ABSTRACT
The intestinal mucosa contributes to homeostasis by preventing the entrance of biological and chemical agents across the epithelium that could alter the stability of the system. This protective function is especially important at the time of weaning, when animals are exposed to infectious agents and to numerous stresses such as the change of environment and diet. Diets supplemented with spray-dried plasma or plasma protein fractions have been shown to improve growth performance of farm animals and have been proposed as an alternative to antibiotics. In this review, we summarize our findings on the mechanism of action of dietary plasma proteins using a rat model of intestinal inflammation, based on the administration of Staphylococcus aureus enterotoxin B (SEB). Staphylococcal enterotoxin B activates the gut-associated lymphoid tissue (GALT), increasing T-lymphocytes in Peyer's patches and the number of activated T lymphocytes in mesenteric lymph nodes (organized GALT). In the lamina propria SEB increased cytotoxic T δγ and natural killer cell populations of the diffuse GALT. Staphyloccocal enterotoxin B significantly increased proinflammatory cytokines in Peyer's patches and mucosa. Plasma protein supplements modulated the mucosal immune response in organized and diffuse GALT, protecting GALT from possible excessive activation by the SEB challenge. These effects are accompanied by a reduction of proinflammatory cytokine production, supporting the view that changes in cytokine production mediate the effects of dietary plasma proteins during intestinal inflammation. The increase in mucosal permeability and intestinal secretion induced by SEB was associated with decreased expression of mucosal tight-junction and adherent-junction proteins. Both plasma and plasma protein fractions prevented the effects of SEB on intestinal permeability, thus reducing the exposure of the host to microbial and food antigens across the interstitial space. These findings indicate that dietary plasma proteins modulate functional and structural properties of the intestinal mucosa.
Keywords: gut-associated lymphoid tissue, immunoglobulin, intestinal inflammation, nutrient absorption, spray-dried plasma, Staphylococcus aureus enterotoxin B
INTRODUCTION
Spray-dried plasma (SDP) is an ingredient that, when included in the diet of weanling pigs, improved daily gain, feed intake, and feed efficiency, and reduced the extent and severity of diarrhea (Coffey and Cromwell, 2001; van Dijk et al., 2001). Numerous studies involving challenges with pathogenic bacteria (Escherichia coli, Salmonella, Pasteurella multocida), viruses (rotavirus, coronavirus, white spot syndrome virus), or protozoa (Cryptosporidium parvum) demonstrated reduced mortality and morbidity when feeding spray-dried bovine or porcine plasma to various animal species, indicating that plasma supplements can be considered as an alternative to the use of antibiotics (Quigley and Drew, 2000; Bikker et al., 2004; Campbell et al., 2004; Torrallardona et al., 2007).
In an epidemiological study of Canadian swine farms, feeding SDP was associated with reduced mortality due to porcine reproductive and respiratory syndrome and porcine circovirus type 2 (Dewey et al., 2006). A protein fraction isolated from bovine plasma containing approximately 50% IgG consumed orally has been shown to improve viral gastroenteritis in children (Guarino et al., 1994) and has been used in the treatment of diarrhea in acquired immune deficiency syndrome patients infected with C. parvum (Greenberg and Cello, 1996). It has been speculated that IgG is responsible for the SDP effect (van Djik et al., 2001). However, over 250 peptides have been identified in human plasma (Anderson and Anderson, 2002). Many proteins in SDP were shown to retain biological function after spray drying (Borg et al., 2002). Although it is logical that IgG contributes to the effects of SDP, it is also likely that many of the other plasma compounds also contribute to the various responses that have been attributed to the oral consumption of SDP.
To better understand the effects of SDP on the intestinal immune system and on intestinal barrier function, a series of experiments was conducted utilizing a rat model of mild intestinal inflammation. The model is based on the systemic administration of Staphylococcus aureus enterotoxin B (SEB), an enterotoxin that activates the mucosal immune system affecting both organized and diffuse gut-associated lymphoid tissue (GALT). Staphylococcal enterotoxin B also increases mucosal permeability, increases luminal water secretion, and reduces the absorption of glucose across the small intestine. Our main interest was to better understand the mechanism of action of SDP at the mucosal level and potential modulation of the intestinal inflammatory responses. A second objective was to compare the effects of SDP with a full plasma protein fraction (PPF) isolated from plasma containing approximately 50% IgG (plasma with fibrin and albumin mostly removed).
INTESTINAL IMMUNE SYSTEM
The immune response is divided into innate and acquired immunity. Innate immunity consists of those functions that are nonspecific in nature and with which the host is born. The innate immune system acts as a first line of defense by preventing the entry of infectious agents or by eliminating invading pathogens. It comprises physical barriers (e.g., skin or mucous membranes), cells in blood and tissues (e.g., phagocytes and natural killer cells), and soluble mediators (e.g., complement proteins and cytokines). While protection by innate immunity is effective, some pathogens have developed the ability to escape detection or clearance by this system, leading to the activation of the acquired immune system. This system consists of 2 major cell types, the T- and B-lymphocytes, which enable the specific recognition of and response to invaders. The T-lymphocytes mediate mainly cellular acquired immunity, whereas B lymphocytes are involved in humoral acquired immunity (Janeway, 2001).
The largest immune organ is situated in the gut where the mucosal immune system interacts with host and intestinal microbiota, playing an important role in protecting against pathogenic microorganisms. The mucosal immune system maintains homeostasis by innate and acquired immunity along the epithelial surface (Kiyono and Fukuyama, 2004). Gut-associated lymphoid tissue accounts for up to 80% of the mucosal immune system and is distributed throughout the intestine as organized entities, including Peyer's patches, isolated follicles, and mesenteric lymph nodes, or as diffuse GALT consisting of lymphocytes scattered along the epithelium and the lamina propria (Granger et al., 2006). Both compartments are part of a regulatory system with specific roles; organized GALT is the inductor site of the immune response and diffuse GALT is the effector site.
The primary role of the immune response is to recognize, destroy, and eliminate antigens and pathogens. However, if the immune system is stimulated and the response is not controlled, it can lead to tissue damage and pathology (McKay et al., 1998). In addition, stimulation of the immune system shifts available energy and nutrients from growth and other productive functions and diverts them to support the immune system (Demas et al., 1997; Johnson, 1997; Klasing and Korver, 1997).
The results described in this review were obtained from a rat model of mild intestinal inflammation induced by SEB. Wistar-Lewis rats receive an intraperitoneal injection of SEB on d 30 and 33 of age resulting in intestinal inflammation characterized by activation of T helper lymphocytes and an increase of several cell populations involved in inflammatory states (Pérez-Bosque et al., 2004, 2008b). In all experiments, animals were assigned to dietary treatment at weaning (d 21) and continued until d 35, when they were killed.
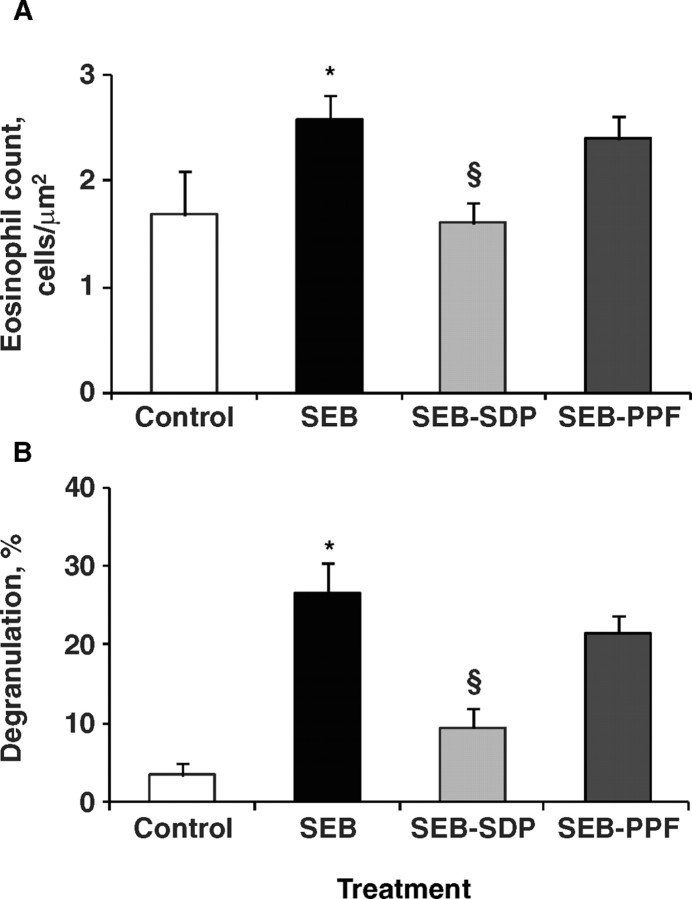
Staphylococcal enterotoxin B induced recruitment of immune cells belonging to the innate immune system, neutrophils (Pérez-Bosque et al., 2004) and eosinophils (M. Moretó and A. Pérez-Bosque, unpublished results). Addition of SDP to the diet did not modify the SEB-induced effects on neutrophil infiltration, but reduced eosinophil infiltration and the degree of degranulation (Figure 1). Dietary supplementation with SDP or PPF prevented the SEB-induced activation of CD4 cells (i.e., T-helper lymphocytes) in Peyer's patches, and in the lamina propria and intraepithelial compartments (Pérez-Bosque et al., 2004, 2008b; Figure 2). These results indicate that feeding rats with SDP or PPF reduces the SEB-induced activation of T-helper cells. Activation of T-helper lymphocyte produces a cytokine release that amplifies the innate and the acquired immune system (Mowat, 2003).
Figure 1.
Effects of dietary supplementation with plasma concentrate (spray-dried plasma, SDP) and plasma protein fraction (PPF) obtained from porcine blood, on lamina propria eosinophils in a rat model of mild intestinal inflammation. Panel A shows the effect on cell number. Panel B shows the effects on eosinophil activation as indicated by the degree of degranulation. Inflammation was induced by intraperitoneal administration of 50 μ g of Staphylococcus aureus enterotoxin B (SEB) on d 30 and 33 of age. Animals were fed experimental diets for 14 d. Results are mean ± SEM of 5 animals. Symbols indicate significant differences (P < 0.05): *vs. control group; §vs. SEB group (unpublished data).
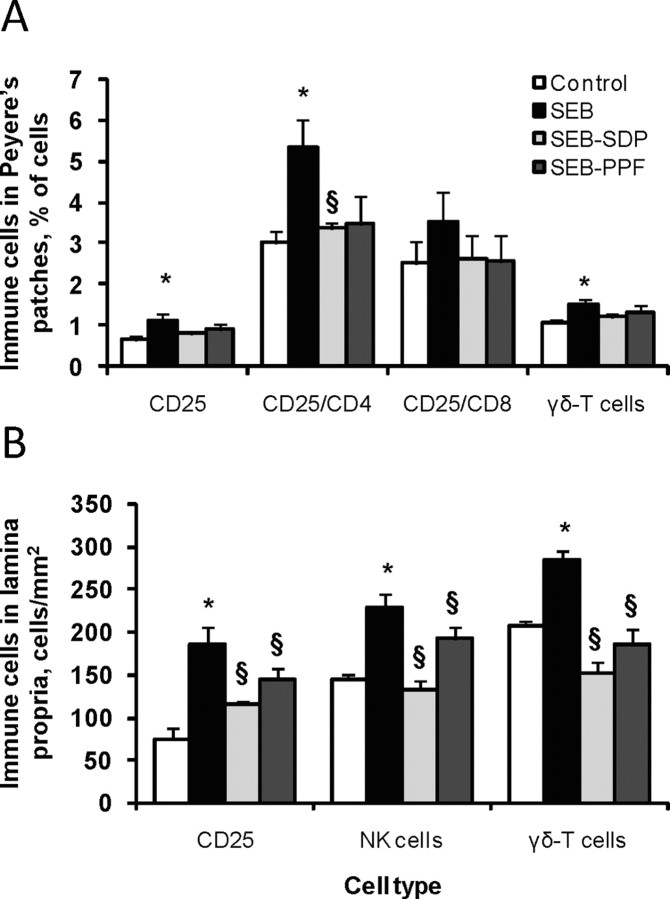
Figure 2.
Effects of dietary supplementation with plasma concentrate (spray-dried plasma, SDP) and the plasma protein fraction (PPF) on activated T lymphocytes and cytotoxic cell populations in Peyer's patches (PP; panel A) and lamina propria (LP; panel B) in a rat model of mild intestinal inflammation. Inflammation was induced by intraperitoneal administration of 50 μ g of Staphylococcus aureus enterotoxin B (SEB) on d 30 and 33 of age. Animals were fed experimental diets for 14 d. The CD25 (activated T lymphocytes), CD25/CD4 (as the fraction of activated T helper lymphocytes), CD25/CD8 (as the fraction of activated suppressor/cytotoxic T lymphocytes), natural killer (NK) cells, and δγ-T cells (T lymphocytes that express the δγ-T cell receptor) as percentages respect to total number of lymphocytes (A) and as number of cells per mm2 (B). Results are mean ± SEM of 8 to 10 animals in PP and 5 to 8 animals in LP. Symbols indicate significant differences (P < 0.05): *vs. control group; §vs. SEB group (from Pérez-Bosque et al., 2004, 2008b).
The δγ-T cell subset modulates the inflammatory response by promoting influx of lymphocytes and monocytes to mucosal surfaces (Soltys and Quinn, 1999). Staphylococcal enterotoxin B administration increased the δγ-T lymphocyte population in Peyer's patches, lamina propria, and intraepithelial lymphocytes. Plasma protein prevents the increase in the δγ-T lymphocytes in Peyer's patches and in lamina propria, which supports the hypothesis that a SDP-supplemented diet can play a role in the modulation of the immune response. In contrast, the PPF supplement only prevented the SEB effects in the lamina propria (Pérez-Bosque et al., 2004, 2008b).
Nofrarías et al. (2006) reported similar results from studies with pigs, showing that SDP can reduce the number of δγ-T cells in ileocolic lymph nodes and decrease leukocyte infiltration in intestinal mucosa. These findings agree with the decreased cell density in intra-villous lamina propria described by Jiang et al. (2000). Nofrarias et al. (2006) found evidence that the effects of SDP on GALT were also observable in nonchallenged pigs at weaning, a stressful event involving complex social, environmental, and nutritional changes for piglets (Pluske et al., 1997) that is associated with intestinal and systemic inflammatory responses (McCracken et al., 1999; Jiang et al., 2000; Pié et al., 2004).
The release of numerous proinflammatory cytokines is upregulated in response to an infection. These proinflammatory cytokines behave as chemoattractants and induce expression of adhesion molecules, which favors localization of immune cells responding to infection. In addition, cytokines stimulate functional maturation of immune cells, enabling their response to (or recognition of) pathogens. To balance the proinflammatory cytokines, a group of antiinflammatory cytokines dampens the immune response to prevent injury to the host by its own immune system (Lippolis, 2008).
In the mild intestinal inflammation rat model, SEB administration increased the release of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) in the jejunal mucosa (Moretó et al., 2008). Both SDP and PPF prevented the effects of SEB on the release of proinflammatory cytokines, indicating that the effects of plasma supplements during an inflammatory event involve changes in the mucosal cytokine balance. Bosi et al. (2004) also showed that SDP can reduce proinflammatory cytokine expression in the gut of early weaned piglets challenged with enterotoxigenic E. coli K88. Both observations are consistent with the view that SDP can limit the production of cytokines by modulating the immune response.
Immunosuppressive functions are also attributed to regulatory T-cells (T-reg lymphocytes) with a cytokine profile distinct from either Th1 or Th2 cells (e.g., transforming growth factor-β and IL-10). After antigenic stimulation, T-reg lymphocytes can specifically inhibit the immune response of T-helper cells (Iijima et al., 2001; Janeway, 2001). Recent results in our laboratory showed that SDP supplementation increased IL-10 production in SEB-challenged rats at the intestinal (Peyer's patches and intestinal mucosa) as well as at the systemic level (Moretó et al., 2008). These results support the view that the mechanism of action of SDP involves modulating the immune response to toxins and other pathogenic agents by regulating the balance of proinflammatory and antiinflammatory cytokines.
BARRIER FUNCTION
A key function of the intestinal epithelium is to serve as a selective barrier allowing the uptake of nutrients while excluding toxins and microorganisms. However, the epithelium is not completely impermeable to luminal contents, and for this reason, the mucosal to serosal movement of solvents and solutes is closely regulated. Mucosal permeability mainly depends on the capacity of tight junctions to efficiently seal the apical poles of epithelial cells. The space between cells is occupied by interlocking proteins, called claudins, that bind scaffolding proteins, such as ZO-1, which in turn link them to the cellular cytoskeleton (Barrett, 2008). An acute change of the intestinal barrier function contributes to disease pathogenesis, especially when the intestine is challenged by luminal antigens.
Permeability of the intestinal epithelium is regulated by several stimuli. Increased permeability is associated with secretory diarrhea (Toivola et al., 2004). Toxins from Clostridium and Vibrio change the localization of several tight junction proteins (Chen et al., 2002) or reduce the number of strands in the tight junction (Sonoda et al., 1999). Moreover, enterotoxins can also have indirect effects by inducing the release of proinflammatory cytokines, such as INF-γ and TNF-α. Both cytokines increase epithelial permeability by reducing the expression of ZO-1 and occludin (Tsukita et al., 2002; Bruewer et al., 2003). Staphylococcal enterotoxin B can also stimulate the secretion of IFN-γ and TNF-α from lymphocytes, which can disassemble tight junction protein complexes and enhance paracellular permeability of micro vascular endothelial cells (Nusrat et al., 2000).
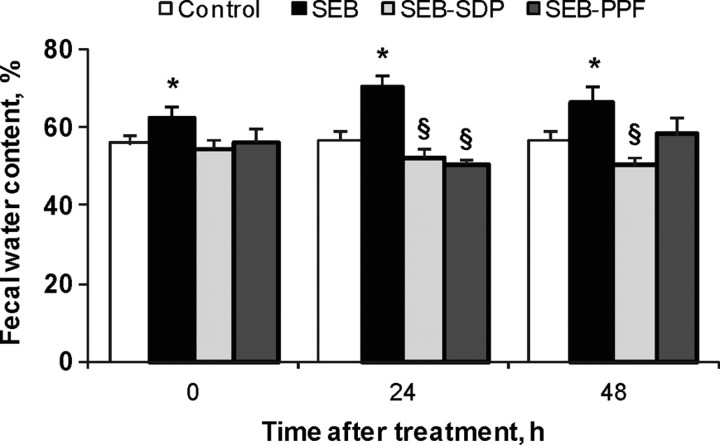
We observed that SEB increases luminal water contents, indicating an effect of SEB on the absorptive and secretory water balance (Pérez-Bosque et al., 2004). Figure 3 shows that dietary supplementation with SDP and PPF prevented the effect of the enterotoxin 24 h after the second SEB dose and that SDP was still effective after 48 h. Other studies performed with plasma supplements showed positive health effects; for example, oral supplementation of calves with bovine serum ameliorated C. parvum-induced diarrhea (Hunt et al., 2002) and decreased the severity of disease in young calves exposed to coronavirus (Arthington et al., 2002).
Figure 3.
Effects of dietary supplementation with plasma concentrate (spray-dried plasma, SDP) and plasma protein fraction (PPF) on water content in feces (expressed as percentage of wet weight) in a rat model of mild intestinal inflammation. Inflammation was induced by intraperitoneal administration of 50 μ g of Staphylococcus aureus enterotoxin B (SEB) on d 30 and 33 of age. Animals were fed experimental diets for 14 d. Results are mean ± SEM of 8 to 10 animals. Symbols indicate significant differences (P < 0.05): *vs. control group; §vs. SEB group (from Pérez-Bosque et al., 2006).
Increased luminal water may be secondary to changes in luminal osmolality (e.g., due to reduced nutrient absorption or secretion) or to changes in mucosal permeability. Because the effects on the absorption of glucose and amino acids are small (see discussion below), we wanted to know if these effects on luminal water contents could be explained by changes in paracellular permeability. To this end, we measured the transmural flux of tracers and the expression of tight junction proteins that are known to control paracellular permeability. Two tracers were utilized, fluorescein isothiocyanate-Dextran (FD, 4 kD) and horseradish peroxidase (HRP) with a molecular mass of 40 kD, similar to those of many antigenic food proteins.
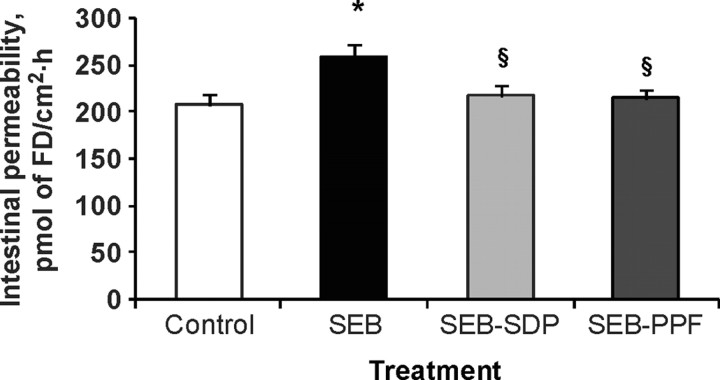
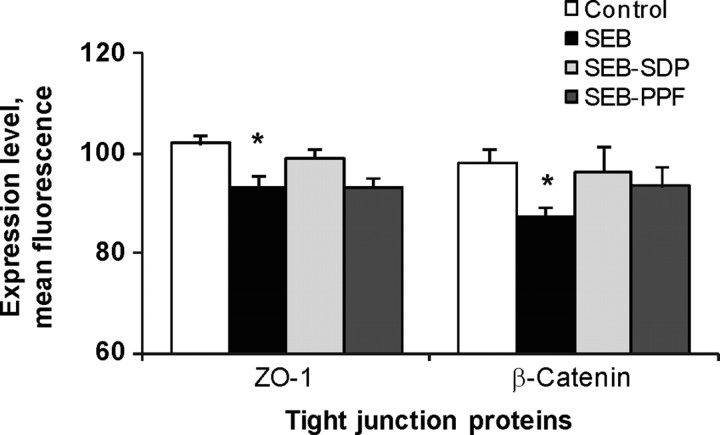
Staphylococcal enterotoxin B treatment significantly increased the flux of FD across the intestinal wall, and the SDP and PPF supplements prevented this effect (Figure 4). Staphyloccocal enterotoxin B also enhanced the HRP movement across the mucosa, and the supplements showed a tendency to prevent this effect. However, there was a significant positive correlation between FD and HRP flux values, indicating that the paracellular route contributes to increased protein translocation across the intestine in animals challenged with the enterotoxin. Staphyloccocal enterotoxin B reduced the expression of ZO-1 and β-catenin, and both proteins tended to be negatively correlated with FD flux, indicating that the increases in dextran flux are paralleled by reductions in ZO-1 and β-catenin expression at the epithelial level (Figure 5). These results indicate that the increase in FD flux induced by SEB treatment is associated with a reduction in the tightness of the epithelial junction complex and that both dietary supplements resulted in some recovery (Figure 5). The effects of SEB were similar to those described in SEB-injected mice (Lu et al., 1998). The effects of SDP and PPF supplementation on reducing toxin-induced increase in mucosal permeability may prevent the passage of microbial and food antigens to the interstitial space, thereby blocking local inflammation (Santos et al., 2001).
Figure 4.
Effects of dietary supplementation with plasma concentrate (spray-dried plasma, SDP) and plasma protein fraction (PPF) on intestinal permeability of jejunum in a rat model of mild intestinal inflammation. Inflammation was induced by intraperitoneal administration of 50 μ g of Staphylococcus aureus enterotoxin B (SEB) on d 30 and 33 of age. Animals were fed experimental diets for 14 d. Intestinal permeability was estimated from the flux of FITC-dextran 4kDa (FD) across the whole intestinal wall in Ussing chambers. Results are mean ± SEM of 8 to 10 animals. Symbols indicate significant differences (P < 0.05): *vs. control group; §vs. SEB group (from Pérez-Bosque et al., 2006).
Figure 5.
Effects of dietary supplementation with plasma concentrate (spray-dried plasma, SDP) and plasma protein fraction (PPF) on epithelial expression of ZO-1 and β-Catenin at intestinal level in a rat model of mild intestinal inflammation. Inflammation was induced by intraperitoneal administration of 50 μ g of Staphylococcus aureus enterotoxin (SEB) on d 30 and 33 of age. Animals were fed experimental diets for 14 d. Results are mean ± SEM of 8 to 10 animals. Symbols indicate significant differences (P < 0.05): *vs. control group; §vs. SEB group (from Pérez-Bosque et al., 2006).
Proinflammatory cytokines are the main candidates for mediating these effects because they are induced by SEB (Huang and Koller, 1998; McKay et al., 1998) and facilitate antigen flux across the paracellular space (Toivola et al., 2004). Consistent with these observations, toxins like SEB stimulate lymphocytes to secrete INF-γ and TNF-α, which can disassemble tight junction proteins (Nusrat et al., 2000) or reduce their expression (Tsukita et al., 2002; Bruewer et al., 2003). The resulting increased intestinal permeability can enhance local inflammation, as happens in ulcerative colitis and inflammatory bowel disease (McKay, 2001).
NUTRIENT ABSORPTION
In farm animals, enteric infections may cause intestinal inflammation, villous atrophy, and malabsorption that contribute to high rates of mortality (van Dijk et al., 2002). The effects of intestinal inflammation on nutrient absorption have been studied in different animal models. In neonatal rats, C. parvum infection does not affect peptide transport (Marquet et al., 2007) but disrupts AA absorption, leading to undernutrition and growth reduction that is not restored by spontaneous clearance of the parasite (Topouchian et al., 2001). Lipopolysaccharide (LPS) inhibits intestinal absorption of D-galactose via a complex cellular mechanism that could involve posttranscriptional regulation of the brush-border Na+-coupled glucose and galactose transporter [SGLT1 (Amador et al., 2007)]. Lipopolysaccharide also reduces the expression of mucosal glucose transporter 5, the brush-border specific fructose transporter, by a mechanism involving TNF-α (García-Herrera et al., 2008). Tumor necrosis factor-α inhibits D-fructose uptake in rabbit intestine (García-Herrera et al., 2004), whereas IFN-γ downregulates D-glucose transport in T84 cells (Yoo et al., 2000). These results support the view that cytokines produced during inflammatory processes are involved in control of expression of nutrient transport proteins.
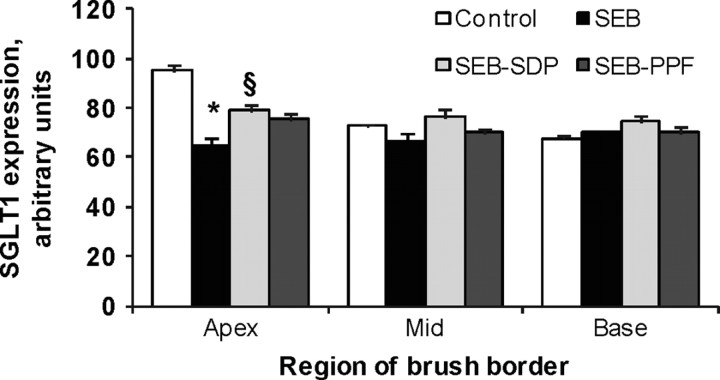
Our results using the SEB model show that the enterotoxin did not affect the uptake rates of l-leucine, l -methionine, and l -lysine but reduced the capacity of the jejunum to transport D-glucose through SGLT1 (Garriga et al., 2005). This effect was attributed to a reduction in the expression of D-glucose transporters in the brush border membrane, as indicated by the decreased Vmax (the maximal transport capacity kinetic constant). This observation was supported by results from immunohistochemical analysis of SGLT1. Consistent with Ferraris et al. (1990), immune staining of SGLT1 showed that the protein is present in absorptive epithelial cells along the crypt-villous axis, with greater expression in the upper villous region than in the mid and basal regions (Figure 6). Therefore, it was concluded that the changes in D-glucose transport kinetics observed in rats challenged with SEB were due to specific effects on the function of SGLT1. In rats challenged with SEB, SDP increased SGLT1 maximal transport capacity. The effect of PPF was intermediate. This effect of SDP on SGLT1 expression was further confirmed by Western blot analysis (Garriga et al., 2005).
Figure 6.
Effects of dietary supplementation with plasma concentrate (spray-dried plasma, SDP) and plasma protein fraction (PPF) on brush border expression of Na+-glucose transporter-1 (SGLT1) in the jejunum, in a rat model of mild intestinal inflammation. Inflammation was induced by intraperitoneal administration of 50 μ g of Staphylococcus aureus enterotoxin B (SEB) on d 30 and 33 of age. Animals were fed experimental diets for 14 d. Results are mean ± SEM of 8 to 10 animals. Symbols indicate significant differences (P < 0.05): *vs. control group; §vs. SEB group (from Garriga et al., 2005).
Although the mechanism of action of SDP is controversial, there is evidence that it reduces proinflammatory cytokine expression in GALT of early weaned piglets challenged with enterotoxigenic E. coli K88 (Bosi et al., 2004). Furthermore, results from our laboratory indicate that plasma supplements may alter the mucosal balance of pro- and antiinflammatory cytokines (Moretó et al., 2008). These results indicate that the effects of SDP on SGLT1 expression could be due to a reduction in mucosal proinflammatory cytokines. Mucosal proinflammatory cytokines have been shown to reduce SGLT1 expression in vivo (García-Herrera et al., 2004, 2008) and in vitro (Yoo et al., 2000).
Garriga et al. (2005) estimated that in control rats, at a physiological luminal D-glucose concentration of 50 mmol/L, 25% of total uptake would take place by the mediated mechanism and 75% by simple diffusion, whereas in SEB-challenged rats, these values would be 6 and 94%, respectively. If we assume that during interdigestive periods, typical luminal D-glucose concentration values are around 0.2 mmol/L (Ferraris et al., 1990), it can be calculated that SDP will increase the intestinal capacity to absorb D-glucose by 8 to 9%. This small but significant effect may contribute to the increase in growth and feed efficiency observed in farm animals fed SDP (Coffey and Cromwell, 2001; van Dijk et al., 2001).
MODE OF ACTION
The effects of SDP may be a direct effect improving food intake (e.g., by the greater palatability leading to increased feed intake; Ermer et al., 1994) or the effect of SDP may be more indirect by other mechanisms at luminal, mucosal, and systemic levels. Luminal effects were suggested by van Dijk et al. (2001) who reported an improvement in immunocompetence or a reduction in mucosal binding of luminal antigens by plasma glycoproteins.
More recent evidence indicates that the mechanism of action of SDP on growth performance involves regulation of GALT. In challenged pigs, Bosi et al. (2004) observed that the expression of proinflammatory cytokines was less in animals fed SDP. Results from our laboratory indicate that plasma supplements reduce the expression of mucosal proinflammatory cytokines (Moretó et al., 2008) and the activation of T-helper subsets in the lamina propria and epithelium (Pérez-Bosque et al., 2008b). These effects on mucosal cytokine profile can explain the observed reduction of mucosal inflammation by plasma supplements and the preventive effects of SDP and PPF on changes in mucosal permeability (Pérez-Bosque et al., 2004), tight junction protein expression (Pérez-Bosque et al., 2006), and nutrient absorption (Garriga et al., 2005) induced by SEB.
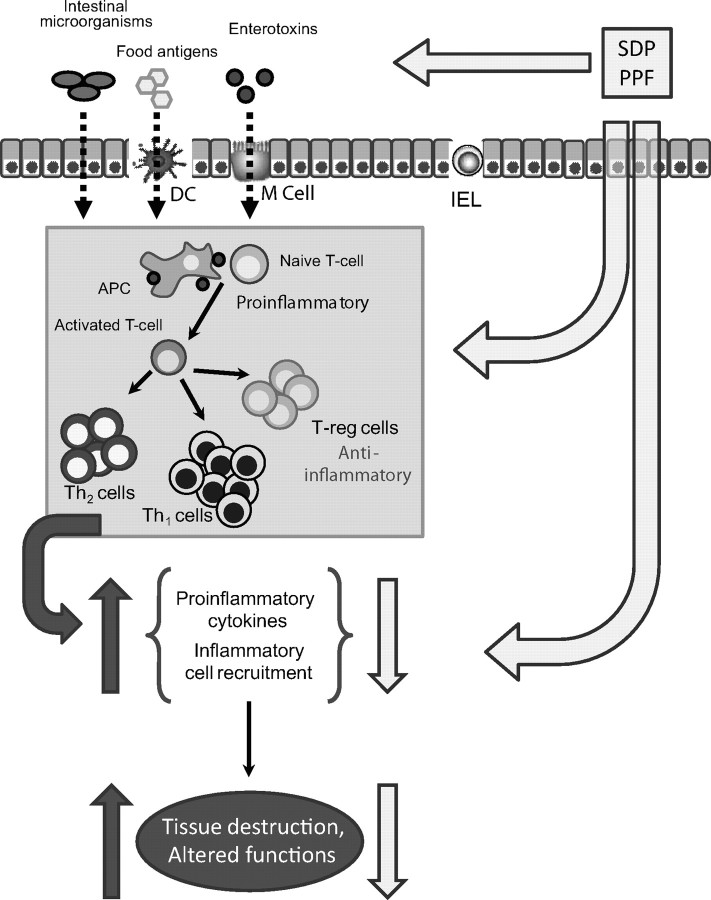
There are several possibilities by which SDP and PPF can modulate intestinal immune response. Plasma concentrate is a complex mixture of growth factors, cytokines, and other biologically active compounds. These proteins can interact with immune cells present in the mucosa, thus changing the cytokine environment. Finally, SDP affected more of the variables measured than did PPF, supporting the hypotheses that several of the proteins in SDP contribute to the effects of SDP (Figure 7).
Figure 7.
Diagram summarizing the mode of action of plasma supplements [spray-dried plasma, (SDP) or plasma protein fraction (PPF)]. Food proteins and products from commensal bacteria can be taken up by M cells present in Peyer's patches, by dendritic cells (DC) of the lamina propria, or by transcytosis (especially at early stages of development). Antigens are then presented to naive CD4 T cells and activated. Activated lymphocytes produce proinflammatory cytokines that amplify the immune response and induce inflammatory cell recruitment. In an inflammatory state, immune cells release reactive oxygen species (ROS) to eliminate invaded pathogens; however, these agents also induced gut alterations and tissue destruction. Plasma protein supplementation can modulate the immune response at luminal level (e.g., modifying the microbiota profile, pathogen binding); biologically active compounds present in plasma supplements can also interact directly with mucosal immune cells. The plasma-induced changes in mucosal cytokine profile will prevent or reverse deleterious effects resulting from immune system activation. IEL = intraepithelial lymphocytes; T-reg = regulator T-lymphocytes; APC = antigen presenting cells; Th1 = T helper type 1; Th2 = T helper type 2.
SUMMARY AND CONCLUSIONS
Spray-dried plasma reduces the overstimulation of the immune response in animals (e.g., pigs and rats) allowing more of the available energy and nutrients to be used for growth and other productive functions rather than being diverted to support the immune response. In the SEB-challenged rat experiencing mild intestinal inflammation, SDP reduced the production of mucosal proinflammatory cytokines and increased production of antiinflammatory cytokine IL-10. This supports the hypotheses that SDP reduces overstimulation of the mucosal immune response by enhancing IL-10 secretion.
It is worth noting that SDP also has systemic effects. For example, it can reduce the expression of proinflammatory cytokines in peripheral tissues of LPS-challenged pigs (Touchette et al., 2002) and prevent the increase in activated lymphocyte populations in a LPS-induced lung inflammation model (Pérez-Bosque et al., 2008a). Because organized GALT is an inductive site that connects with both local and peripheral effecter sites (respiratory tracts, glandular tissues and the uterus mucosa), it can be further hypothesized that SDP may reduce overstimulation of the broader common mucosal immune system.
LITERATURE CITED
- Amador P., García-Herrera J., Marca M. C., de la Osada J., Acín S., Navarro M. A., Salvador M. T., Lostao M. P., and Rodríguez-Yoldi M. J.. 2007. Intestinal D-galactose transport in an endotoxemia model in the rabbit. J. Membr. Biol. 215:125–133. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., and Anderson N. G.. 2002. The human plasma proteome. Mol. Cell. Proteomics 1:845–867. [DOI] [PubMed] [Google Scholar]
- Arthington J. D., Jaynes C. A., Tyler H. D., Kapil S., and Quigley J. D. III.. 2002. The use of bovine serum protein as an oral support therapy following coronavirus challenge in calves. J. Dairy Sci. 85:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. E. 2008. New ways of thinking about (and teaching about) intestinal epithelial function. Adv. Physiol. Educ. 32:25–34. [DOI] [PubMed] [Google Scholar]
- Bikker P., van Dijk A. J., Dirkzwager A., Fledderus J., Ubbink-Blanksma M., and Beynen A. C.. 2004. The influence of diet composition and an anti-microbial growth promoter on the growth response of weaned piglets to spray dried animal plasma. Livest. Prod. Sci. 86:201–208. [Google Scholar]
- Borg B. S., Campbell J. M., Polo J., Russell L. E., Rodríguez C., and Ródenas J.. 2002. Evaluation the chemical and biological characteristics of spray-dried plasma protein collected from various locations around the world. Pages 97–100 in Proc. Am. Assoc Swine Vet. March 2 to 5, 2002. Am. Assoc. Swine Vet., Kansas City, MO. [Google Scholar]
- Bosi P., Casini L., Finamore A., Cremokolini C., Merialdi G., Trevisi P., Nobili F., and Mengheri E.. 2004. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 82:1764–1772. [DOI] [PubMed] [Google Scholar]
- Bruewer M., Luegering A., Kucharzik T., Parkos C. A., Madara J. L., Hopkins A. M., and Nusrat A.. 2003. Pro-inflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 171:6164–6172. [DOI] [PubMed] [Google Scholar]
- Campbell J. M., Quigley J. D. III, Russell L. E., and Koehnk L. D.. 2004. Efficacy of spray-dried bovine serum on health and performance of turkeys challenged with Pasteurella multocida.J. Appl. Poult. Res. 13:388–393. [Google Scholar]
- Chen M. L., Pothoulakis C., and LaMont J. T.. 2002. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J. Biol. Chem. 277:4247–4254. [DOI] [PubMed] [Google Scholar]
- Coffey R. D., and Cromwell G. L.. 2001. Use of spray-dried animal plasma in diets for weanling pigs. Pig News Inf. 22:39N–48N. [Google Scholar]
- Demas G. E., Chefer V., Talan M. I., and Nelson R. J.. 1997. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. 273:R1631–R1637. [DOI] [PubMed] [Google Scholar]
- Dewey C. E., Johnston W. T., Gould L., and Whiting T. L.. 2006. Postweaning mortality in Manitoba swine. Can. J. Vet. Res. 70:161–167. [PMC free article] [PubMed] [Google Scholar]
- Ermer P. M., Miller P. S., and Lewis A. J.. 1994. Diet preference and meal patterns of weanling pigs offered diets containing either spray-dried porcine plasma or dried skim milk. J. Anim. Sci. 72:1548–1554. [DOI] [PubMed] [Google Scholar]
- Ferraris R. P., Yasharpour S., Lloyd K. C., Mirzayan R., and Diamond J. M.. 1990. Luminal glucose concentrations in the gut under normal conditions. Am. J. Physiol. 259:G822–G837. [DOI] [PubMed] [Google Scholar]
- García-Herrera J., Marca M. C., Brot-Laroche E., Guillén N., Acin S., Navarro M. A., de la Osada J., and Rodríguez-Yoldi M. J.. 2008. Protein kinases, TNF-α and proteasome contribute in the inhibition of fructose intestinal transport by sepsis in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G155–G164. [DOI] [PubMed] [Google Scholar]
- García-Herrera J., Navarro M. A., Marca N. C., de la Osada J., and Rodríguez-Yoldi M. J.. 2004. The effect of tumor necrosis factor-α on D-fructose intestinal transport in rabbits. Cytokine 25:21–30. [DOI] [PubMed] [Google Scholar]
- Garriga C., Pérez-Bosque A., Amat C., Campbell J. M., Russell L., Polo J., Planas J. M., and Moretó M.. 2005. Spray-dried porcine plasma reduces the effects of staphylococcal enterotoxin B on glucose transport in rat intestine. J. Nutr. 135:1653–1658. [DOI] [PubMed] [Google Scholar]
- Granger N., Kevil C. G., and Grisham M. B.. 2006. Recruitment of inflammatory and immune cells in the gut: Physiology and pathophysiology. Pages 1137–1162 in Physiology of the Gastrointestinal Tract. Johnson L. R., Barrett K. E., Merchant J. L., Ghishan F. K., Said H. M., and Wood J. D. ed. Elsevier, Amsterdam, the Netherlands. [Google Scholar]
- Greenberg P. D., and Cello J. P.. 1996. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:348–354. [DOI] [PubMed] [Google Scholar]
- Guarino A., Canani R. B., Russo S., Albano F., Canani M. B., Ruggeri F. M., Donelli G., and Rubino A.. 1994. Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics 93:12–16. [PubMed] [Google Scholar]
- Huang W., and Koller L. D.. 1998. Superantigen activation and kinetics of cytokines in the Long-Evans rat. Immunology 95:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt E., Fu Q., Armstrong M. U., Rennix D. K., Webster D. W., Galanko J. A., Chen W., Weaver E. M., Argenzio R. A., and Rhoads J. M.. 2002. Oral bovine serum concentrate improves crytosporidial enteritis in calves. Pediatr. Res. 51:370–376. [DOI] [PubMed] [Google Scholar]
- Iijima H., Takahashi I., and Kiyono H.. 2001. Mucosal immune network in the gut for the control of infectious diseases. Rev. Med. Virol. 11:117–133. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr 2001. How the immune system protects the host from infection. Microbes Infect. 3:1167–1171. [DOI] [PubMed] [Google Scholar]
- Jiang R., Chang X., Stoll B., Fan M. Z., Arthington J., Weaver E., Campbell J., and Burrin D. G.. 2000. Dietary plasma protein reduces small intestinal growth and lamina propria cell density in early weaned pigs. J. Nutr. 130:21–26. [DOI] [PubMed] [Google Scholar]
- Johnson R. 1997. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 75:1244–1255. [DOI] [PubMed] [Google Scholar]
- Kiyono H., and Fukuyama S.. 2004. NALT-versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K. C., and Korver D. R.. 1997. Leukocytic cytokines regulate growth rate and composition following activation of the immune system. J. Anim. Sci. 75:58–67. [Google Scholar]
- Lippolis J. D. 2008. Immunological signaling networks: Integrating the body's immune response. J. Anim. Sci. 86:E53–E63. [DOI] [PubMed] [Google Scholar]
- Lu J., Philpott D. J., Saunders P. R., Perdue M. H., Yang P. C., and McKay D. M.. 1998. Epithelial ion transport and barrier abnormalities evoked by superantigen-activated immune cells are inhibited by interleukin-10 but not interleukin-4. J. Pharmacol. Exp. Ther. 287:128–136. [PubMed] [Google Scholar]
- Marquet P., Barbot L., Planté A., Franc J., Huneau J. F., Gobert J. G., and Kapel N.. 2007. Cryptosporidiosis induces a transient upregulation of the oligopeptides transporter (PepT1) activity in neonatal rats. Exp. Biol. Med. 232:454–460. [PubMed] [Google Scholar]
- McCracken B. A., Spurlock M. E., Roos M. A., Zuckermann F. A., and Gaskins H. R.. 1999. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J. Nutr. 129:613–619. [DOI] [PubMed] [Google Scholar]
- McKay D. M. 2001. Bacterial superantigens: Provocateurs of gut dysfunction and inflammation? Trends Immunol. 22:497–501. [DOI] [PubMed] [Google Scholar]
- McKay D. M., Benjamin M. A., and Lu J.. 1998. CD4 T cells mediate superantigen induced abnormalities in murine jejunal ion transport. Am. J. Physiol. 275:G29–G38. [DOI] [PubMed] [Google Scholar]
- Moretó M., Miró L., Polo J., Russell L., Campbell J., Weaver E., Crenshaw J., and Pérez-Bosque A.. 2008. Oral porcine plasma proteins prevent the release of mucosal pro-inflammatory cytokines in rats challenged with S. aureus enterotoxin B. Digestive Disease Week, San Diego, CA, May 17–22, 2008. [Google Scholar]
- Mowat A. M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331–341. [DOI] [PubMed] [Google Scholar]
- Nofrarías M., Manzanilla E. G., Pujols J., Gibert X., Majó N., Segalés J., and Gasa J.. 2006. Effects of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J. Anim. Sci. 84:2735–2742. [DOI] [PubMed] [Google Scholar]
- Nusrat A., Turner J. R., and Madara J. L.. 2000. Molecular physiology and pathophysiology of tight junctions IV. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines, and immune cells. Am. J. Physiol. 279:G851–G857. [DOI] [PubMed] [Google Scholar]
- Pérez-Bosque A., Amat C., Polo J., Campbell J. M., Crenshaw J., Russell L., and Moretó M.. 2006. Spray-dried animal plasma prevents the effects of Staphylococcus aureus Enterotoxin B on intestinal barrier function in weaned rats. J. Nutr. 136:2838–2843. [DOI] [PubMed] [Google Scholar]
- Pérez-Bosque A., Maijó M., Miró L., Polo J., Russell L., Campbell J., Weaver E., Crenshaw J., and Moretó M.. 2008a. Effects of spray-dried porcine plasma on nasal associated lymphoid tissue in a lung inflammation model in mice. J. Anim. Sci. 86 (E-Suppl. 2):402. (Abstr.) [Google Scholar]
- Pérez-Bosque A., Miró L., Polo J., Russell L., Campbell J., Weaver E., Crenshaw J., and Moretó M.. 2008b. Dietary plasma proteins modulate the immune response of diffuse gut-associated lymphoid tissue in rats challenged with Staphylococcus aureus enterotoxin B. J. Nutr. 138:533–537. [DOI] [PubMed] [Google Scholar]
- Pérez-Bosque A., Pelegrí C., Vicario M., Castell M., Russell L., Campbell J. M., Quigley J. D. I. I. I., Polo J., Amat C., and Moretó M.. 2004. Dietary plasma protein affects the immune response of weaned rats challenged with S. aureus superantigen B. J. Nutr. 134:2667–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pié S., Lallès J. P., Blaszy F., Laffitte J., Sève B., and Oswald I. P.. 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 134:641–647. [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. 1997. Factors influencing the structure and function of the small intestine in the weaned pigs: A review. Livest. Prod. Sci. 51:215–236. [Google Scholar]
- Quigley J. D. III, and Drew M. D.. 2000. Effects of oral antibiotics or IgG on survival, health and growth in dairy calves challenged with Escherichia coli. Food Agric. Immunol. 12:311–318. [Google Scholar]
- Santos J., Yang P. C., Soderholm J. D., Benjamin M., and Perdue M. H.. 2001. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 48:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys J., and Quinn M. T.. 1999. Selective recruitment of T-cell subsets to the udder during staphylococcal and streptococcal mastitis: Analysis of lymphocyte subsets and adhesion molecule expression. Infect. Immun. 67:6293–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda N., Furuse M., Sasaki H., Yonemura S., Katahira J., Horiguchi Y., and Tsukita S.. 1999. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 147:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivola D. M., Krishnan S., Binder H. J., Singh S. K., and Omary M. B.. 2004. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J. Cell Biol. 164:911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topouchian A., Kapel N., Huneau J. F., Barbot L., Magne D., Tomé D., and Gobert J. G.. 2001. Impairment of aminoacid absorption in suckling rats infected with Cryptosporidium parvum.Parasitol. Res. 87:891–896. [DOI] [PubMed] [Google Scholar]
- Torrallardona D., Conde M. R., Badiola I., and Polo J.. 2007. Evaluation of spray dried animal plasma and calcium formate as alternatives to colistin in piglets experimentally infected with Escherichia coli K99. Livest. Sci. 108:303–306. [Google Scholar]
- Touchette K. J., Carroll J. A., Allee G. L., Matteri R. L., Dyer C. J., Beausang L. A., and Zannelli M. E.. 2002. Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: I. Effects on the immune axis of weaned pigs. J. Anim. Sci. 80:494–501. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., and Itoh M.. 2002. The tight junction. Pages 369–395 in Cell Adhesion. Berkeley M. C. ed. Oxford University Press, New York, NY. [Google Scholar]
- van Dijk A. J., Everts H., Nabuurs M. J. A., Margry R. J. C. F., and Beynen A. C.. 2001. Growth performance of weanling pigs fed spray-dried animal plasma: A review. Livest. Prod. Sci. 68:263–274. [Google Scholar]
- van Dijk A. J., Niewold T. A., Nabuurs M. J., Van Hees J., De Bot P., Stockhofe-Zurwieden N., Ubbink-Blanksma M., and Beynen A. C.. 2002. Small intestinal morphology and disaccharidase activities in early-weaned piglets fed a diet containing spray-dried porcine plasma. J. Vet. Med. A Physiol. Pathol. Clin. Med. 49:81–86. [DOI] [PubMed] [Google Scholar]
- Yoo D., Lo W., Goodman S., Ali W., Semrad C., and Field M.. 2000. Interferon-gamma downregulates ion transport in murine small intestine cultured in vitro. Am. J. Physiol. 279:G1323–G1332. [DOI] [PubMed] [Google Scholar]