Abstract
CEACAM10 was purified from mouse seminal vesicle secretions by a series of purification steps that included ion exchange chromatography on a DEAE-Sephacel column and ion exchange high-performance liquid chromatography on a sulfopropyl column. It was shown to be a 36-kDa glycoprotein with an N-linked carbohydrate moiety. The circular dichromoism spectrum of CEACAM10 in 50 mM phosphate buffer at pH 7.4 appeared as one negative band arising from the β form at 217 nm. CEACAM10 was expressed predominantly in seminal vesicles of adult mice. Both CEACAM10 and its mRNA were demonstrated on the luminal epithelium of the mucosal folds in the seminal vesicle. The amount of Ceacam10 mRNA in the seminal vesicle was correlated with the stage of animal maturation. Castration of adult mice resulted in cessation of Ceacam10 expression, while treatment of castrated mice with testosterone propionate in corn oil restored Ceacam10 expression in the seminal vesicle. During the entire course of pregnancy, Ceacam10 might be silent in the embryo. A cytochemical study illustrated the presence of the CEACAM10 binding region on the entire surface of mouse sperm. CEACAM10-sperm binding greatly enhanced sperm motility in vitro.
Keywords: male reproductive tract, seminal vesicles, sperm motility and transport
Introduction
Mammalian sperm display an intriguing sense of timing in undergoing some modification during their transit in the reproductive tract before encountering an egg. Studying how the lumen of the reproductive tract affects sperm function is a prerequisite to unraveling the molecular mechanisms underlying the complex modification of sperm. Factors that affect sperm motility have been reported in the seminal plasma of several mammals including the pig [1– 3], bovine [4], mouse [5], and human [6].
The seminal vesicle is a male accessory sexual gland found in many species of more than 4000 mammalian species alive on the earth today. After puberty, the gland secretes a fluid called seminal vesicle secretion (SVS), which accumulates in its lumen. SVS contains both protein and nonprotein components. When ejaculated, SVS squirts into the urethra, contributing the major part of the liquid portion of seminal plasma, which is the complex biological fluid formed from mixing of various fluid in the male reproductive tract. It has been found that extirpation of the seminal vesicle from mice and rats greatly reduces fertility [7, 8], demonstrating the importance of SVS to sperm modification under natural circumstances. SVS differs extensively in terms of volume and composition in various species of mammals. However, rodents have proven to be good experimental animals for the molecular study of mammalian reproduction, so attempts have been made to isolate the proteins involved in sperm modification by mouse SVS, which contains several minor proteins and seven well-defined major proteins designated SVS I–VII, named in decreasing order of molecular mass according to their mobility in SDS-PAGE [9]. Previously, we demonstrated that SVS VII enhances sperm motility [10], and two of the minor proteins modulate sperm activity. One is a caltrin-like trypsin inhibitor/P12, which suppresses Ca2+ uptake by sperm [11], and the other is a seminal vesicle autoantigen, which serves as a decapacitation factor [12, 13].
Here we report the purification and identification of an androgen-stimulated 36-kDa glycoprotein, a minor protein component of mouse SVS that is able to enhance sperm motility in vitro. We have demonstrated that its core protein is derived from the Ceacam10 gene [14], which is a member of the cell adhesion molecule (CAM) subgroup belonging to the carcinoembryonic antigen (CEA) family.
Materials and Methods
Materials
The following materials were obtained from commercial sources: DEAE-Sephacel (Amersham Pharmacia Biotech, Uppsala, Sweden); Protein PAK SP 5PW column (Waters, Milford, MA); Vydac 218TP54 C18 column (Separations Group, Hesperia, CA); AminoLink coupling gel, bicinchoninic protein assay kit (Pierce, Rockford, IL); testosterone propionate, nitroblue tetrazolium, 5-bromo-4-chloro-3-indolyl phosphate (BCIP), PMSF, periodic acid Schiff reagent, and silanated glass slides (Sigma Chemical Co., St Louis, MO); cDNA integrity kit, alkaline phosphatase-conjugated streptavidin, and biotin-conjugated goat anti-rabbit immunoglobulin G (IgG; Kirkegaard & Perry Laboratories, Gaithersburg, MD); rhodamine-conjugated goat anti-rabbit IgG (Zymed Laboratories, San Francisco, CA); Nuclear Fast Red (Vector Laboratories, Burlingame, CA); enhanced chemiluminescent substrate and [α-32P]dATP (NEN Life Science Products, Boston, MA); Tissue-Tek OCT medium (Miles Inc., Elkhart, IN); HistoGene laser capture microdessection (LCM) frozen section staining kit, CapSure HS LCM caps, Picopure RNA Isolation kit (Arcturus Engineering, Mountain View, CA); l-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK) modified trypsin, DNase I, Prime-a-Gene kit and pGEM-T-easy vector (Promega, Madison, WI); Ultraspec-II RNA isolation kit (Biotecx Laboratories, Inc., Houston, TX); Thermoscript reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA); N-glycosidase F, Taq DNA polymerase (TaKaRa, Shiga, Japan); and mouse embryo-stage blots designed to observe gene expression during pregnancy (Seegene, Inc., Korea). All other chemicals were reagent grade.
Animals and Hormone Treatment
Outbred ICR mice were purchased from Charles River Laboratories (Wilmington, MA) and were maintained and bred in the animal center at the College of Medicine, National Taiwan University. Animals were treated according to institutional guidelines for the care and use of experimental animals. They were housed under controlled lighting (14L:10D) at 21– 22°C and were provided with water and NIH-31 laboratory mouse chow ad libitum.
For investigation of androgenic effects, 8-wk-old adult male mice, which had been castrated 3 wk earlier, received a daily s.c. injection of testosterone propionate in corn oil (5 mg/kg body weight) for 8 consecutive days. Control animals received corn oil only. Seminal vesicles were removed from the animals 12 h after the last injection.
Protein Purification
Normal adult mice (8 to 12 wk old) were killed by cervical dislocation. The seminal vesicles of 30 mice were carefully dissected to free them from the adjacent coagulating glands, and the secretions were squeezed directly into 50 ml of ice-cold 10 mM Tris-HCl in the presence of 1 mM PMSF at pH 8.0. After centrifugation at 10 000 × g for 15 min, the supernatant was resolved by ion exchange chromatography on a DEAE-Sephacel column (12 × 2.6 cm) that had been pre-equilibrated with 10 mM Tris-HCl at pH 8.0. After the nonretarded fractions were washed out, the column was eluted with 0.5 M NaCl in the same buffer at a flow rate of 18 ml/h. Fractions (4 ml) were collected, and their absorbance at 280 nm was recorded (Fig. 1A). Fraction III was further subjected to ion-exchange high-performance liquid chromatography (HPLC) on a sulfopropyl (SP) column (7.5 cm × 7.5 mm). The column was sequentially eluted with three linear gradients, including 0%–15%, 15%–40%, and 40%–80% of 0.6 M NaCl in 20 mM sodium acetate at pH 6.0 at a flow rate of 1.0 ml/min for 60 min (see Fig. 1B). Thirty micrograms of each peak (a–d) in 50 μl of 100 mM Tris-HCl pH 8.6 were digested with 0.6 μg of trypsin-TPCK at 37°C for 18 h. The reaction was stopped by adding 100 μl of 0.1% trifluoroacetic acid and the reaction mixture was resolved by a reverse-phase C18 column (4.6 × 250 mm). The column was eluted with a linear gradient of 0%–80% acetonitrile at a flow rate of 1.0 ml/min for 60 min (see Fig. 2A).
Fig. 1.
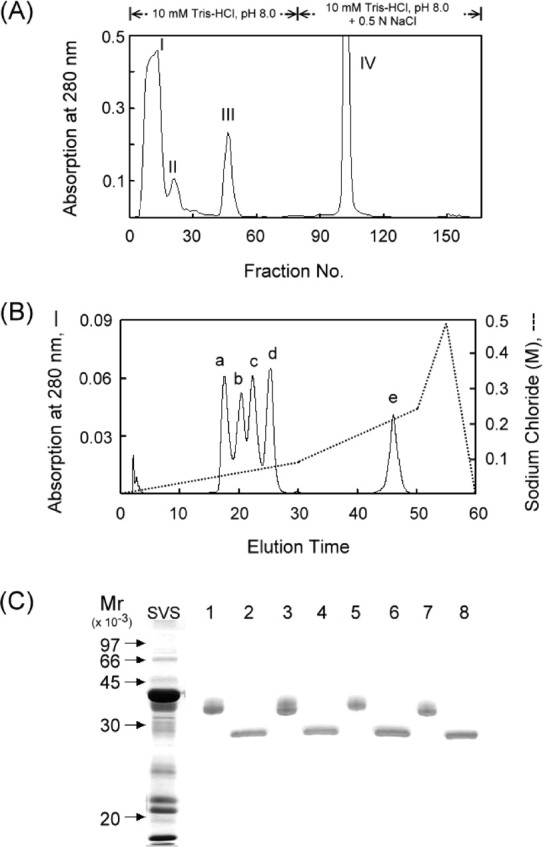
Purification of 36-kDa glycoproteins from mouse SVS proteins. A) Fractionation of soluble mouse SVS proteins by ion exchange chromatography on a DEAE-Sephacel column. B) Resolution of fraction III sample from A by ion-exchange HPLC on an SP column. C) Demonstration of the glycoprotein nature. Each of the a-to-d peaks from B were digested with N-glycosidase F. The parent proteins (lane 1, peak a; lane 3, peak b; lane 5, peak c; lane 7, peak d) and their deglycosylated forms (lanes 2, 4, 6, and 8) were identified by SDS-PAGE on a 12% polyacrylamide gel slab. The proteins in the gel were stained with Coomassie brilliant blue
Fig. 2.
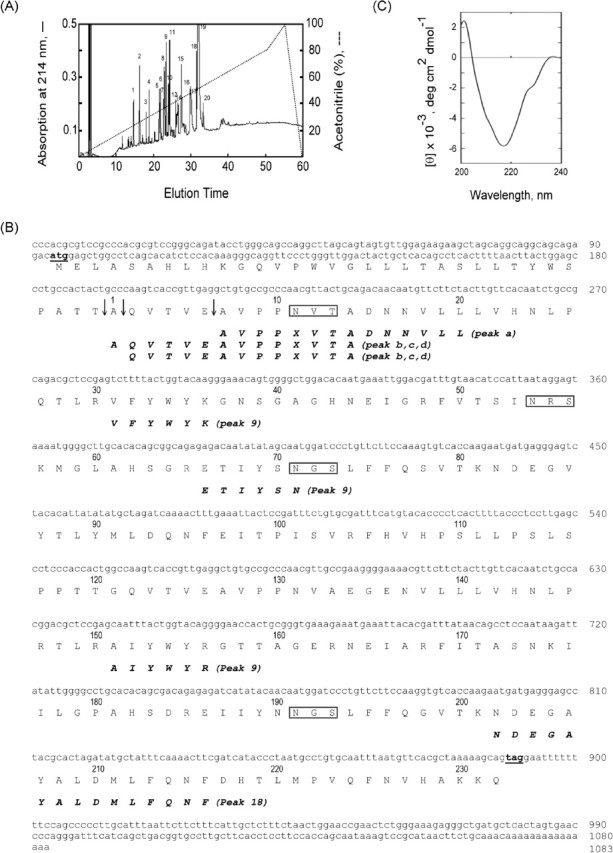
Identification of the 36-kDa glycoprotein derived from Ceacam10 and its circular dichroism. A) The trypsin-digested sample of peak c from Figure 1B was resolved by reverse-phase HPLC on a C18 column (see Materials and Methods). B) The protein sequence was deduced from the reading frame of Ceacam10 cDNA (GenBank accession number NM_007675). The initial and stop codons are underlined. The potential N-linked glycosylation sites are denoted by open boxes. The deduced protein sequence and the amino acid sequences determined directly from protein analysis for peaks a to d in Figure 1B and peaks 9 and 18 in Figure 2A agree in all positions except that Asn11 from the cDNA-deduced protein was not identified in protein sequencing. The cleavage points for the generation of mature protein are indicated by an arrow. C) Circular dichroism of CEACAM10 in 50 mM phosphate buffer at pH 7.4 at room temperature
Protein Analysis
The N-glycoconjugate was removed from a glycoprotein by following the method described by Tarentino and Plummer [15]. The protein was boiled in 1.0% SDS and incubated with N-glycosidase F (40 U/mg of protein) in 20 mM sodium phosphate at pH 7.2 in the presence of 50 mM EDTA, 0.5% Nonidet P-40, and 10 mM sodium azide for 16 h at 37°C. The concentration of CEACAM10 was determined using the bicinchoninic acid protein assay [16] according to the manufacturer’s instructions. The amino acid sequence was determined using automated Edman degradation with a 492 protein sequencer with an online 140 C analyzer (Applied Biosystems, Foster City, CA). The circular dichromoism (CD) spectra were measured with a Jasco J-700 spectropolarimeter under constant flushing with N2 at room temperature. The mean residue elipticity (θ) was estimated from the mean residue weight, which was calculated from the primary structure.
Western Blot Analysis and Immunohistochemical Staining
Antisera against CEACAM10 were raised in New Zealand White rabbits. The anti-CEACAM10 antibody was purified from the antiserum on a column (2.5 × 1.0 cm) of AminoLink gel coupled with the purified antigen according to our previously described method [17].
Proteins were resolved using SDS-PAGE on a 12% gel slab (8.2 × 7.3 × 0.075 cm) according to the method described by Laemmli [18]. The proteins on the gel were stained with Coomassie brilliant blue or transferred to a nitrocellulose membrane using an electroblotting method, which was conducted at 35 V at 4°C for 18 h in a solution of 25 mM Tris-HCl, 197 mM glycine, and 13.3% methanol. Membranes were blocked with 10% normal goat serum in PBS for 2 h, and then incubated with anti-CEACAM10 antibody (0.5 μg/ml) in the blocking solution for 1 h at room temperature. After gently agitating in four changes of PBS for 15 min each, they were immunoreacted with horseradish peroxidase-conjugated goat anti-rabbit IgG diluted to 1:15 000 in the blocking solution for 1 h. Immunoreactive bands were revealed using an enhanced chemiluminescent substrate according to the manufacturer’s instructions.
Mouse seminal vesicles were fixed in Bouin solution, embedded in paraffin, and 8-μm serial cross-sections were mounted on silanated glass slides. Deparaffinized sections were blocked in blocking solution for 1 h at room temperature and then incubated with affinity-purified anti-CEACAM10 antibody at a concentration of 1 μg/ml in the blocking solution for 1 h. The slides were gently agitated in three changes of washing solution for 10 min each and then treated with biotin-conjugated goat anti-rabbit IgG (1 μg/ml) in the blocking solution for 1 h at room temperature. The slides were washed again as described above and then incubated with alkaline phosphatase-conjugated streptavidin (1 μg/ml) in blocking solution for 1 h at room temperature. Protein signals were observed after the slides were incubated for 10 min with 0.0375% nitroblue tetrazolium and 0.0188% BCIP in a solution of 100 mM Tris-HCl, 100 mM NaCl, and 5 mM MgCl2 at pH 9.5. The slides were washed in three changes of water for 3 min each and then counterstained with Nuclear Fast Red for 3 min. Finally, the slides were briefly washed with water and photographed using a brightfield microscope (AH3-RFCA; Olympus, Tokyo, Japan).
Laser Capture Microdissection and Detection of Ceacam10 mRNA
Seminal vesicle tissues were oriented in a small aluminum foil cup and frozen immediately in Tissue-Tek OCT medium. Embedded samples were then stored at −80°C before further processing. Serial 8-μm cryostat sections were mounted on uncoated glass slides and stored at −80°C. Before use, sections were fixed in 70% ethanol and stained with a HistoGene LCM frozen section-staining kit following the supplier’s protocol. Slides were dipped in xylene twice for 5 min each time and then air-dried. Mucosal folds or smooth muscle cells were harvested using a PixCell II LCM system (Arcturus Engineering, Mountain View, CA). Captured tissues from three sections were collected on CapSure HS LCM caps containing transfer film. Tissue samples from three different animals were pooled for subsequent analysis.
Total RNA was extracted from the captured cells by using the Picopure RNA Isolation kit, followed by treatment with DNase I before cDNA synthesis. The total RNA was reverse-transcribed using Thermoscript reverse transcriptase for first-strand cDNA synthesis according to the manufacturer’s instructions. A cDNA integrity kit was employed to examine the quality of the cDNA. The qualified cDNA samples were used as templates for PCR. The primer pair for amplification of a 237-base pair (bp) Ceacam10 gene fragment was a forward primer (5′-TATGCTATTTCAAAACTTCGATCAT-3′), which corresponds to sequence 822–846, and a reverse primer (5′-GTTATGCGGACTTTATTG-3′), which corresponds to sequence 1058–1041 (GenBank accession number NM_007675). The primer pair for amplification of a 557-bp mouse glyceraldehyde-3-phosphate dehydrogenase (Gapd) DNA fragment was a forward primer (5′-CGGCAAATTCAACGGCACAGT-3′), which corresponds to sequence 199–219, and a reverse primer (5′-TGGGGGTAGGAACACGGAAGG-3′), which corresponds to sequence 755–735 (GenBank accession number XM_194302). PCR reaction mixtures consisted of 2 μl of template, 1.25 units of Taq DNA polymerase, 0.2 mM dNTP, and 0.2 μM primer pair in a 50-μl final solution of 10 μM Tris-HCl, 50 μM KCl, and 1.5 mM MgCl2 at pH 8.3. PCR was performed in a GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer, Boston, MA) with the following parameters: 3 min at 94°C followed by 30 cycles of melting for 30 sec at 95°C, annealing for 30 sec at 53°C, extension for 30 sec at 72°C, and a final extension for 7 min at 72°C. The polymerase chain reaction (PCR) products were analyzed by electrophoresis on a 2.0% agarose gel. The identity of PCR products was confirmed by cloning and sequencing. DNA sequencing was carried out with an ABI PRISM 377-96 DNA sequencer using the ABI PRISM BigDye Terminator cycle sequencing ready reaction kit (Applied Biosystems).
RNA Isolation and Northern Blot Analysis
Total RNA was extracted from tissue homogenates using an Ultraspec-II RNA isolation kit. A PCR-amplified fragment of Ceacam10 cDNA (237 bp), which was inserted in pGEM-T-easy, and a cDNA fragment of the mouse Gapd gene (1233 bp), which was inserted in pGEM3 vector, were used as a template to prepare a 32P-labeled cDNA probe using a Promega random-priming kit. RNA samples (20 μg) were subjected to denaturing by 1.0% agarose-formaldehyde gel electrophoresis and then blotted onto nylon membranes by capillary transfer as previously described [19]. After incubation with the prehybridization buffer (50% deionized formamide, 6× SSC, 5× Denhardt solution, 1.0% SDS, and 100 μg/ml of sheared salmon sperm DNA) for 2 h at 50°C, the membranes were hybridized with one labeled probe overnight at 50°C. Following hybridization, the membranes were washed using standard procedures. RNA messages on one filter membrane were observed after autoradiography and the probes were removed from the membranes as previously described [19]. The same membrane was then hybridized with another labeled probe. Thus, hybridization with Ceacam10 or Gapd cDNA probe was performed on the same filter membrane.
Cytological Observation and Assay of Sperm Motility
In accordance with a method previously used [20], a modified Tyrode buffer, which consisted of 124.7 mM NaCl, 2.7 mM KCl, 0.5 mM MgCl2, 0.4 mM NaH2PO4, 5.6 mM glucose, 0.5 mM sodium pyruvate, 15 mM NaHCO3, 10 mM Hepes, 100 IU/ml penicillin, and 100 μg/ml streptomycin was adjusted to pH 7.3–7.4 by aeration with humidified air/CO2 (19:1) in an incubator for 48 h at 37°C before use. Mouse epididymides were removed and immersed in the medium. After careful dissection from the connective tissue, spermatozoa were extruded from the distal portion of the tissues for 10 min at 37°C. The cells were gently filtered through two layers of nylon gauze, layered on top of a linear gradient of 20%– 80% Percoll (v/v), and centrifuged at 275 × g for 30 min at room temperature [21, 22]. Three distinct cell layers formed. The lowest layer, which contained cells with progressive motility, was washed with three volumes of the medium and collected using centrifugation at 60 × g for 10 min at room temperature. The sperm were resuspended and centrifuged two more times in a similar manner. The cell pellets were resuspended, and CaCl2 was added to the culture medium at a final concentration of 1.8 mM before the sperm were assayed.
Freshly prepared epididymal spermatozoa (106 cells/ml) were blocked in PBS containing 10% normal goat serum for 30 min at room temperature. The cells were further incubated with 1 μM CEACAM10 for 1 h. At the end of incubation the cells were centrifuged and the cell pellets were washed with PBS to remove the unbound ligands. The cells were air-dried on a glass slide and washed twice with PBS. The slides were incubated with the affinity-purified anti-CEACAM10 antibody at a concentration of 1 μg/ml in blocking solution for 1 h. The slides were washed three times with PBS to remove excess antibody before they were incubated with rhodamine-conjugated goat anti-rabbit IgG diluted to 1:500 in blocking solution for 40 min. All slides were then washed with PBS, covered with 50% (v/v) glycerol in PBS, and photographed with a fluorescence microscope (Axioplan 2 Imaging; Carl Zeiss, Oberkochen, Germany).
Ejaculated sperm were collected from semen that existed in the uterine cavity of three vagina-plugged female mice. After extensive washing with PBS the ejaculated sperm without incubation with the exogenous CEACAM10 were smeared onto slides for immunolocalization of CEACAM10 as mentioned above.
Sperm motility was determined using a computer-assisted sperm assay (CASA) with a sperm motility analyzer (IVOS version 10; Hamilton-Thorne Research, Beverly, MA). A 10-μl sample was placed in a 10-μm-deep Makler chamber at 37°C. The analyzer was set as follows: negative phase-contrast optics and recording at 60 frames/sec; minimum contrast, 40; minimum cell size, 4 pixels; low-size gate, 0.2; high-size gate, 1.5; low-intensity gate, 0.5; high-intensity gate, 1.5; nonmotile head size, 29; nonmotile head intensity, 76; medium average path velocity, 50 μm/sec; low path velocity, 7.0 μm/sec; slow motile cells, yes; and threshold straightness, greater than 80%. Ten fields were assessed for each sample.
Results
Protein Characterization of CEACAM10 Purified From Mouse SVS
The fresh preparation of soluble SVS was divided into fractions. I to IV by ion exchange chromatography on a DEAE-Sephacel column (Fig. 1A). The fraction III sample was further resolved into peaks a–e (Fig. 1B) by ion exchange HPLC on an SP column. Peak e was a FAD-dependent sulfhydryl oxidase (unpublished observation). On reducing SDS-PAGE gel, each of the a-to-d peak samples gave one rather broad 36-kDa band that could be stained with either Coomassie brilliant blue or periodic acid-Schiff reagent, demonstrating their glycoprotein nature (Fig. 1C, lanes 1, 3, 5, and 7). Each protein sample could be deglycosylated either by trifluoromethane sulfonic acid or exhaustive digestion with N-glycosidase F to a core protein that was identified as a sharp band between 26 and 28 kDa by SDS-PAGE (Fig. 1C, lanes 2, 4, 6 and 8), indicating a similar molecular mass of the protein cores. Apparently, peaks a–d were glycoproteins with an N-linked carbohydrate moiety. They were purified to homogeneity.
Automated Edman degradation for each a-to-d peak samples for 14 cycles gave reliable data, which were assembled to the N-terminal sequences. AVPPXVTADNNVLL was determined from the peak a sample. Two amino acids were detected in each cycle during protein analysis for each peak (b to d). The actual yield of the two sequences in an individual cycle was such that the ratio of the major sequence to the minor one was estimated to be 2.5–3.0:1. Assembly of the major and minor sequences gave a peptide sequence of AQVTVEAVPPXVTA and QVTVEAVPPXVTA, respectively. The three N-terminal peptides were completely confirmed in the Ceacam10-deduced protein consisting of 265 amino acid residues in all positions except that X (asparagine), one potential site for an N-linked carbohydrate in CEACAM10, was not identified in the protein sequencing (Fig. 2B). The post-translational cleavage at the peptide bond between Glu and Ala, Thr and Ala, or Ala and Gln in the signal peptide of the putative CEACAM10 sequence gives rise to a peak a protein or to peak b-to-d proteins. As a result, peaks a to d share a very similar protein core with a slight difference in their N-terminal sequences. Thereafter, we combined them for further study. Among the SVS protein components on SDS-PAGE gel, antibody against CEACAM10 immunoreacted only to a 36-kDa protein band corresponding to the antigen, showing high specificity of the antibody. Taken together, these data indicate that peaks a to d are translational products of the Ceacam10 gene.
Each peak (a to d) was digested with trypsin, and the digests were subjected to HPLC on a C18 column. The chromatographic patterns of the four trypsin-digested samples were very similar. One representative chromatogram is shown in Figure 2A. Three amino acids were identified in each cycle of automated Edman degradation of peak 9 on Figure 2A. These data could be assembled to three peptide sequences of VFYWYK, ETIYSN, and AIYWYR in CEACAM10. The peptide sequence of NDEGAYALDMLFQNF in CEACAM10 was completely confirmed by automated Edman degradation of peak 18 (see Fig. 2B).
CEACAM10 was stable in 10 mM Tris-HCl at pH 8.0, but it was degraded to an 18-kDa protein component in 5% acetic acid (not shown). The CD spectrum of this protein at pH 7.4 shows a negative band with a minimum mean residue ellipticity of 12 000 deg cm2 dmol−1 at 217 nm (Fig. 2C). In addition, a positive band appeared as the CD profile extending below 200 nm. The spectral profile in the UV region shows some resemblance to that of the β form of protein conformation [23–26], suggesting the presence of a considerable amount of β form, a β turn, or both in the protein molecule.
Predominant Ceacam10 Expression in the Luminal Epithelium of the Seminal Vesicle
We examined the distribution of CEACAM10 and its RNA message in the tissue homogenates of reproductive glands, including the seminal vesicle, epididymis, testis, coagulating gland, vas deferens, prostate, uterus, and ovary. The RNA message was predominantly detected in the seminal vesicles (Fig. 3A). This was confirmed by the results of Western blot analysis showing that CEACAM10 was abundant in the seminal vesicle; a trace appeared in the epididymis and prostate only after over autoradiography (Fig. 3B). When equal amounts of total RNA from the homogenate of a nonreproductive organ were compared with those of the seminal vesicle, very litter to no Ceacam10 mRNA was found in brain, heart, lung, liver, spleen, kidney, stomach, small intestine, muscle, skin, and thymus (not shown).
Fig. 3.
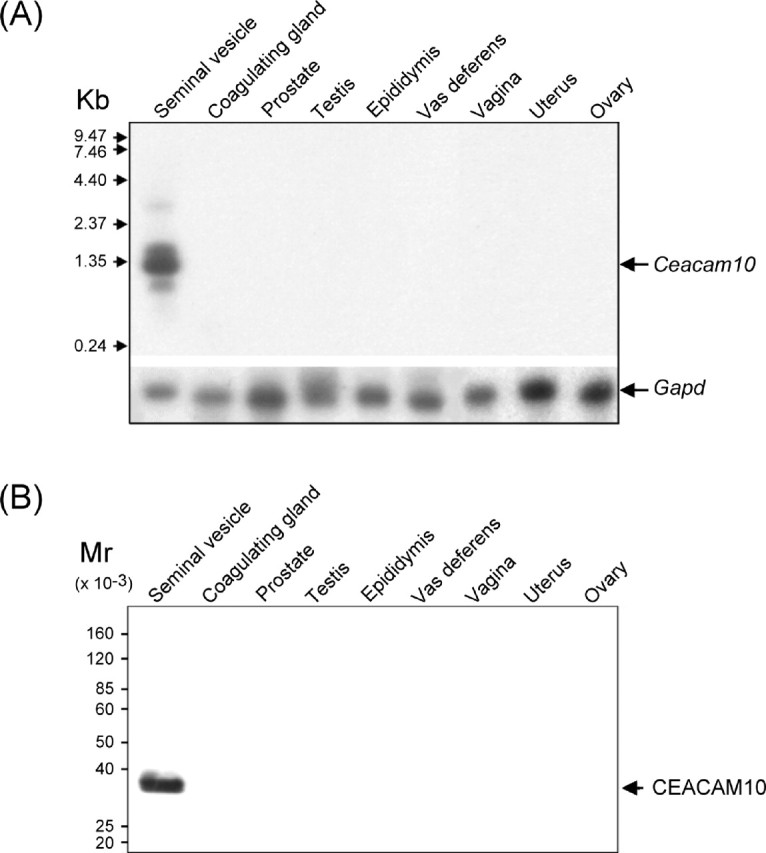
Distribution of Ceacam10 and its protein among the reproductive glands. Total RNA (20 μg) or protein extract (50 μg) prepared from the homogenates of each sexual gland were analyzed by Northern blot procedure (A) or Western blot procedure (B) (see Materials and Methods)
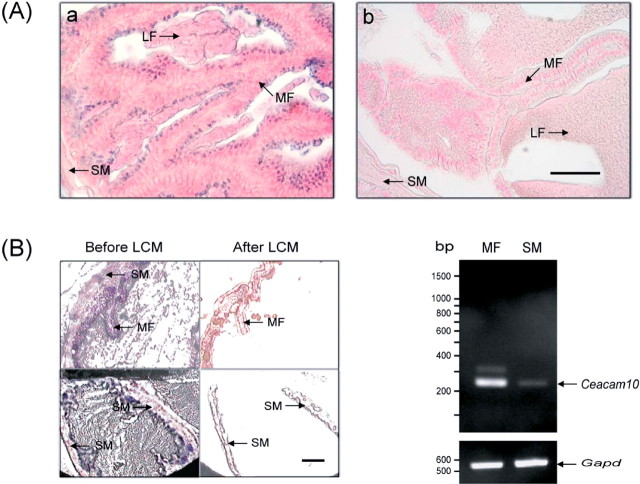
CEACAM10 was immunolocalized primarily to the luminal epithelium of the mucosal folds in the seminal vesicle slides of adult mice (Fig. 4A). The smooth muscle layer contained almost none. The strong immunochemical staining in the lumen supports the view that CEACAM10 accumulates in the lumen as a result of its secretion from the luminal epithelium. Further, we separated mucosal epithelial cells and smooth muscle cells from the tissue slices by LCM. Ceacam10 transcripts were relatively abundant in the mucosal cells, but only trace amounts of the RNA message appeared in the smooth muscle cells (Fig. 4B).
Fig. 4.
Ceacam10 expression in the luminal epithelium of the seminal vesicle. A) Immunolocalization of CEACAM10 to the luminal epithelium of the seminal vesicle. Tissue slices were histochemically stained for CEACAM10 with antibody against the protein, biotin-conjugate goat anti-rabbit IgG, and alkaline phosphatase-conjugated streptavidin a. The specimens were stained as in a except that the antibody was replaced by normal serum (b). For contrast, the specimens were further stained with Nuclear Fast Red. Photographs were taken with brightfield illumination. MF, mucosal fold; SM, smooth muscle; LF, luminal fluid. The staining of Nuclear Fast Red is in pink and the signals of CEACAM10 protein, demonstrated by staining of alkaline phosphatase activity, are in dark blue. Bar = 20 μm. B) Demonstration of Ceacam10 mRNA in the luminal epithelial cells of the seminal vesicle. The epithelial cells of MF or SM cells in a tissue slice (8 μm) of mouse seminal vesicle were selectively captured and transferred to films by LCM. The tissue slides before and after LCM were stained and observed (see text for details). A Ceacam10 cDNA fragment (237 bp) or a Gapd cDNA fragment (557 bp) was amplified from the total RNA of MF or SM by reverse transcription-polymerase chain reaction. The level of Gapd mRNA was used as an internal control. Bar = 100 μm
Developmental Profiles of Ceacam10 mRNA in Seminal Vesicles and Embryos
The amounts of Ceacam10 mRNA in the seminal vesicles of mice at different ages were compared. The RNA message first appeared at a considerable level in 3-wk-old mice. Thereafter, the amount of transcript began increasing rapidly at 4 wk and reached a maximum in 7-wk-old mice (Fig. 5A).
Fig. 5.
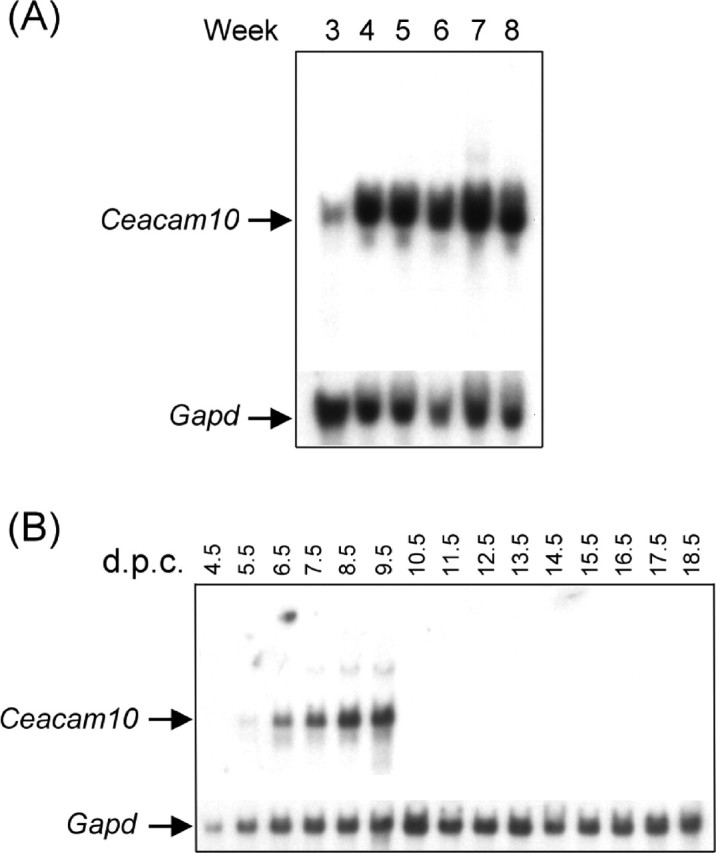
Developmental profile of Ceacam10 mRNA in embryos and seminal vesicles. Ceacam10 mRNA and Gapd mRNA in the total RNA prepared from mouse seminal vesicles at different ages (A) or in mouse embryos collected on various days postcoitus (B). The RNA messages were measured by Northern blot as described in the text
We analyzed mouse embryos from 4.5 to 18.5 days postcoitus (d.p.c.). The 4.5–6.5 d.p.c. samples included early stage embryos, extraembryonic tissue, and maternal uterus; the 7.5–9.5 d.p.c. samples included embryos and extraembryonic tissues, and the 10.5–18.5 d.p.c. samples were solely embryos. The RNA message in the embryo samples was present in trace amounts on 5.5 d.p.c, increased remarkably from 6.5 d.p.c. to a maximum on 9.5 d.p.c., and rapidly declined thereafter to an almost undetectable level until delivery (Fig. 5B).
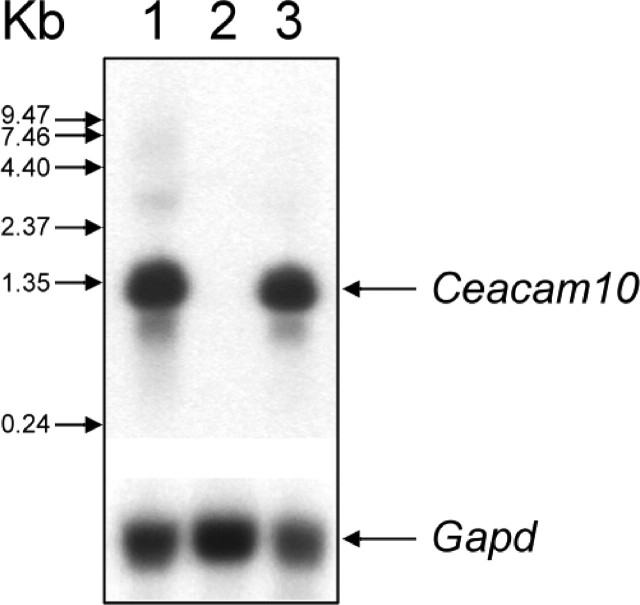
Because seminal vesicle growth is known to be androgen-dependent, we examined how androgen influenced Ceacam10 expression in the seminal vesicles of adult mice that had been castrated 3 wk earlier (Fig. 6). Ceacam10 mRNA was undetectable in the total RNA prepared from the control castrates that had received a daily injection of corn oil only compared with that of normal adults. Induction of Ceacam10 mRNA was observed in the castrates treated with testosterone (5 mg/kg per day) for 8 consecutive days.
Fig. 6.
Androgen dependence of Ceacam10 mRNA expression in seminal vesicles of adult mice. Northern blot analysis for 1.1-kilobase Ceacam10 mRNA in total RNA from seminal vesicles from normal adult mice (lane 1), adults castrated 3 wk previously and treated only with corn oil (lane 2), and adults castrated 3 wk previously and treated testosterone propionate in corn oil for 8 consecutive days (lane 3). Total RNA (20 μg) was used for each experiment. Gapd mRNA was used as an internal control
Enhancement of Sperm Motility by CEACAM10 In Vitro
Figure 7A shows micrographs of epididymal spermatozoa with indirect fluorescence staining. No fluorescence was observed on the cells after they were treated successively with the CEACAM10 antibody and rhodamine-conjugated anti-rabbit IgG, demonstrating a lack of CEACAM10 on the cell surface. When spermatozoa were preincubated with 1 μM CEACAM10 in a blocking solution at room temperature for 45 min, rhodamine fluorescence was prominent on the middle piece, relatively weak on the tail, and faint on the head (Fig. 7A, d). Apparently, sperm have CEACAM10-binding sites that cover the entire cell surface. To access the binding of CEACAM10 to epididymal sperm upon ejaculation, the ejaculated sperm were directly stained with the antibody. CEACAM10 was immunodetected on the surface of the ejaculated sperm, in spite of high fluorescence background due to the free CEACAM10 that was difficult to removed completely during cell preparation (Fig. 7B, d).
Fig. 7.
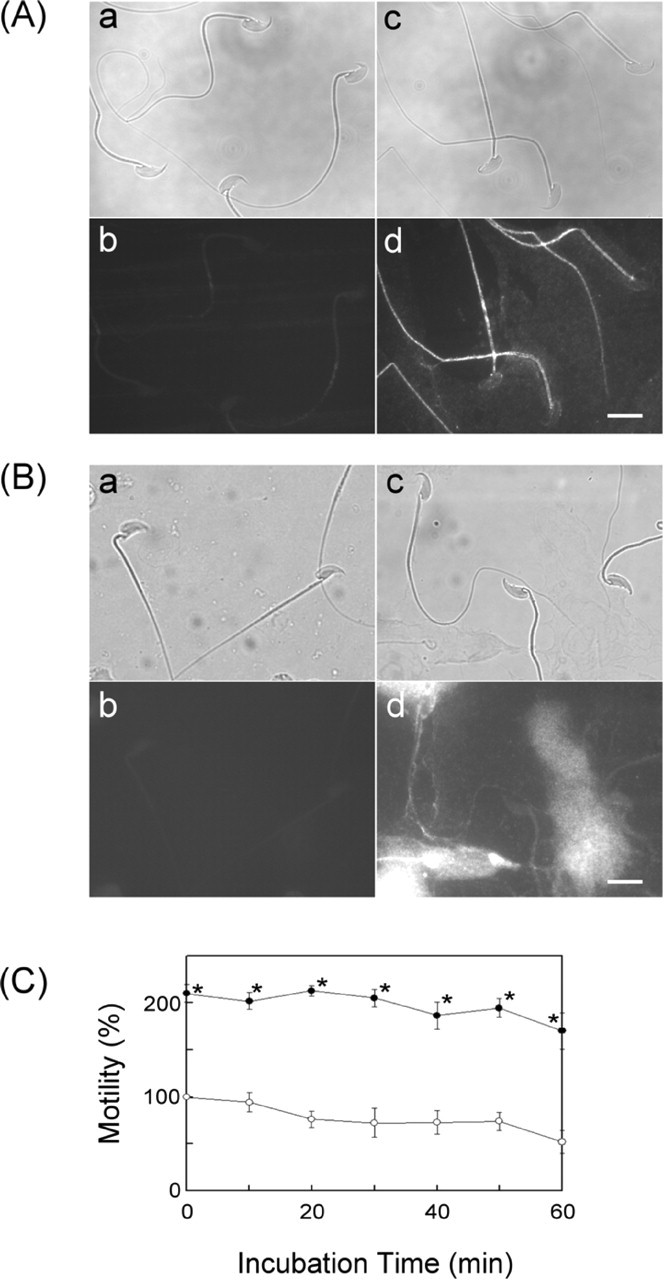
Analysis of sperm motility under the influence of CEACAM10. A) Demonstration of the CEACAM10-binding zone on epididymal spermatozoa. Fresh sperm were incubated with or without CEACAM10 as described in Materials and Methods. The cells on slides were incubated with normal serum (a and b) or affinity-purified anti-CEACAM10 antibody (c and d). The slides were then incubated with rhodamine-conjugated anti-rabbit IgG and observed via light microscopy (a and c) or fluorescence microscopy (b and d). Bar = 10 μm. B) Illustration of CEACAM10 on ejaculated sperm. Freshly prepared cells (see Materials and Methods) on slides were incubated with normal serum (a and b) or the CEACAM10 antibody (c and d) and followed by incubation with rhodamine-conjugated anti-rabbit IgG. The slides were observed via light microscopy (a and c) or fluorescence microscopy (b and d). Bar = 10 μm. C) Freshly prepared mouse spermatozoa in modified Tyrode solution (105 cells/ml) containing 1.8 mM CaCl2 were incubated alone (○) or in the presence of 90 μM CEACAM10 (•) at 37°C for 0 to 60 min. Cell motility determined at each specified incubation time was expressed as a percentage of control cell motility at time zero. Points are mean ± SD for three determinations. *P < 0.01 in a paired statistical comparison with the corresponding control. Values were evaluated using one-way analysis of variance
Most spermatozoa freshly retrieved from the caudal epididymis of mice in modified Tyrode buffer were mobile with visible tail beating. The result of CASA for the cell incubation at specified conditions revealed that 90.0 μM CEACAM10 in cell culture greatly enhanced sperm motility relative to the motility of control cells at any incubation time (Fig. 7C).
Discussion
This work is the first to purify CEACAM10 from mouse SVS. We demonstrated it to be a 36-kDa protein with an N-linked glycoconjugate. Asn11, Asn54, Asn71, and Asn191, each being part of consensus Asn-Xaa-(Ser/Thr) [27, 28] in the protein molecule, are the potential acceptor sites for the attachment of the carbohydrate moieties. Our results of Edman degradation support Asn11 as being one N-glycosylated site but rule out Asn71 in that role. Removal of the hydrophobic leader sequence from the putative form of this protein gives a protein core consisting 226, 231, or 232 amino acid residues that sum to have a molecular mass of 25 270, 25 827 or 25 898 daltons, which is close to the molecular size of the deglycosylated proteins as determined by SDS-PAGE (Fig. 1C).
The CEA family consists of CEACAM and pregnancy-specific glycoprotein subfamilies. These evolutionarily and structurally divergent glycoproteins of mammals share many common structural features [29]. They are characterized by the assembly of immunoglobulin variable (IgV)-like domain and immunoglobulin constant (IgC)-like domains in each member of the family. According to the molecular model established by Watt et al. [30] and Tan et al. [31], there are nine β strands in one immunoglobulin-like domain involved in the maintenance of the three-dimensional architecture. In the CEACAM10 molecule, residues 2–96 form one IgV-like domain and residues 122–216 form another [14]. This may account for the characteristic CD shown in Figure 2C. Many members of the murine and human CEACAM family contain either a transmembrane domain or a glycosyl PtdIns moiety, but no such structural element is present in the Ceacam10-deduced protein sequence, suggesting that CEACAM10 is not a membrane-bound protein [32]. This is substantiated by our demonstration of its secretion from the luminal epithelium of seminal vesicle (Fig. 4).
In the sexual glands of adult mice, the Ceacam10 gene is predominantly transcribed and translated in the seminal vesicle. On ejaculation, the CEACAM10-sperm binding may take place in the semen to enhance sperm motility. Finkenzeller et al. [33] demonstrated that Ceacam10−/− male and female mice developed indistinguishably from wild-type litter mates with respect to sex ratio, weight gain, and fertility, but a significant reduction by 23% of litter size was observed in Ceacam10−/− mating. Although this may be partially attributed to the lack of CEACAM10 in the semen of Ceacam10-inactivated males, it remains arguable whether in vitro CEACAM10-enhanced sperm motility may play a significant role after coitus under natural circumstances.
The maternal decidua surrounding the implantation site was not removed from the mouse conceptus that was used to prepare the commercially available embryo-stage blot for the observation of gene expression during pregnancy. As a result, the appearance of Ceacam10 mRNA in embryo samples at the blastula or gastrula stage (6.5–9.5 d.p.c.) (Fig. 5B) may arise from the maternal decidua as suggested in the study by Finkenzeller et al. [33]. This finding, together with the lack of an RNA message in embryo samples at 4.5 and 10.5–18.5 d.p.c. (Fig. 5B) implies that Ceacam10 might be weakly expressed if not silent during the entire course of embryonic development. In fact, we were unable to immunodetect the presence of CEACAM10 in the embryo samples obtained by carefully microdissecting the extraembryonic tissues from the conceptus at 8.5 to 18.5 d.p.c. Cytochemical observations shown in Figure 7 suggest the presence of CEACAM10-binding sites on the entire sperm surface. Considering the presence of CEACAM1 on human sperm cells [34], it raises a possibility that heterophilic adhesion exists between CEACAM10 and other CEACAM molecules on the mouse sperm surface. This is unlike the action of other sperm motility effectors in the mouse SVS, such as SVS VII, which binds neutral phospholipid to enhance sperm motility [10], and SVA, which predominantly binds membrane phosphatidylcholine to suppress sperm motility [12].
References
- 1. Nichol R, Hunter RH, de Lamirande E, Gagnon C, Cooke GM. Motility of spermatozoa in hydrosalpingeal and follicular fluid of pigs. J Reprod Fertil 199710:79–86 [DOI] [PubMed] [Google Scholar]
- 2. Iwamoto T, Tsang A, Luterman M, Dickson J, de Lamirande E, Okuno M, Mohri H, Gagnon C. Purification and characterization of a sperm motility-dynein ATPase inhibitor from boar seminal plasma. Mol Reprod Dev 199231:55–62 [DOI] [PubMed] [Google Scholar]
- 3. Jeng H, Liu KM, Chang WC. Purification and characterization of reversible sperm motility inhibitors from porcine seminal plasma. Biochem Biophys Res Commun 1993191:435–440 [DOI] [PubMed] [Google Scholar]
- 4. Al-Somai N, Vishwanath R, Shannon P, Molan PC. Low molecular weight components in bovine semen diffusate and their effects on motility of bull sperm. Reprod Fertil Dev 19946:165–171 [DOI] [PubMed] [Google Scholar]
- 5. Peitz B. Effects of seminal vesicle fluid components on sperm motility in the house mouse. J Reprod Fertil 198883:169–176 [DOI] [PubMed] [Google Scholar]
- 6. Robert M, Gagnon C. Purification and characterization of the active precursor of a human sperm motility inhibitor secreted by the seminal vesicles: identity with semenogelin. Biol Reprod 199655:813–821 [DOI] [PubMed] [Google Scholar]
- 7. Pang SF, Chow PH, Wong TM. The role of the seminal vesicles, coagulating glands and prostate glands on the fertility and fecundity of mice. J Reprod Fertil 197956:129–132 [DOI] [PubMed] [Google Scholar]
- 8. Peitz B, Olds-Clarke P. Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol Reprod 198635:608–617 [DOI] [PubMed] [Google Scholar]
- 9. Chen YH, Pentecost BT, McLachlan JA, Teng CT. The androgen-dependent mouse seminal vesicle secretory protein IV: characterization and complementary deoxyribonucleic acid cloning. Mol Endocrinol 19871:707–716 [DOI] [PubMed] [Google Scholar]
- 10. Luo CW, Lin HJ, Chen YH. A novel heat-labile phospholipid-binding protein, SVS VII, in mouse seminal vesicle as a sperm motility enhancer. J Biol Chem 2001276:6913–6921 [DOI] [PubMed] [Google Scholar]
- 11. Chen LY, Lin YH, Lai ML, Chen YH. Developmental profile of a caltrin-like protease inhibitor, P12, in mouse seminal vesicle and characterization of its binding sites on sperm surface. Biol Reprod 199859:1498–1505 [DOI] [PubMed] [Google Scholar]
- 12. Huang YH, Chu ST, Chen YH. Seminal vesicle autoantigen, a novel phospholipid-binding protein secreted from luminal epithelium of mouse seminal vesicle, exhibits the ability to suppress mouse sperm motility. Biochem J 1999343:241–248 [PMC free article] [PubMed] [Google Scholar]
- 13. Huang YH, Chu ST, Chen YH. A seminal vesicle autoantigen of mouse is able to suppress sperm capacitation-related events stimulated by serum albumin. Biol Reprod 200063:1562–1566 [DOI] [PubMed] [Google Scholar]
- 14. Keck U, Nedellec P, Beauchemin N, Thompson J, Zimmermann W. The cea10 gene encodes a secreted member of the murine carcinoembryonic antigen family and is expressed in the placenta, gastrointestinal tract and bone marrow. Eur J Biochem 1995229:455–464 [DOI] [PubMed] [Google Scholar]
- 15. Tarentino AL, Plummer TH Jr. Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol 1994230:44–57 [DOI] [PubMed] [Google Scholar]
- 16. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 1985150:76–85 [DOI] [PubMed] [Google Scholar]
- 17. Li SH, Chen YH. Various forms of mouse lactoferrins: purification and characterization. J Chromatogr B 1999762:45–52 [DOI] [PubMed] [Google Scholar]
- 18. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970227:680–685 [DOI] [PubMed] [Google Scholar]
- 19. Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989:7.46, 9.58 [Google Scholar]
- 20. Bellve AR, Zheng W, Martinova YS. Recovery, capacitation, acrosome reaction, and fractionation of sperm. Methods Enzymol 1993225:113–136 [DOI] [PubMed] [Google Scholar]
- 21. Ruiz-Romero J, Antich M, Bassas L. Choosing among different technical variations of Percoll centrifugation for sperm selection. Andrologia 199527:149–153 [DOI] [PubMed] [Google Scholar]
- 22. Miyake M, Coney P, Iritani A, Kling OR. Motility and fertilizing ability of rat epididymal spermatozoa washed by a continuous gradient of Percoll. Gamete Res 198924:49–57 [DOI] [PubMed] [Google Scholar]
- 23. Chen YH, Yang JT, Martinez HM. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry 197211:4120–4131 [DOI] [PubMed] [Google Scholar]
- 24. Chen YH, Yang JT, Chau KH. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry 197413:3350–3359 [DOI] [PubMed] [Google Scholar]
- 25. Chang CT, Wu CS, Yang JT. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem 197891:13–31 [DOI] [PubMed] [Google Scholar]
- 26. Perczel A, Park K, Fasman GD. Analysis of the circular dichroism spectrum of proteins using the convex constraint algorithm: a practical guide. Anal Biochem 1992203:83–93 [DOI] [PubMed] [Google Scholar]
- 27. Marshall RD. Glycoproteins. Annu Rev Biochem 197241:673–702 [DOI] [PubMed] [Google Scholar]
- 28. Gavel Y, von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng 19903:433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 19999:67–81 [DOI] [PubMed] [Google Scholar]
- 30. Watt SM, Teixeira AM, Zhou GQ, Doyonnas R, Zhang Y, Grunert F, Blumberg RS, Kuroki M, Skubitz KM, Bates PA. Homophilic adhesion of human CEACAM1 involves N-terminal domain interactions: structural analysis of the binding site. Blood 200198:1469–1479 [DOI] [PubMed] [Google Scholar]
- 31. Tan K, Zelus BD, Meijers R, Liu JH, Bergelson JM, Duke N, Zhang R, Joachimiak A, Holmes KV, Wang JH. Crystal structure of murine sCEACAM1a[1,4]: a coronavirus receptor in the CEA family. Embo J 200221:2076–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlson A, Kuroki M, Lin SH, Lucka L, Najjar SM, Neumaier M, Öbrink B, Shively JE, Skubitz KM, Stanners CP, Thomas P, Thompson JA, Virji M, von Kleist S, Wagener C, Watt S, Zimmermann W. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res 1999252:243–249 [DOI] [PubMed] [Google Scholar]
- 33. Finkenzeller D, Fischer B, Lutz S, Schrewe H, Shimizu T, Zimmermann W. Carcinoembryonic antigen-related cell adhesion molecule 10 expressed specifically early in pregnancy in the decidua is dispensable for normal murine development. Mol Cell Biol 200323:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Draberova L, Cerna H, Brodska H, Boubelik M, Watt SM, Stanners CP, Draber P. Soluble isoforms of CEACAM1 containing the A2 domain: increased serum levels in patients with obstructive jaundice and differences in 3-fucosyl-N-acetyl-lactosamine moiety. Immunology 2000101:279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]