Abstract
Rhinoviruses and coronaviruses are recognized as the major causes of the common cold syndrome. The role of these viruses in more serious respiratory illnesses resulting in hospitalization is less well defined. During a winter when influenza A infection was prevalent, 100 elderly adults hospitalized because of cardiopulmonary illnesses were evaluated for rhinovirus and coronavirus infection. Patients who tested negative for influenza or respiratory syncytial virus had nasal swab samples tested for rhinovirus, coronavirus OC43, and coronavirus 229E by reverse-transcription polymerase chain reaction and for coronaviruses by serologic testing. Twelve percent of patients had rhinovirus or coronavirus identified (rhinovirus, 4 patients; coronavirus 229E, 4 patients; coronavirus OC43, 3 patients; and mixed rhinovirus/coronavirus 229E infection, 1 patient). All patients had significant underlying diseases. Although all patients recovered, the mean length of stay was 8 days; 4 persons had pneumonia, and 1 required ventilator support. These data suggest that rhinoviruses and coronaviruses may be associated with serious respiratory illnesses in frail older adults.
Influenza virus and respiratory syncytial virus (RSV) have been recognized as important causes of hospitalization in elderly adults during the winter months. However, the role of other respiratory viruses has been less well defined. Rhinoviruses and coronaviruses cause the majority of common cold syndromes [1, 2]. With the exception of severely immunocompromised hosts, these viruses are uncommonly associated with severe illnesses resulting in hospitalizations [3, 4]. This phenomenon may be due to the limited virulence of these pathogens and the pathogenesis of their infections, but it may also be due to the lack of detection, either because of the failure to perform appropriate tests or the difficulty in identifying these organisms using standard viral culture and serologic techniques. Several reports describe the effects of coronavirus and rhinovirus infection in elderly persons in day care or long-term care [5, 6]. Although lower respiratory signs and symptoms were common, serious sequelae were not often observed. Rhinoviruses and coronaviruses also have been implicated in exacerbations of chronic obstructive pulmonary disease (COPD) [7, 8]. However, most studies used viral culture or serologic testing to diagnose infections and did not comment on hospitalization rates. A recent large study of patients of all ages hospitalized with respiratory illnesses demonstrated that rhinoviruses and coronaviruses accounted for 5.1% of illnesses [9]. Because culture and serologic testing, rather than new molecular techniques, were used for diagnosis, the authors postulated that the incidence of disease may have been significantly underestimated. Reverse-transcription (RT) polymerase chain reaction (PCR) is a very sensitive and specific method of diagnosis that has been used with success to accurately define the true burden of disease due to these common viruses [10, 11]. The purpose of this study was to determine whether rhinoviruses and coronaviruses, as diagnosed by RT-PCR and serologic testing, are associated with hospitalization in elderly adults during the winter months and to describe the presenting signs and symptoms of these illnesses.
Patients and Methods
Study protocol. Active surveillance for respiratory infections was performed at Rochester General Hospital (Rochester, New York) from 15 November 1999 to 15 April 2000 as part of an ongoing study to identify influenza and RSV infections. All patients >65 years old and those with underlying heart or lung conditions admitted with a clinical diagnosis of acute respiratory infection, bronchitis, exacerbation of COPD, congestive heart failure (CHF), influenza, or pneumonia were invited to participate. Demographic and clinical information was collected during patient interviews and from medical records. Combined nasal and pharyngeal swab specimens were tested for influenza virus and RSV by culture and RT-PCR. Acute and convalescent blood samples (at ~4 weeks) were obtained for serologic testing in as many cases as possible. During the 5 months of surveillance, 332 illnesses in 316 hospitalized persons were evaluated. Sixty-one cases of influenza A infection and 22 cases of RSV infection were identified and excluded from subsequent analysis. Of the 249 remaining cases, 100 samples were randomly selected for rhinovirus and coronavirus testing.
Laboratory methods for rhinovirus and coronavirus RT-PCR. RTPCR for human rhinovirus (HRV) and human coronaviruses (HCV) was done by methods described elsewhere, with minor modifications [12, 13]. Total RNA was extracted from 200 µL of sample by matrix affinity chromatography (QIAamp DNA blood kit; Qiagen). The eluted RNA was transcribed into cDNA with murine reverse transcriptase (MMLV-RT; Gibco BRL) and virus specific oligonucleotide primer, for 1 h at 37°C. After MMLV-RT denaturation at 95°C, virus-specific 5′ biotinylated oligonucleotide primer and Taq polymerase (Applied Biosystems) were added, and 35 cycles of PCR were run, consisting of denaturation (1 min at 95°C for HRV; 2 min at 95°C for HCV), annealing (1.5 min at 48.2°C for HRV; 1 min at 60°C for HCV), and DNA extension for 1 min at 72°C. The pairs of primers for coronaviruses OC43 and 229E were used in the same reaction in a multiplex format. Excess primers, dNTPs, Taq DNA polymerase, and salts were removed by adsorbing the amplified product to the QIAquick silica-gel membrane (QIAquick PCR purification kit; Qiagen). The presence of the PCR product was detected by microplate hybridization with digoxigenin-labeled virusspecific probes, as described elsewhere [13]. Positive and negative controls were previously tested nasal washings either containing or lacking specific HRV or HCV RNA.
Laboratory methods for coronavirus serologic testing. Serologic evidence of coronavirus infection was defined as a ⩾3-fold increase in coronavirus-specific IgG, as measured by EIA. Coronavirus 229E antigens were prepared from infected WI-38 cell lysates and OC43 antigens in suckling mouse brains. EIA plates were coated with either 229E or OC43 antigens to Nunc flat-bottom plates in bicarbonate buffer. Control plates were prepared by using uninfected WI-38 cell lysates or mouse brain suspensions. Serum samples obtained during acute and convalescent illness (acute and convalescent serumsamples) were added to wells in duplicate in serial 2-fold dilutions from 1:400 to 102:400. Serum IgG was detected with alkaline phosphatase-conjugated goat anti-human antibody, followed by substrate. The end-point titer was defined as the highest dilution with an optical density ⩾0.100 that was at least twice that of the control plate [6].
Statistical methods. Means were compared with Student's t test, and proportions were compared using χ2 and Fisher's exact tests, as appropriate.
Results
Of the 100 cases evaluated, rhinovirus and/or coronavirus were identified as a pathogen in 12 of them. Four nasal specimens were RT-PCR positive for rhinovirus, 4 were positive for coronavirus 229E, 1 was positive for coronavirus OC43, and 1was positive for both rhinovirus and coronavirus 229E. Of the 100 cases, acute and convalescent serum samples were available for 88 of them, from which 2 additional OC43 infections were identified. The 6 patients who tested positive by PCR for coronaviruses were seronegative for coronavirus.
Rhinovirus infections occurred sporadically throughout the winter, although coronavirus OC43 infections occurred primarily in December and January and coronavirus 229E infections occurred primarily in March and April. The mean (±SD) age of patients was 74 ± 11 years, with similar numbers of men and women in both groups (table 1). All patients had significant underlying chronic medical conditions, primarily CHF and COPD, and 1 patient had chronic lymphocytic leukemia (CLL). Consistent with the high rate of lung disease, 92% were active or previous smokers, 33% took corticosteroids daily, and 50% received oxygen at home. Seventy-five percent had exposure to young children. Compared with patients infected with influenza during the same period, the rhinovirus/coronavirus group had significantly more lung disease. Nasal congestion and cough were common symptoms (67% and 78%, respectively), as were lower respiratory tract symptoms of dyspnea (83%) and sputum production (58%). Constitutional symptoms, such as fatigue and myalgias, occurred in only 33% of patients with HRV or HCV, compared with 54% in influenza virus-infected patients. Fever was significantly less common in the HRV and HCV group than in the influenza group (mean temperature, 37:4°C±1:0°C vs. 38:4°C±1:1°C; P = .005). Patients were quite ill at admission, with 75% demonstrating wheezing and rales by auscultation. Supplemental oxygen was required for all but 1 individual. Most patients received steroids (75%), bronchodilators (83%), and antibiotics (83%). All patients eventually recovered and were discharged after a mean length of stay of 8 days. There were no distinct features of rhinovirus or coronavirus infection.
Table 1.
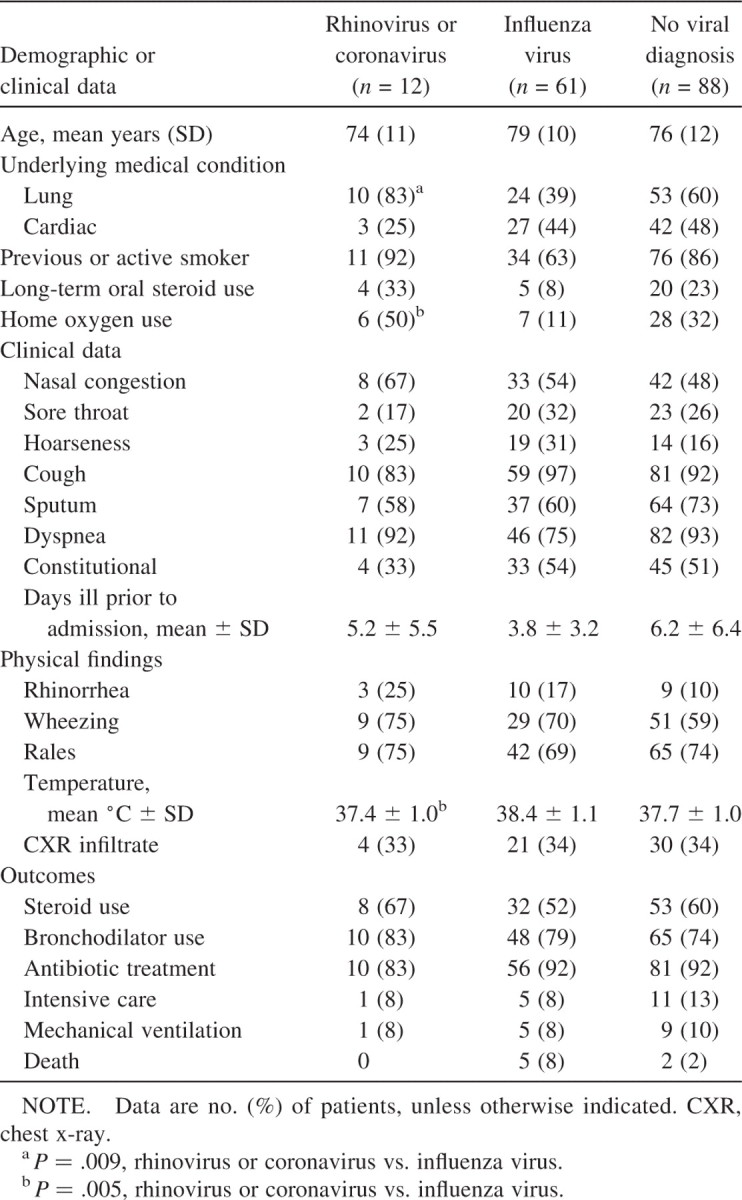
Demographic and clinical data among elderly patients hospitalized for cardiopulmonary illness.
Four patients had radiographically proven pneumonia (2 with coronavirus and 2 with rhinovirus infection). One 89-year-old woman with rhinovirus infection had a history of COPD and presented with a temperature of 39.3°C, an SaO2 level of 57%, and an infiltrate on chest X-ray.No blood or sputumcultures were obtained. The other rhinovirus-positive patient with pneumonia had CLL, a temperature of 39.0°C, and blood and sputum cultures that grew no pathogens. Both patients with coronavirus infection and pneumonia were elderly women with CHF and presented with nasal congestion, cough, and dyspnea and had visible rhinorrhea at examination. Blood cultures were sterile, and 1 patient tested positive for Pseudomonas aeruginosa in an inadequate sputum sample. Two individuals without pneumonia had evidence of concurrent bacterial infection: 1 patient with positive coronavirus serologic test results had Streptococcus pneumoniae bacteremia, and Staphylococcus aureus was isolated from the sputum of 1 person with coronavirus 229E.
Discussion
During a winter season when influenza was prevalent and accounted for a substantial number of hospitalizations, rhinoviruses and coronaviruses were diagnosed by RT-PCR and serologic testing in 12% of patients without influenza or with RSV. These data provide more evidence that not all serious respiratory illnesses that occur during the winter months are always “the flu.” Our results are consistent with a recent study by El-Sahly et al. [9], documenting coronavirus/rhinovirus infections in 26 (4.7%) of 546 persons #x003E;35 years old who were hospitalizedwith cardiopulmonary illnesses, and with a 4-year study by Glezen et al. [4], in which viruses were found to be a common trigger for acute decompensation leading to hospitalization in persons with preexisting lung diseases. In the latter study of 417 adults ⩾45 years old who were hospitalized for respiratory infections, influenza viruses were detected most frequently in 8% of patients, whereas rhinoviruses were detected in 2% and coronaviruses in 3% of patients. However, this study did not use RT-PCR to detect viruses, and rates likelywould have been higher if more-sensitive diagnostic tools were available. In a study of inner-city adults with asthma, rhinoviruses and coronaviruses were more common than influenza in patients presenting to an emergency room [10]. Sixty percent of rhinovirus and 71% of coronavirus infections were detectable only by RT-PCR.
The higher rate of infection in our study likely reflects the use of RT-PCR, rather than culture, for diagnosis, aswell as the months studied and the elderly population. A limitation of this study is the lack of a control group of hospitalized elderly patients without respiratory illnesses. Previous studies have shown that rhinoviruses may be detected in 3% of asymptomatic elderly adults [11]. The use of a method of diagnosis that detects minute quantities of RNA raises the question of the role of the virus in causality of the illness. Unfortunately, samples from a control group were not available. It was not unexpected that there were patients with positive coronavirus PCR results but negative serologic test results, since antibody responses may be impaired in frail elderly persons.
Of note, persons hospitalized because of rhinovirus/coronavirus infections were more frail than those with influenza, with all persons having either significant cardiopulmonary disease or cancer. This finding is consistent with a study by Nicholson et al. [11], in which the presence of chronic medical conditions or smoking increased the risk of lower respiratory tract disease by ~40% in elderly adults with rhinovirus infection. In the study by El-Sahly et al. [9], asthma, COPD, or CHF was present in 73% of patients ⩾35 years old who were hospitalized because of rhinovirus or coronavirus infections. In addition,Wald et al. [5] found that rhinovirus infections produced more-protracted lower respiratory tract symptoms in nursing home residents with COPD or a history of smoking.
Illnesses were significant, with the mean length of stay >1 week, and nearly all patients received supplemental oxygen, steroids, and antibiotics. One patient required intensive care, but all survived.Of interest, 2 patientswith rhinovirus infections had high fevers and radiographic evidence of pneumonia. Rhinoviruses are rarely implicated as a cause of pneumonia, which is believed to reflect their biologic characteristics [14]. However, recent data from Papadopoulos et al. [15] suggest that infection of the lower airways is possible and that rhinoviruses may have direct effects on the lower respiratory tract epithelium. The high frequency of lower respiratory tract symptoms in ambulatory elderly persons with rhinovirus infections and the recovery of rhinovirus from expectorated sputum samples are additional evidence for viral invasion of the lower airways [5, 11]. It is unknown how often bacterial suprainfection in the lower respiratory tract follows HRV or HCV infection. Despite these uncertainties, mixed viralbacterial infection in our patients seems likely, although one person had CLL, thus making a viral pneumonia a possibility.
In summary, infectionwith agents that typically cause colds in young adults may lead to hospitalization in frail elderly persons with underlying heart and lung problems. It does not appear that healthy elderly persons are at high risk from these agents. These viruses, although not as common as influenza and RSV among hospitalized adults, also circulate during the winter months, producing similar clinical syndromes. The use of RT-PCR improves the ability to identify these previously difficult to detect viruses, but further studies with control groups are needed to fully define the impact of HCV and HRV in hospitalized elderly adults.
Footnotes
The study was approved by the Rochester General Hospital institutional review board, and informed consent was obtained from all patients or their legal guardians.
Financial support: National Institute of Allergy and Infectious Diseases (grant RO-145969).
The authors have no associations that pose a conflict of interest.
Reference
- 1.Gwaltney JM, Hendley JO, Simon G, Jordan WS. Rhinovirus infections in an industrial population. I. The occurrence of illness. N Engl J Med. 1966;275:1261–8. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- 2.Hendley JO, Fishburne HB, Gwaltney JM., Jr Coronavirus infections in working adults. Am Rev Respir Dis. 1972;105:805–11. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S, Champlin R, Couch R, et al. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:553–5. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 4.Glezen PW, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 5.Wald TG, Shult P, Krause P, Miller BA, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long-term care facility. Ann Intern Med. 1995;123:588–9. doi: 10.7326/0003-4819-123-8-199510150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, McCann RM, Hall WJ, et al. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc. 1997;45:706–11. doi: 10.1111/j.1532-5415.1997.tb01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh EE, Falsey AR, Hennessey PA. Respiratory syncytial virus and other infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care Med. 1999;160:791–5. doi: 10.1164/ajrccm.160.3.9901004. [DOI] [PubMed] [Google Scholar]
- 8.Fagon J, Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Semin Respir Infect. 1996;11:109–18. [PubMed] [Google Scholar]
- 9.El-Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atmar R, Guy E, Guntupalli K, et al. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–9. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson KG, Kent J, Hammersley V, Esperanza C. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–4. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitkaranta A, Arruda E, Malmberg H, Hayden FG. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–3. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arruda E, Hayden FG. Detection of human rhinovirus RNA in nasal washings by PCR. Mol Cell Probes. 1993;7:373–9. doi: 10.1006/mcpr.1993.1055. [DOI] [PubMed] [Google Scholar]
- 14.Naclerio RM, Proud D, Lichtenstein LM, Kagey-Sobotka A, Sorrentino J, Gwaltney JM. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988;157:133–42. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]


