Abstract
Hepatitis C virus (HCV) has evolved complex strategies to evade host immune responses and establish chronic infection. Since human Vγ9Vδ2 T lymphocytes play a critical role in the immune response against viruses, we analyzed their antiviral functions on Huh7 hepatoma cells carrying the subgenomic HCV replicon (Rep60 cells). In a transwell culture system, Rep60 cells were co-cultured with either PBMCs or highly purified γδ T cells stimulated by non-peptidic antigens. Vγ9Vδ2 T cell activation was associated with a dramatic reduction of HCV RNA levels. Neutralizing antibodies targeting IFN-γ revealed a critical role for this cytokine in the inhibition of HCV replication. Interestingly, drugs already in clinical use, such as Phosphostim and Zoledronate, known to activate γδ T cells, were shown to induce the inhibition of HCV replication mediated by Vγ9Vδ2 T cells of HCV patients. Our data suggest that the therapeutic activation of Vγ9Vδ2 T lymphocytes may represent an additional strategy to inhibit HCV replication and to restore a Th1-oriented immune response in HCV-infected patients.
Keywords: hepatitis C, IFN-γ, natural immunity, γδ T cells
Keywords: BrHPP bromohydrin pyrophosphate, CBA cytometric bead assay, FBS fetal bovine serum, HBV hepatitis B virus, HCV hepatitis C virus, IPP isopentenyl pyrophosphate, MIP1α/β macrophage inflammatory protein 1 α/β, NU neutralization unit, RANTES regulation on activation normal T expressed and secreted, RT reverse transcription, TNF tumor necrosis factor, UNG uracil-N-glycosylase, ZOL Zoledronate
Introduction
Hepatitis C virus (HCV) is a major causative agent of liver disease worldwide and the majority of infected people develop a lifelong chronic infection (1, 2). The current therapeutic protocols, based on the combined administration of IFN-α and ribavirin, are unable to eradicate the virus in a significant number of patients (3). Thus, it is crucial to improve the current protocols investigating new therapeutical approaches.
In viral infections, both specific and natural immunity cooperate to provide host defense. In viral hepatitis, the interaction between immune response and virus-infected hepatocytes represents a key event for the initial control of viral replication, the protection and the development of the disease (4, 5). Several data indicate that control and clearance of hepatitis B virus (HBV) and HCV infections may be provided by the direct non-cytolytic antiviral activity of soluble factors (6, 7). Among the different kinds of cells involved in the early immunity, γδ T lymphocytes are known to exert a broad antiviral activity against different viruses such as retrovirus, flavivirus, paramyxovirus, orthomyxovirus, picornavirus, coronavirus, arenavirus, herpesvirus, hepadnavirus and orthopox virus (8).
Intrahepatic T lymphocytes of chronic hepatitis C patients, with a higher degree of necroinflammatory liver disease, belong to the Vδ1 T cell subset (9). These cells are polyclonally activated and recruited in the liver, suggesting their involvement in the HCV liver pathology (5). Differently, a decrease of the Vγ9Vδ2 T cell subset was observed in the peripheral blood of patients with chronic hepatitis C when compared with those of either controls or responders to IFN-α therapy (10), suggesting a Vγ9Vδ2 T cell-specific involvement in the antiviral immune response. Interestingly, Vγ9Vδ2 T cells can be activated by several non-peptidic antigens such as pyrophosphomonoesters (11–14), alkylamines (15) and N-containing bisphosphonates (16, 17). Moreover, the N-containing bisphosphonate drug known as Zoledronate (ZOL) and the bromohydrin pyrophosphate (BrHPP) drug called Phosphostim are currently used in oncology for therapy or pre-clinical trials (18–20).
In this work, we analyzed the ability of activated Vγ9Vδ2 T lymphocytes to exert a non-cytolytic antiviral activity against HCV using a model for subgenomic HCV replication in hepatoma cells (21, 22). We observed that the activation of peripheral Vγ9Vδ2 T lymphocytes by non-peptidic antigens induces a non-cytolytic inhibition of subgenomic HCV replication. Neutralization experiments have shown that this effect is mediated by IFN-γ, indicating that modulation of Vγ9Vδ2 T lymphocyte-mediated non-cytolytic antiviral activity by non-peptidic drugs may provide a novel approach for the immunotherapy of HCV infection.
Methods
HVC replicon cell culture
Rep60 is a human hepatoma Huh7 cell line harboring HCV replicon as previously described (23). HCV replicon present in Rep60 cells carries the previously described adaptative mutation A2199T (24), as determined by sequencing. Rep60 cells and the parental cell line Huh7 (i.e. not containing the HCV replicon) were maintained in DMEM (Life Technologies, Milan, Italy) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37°C in 5% CO2. Recombinant human IFN-γ (PeproTech, London, UK) and IFNα2b (Intron-A3, Schering-Plough, Kenilworth, NJ, USA) were used to induce HCV replicon clearance as previously described (25, 26).
Lymphocyte isolation
Blood samples were obtained from either healthy donors or HCV patients and PBMCs were isolated by Ficoll-Hypaque gradients (Pharmacia Biotech, Piscataway, NJ, USA). γδ T cells were purified from PBMCs by immunomagnetic separation using anti-γδ-conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of cell fraction was ≥95% in all experiments as measured by flow cytometric analysis (data not shown). Before co-cultures, cells were maintained in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 50 U ml−1 penicillin and 50 μg ml−1 streptomycin. Vγ9Vδ2 T cell lines were obtained by stimulating PBMCs of healthy donors with isopentenyl pyrophosphate (IPP) (3 μg ml−1) and IL-2 (100 U ml−1) for 10 days.
Co-cultures of PBMCs or γδ T cells and Rep60
Rep60 cells (3 × 104 cells per well) were co-cultured in flat-bottom 24-well plates in the presence of either PBMCs (1 × 106 cells per well) or purified γδ T cells (5 × 105 cells per well) in a RPMI/DMEM medium (1:1 ratio) supplemented as described above. To avoid heterologous cell–cell contacts, PBMCs or isolated γδ T cells were cultured in a 0.4-μm pore size semi-permeable polycarbonate membrane transwell chamber (BD Labware, Franklin Lakes, NJ, USA). The co-cultures were left untreated or stimulated with IPP (3 μg ml−1, Sigma, St Louis, MO, USA), zoledronic acid (ZOL) (2 μM, Novartis, Basel, Switzerland) or BrHPP (160 ng ml−1, kindly provided by Innate-Pharma, Marseille, France). For the analysis of cytokine production, supernatants were collected after 24 h of co-culture.
In some experiments, Rep60 cells or the parental cell line Huh7 were pre-treated with different doses of ZOL (1, 5, 80 and 160 μM) for 2 h, washed twice and co-cultured with Vγ9Vδ2 T cell lines in an RPMI/DMEM medium (1:1 ratio) for 16 h. IFN-γ production was evaluated by intracellular staining and flow cytometry.
Flow cytometry
PBMCs or γδ T cells were washed in PBS containing 1% BSA and 0.1% sodium azide and were incubated for 15 min at 4°C with the following mAbs: anti-Vδ2 mAb (IgG1, clone B6.1) coupled with FITC, anti-CD25 (IgG1, clone M-A251), anti-CD69 (IgG1, clone L78) and anti-HLA-DR (IgG2b, clone TU36) coupled with PE (BD Biosciences, Mountain View, CA, USA). Samples were washed twice in PBS, 1% BSA, fixed in 4% PFA and acquired by a FACSCalibur flow cytometer (BD Biosciences). A total of 50 000 events was acquired for each sample and analyzed with CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA).
The analysis of cytokines production was performed using the cytometric bead assay (CBA). Using the human Th1/Th2 CBA kit (BD Biosciences) co-cultures, supernatants were collected after 24 h and analyzed for the production of IL-2, IL-4, IL-5, IL-10, tumor necrosis factor-α (TNF-α) and IFN-γ, according to the manufacturer's instructions. Data were analyzed by CBA software (Becton Dickinson).
The frequency of TNF-α- and IFN-γ-producing Vδ2 T cells was examined by single-cell analysis and flow cytometry as previously described (27). PBMCs were stimulated overnight with IPP (3 μg ml−1), ZOL (2 μM) or BrHPP (160 ng ml−1) and Brefeldin A (10 μg ml−1, Serva Heidelberg, Germany) was added 2 h after stimulation to block intracellular transport. In the co-culture experiments between hepatoma cells and Vγ9Vδ2 T cell lines, Brefeldin A (10 μg ml−1) was added 2 h after the beginning of co-cultures. Cells (PBMCs or Vγ9Vδ2 T cell lines) were washed twice in PBS, 1% BSA and 0.1% sodium azide and stained with anti-Vδ2 mAb (IgG1, clone B6.1) for 15 min at 4°C. Samples were then fixed in 1% PFA for 10 min at 4°C and incubated with anti-cytokine-specific mAbs (TNF-α–PE: IgG1, clone MP6-XT22; IFN-γ–allophycocyanin: IgG2b, clone 2573.11) diluted in PBS, 1% BSA and 0.5% saponin. The cells were finally washed twice in PBS, 1% BSA and 0.1% saponin and acquired on a FACSCalibur (Becton Dickinson).
RNA isolation, reverse transcription–PCR and real-time PCR
Total RNA was extracted with TRIzol reagent (GIBCO BRL, Life Technology, NY, USA) according to the manufacturer's instructions. Reverse transcription (RT)–PCR amplification for HCV NS4A and β-actin was performed as previously described (23).
The number of HCV replicon copies was measured by real-time quantitative PCR targeting the 5′-untranslated region of HCV, using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Applera Italia, Italy). One microgram of total RNA was used for RT reaction using MultiScribe reverse transcriptase random hexamers method (Applied Biosytems). Primers and probe were the following: forward primer FHCV139R, reverse primer RHCV85F and TaqMan probe (FAM) HCV105MGB (Applied Biosystems).
The amplification was performed in a 50-μl reaction mixture containing 10 μl of template, 25 μl of 2× TaqMan Universal PCR Master Mix (Applied Biosystems), 900 nM of each primer and 300 nM of probe. Following activation of the uracil-N-glycosylase (UNG) (2 min, 50°C) and activation of the AmpliTaq Gold for 10 min at 95°C, 40 cycles of amplification (15 s at 95°C and 1 min at 60°C) were performed. The standard curve has been obtained using known concentrations of HCV replicon-encoding plasmid.
Neutralizing antibody assay
Cytokine neutralization assay was achieved using rabbit polyclonal antiserum against either IFN-α [original titer: 5 × 106 neutralization units (NU) ml−1] or IFN-γ (original titer: 5 × 104 NU ml−1), obtained in our laboratory (28). A total of 1 × 104 NU ml−1 of neutralizing antibodies was incubated with either the supernatants of PBMCs stimulated with IPP (3 μg ml−1) for 24 h, or different doses of recombinant IFN-α or IFN-γ. After 30 min, these neutralized supernatants were added to Rep60 cell cultures and the presence of HCV RNA was analyzed after 4 days by RT–PCR, as previously described (23).
Results
Selective activation of Vγ9Vδ2 T cells induces HCV replication inhibition
To explore if soluble factors released by activated γδ T cells exert an antiviral activity against the HCV replicon, PBMCs isolated from two healthy donors were co-cultured with Huh7 cells carrying the subgenomic HCV replicon [Rep60; (23)] in a cell-contact-free culture system, using transwell inserts. In order to specifically activate Vγ9Vδ2 T cells, we used IPP, a non-peptidic compound known to selectively trigger this population (12, 13). After 4 days of co-culture, total RNA was extracted and the levels of HCV RNA were determined by RT–PCR analysis. Notably, as shown in Fig. 1(A), IPP stimulation of co-cultures induced a dramatic reduction of HCV RNA levels in Rep60 cells (lane 5 and lane 7 for donors 0502 and 2902, respectively) independently of cell–cell contact, while IPP does not affect per se HCV replication (lane 2). On the other hand, no effects on HCV RNA levels were observed with unstimulated PBMCs (lane 4 and lane 6 for donors 0502 and 2902, respectively), indicating that IPP activation is necessary to induce the inhibition of HCV replication and suggesting, therefore, the direct involvement of Vγ9Vδ2 T cells. IFN-α, known to inhibit subgenomic HCV replication (26), was used as control (lane 3). Similar results were obtained using PBMCs isolated from other 15 healthy donors (data not shown). Notably, flow cytometric analysis of PBMCs confirmed that IPP stimulation induces the expression of different activation markers (CD25, CD69 and HLA-DR) exclusively on the Vδ2 T lymphocyte surface from all donors analyzed (data not shown).
Fig. 1.
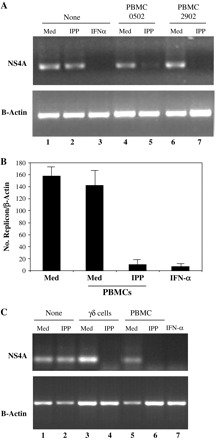
Soluble factors released by Vγ9Vδ2 T cells are able to inhibit HCV replication. (A) Detection of HCV replicon presence in Rep60 cells co-cultured with PBMCs. Rep60 cells were co-cultured with PBMCs isolated from two healthy donors (0502 and 2902) either in the absence (Med) or in the presence of IPP. After 4 days of co-culture, total RNA was extracted and HCV RNA was detected by conventional RT–PCR, using specific oligos for NS4A region. As a loading control, levels of β-actin mRNA were analyzed. IFN-α was used to induce HCV replicon clearance. (B) Measurement of the HCV replicon copies number in Rep60 cells by quantitative real-time PCR. Rep60 cells were left untreated (Med), IFN-α treated or co-cultured with PBMCs, either unstimulated (Med) or IPP stimulated (IPP). Total RNA was extracted and quantitative analysis of the HCV RNA levels was performed by real-time PCR, targeting the 5′-untranslated region of the HCV replicon. Expression levels of HCV normalized for β-actin expression are reported as mean values + SD of three experiments. (C) Detection of HCV replicon presence in Rep60 cells co-cultured with purified γδ T cells. Rep60 cells were co-cultured with either PBMCs or purified γδ T cells (≥95% pure population) of the same healthy donor, either in the absence (Med) or in the presence of IPP. Total RNA was extracted and HCV RNA was analyzed as in (A).
Since conventional RT–PCR is only a semi-quantitative technique, we quantified the extent of HCV replicon inhibition induced by IPP-stimulated PBMCs by real-time PCR. As shown in Fig. 1(B), IPP stimulation of PBMCs induced a 15-fold reduction of HCV RNA levels similar to IFN-α (18-fold reduction), thus confirming the results obtained by semi-quantitative RT–PCR.
To test whether the antiviral activity was directly mediated by γδ T cells or was due to a bystander activation of other cells, γδ T cells were purified by magnetic beads and used in the co-cultures. Total PBMCs or ≥95% pure γδ T cells from the same donors were co-cultured with Rep60 cells, either in the presence or absence of IPP. As shown in Fig. 1(C), purified γδ T cells were able to inhibit subgenomic HCV replication at a similar extent of total PBMC population, only when stimulated by IPP (lanes 4 and 6, respectively).
Altogether, these data demonstrate that the γδ T cell subset, when stimulated by the non-peptidic antigen IPP, exerts a direct antiviral activity mediated by soluble factors.
IPP-activated Vγ9Vδ2 T cells release IFN-γ, TNF-α and IL-10
In order to understand which is the soluble factor responsible for HCV replication inhibition, we analyzed the presence of a subset of cytokines released by IPP-activated Vγ9Vδ2 T cells. Supernatants from Rep60 and PBMC co-cultures stimulated with IPP were collected after 24 h and different cytokines were simultaneously analyzed by the CBA. Figure 2(A) shows the results obtained from 12 different experiments. While unstimulated PBMCs produced none of the cytokines detectable by the CBA system (IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ), the antigen stimulation induced a drastic increase of IL-10, TNF-α and IFN-γ production (IL-10: 284.5 ± 81.7 pg ml−1; TNF-α: 1803.9 ± 485.3 pg ml−1 and IFN-γ: 1227.1 ± 567.8 pg ml−1). These cytokines were mainly released by Vδ2 T cells. In fact, intracellular staining assays of PBMCs cultured for 6 h in the presence of IPP showed that this stimulation induced a high frequency of TNF-α- and IFN-γ-producing Vδ2+ cells (Fig. 2B and C; 28.5 and 30% of the total Vδ2+ cells, respectively). In contrast, bystander production of these cytokines by other cells was not significant (0.6 and 0.2%, respectively), confirming that IPP selectively activates Vγ9Vδ2 T cells.
Fig. 2.

IPP induces TNF-α and IFN-γ production by Vγ9Vδ2 T cells. (A) Quantitative analysis of cytokines released by IPP-stimulated PBMCs. The presence of IL-4, IL-5, IL-10, TNF-α and IFN-γ in the supernatants of either unstimulated (white bars, all below 15 pg/ml) or 24-h IPP-stimulated (black bars) PBMCs of healthy donors (n = 12) was simultaneously measured by CBA. Data are shown as the arithmetic mean + SD. (B, C) IPP treatment induces TNF-α and IFN-γ production by Vγ9Vδ2 cells. Two-color intracellular flow cytometric analysis was used to measure the frequency of (B) TNF-α- and (C) IFN-γ-producing Vδ2 T lymphocytes present in PBMCs treated with IPP for 24 h. The percentage of positive cells is reported. In parentheses the percentage of cytokine-producing Vδ2 T cells among total Vδ2 T cells is reported. A representative analysis from one experiment is shown (n = 12).
IPP-stimulated Vγ9Vδ2 T cells mediate anti-HCV activity through IFN-γ production
To determine the specific role of IPP-induced IFN-γ and TNF-α production in the inhibition of HCV replication, we performed antibody-blocking assays. For this experiment we used cell-free supernatants. These supernatants, obtained from Vγ9Vδ2 T cells stimulated with IPP for different periods of time, were able to inhibit HCV replication in Rep60 cells at a similar extent of co-cultures (Fig. 3, lane 2, and data not shown), thus confirming that the IPP-stimulated γδ T cells produce cytokines which exert antiviral activity. This hypothesis was supported by the observation that the block of IFN-γ activity by specific neutralizing antibodies (28) significantly reduced the HCV replication inhibition (Fig. 3, lane 6). On the contrary, no impairment of the HCV-inhibitory activity was observed using neutralizing antibodies against either TNF-α (data not shown) or IFN-α (Fig. 3, lane 4). As control for the neutralizing activity of mAbs used in our experiments, Rep60 cells were treated with IFN-α or IFN-γ, two known inhibitors of HCV replication (25, 26), either in the presence or absence of the specific neutralizing mAbs (Fig. 3, lanes 8–13 and lanes 14–19, respectively). As expected, both anti-IFN-α and anti-IFN-γ mAbs were able to block HCV replicon clearance even at high doses of cytokines (Fig. 3, lane 13 and lane 19, respectively). It is worthy to note that high concentrations of recombinant TNF-α were not able to influence HCV RNA levels (data not shown). Our data demonstrate that antiviral activity of IPP-activated Vγ9Vδ2 T cells is mainly mediated through IFN-γ production.
Fig. 3.

IFN-γ mediates the IPP-induced anti-HCV activity. The role of IFN-α and IFN-γ in the inhibition of HCV replication was analyzed by antibody-blocking assay. Rep60 cells were cultured with IPP-stimulated PBMC supernatants neutralized for either IFN-α (lanes 3 and 4) or IFN-γ (lanes 5 and 6). PBMC supernatants were pre-incubated with neutralizing antibodies for 30 min before being added to Rep60 cell cultures. Increasing doses of either IFN-α (lanes 8–10 and 11–13) or IFN-γ (lanes 14–16 and 17–19) were used to determine the neutralization ability of the mAbs to IFN-α (lanes 11–13) or IFN-γ (lanes 17–19). After 4 days of culture, the HCV RNA was analyzed by RT–PCR as described in Fig. 1(A).
ZOL and Phosphostim drugs are able to stimulate the Vγ9Vδ2 T cell-mediated inhibition of HCV replication
It is known that the N-containing bisphosphonate ZOL and the pyrophosphomonoester Phosphostim (BrHPP), two drugs used in clinical therapies or trials, respectively, activate Vγ9Vδ2 T cells (14, 19). We asked, therefore, whether these drugs are able to induce Vγ9Vδ2 T cell-mediated HCV clearance. As shown in Fig. 4(A), PBMC/Rep60 co-cultures treated with ZOL or BrHPP inhibited subgenomic HCV replication (lanes 5 and 6, respectively) at a similar extent to IPP stimulation (lane 4). This antiviral activity was mediated by PBMC activation since no effects on viral replication per se was observed (data not shown). Notably, peripheral Vγ9Vδ2 T cells release IFN-γ in response to either ZOL or BrHPP (Fig. 4B), indicating that these drugs, as well as for IPP stimulation, may induce HCV replicon clearance through IFN-γ production.
Fig. 4.

Drugs containing non-peptidic antigens induce HCV replication inhibition and Vγ9Vδ2 T cell-mediated IFN-γ production. (A) Detection of the presence of HCV replicon in Rep60 cells. Rep60 cells were co-cultured with PBMCs, isolated from healthy donors, either in the absence (Med) or in the presence of IPP, ZOL or Phosphostim (BrHPP). After 4 days of co-culture, the HCV RNA was analyzed by RT–PCR as described in Fig. 1(A). A representative experiment is shown (n = 5). (B) ZOL and BrHPP treatments induce IFN-γ production by Vγ9Vδ2 T cells. PBMCs isolated from healthy donors were treated with either ZOL or BrHPP for 24 h and two-color intracellular flow cytometric analysis was performed as described in Fig. 2(C). The percentage of positive cells is reported. In parentheses the percentage of IFN-γ-producing Vδ2 T cells among total Vδ2 T cells is reported. A representative analysis of one experiment is shown (n = 5). (C) IFN-γ production by Vγ9Vδ2 T cell lines co-cultured with Rep60 and Huh7 cells in a cell–cell contact system. Vγ9Vδ2 T cell lines, >80% pure population obtained as described in Methods, were co-cultured in a cell–cell contact system with Rep60 cells (black circle) or the parental cell line Huh7 (white circle; i.e. not containing the HCV replicon) either untreated (−) or pre-treated for 2 h with ZOL at different doses as indicated. After 16 h of co-culture, the IFN-γ production by Vδ2 T cells was evaluated by two-color intracellular flow cytometric analysis, as described in Fig. 2(C). A representative experiment is shown (n = 3).
Since it is known that ZOL can induce the recognition of target cells by γδ T lymphocytes, we asked whether the presence of HCV replicon could affect this mechanism. In a cell–cell contact system, HCV replicon-carrying cells and the parental cell line Huh7 (i.e. not containing HCV replicon) were either left untreated or pre-treated with increasing doses of ZOL (1, 5, 80 and 160 μM) for 2 h, extensively washed and co-cultured with Vγ9Vδ2 T cell lines (purity >80%). After 16 h, IFN-γ production by Vγ9Vδ2 cells was analyzed by intracellular staining assay. Notably, as shown in Fig. 4(C), in the absence of ZOL stimulation, Rep60 cells, as well as the parental cell line Huh7, did not induce a significant IFN-γ production by Vγ9Vδ2 T cells (0.44%), thus indicating that HCV replicon per se was not able to trigger Vγ9Vδ2 T lymphocytes activation. On the other hand, ZOL pre-treated Rep60 and Huh7 cells induced a similar IFN-γ production by Vγ9Vδ2 T cells, thus suggesting that ZOL induces Vγ9Vδ2 T cell activation through an HCV-independent indirect mechanism, probably by an accumulation of mevalonate metabolites in the cells.
Non-peptidic antigens are able to induce IFN-γ production and antiviral activity in Vγ9Vδ2 T cells of HCV patients
To test if Vγ9Vδ2 T cells of HCV patients could be activated by non-peptidic antigens, we analyzed drug-stimulated HCV+ PBMCs for both IFN-γ production and HCV replicon clearance induction. As showed in Fig. 5(A), IPP, BrHPP and ZOL were able to induce the release of IFN-γ by Vγ9Vδ2 T cells of HCV patients at a similar extent to those observed for healthy donors. To verify if the IFN-γ released by HCV+ Vγ9Vδ2 T cells was sufficient and biologically active to induce the HCV replicon clearance, we co-cultivated Rep60 and HCV+ PBMCs in the presence of IPP, BrHPP or ZOL. After 4 days of co-culture, the presence of HCV RNA was analyzed by RT–PCR. The representative experiment shown in Fig. 5(B) demonstrated that the PBMCs of HCV patients stimulated with IPP, BrHPP and ZOL are able to induce the clearance of HCV replicon (lanes 4, 5 and 6, respectively). Altogether, these data demonstrate that the Vγ9Vδ2 T cells present in the peripheral blood of HCV patients could be stimulated by non-peptidic antigens to exert an antiviral activity.
Fig. 5.

Non-peptidic antigens induce HCV replication inhibition and IFN-γ production in Vγ9Vδ2 T cells of HCV patients. (A) Vγ9Vδ2 T cells of HCV patients are activated by non-peptidic antigens. PBMCs were isolated from healthy donors (white bars; n = 5) or HCV patients (striped bars; n = 5) and stimulated with IPP, BrHPP or ZOL for 24 h. The IFN-γ production by Vδ2 T cells was evaluated by two-color intracellular flow cytometric analysis, as described in Fig. 2(C). The bars encompass the middle 50% of the individual measurements and the horizontal bar-dividing line indicates the median value. The vertical lines span the range of the lowest and highest measurements. Differences were not statistically significant. (B) Detection of the presence of HCV replicon in Rep60 cells. Rep60 cells were co-cultured with PBMCs, isolated from HCV patients (PBMC/HCV+), either in the absence (Med) or in the presence of IPP, ZOL or Phosphostim (BrHPP). After 4 days of co-culture, the HCV RNA was analyzed by RT–PCR as described in Fig. 1(A). A representative experiment is shown (n = 3).
Discussion
In HCV patients, both specific and innate immunities are frequently unable to eradicate the infection. Moreover, the current regimens based on IFN-α and ribavirin are not effective in a considerable number of HCV patients. Therefore, the development of additional therapeutical approaches is needed. In this study, taking advantage of the subgenomic HCV replicon system in hepatoma cells (21, 22), we analyzed the anti-HCV activity of γδ T cells. The major contribution of this work is the finding that Vγ9Vδ2 T lymphocytes exert an antiviral activity against HCV when activated by non-peptidic antigens. We found that IPP stimulation of either PBMCs or highly purified γδ T lymphocytes induced a drastic inhibition of HCV replication. This antiviral activity was mediated by the release of non-cytolytic antiviral factors since it was independent of cell–cell contacts and obtained using the supernatants of IPP-stimulated cultures. By the use of neutralizing antibodies we have demonstrated that, in our cell-culture system, the inhibition of HCV replication is exerted through the release of IFN-γ. This result is in line with the ability of recombinant IFN-γ to inhibit HCV replicon replication (25). We identified the Vγ9Vδ2 T population as the effector cells of the IPP-mediated antiviral activity. Although, we cannot completely rule out a possible effect of other contaminating effector cells, intracellular staining experiments demonstrated that the main IFN-γ-producing cells are Vδ2 T lymphocytes. Moreover, the ability of highly purified γδ T lymphocytes to inhibit HCV replication strongly suggested that this cell population is likely to be the main mediator of the antiviral activity. It has been shown that HCV-specific CD8 T cells are able to inhibit HCV replication by both direct cytolytic effects and cytokine-mediated activity (7). Our findings extend to the cells of innate immunity the capability to induce an activity against HCV. Evidences that γδ T cells mediate antiviral activity through the release of non-cytolytic soluble factors have been already reported. In human immunodeficiency virus infection, several data indicate that Vγ9Vδ2 T cells exert both cytolytic (29, 30) and non-cytolytic antiviral activity through the release of β-chemokines [macrophage inflammatory protein 1 α/β (MIP1α/β) and regulation on activation normal T expressed and secreted (RANTES)] (31–33). Considering another flavivirus such as West Nile virus, the adoptive transfer of γδ T cells to TCRδ−/− mice reduced the susceptibility of these mice to the virus (34). As for our in vitro co-culture system, this protective effect in vivo was primarily due to IFN-γ production.
IFN-γ is a key molecule in the immune response against viral infections, modulating both innate and adaptive immune responses and stimulating several intracellular pathways that directly suppress viral replication without killing the host cells (35, 36). In HCV infections, the ability of T cells to produce IFN-γ in the acute phase has been associated to viral clearance in humans (37). In chronic HCV-infected patients, an impairment of peripheral-specific T cells to produce IFN-γ and hepatic expansion of HCV-specific CD8+ T cells with regulatory phenotype have been reported (38). Moreover, in chimpanzees the expansion of a Th1-oriented, multi-specific and sustained T cell response was associated with the resolution of HCV infection (39). These data indicate that the establishment of chronic infection may be associated to a status of low inflammation level. This could lead to an inhibition of Th1-specific immune response and to an expansion of IL-10-producing T cells, thus suggesting that IFN-γ production could be one of the key events for the resolution of HCV infection.
Utilizing cell–cell contact culture conditions, we showed that HCV replicon-carrying cells are not able to stimulate IFN-γ production by Vγ9Vδ2 T cells. However, since we utilized the subgenomic HCV replicon that lacks structural proteins, we cannot completely rule out that Vγ9Vδ2 T cells are not involved in the recognition of HCV-infected hepatocytes. Our data suggest that γδ T cells, in spite of their apparent lack of specificity in virus recognition, could play an important role in the immune response against viruses by releasing IFN-γ and, likely, other non-cytolytic soluble factors. An interesting feature of the γδ T cell biology is the possibility to induce their activation by non-peptidic antigens. Vγ9Vδ2 T cells were shown to release Th1 cytokines and to mediate a cytoreductive effect on myeloma cells when activated by N-containing bisphosphonates (16). Moreover, Phosphostim-activated Vγ9Vδ2 T cells kill autologous metastatic renal carcinoma (20). We showed that the non-peptidic antigens ZOL and BrHPP were able to induce HCV replicon clearance mediated by PBMCs derived from both healthy donors and, more importantly, HCV patients. Vγ9Vδ2 T cells released IFN-γ when directly stimulated by non-peptidic antigens, indicating that, at least in the peripheral blood, γδ T cells of HCV patients could be specifically activated by these drugs. Interestingly, some of these non-peptidic antigens are already in clinical use (18), thus suggesting the feasibility of this approach in humans.
Altogether, our data suggest that γδ T cells of HCV patients could be stimulated to release soluble factors useful for both non-cytolytic antiviral activity and immunoregulatory functions important for the adaptive response. Therefore, a cell-target immunotherapy aimed to boost γδ T cells may complement the traditional therapeutic regimen currently in use for treating chronic HCV infection.
Acknowledgments
We thank F. Romagnè (Innate-Pharma) for providing us the Phosphostim (BrHPP) used in this study. The work was supported by grants from Ricerca Corrente and Ricerca Finalizzata of the Italian Ministry of Health.
Footnotes
These authors contributed equally to this work.
Transmitting editor: E. Vivier
References
- 1.Lauer, G. M. and Walker, B. D. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41. [DOI] [PubMed] [Google Scholar]
- 2.Ippolito, G. and Craxi, A. 2003. Hepatitis C: virus, host, disease. Cell Death Differ. 10(Suppl. 1):S1. [DOI] [PubMed] [Google Scholar]
- 3.Capobianchi, M. R., Abbate, I., Cappiello, G. and Solmone, M. 2003. HCV and interferon: viral strategies for evading innate defence mechanisms in the virus-host battle. Cell Death Differ. 10(Suppl. 1):S22. [DOI] [PubMed] [Google Scholar]
- 4.Willberg, C., Barnes, E. and Klenerman, P. 2003. HCV immunology—death and the maiden T cell. Cell Death Differ. 10(Suppl. 1):S39. [DOI] [PubMed] [Google Scholar]
- 5.Poccia, F. and Agrati, C. 2003. Intrahepatic natural immunity and HCV immunopathogenesis. Cell Death Differ. 10(Suppl. 1):S9. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti, L. G., Rochford, R., Chung, J., Shapiro, M., Purcell, R. and Chisari, F. V. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825. [DOI] [PubMed] [Google Scholar]
- 7.Liu, C., Zhu, H., Tu, Z., Xu, Y. L. and Nelson, D. R. 2003. CD8+ T-cell interaction with HCV replicon cells: evidence for both cytokine- and cell-mediated antiviral activity. Hepatology 37:1335. [DOI] [PubMed] [Google Scholar]
- 8.Poccia, F., Agrati, C., Martini, F., Capobianchi, M. R., Wallace, M. and Malkovsky, M. 2005. Antiviral reactivities of γδ T cells. Microbes Infect.7:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonekura, K., Ichida, T., Sato, K. et al.2000. Liver-infiltrating CD56 positive T lymphocytes in hepatitis C virus infection. Liver 20:357. [DOI] [PubMed] [Google Scholar]
- 10.Par, G., Rukavina, D., Podack, E. R. et al.2002. Decrease in CD3-negative-CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J. Hepatol. 37:514. [DOI] [PubMed] [Google Scholar]
- 11.Constant, P., Davodeau, F., Peyrat, M. A. et al.1994. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science 264:267. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka, Y., Morita, C. T., Tanaka, Y., Nieves, E., Brenner, M. B. and Bloom, B. R. 1995. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375:155. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka, Y., Brenner, M. B., Bloom, B. R. and Morita, C. T. 1996. Recognition of nonpeptide antigens by T cells. J. Mol. Med. 74:223. [DOI] [PubMed] [Google Scholar]
- 14.Espinosa, E., Belmant, C., Pont, F. et al.2001. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J. Biol. Chem. 276:18337. [DOI] [PubMed] [Google Scholar]
- 15.Bukowski, J. F., Morita, C. T. and Brenner, M. B. 1999. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity 11:57. [DOI] [PubMed] [Google Scholar]
- 16.Kunzmann, V., Bauer, E., Feurle, J., Weissinger, F., Tony, H. P. and Wilhelm, M. 2000. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 96:384. [PubMed] [Google Scholar]
- 17.Kunzmann, V., Bauer, E. and Wilhelm, M. 1999. Gamma/delta T-cell stimulation by pamidronate. N. Engl. J. Med. 340:737. [DOI] [PubMed] [Google Scholar]
- 18.Li, E. C. and Davis, L. E. 2003. Zoledronic acid: a new parenteral bisphosphonate. Clin. Ther. 25:2669. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm, M., Kunzmann, V., Eckstein, S. et al.2003. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102:200. [DOI] [PubMed] [Google Scholar]
- 20.Viey, E., Fromont, G., Escudier, B. et al.2005. Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J. Immunol. 174:1338. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann, V., Korner, F., Koch, J., Herian, U., Theilmann, L. and Bartenschlager, R. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110. [DOI] [PubMed] [Google Scholar]
- 22.Blight, K. J., Kolykhalov, A. A. and Rice, C. M. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972. [DOI] [PubMed] [Google Scholar]
- 23.Fimia, G. M., Evangelisti, C., Alonzi, T. et al.2004. Conventional protein kinase C inhibition prevents alpha interferon-mediated hepatitis C virus replicon clearance by impairing STAT activation. J. Virol. 78:12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, E. M., Grobler, J. A., Markel, E. J. et al.2003. Persistent replication of hepatitis C virus replicons expressing the beta-lactamase reporter in subpopulations of highly permissive Huh7 cells. J. Virol. 77:2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frese, M., Schwarzle, V., Barth, K. et al.2002. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 35:694. [DOI] [PubMed] [Google Scholar]
- 26.Guo, J. T., Bichko, V. V. and Seeger, C. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battistini, L., Borsellino, G., Sawicki, G. et al.1997. Phenotypic and cytokine analysis of human peripheral blood gamma delta T cells expressing NK cell receptors. J. Immunol. 159:3723. [PubMed] [Google Scholar]
- 28.Capobianchi, M. R., Ameglio, F., Cordiali, F. P. et al.1993. Coordinate induction of interferon alpha and gamma by recombinant HIV-1 glycoprotein 120. AIDS Res. Hum. Retrovir. 9:957. [DOI] [PubMed] [Google Scholar]
- 29.Wallace, M., Bartz, S. R., Chang, W. L., Mackenzie, D. A., Pauza, C. D. and Malkovsky, M. 1996. Gamma delta T lymphocyte responses to HIV. Clin. Exp. Immunol. 103:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poccia, F., Cipriani, B., Vendetti, S. et al.1997. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive V gamma 9V delta 2 T lymphocytes. J. Immunol. 159:6009. [PubMed] [Google Scholar]
- 31.Poccia, F., Battistini, L., Cipriani, B. et al.1999. Phosphoantigen-reactive Vgamma9Vdelta2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J. Infect. Dis. 180:858. [DOI] [PubMed] [Google Scholar]
- 32.Levy, J. A., Mackewicz, C. E. and Barker, E. 1996. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today 17:217. [DOI] [PubMed] [Google Scholar]
- 33.Cipriani, B., Borsellino, G., Poccia, F. et al.2000. Activation of C–C beta-chemokines in human peripheral blood gammadelta T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood 95:39. [PubMed] [Google Scholar]
- 34.Wang, T., Scully, E., Yin, Z. et al.2003. IFN-gamma-producing gamma delta T cells help control murine West Nile virus infection. J. Immunol. 171:2524. [DOI] [PubMed] [Google Scholar]
- 35.Goodbourn, S., Didcock, L. and Randall, R. E. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341. [DOI] [PubMed] [Google Scholar]
- 36.Grandvaux, N., tenOever, B. R., Servant, M. J. and Hiscott, J. 2002. The interferon antiviral response: from viral invasion to evasion. Curr. Opin. Infect. Dis. 15:259. [DOI] [PubMed] [Google Scholar]
- 37.Lechner, F., Wong, D. K., Dunbar, P. R. et al.2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francavilla, V., Accapezzato, D., De Salvo, M. et al.2004. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: exploring the immunological mechanisms. Eur. J. Immunol. 34:427. [DOI] [PubMed] [Google Scholar]
- 39.Cooper, S., Erickson, A. L., Adams, E. J. et al.1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439. [DOI] [PubMed] [Google Scholar]


