in Ditan Hospital, Beijing, China. The case numbers were derived from the Department of Pathology, Peking University, and the gaps in numbers were female patients who were not included in this study. The patients’ ages ranged from 20 to 58 yr old (average 39 yr). All six patients met the diagnostic criteria for SARS defined by the World Health Organization (WHO) [12]. The average course of disease was 43 days, ranging from 21 to 62 days. Five of these patients were treated with steroids, except case S05. More clinical data are presented in Table 1.
Table 1.
Clinical data for the 6 SARS cases.
| Cases | Age (yr) | Fever (initial temp. [°C]) | Course of disease (days) | Steroid treatmenta | SARS pathogen (real-time PCR or ISH)b | Fathered childrenc |
|---|---|---|---|---|---|---|
| S01 | 51 | 38 | 45 | + | + | + |
| S03 | 50 | 39 | 33 | + | + | + |
| S05 | 31 | 38.5 | 35 | - | + | NA |
| S08 | 24 | 39 | 21 | + | + | - |
| S11 | 20 | 38 | 62 | + | + | - |
| S15 | 58 | 38 | 62 | + | + | + |
Treatment with (+) or without (—) steroids.
Positive for SARS pathogen indicated by +.
Child indicated by +; no child indicated by —; NA, information not available.
Four non-SARS specimens of testis were taken as controls (C01–C04). Cases C01 and C02 died of accidents (aged 30 and 42 yr). Case C03 died of a disease with high fever and was treated with steroids before death (aged 38 yr). The specimens of case C04 were collected from a person during his/her transsexual operation (aged 28 years and the patient was routinely treated with estrogen before operation). All tissues were fixed in 10% formaldehyde and embedded in paraffin.
The use of the specimens and related ethical issues were reviewed and approved by the Research Administration Committee of the Peking University.
Morphological Analysis
Six-micrometer paraffin sections were cut and stained with hematoxylin–eosin. Apoptotic cells were detected by TUNEL assay using TdT-Frag EL DNA Fragmentation Detection Kit, according to the instructions provided by the supplier (Calbiochem, San Diego, CA). In brief, after dewaxing and rehydration, the sections were covered with 1× TdT balance buffer and incubated at room temperature for 15–30 min, followed by addition of TdT-labeled reaction mixture with enzyme, and incubated at 37°C for 1 h. TBS was applied instead of TdT enzyme for negative control. These sections were rinsed with TBS between incubations at room temperature with stop solution for 5 min, 3% H2O2 for 5 min, and blocking buffer for 10 min. Finally, peroxidase-streptavidin conjugate was added at room temperature for 30 min. After another rinse with TBS, the sections were incubated for color reaction with DAB solution at room temperature for 10–15 min. All slides were counterstained with Mayer hematoxylin for 30 sec.
Slide evaluation and image analysis were performed under a light microscope (Nikon, E800) at 200× magnification.
Immunohistochemistry
Sections were dewaxed and rehydrated and then heated in glycine buffer (glycine 3.75 g, EDTA 0.1 g, in 1 L distilled water, pH: 3.6) in a microwave oven for 10 min × 2, cooled to room temperature before washing with distilled water. The sections were then treated with 1% H2O2/methanol for 10 min, washed with PBS, and then incubated with blocking solution for 1 h at 4°C in a humidified chamber. Specific mouse monoclonal antibodies against CD3 and CD68 (Zymed Lab, San Francisco, CA) diluted in blocking solution to 5μg/ml, and HRP (horseradish peroxidase)-conjugated goat anti-human IgG (Jackson ImmunoResearch Lab, West Grove, PA) diluted in blocking solution to 3μg/ml were applied to the sections and incubated overnight at 4°C. Slides for CD3 and CD68 were washed with PBS and biotinylated anti-mouse IgG antibody (Vector Labs, Burlingame, CA) in 1:200 was applied for 1 h, then washed in PBS, followed by avidin-biotin complex (Vector Labs) for 30 min at 4°C. After washing with PBS, the sections were incubated with DAB solution (Zymed Lab) for 5–10 min. Slides for IgG were incubated in 3-Amino-9-ethylcarbazole (Sigma, St. Louis, MO) for 5–10 min.
For negative control, the CD3 or CD68 antibody was replaced with the blocking solution. To avoid the possible unspecific reaction between the goat IgG and human tissue, an additional control (isotype control) was performed in which HRP-conjugated goat anti-human IgG was replaced with HRP-conjugated goat IgG (isotype control antibody, Jackson ImmunoResearch Lab, West Grove, PA).
All slides were counterstained with Mayer hematoxylin for 30 sec. The positive reaction for CD3 or CD68 was brown and IgG was red.
In Situ Hybridization
Probe preparation
Sense and antisense digoxigenin-labeled RNA probes were kindly provided by Dr. Bo Zhang [13]. Briefly, a pair of primers were designed based on SARS coronavirus genome sequence (GenBank, Accession AY274119). The sequences of the primers were: A: 5′-GCGCAAGTATTAAGTGAGATG-3′ (15348–15368 nt); B: 5′-GAAGTGCATTTACATTGGC-3′ (15 473–14 492 nt). Total RNA was extracted from peripheral blood of a SARS patient with TRIZOL reagent (Roche). The RT-PCR products were purified and confirmed by sequencing. Then the RNA probe was prepared by in vitro transcription with the label of digoxigenin. The concentration of probes was estimated at about 50 μg/ml.
In situ hybridization staining
The paraffin-embedded sections were dewaxed, rehydrated, and then immersed in 0.1 N HCl for 10 min before digestion with proteinase K (50 μg/ml) at 37°C for 20 min. They were then postfixed in 4% paraformamidehyde-PBS at room temperature for 10 min, and then dehydrated in a series of increasing concentrations of ethanol and air dried. Then 20 μl of hybridization solution, consisting of 50 ng labeled probe, 50% formamid, 100 μg/ml yeast tRNA, 0.1 M DTT, 5× Denhardt, 10% dextran sulfate, and 2× SSC, was added to the sections and incubated at 55°C for 16 h. The washes for posthybridization were carried out in 50% formamid/2× SSC at 55°C for 30 min, three times in 2× SSC at 55°C, and 0.1× SSC for 20 min at room temperature. After blocking with horse serum (1:100) for 60 min, AP-labeled anti-Dig antibody (1:500) was added for 60 min at room temperature. Color reaction was achieved with NBT/BCIP and counterstained with methyl-green. The negative control was performed without any probe, and the sections of SARS lungs were stained as positive controls.
Histomorphological Evaluation
Each experiment for every specimen was repeated at least three times. Five random fields were picked per section using the 20× objective on a light microscope (E800; Nikon). Tubules that contained positively stained cells were recorded as positive tubules. The total numbers of positively and negatively stained cells in tubules and interstitial tissue in the fields captured were counted, and the percentage of positive cells was derived from total cell counts. The difference between mean values of SARS and control groups was analyzed by Student t-test (SAS software), and the level of significance was determined as P < 0.05. All assessments were made in a double-blinded fashion.
Results
Histomorphology
In the control group, testes of cases C01, C02, and C04 displayed normal morphology (Fig. 1A). Testis of case C03 showed no significant germ cell loss. There was minimal peritubular fibrosis and vascular congestion in the interstitial tissue (Fig. 1B). All SARS testes demonstrated extensive germ cell destruction, with few or no spermatozoon in the seminiferous epithelium and the lumen. The basement membrane was thickened, and there was peritubular fibrosis. Leukocyte infiltration and vascular congestions were present in the interstitial tissue (Fig. 1, C and D).
Fig. 1.
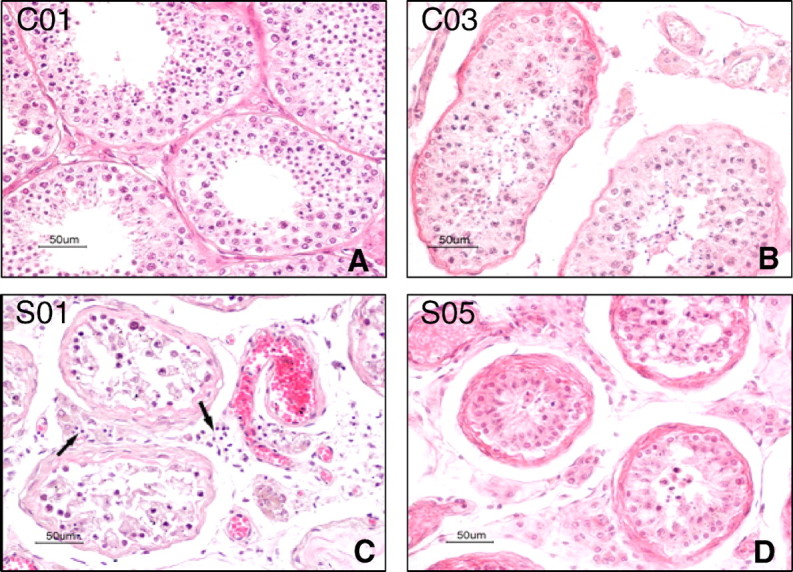
Hematoxylin-eosin stain. A) Testis from the control case C01, showing normal morphology. B) Testis from the control case C03, who died of a disease with high fever and was treated with steroids, showing mild basement membrane thickening and vascular congestion. C) Testis from SARS patient S01, showing loss of germ cells, leukocytes infiltration (arrows), and vascular congestion. D) Testis from SARS patient S05, showing basement membrane thickening, peritubular fibrosis, and vascular congestion. Bar = 50 μm
Pattern of Apoptosis
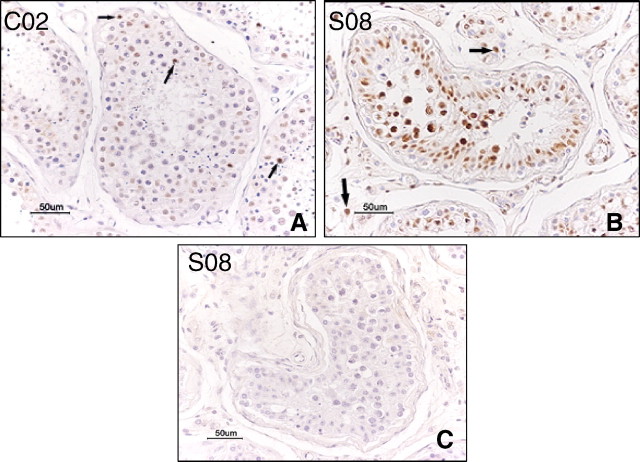
TUNEL assay showed increased apoptotic spermatogenetic cells in SARS (Fig. 2B), and this was found to be statistically different when compared with the control group (Table 2), with P-value < 0.05. Apoptosis was also demonstrated in a few Leydig cells (Fig. 2B, arrows) in SARS testes, but this is not statistically different when compared with the control group (0.8% of control group and 1.5% of SARS, respectively; P > 0.05).
Fig. 2.
TUNEL stain. A) Testis from the control case C02, showing a few apoptotic spermatogenetic cells in the tubules (arrows). B) Testis from SARS patient S08, showing increased apoptotic spermatogenetic cells and a few positive Leydig cells (arrows). C) Negative control stain of SARS sample S08 without TdT in the staining process, no positive stain was seen. Bar = 50 μm
Table 2.
Analysis of TUNEL staining of SARS and control cases.
| SARS (n = 6) | Control (n = 4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S01 | S03 | S05 | S08 | S11 | S15 | C01 | C02 | C03 | C04 | |
| Positive cells (%) | 1.5 | 5.5 | 12.6 | 2.7 | 3.9 | 9.5 | 1.7 | 2.9 | 1.9 | 2.8 |
| Mean value (%) | 5.95 ± 3.23a | 2.33 ± 0.661a | ||||||||
Percentage of positive cells, SARS compared to control (P < 0.05).
Leukocyte Infiltration and Autoimmune Antibody
With immunohistochemistry (IHC), the quantities of CD3+ T lymphocytes and CD68+ macrophages found in the control testes were 0.65% and 2.11%, respectively (Table 3). These cells were absent in the tubules (Fig. 3, A and B). In SARS testes, the numbers of both cell types were increased, with 4.49% of T lymphocytes and 11.72% of macrophages (Table 3). The difference between the SARS group and the control group was significant (P < 0.05). Both cell types were observed to be present in the seminiferous tubules in the SARS cases (Fig. 3, C and D, arrowhead) and 5.43% and 7.03% of tubules were found to contain T lymphocytes and macrophages, respectively (Table 3).
Table 3.
Analysis of CD3 and CD68 positivities in SARS and control cases.
| SARS (n = 6) | Control (n = 4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S01 | S03 | S05 | S08 | S11 | S15 | C01 | C02 | C03 | C04 | |
| CD3 | ||||||||||
| Positive tubule (%) | 9.3 | 9.3 | 3.7 | 2.3 | 5.9 | 2.1 | 0 | 0 | 0 | 0 |
| Mean value (%) | 5.43 ± 3.2 | 0 | ||||||||
| Positive cell (%)a,b | 3.95 | 3.77 | 6.73 | 6.61 | 2.67 | 3.21 | 0.15 | 0.31 | 0.21 | 1.94 |
| Mean value (%) | 4.49 ± 2.68 | 0.65 ± 0.23 | ||||||||
| CD68 | ||||||||||
| Positive tubule (%) | 5.9 | 13.5 | 5.4 | 9.5 | 4.2 | 3.7 | 0 | 0 | 0 | 0 |
| Mean value (%) | 7.03 ± 3.4 | 0 | ||||||||
| Positive cell (%)a,b | 4.88 | 6.65 | 44.61 | 3.89 | 4.36 | 5.93 | 1.25 | 2.94 | 2.11 | 2.12 |
| Mean value (%) | 11.72 ± 2.35 | 2.11 ± 1.69 | ||||||||
Percentage of positive cells, SARS compared to control (P < 0.05).
In the control cases, the positive cells were all in the interstitial tissue.
Fig. 3.
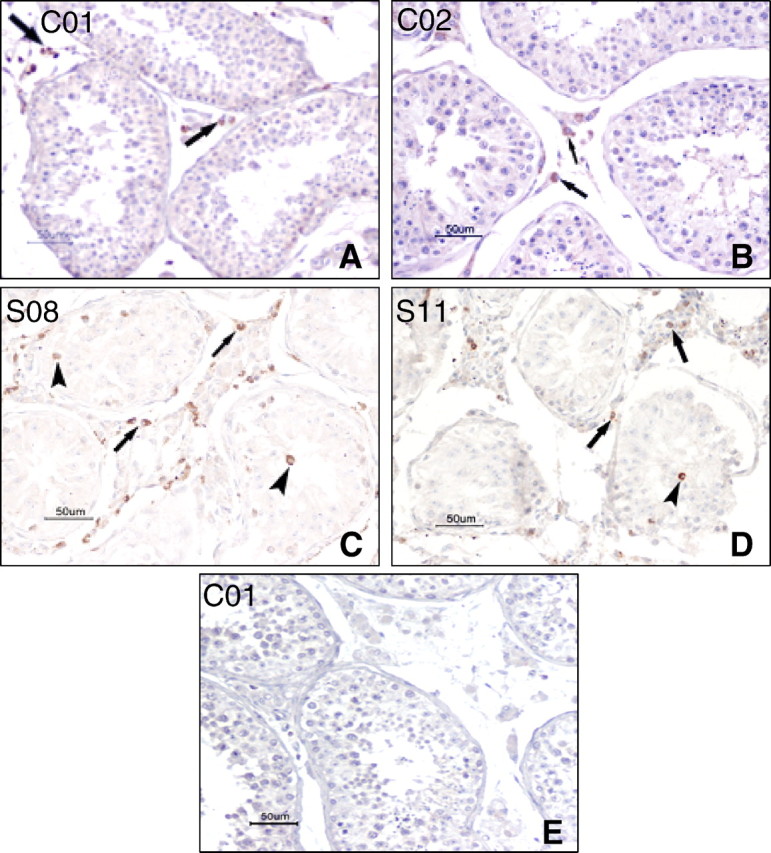
IHC stain with CD68 (A and C) and CD3 (B and D). A) Testis from the control case C01, showing a few CD68+ macrophages in the interstitial tissue (arrows) and no macrophage in the tubule. B) Testis from the control case C02, showing a few CD3+ T lymphocytes in the interstitial tissue (arrows), and no positive cell in the tubule. C) Testis from SARS patient S08, showing CD68+ macrophages in the seminiferous tubules (arrow heads) and the interstitial tissue (arrows). D) Testis from SARS patient S11, showing CD3+ T lymphocytes in the seminiferous tubules (arrow head) and the interstitial tissue (arrows). E) Negative control without primary antibody on the case C01. Bar = 50 μm
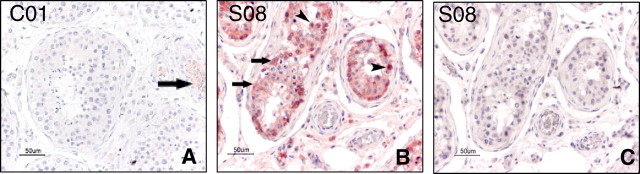
We examined the expression and distribution of IgG in the testes. In the control group, IgG was localized in the lumen of some of the blood vessels (Fig. 4A, arrow). However, in the SARS testes, deposits of extensive IgG immunoreaction were detected in the seminiferous epithelium, interstitium, some degenerated germ cells, and Sertoli cells (Fig. 4B). Isotype control showed negative staining (Fig. 4C).
Fig. 4.
IHC stain with IgG. A) Testis from the control case C01, showing IgG signals in the lumen of blood vessel (arrow). B) Testis from SARS patient S08, showing extensive IgG signals in the seminiferous epithelium, a few positive germ cells (arrow heads) and Sertoli cells (arrows). C) Negative control with isotype control antibody on the serial section of B, no positive signal was seen. Bar = 50 μm
SARS Virus Detection
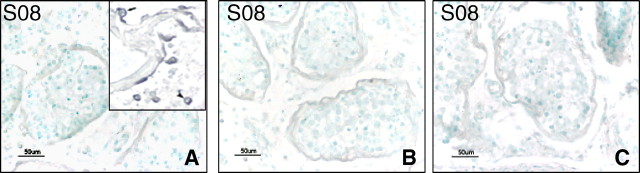
To determine if the SARS virus infected the testis directly, we performed in situ hybridization (ISH) using both sense and antisense RNA probes. There was no positive staining observed in any of the SARS testis sections (Fig. 5). Specific positive signals were obtained in the sections of SARS lungs, which were stained as positive control (Fig. 5A, insert; full report of the lung in SARS patients will be published separately).
Fig. 5.
ISH detection of SARS virus. A) Testis from SARS patient S08 with antisense digoxigenin-labeled RNA probe, showing no positive signals. Insert: SARS lungs stained with the same protocol and probe as a positive control, showing positive type II alveolar cell (arrow) and lymphocytes (arrow head) (will publish separately). B) Testis from SARS patient S08 with sense digoxigenin-labeled RNA probe, showing no positive signals. C) Negative control stain without probe on the same case. Bar = 50 μm
Discussion
The most well-known and extensively studied viruses that cause human testicular disorder are HIV and mumps virus. Autoimmune deficiency syndrome (AIDS) patients often suffer from orchitis, hypogonadism, oligospermia, and, in some cases, testicular germ cell tumor [14–18]. Spermatogenesis dysfunction in male AIDS patients is invariably reported. The major changes include germ cell depletion, presence of Sertoli cell-only pattern, interstitial inflammation, leukocyte infiltration, peritubular fibrosis associated with tubular hyalinization, and thickening of the tubular wall [19–21]. Orchitis caused by mumps virus mainly develops in adult patients [22]. The virus attacks the testis directly, destroying the testicular parenchyma, causing degeneration of germ cells, exudation of leukocytes, and deposition of collagen [23, 24]. Apart from HIV and mumps virus, other viruses, such as Hepatitis B and C viruses, Epstein-Barr virus, and Papilloma virus, were also reported to cause viral orchitis [9]. SARS is a new disease caused by a novel coronavirus. We report here that the SARS virus causes orchitis.
Germ cells must develop at a temperature lower than 37°C. Persistent high fever leads to changes in testicular temperature, contributing to germ cell degeneration and destruction. Previous studies reported that high temperature resulted in meiotic germ cell apoptosis [25]. High fever was also thought to play an important role in mumps orchitis [26]. High fever in SARS might have an indirect effect on testicular dysfunction. However, temperature might not be the only reason. In the control group, there was a case (C03) with lasting high fever, and this testis demonstrated mild fibrosis and congestion with no obvious germ cell loss or leukocyte infiltration. In addition, the treatment of SARS with steroids could also have affected spermatogenesis. Glucocorticoid had been used in five cases (except S05) and it had been reported to induce rat Leydig cell apoptosis [27]. In this study, we did not find an obvious increase of apoptotic Leydig cell in the six SARS cases (P > 0.05 compared with control). Instead, we found mild Leydig cell hyperplasia in some cases. The testis of case S05, who did not take steroids, also displayed changes of orchitis, as did another five SARS cases. Glucocorticoid should not induce leukocyte infiltration and is routinely used to treat orchitis to suppress inflammation. This indicates that steroid treatment might not be the cause for orchitis in SARS, at least not for case S05.
Reports on testicular endocrine function in viral orchitis are rare. Previous studies observed a drop in testosterone level together with an increase in LH and FSH levels in patients suffering from mumps orchitis [28]. Similarly, in some male AIDS cases, low serum testosterone levels associated with high serum LH and FSH levels were also reported [29]. These findings suggest that viruses might act indirectly via changes in the hypothalamic-pituitary-testis axis. However, there were other studies that found no significant abnormality in sex hormone in male patients with HIV orchitis [14]. The absence of clinical information on the levels of testosterone, LH, or FSH in these SARS patients precludes the possibility of a meaningful clinicopathological correlation.
Viruses are known to be capable of infecting the testis directly. For example, mumps viruses were found in human Leydig cells and HIV infects human germ cells. HIV, HBV, HSV, and adenoviruses can also be detected in semen [11]. ACE-2 (angiotensin-converting enzyme 2) was reported as a functional receptor for the SARS virus [30], and three independent groups all identified that ACE-2 was highly expressed in human testis [31–33], which suggested that the testis has the potential to be infected by the SARS virus. We investigated the possibility of direct SARS virus infection in the testis in all six cases by using the ISH method. None showed positive staining. Conflicting reports exist concerning direct infection of testes by the SARS virus. In the study of Zhao et al. [9], it was reported that SARS coronaviruses were detected in testicular epithelial cells and Leydig cells by electron microscopy combined with ISH. However, in another study by Ding et al. [10], testis was negative for the SARS virus, and infections of the heart, the spleen, and the lymph nodes were not established either, yet the spleen and the lymph nodes were reported as being main targets by other groups [7]. Hence, it appears that the SARS virus can infect and damage multiple organs, but direct infection of the testis could not be ascertained at this time.
Leukocytes, especially macrophages, are found within the interstitial tissue of most mammal testes. They may be involved in Leydig cell development, steroidogenesis, and immune response [34]. One of the noticeable phenomena in the SARS testis is leukocyte infiltration. These cells could affect the function of Leydig cells and then production of testosterone, damage the blood-testis barrier, and destroy the seminiferous epithelium directly. More important, these cells and their products, inflammatory cytokines, may activate the autoimmune response and autoantibody development within the tubules. Previous studies demonstrated the deposits of IgG in germinal epithelium, the basement membrane, interstitium, vascular endothelium, and degenerated germ cells in experimental autoimmune orchitis (EAO) [35, 36], and it was reported that serum IgG was increased in SARS patients [37]. In our study, we observed extensive IgG precipitation in the seminiferous epithelium, including in some degenerated germ cells and Sertoli cells. It was very possible that the SARS virus might not infect testis directly but that it triggered a secondary autoimmune response and, like other viral orchitis, SARS orchitis was also autoimmune orchitis.
The earliest sign of orchitis was observed in a patient who died 21 days after the onset of the high fever. Due to the severity of the symptoms of the respiratory tract and the gastrointestinal tract, the earliest symptoms of orchitis in SARS patients were not observed or reported clinically. Mumps orchitis develops within a few days of the disease [23]. EAO in murine developed within 20 days after the treatment [36]. However, due to the lack of clinical data for this cohort of patients, a meaningful comparison between the durations of orchitis of the SARS patients and those of mumps and the experimental allergic cases is not feasible.
In conclusion, orchitis is found to be a complication of SARS. In this study, all six cases had orchitis, including one case (S08) with the course of disease of only 21 days. All cases in this study were fatal and the incidence of orchitis in this cohort of patients was 100%. Although the number of cases in this study is limited, the data indicate that SARS infection affected the testes significantly. Like orchitis associated with HIV, mumps, and HBV, several possible mechanisms may be involved in causing testicular damages in SARS patients. Virus does not only cause orchitis, but also leads to sterility and increased incidence of testicular tumor [15–17]. This is particularly important for SARS patients, as most of them are males in the age range of 20–50 yr. Therefore, SARS orchitis should be a significant concern when evaluating the prognosis of SARS. Findings from this study strongly suggest that the reproductive functions of recovered male SARS patients should be followed and evaluated.
References
- 1. WHO Regional committee, Fifty-fourth session, Manila, Philipines, 8–12 September 2003. http://www.wpro.who.int/sars/docs/RC54–08.pdf, P2 [Google Scholar]
- 2. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, et al A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003348:1953–1966 [DOI] [PubMed] [Google Scholar]
- 3. Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, et al Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003348:1967–1976 [DOI] [PubMed] [Google Scholar]
- 4. Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, Khattra J, Asano JK, Barber SA, Chan SY, Cloutier A, Caughlin SM, et al The genome sequence of the SARS-associated coronavirus. Science 2003300:1399–1404 [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Zhang HT, Xie YQ, Wan JW, Lu ZH, Wang DT, Wang QZ, Xue XH, Si WX, Luo YF, Qiu HM, Morphological study of severe acute respiratory syndrome (SARS). Zhonghua Bing Li Xue Za Zhi 200332:516–520 [PubMed] [Google Scholar]
- 6. Tse GM, To KF, Chan PK, Lo AW, Ng KC, Wu A, Lee N, Wong HC, Mak SM, Chan KF, Hui DS, Sung JJ, et al Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol 200457:260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, et al Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005202:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, Lu Y, Wu D, et al The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003200:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao JM, Zhou GD, Sun YL, Wang SS, Yang JF, Meng EH, Pan D, Li WS, Zhou XS, Wang YD, Lu JY, Li N, et al Clinical pathology and pathogenesis of severe acute respiratory syndrome. Zhonghua Shi Yan he Lin Chang Bing Du Xue Za Zhi 200317:217–221 [PubMed] [Google Scholar]
- 10. Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qui L, Li Z, Geng J, Cai J, et al Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 2004203:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dejucq N, Jégou B, Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev 200165:208–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Scientific Research Advisory Committee on Severe Acute Respiratory Syndrome (SARS). Report of the First Meeting, Geneva, Switzerland; 20–21 October 2003 World Wide Web (URL: http://www.who.int/csr/sars/guidelines/en/)
- 13. Shi X, Gong E, Gao D, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Wu B, Fang W, Liao S, Wang S, et al Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol 2005100:169–176 [DOI] [PubMed] [Google Scholar]
- 14. Poretsky L, Can S, Zumoff B, Testicular dysfunction in human immunodeficiency virus-infected men. Metabolism 199544:946–953 [DOI] [PubMed] [Google Scholar]
- 15. Pudney J, Anderson D, Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am J Pathol 1991139:149–160 [PMC free article] [PubMed] [Google Scholar]
- 16. Tessler AN, Catanese A, AIDS and germ cell tumors of testis. Urology 198730:203–204 [DOI] [PubMed] [Google Scholar]
- 17. Oliver RT, Testis cancer. Curr Opin Oncol 19979:287–294 [DOI] [PubMed] [Google Scholar]
- 18. Feichtinger H, Kaaya E, Putkonen P, Li SL, Ekman M, Gendelman R, Biberfeld G, Biberfeld P, Malignant lymphoma associated with human AIDS and with SIV-induced immunodeficiency in macaques. AIDS Res Hum Retroviruses 19928:339–348 [DOI] [PubMed] [Google Scholar]
- 19. Chabon AB, Stenger RJ, Grabstald H, Histopathology of testis in acquired immune deficiency syndrome. Urology 198729:658–663 [DOI] [PubMed] [Google Scholar]
- 20. Rogers C, Klatt EC, Pathology of the testis in acquired immunodeficiency syndrome. Histopathology 198812:659–665 [DOI] [PubMed] [Google Scholar]
- 21. Yoshikawa Y, Truong LD, Fraire AE, Kim HS, The spectrum of histopathology of the testis in acquired immunodeficiency syndrome. Mod Pathol 19892:233–238 [PubMed] [Google Scholar]
- 22. Freeman R, Hambling MH, Serological studies on 40 cases of mumps virus infection. J Clin Pathol 198033:28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bjorvatn B, Mumps virus recovered from testicles by fine-needle aspiration biopsy in cases of mumps orchitis. Scand J Infect Dis 19735:13–5 [DOI] [PubMed] [Google Scholar]
- 24. Charny CW, Meranze DR, Pathology of mumps orchitis. J Urol 194860:140–143 [DOI] [PubMed] [Google Scholar]
- 25. Xu J, Xu Z, Jiang Y, Qian X, Huang Y, Cryptorchidism induces mouse testicular germ cell apoptosis and changes in bcl-2 and bax protein expression. J Environ Pathol Toxicol Oncol 200019: (1–2) 25–33 [PubMed] [Google Scholar]
- 26. Oliver RT, Atrophy, hormones, genes and viruses in aetiology germ cell tumours. Cancer Surv 19909:263–286 [PubMed] [Google Scholar]
- 27. Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, Hardy MP, Glucocorticoid induces apoptosis in rat Leydig cells. Endocrinology 2002143:1130–138 [DOI] [PubMed] [Google Scholar]
- 28. Adamopoulos DA, Lawrence DM, Vassilopoulos P, Contoyiannis PA, Swyer GI, Pituitary-testicular interrelationships in mumps orchitis and other viral infections. Br Med J 19781:1177–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Croxson TS, Chapman WE, Miller LK, Levit CD, Senie R, Zumoff B, Changes in the hypothalamic-pituitary-gonadal axis in human immunodeficiency virus-infected homosexual men. J Clin Endocrinol Metab 198968:317–321 [DOI] [PubMed] [Google Scholar]
- 30. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003426:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harmer D, Gilbert M, Borman R, Clark KL, Quantitative mRNA expression profiling of ACE2, a novel homologue of angiotensin converting enzyme. FEBS 2002532:107–110 [DOI] [PubMed] [Google Scholar]
- 32. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ, A human homolog of angiotensin-converting enzyme. J Biol Chem 2000275:33238–33243 [DOI] [PubMed] [Google Scholar]
- 33. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Wodf B, Robinson K, Jeyaseelan R, Breitbart RE, Acton S, A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 200087:1–9 [DOI] [PubMed] [Google Scholar]
- 34. Hedger MP, Testicular leukocytes: what are they doing?. Rev Reprod Fertil 19972:38–47 [DOI] [PubMed] [Google Scholar]
- 35. Itoh M, Hiramine C, Tokunaga Y, Mukasa A, Hojo K, A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. II. Immunohistochemical findings of fully-developed inflammatory lesion. Autoimmunity 199110:89–97 [DOI] [PubMed] [Google Scholar]
- 36. Kohno S, Munoz JA, Williams TM, Teuscher C, Bernard CC, Tung KS, Immunopathology of murine experimental allergic orchitis. J Immunol 1983130:2675–2682 [PubMed] [Google Scholar]
- 37. National Research Project for SARS Beijing Group. Dynamic changes of T-lymphocytes and immunoglobulins in patients with SARS. Zhonghua Yi Xue Za Zhi 200383:1014–1017 [PubMed] [Google Scholar]