Abstract
Virus-induced elaboration of proinflammatory cytokines is mediated by virus-induced oxidative stress. The purpose of these studies was to determine the source of the virus-induced oxidative stress. Inhibition of viral replication with antibody to intercellular adhesion molecule-1 had no effect on virus-induced oxidative stress or interleukin-8 (IL-8) response (597 ± 88 vs. 668 ± 78 pg/mL in control cells). Treatment of cells with diphenylene iodonium inhibited virus-induced oxidative stress and IL-8 elaboration, but allopurinol, ibuprofen, and rotenone had no effect. Studies in cell lines produced from a patient with gp91-phox deficiency revealed normal responses. In contrast, the oxidative response was decreased and the IL-8 concentration was 227 ± 36 pg/mL in cells from a patient with p47-phox deficiency, compared with 664 ± 48 pg/mL in control cells. These studies suggest that the stimulation of reactive oxygen species by viral challenge occurs at the cell surface even in the absence of viral replication and involves a flavoprotein that may act in concert with p47-phox.
There is increasing evidence that virus-induced elaboration of proinflammatory cytokines plays a role in the pathogenesis of viral upper respiratory illness. The concentrations of interleukin (IL)-6 and IL-8 increase in the nasal secretions of subjects with symptomatic rhinovirus infection [1–4], and there is a direct correlation between the severity of the common cold symptoms and the concentration of IL-8 and IL-6 in the secretions [2, 3]. It has also been reported that intranasal challenge of normal subjects with IL-8 produces a symptom complex that in some respects mimics the common cold [5].
The cellular mechanisms involved in the elaboration of IL-8 in response to viral challenge appears to involve oxidative stress-induced activation of nuclear factor (NF)-κB [6, 7]. Oxidative stress may occur in a cell either by inhibition of antioxidant enzyme activities or by increased production of oxidants. The Superoxide dismutases detoxify Superoxide (O2−) to hydrogen peroxide, which in turn is detoxified by catalase and glutathione peroxidase. The activities of Superoxide dismutases, catalase, or glutathione peroxidase are not inhibited by rhinovirus infection, suggesting that oxidative stress is a result of increased production of pro-oxidants [8]. Both reactive oxygen species and reactive nitrogen species are pro-oxidants, and an increase in either of these species would potentially result in cellular oxidative stress. Reactive nitrogen species are generated by the action of nitric oxide synthase on L-arginine to produce reactive nitric oxide (NO). Although there are conflicting reports [9], studies in our laboratory find no evidence that NO plays a role in rhinovirus infection or rhinovirus-induced IL-8 elaboration either in vitro or in vivo [10]. In contrast, rhinovirus challenge of fibroblasts or respiratory epithelial cells results in increased concentrations of hydrogen peroxide in the supernatant medium. These observations suggest that cellular oxidative stress in response to rhinovirus infection is due to an increased production of reactive oxygen species.
The purpose of these studies was to examine the virus-cell interactions that result in increased production of reactive oxygen species after rhinovirus challenge. The relationship of production of reactive oxygen species to rhinovirus replication or attachment to intercellular adhesion molecule-1 (ICAM-1), the major cellular receptor for rhinovirus [11, 12], was explored. Diphenylene iodonium (DPI), an inhibitor of flavoproteins, was studied for its inhibitory potential on virus-induced oxidative stress, IL-8 elaboration, and viral replication after challenge with rhinovirus, respiratory syncytial virus, or coronavirus 229E; also, the involvement p47-phox, a cytosolic component of NADPH oxidase, was investigated.
Materials and Methods
Reagents
Monoclonal antibody to ICAM-1 (RR1/1.1.1) was provided by Robert Rothlein (Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT). This antibody was used at a final concentration of 10 ng/mL of medium. DPI and MnTBAP (manganese[III]tetrakis[4-benzoic acid] porphyrin chloride) were purchased from Calbiochem (La Jolla, CA), allopurinol and rotenone were purchased from Sigma (St. Louis), and ibuprofen was purchased from BioMol (Plymouth Meeting, PA).
Cell culture
Human embryonic lung fibroblasts (MRC-5; Biowhittaker, Walkersville, MD) were grown in Eagle MEM (EMEM) supplemented with 10% fetal calf serum, 5 U/mL penicillin G sodium, and 5 μg/mL streptomycin. Cells were used for experiments at passage 21–25 within 2 days of the time the monolayers became confluent. Human bronchial epithelial cells (BEAS-2B; ATCC, Rockville, MD) were grown in bronchial epithelial growth medium (Clonetics, Minneapolis) supplemented with human recombinant epithelial growth factor (0.5 ng/mL), insulin (5 μg/mL), hydrocortisone (0.5 μg/mL), epinephrine (0.5 μg/mL), transferrin (10 μg/mL), gentamicin (50 μg/mL), and amphotericin B (50 ng/mL). All experiments with BEAS-2B cells were done with cells at passage 40–55, when the monolayers were 85%–95% confluent.
Primary skin fibroblast cell lines were prepared from a normal subject and from subjects with chronic granulomatous disease. Skin fibroblast cultures were prepared from biopsy specimens obtained from 1 subject with a deficiency of p47-phox, as demonstrated by Western blotting of neutrophils with antibodies to p47-phox (provided by Thomas L. Leto, NIAID, NIH, Bethesda, MD), and from a second subject with a deficiency of gp91-phox. The skin fibroblasts from these subjects and from the normal subject were used for experiments at passage 5–15. For all experiments, control cells and cells from patients with chronic granulomatous disease were used at the same passage.
Preparation and purification of virus
Rhinovirus type 39 (RV39) was grown in HeLa-I cells, a HeLa cell clone with increased surface expression of ICAM-1 (provided by EG. Hayden, University of Virginia Health Sciences Center, Charlottesville). HeLa-I cells infected with RV39 were mechanically collected and lysed by a freeze-thaw, and the supernatants were clarified by centrifugation at 2000 g (Beckman GPR centrifuge; Beckman Instruments, Palo Alto, CA). The supernatants were then centrifuged at 125,000 g at 4°C for 45 min (Ti45 rotor, Beckman L8–70M centrifuge; Beckman Instruments). Partially purified virus was produced by a modification of a published method [3]. Briefly, after ultracentrifugation, the resulting viral pellet was resuspended in 200 μL of PBS buffer and overlaid onto a 2-layer sucrose cushion containing 60% sucrose in PBS on the bottom layer and 30% sucrose in PBS on the top layer. After centrifugation at 110,000 g (SW28 rotor; Beckman Instruments, Palo Alto, CA) for 135 min at 4°C, the interface containing the virus was collected and resuspended in 50 mL of EMEM. The virus suspension was again centrifuged at 125,000 g for 45 min at 4°C, and the resulting pellet was resuspended in EMEM with 1% bovine serum albumin, and aliquots were snapfrozen in liquid nitrogen and stored at −70°C. The variant strain rhinovirus, respiratory syncytial virus, and coronavirus 229E grown in MRC-5 cells were also partially purified as described.
Viral infection
Cells were grown in 24-well tissue culture plates (∼105 cells/well). Unless specified, the inoculum for viral challenge was 100 TCID50/cell for experiments in BEAS-2B cells and 10 TCID50/cell for experiments in fibroblasts. The cells were challenged with virus in a final volume of 1 mL/well and incubated at 33°C for 1 h to allow for the absorption of virus. The cells were then washed 3 times with medium and further incubated with the fresh medium at 33°C. Supernatants were then collected at the specified time (6, 24, or 48 h) and stored at −70°C until analyzed for IL-8 protein or virus titers. For experiments involving inhibition with antibody or chemical reagents, the inhibitor was incubated on the cells at an appropriate concentration in medium for 30 min at 37°C. Virus was then added and incubated at 33°C for 1 h to allow for the absorption of virus. The cells were then washed 3 times with medium and further incubated with the fresh medium containing the inhibitor until specimens were collected at the specified times. All results are presented as mean ± SD of at least 3 separate experiments.
Cell cytotoxicity
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma) was dissolved in water at a concentration of 5 mg/mL and then syringe-filtered through a 0.2-μm filter and stored at 5°C. After experimental treatment of cells, the medium was poured off, and 50 μL of MTT stock solution in 450 μL of medium was added to each well. The cells were then incubated at 37°C for 3–4 h. The medium was then discarded, the MTT salt was extracted with 500 μL/well acidic isopropanol (0.1 N HCl in absolute isopropanol), and the absorbance of the converted dye was measured at a wavelength of 570 nm with a reference wavelength of 620 nm by means of an automated spectrophotometric plate reader. For all experiments, the absence of cell toxicity at the concentrations of inhibitor that are reported was demonstrated by the absence of cytopathic effect and MTT dye reduction comparable to that of control monolayers.
Measurement of IL-8 protein
The IL-8 concentrations in cell culture supernatant specimens were determined by ELISA with commercially available assays (R&D Systems, Minneapolis). All assays were done in duplicate on an automatic spectrophotometric plate reader (Anthos HTII; Anthos Labtec Instruments, Salzburg, Austria). Sample concentrations were determined from optical density values by use of a standard curve based on a linear regression. All data presented represent the mean ± SD of at least 3 separate experiments.
Quantitative nitroblue tetrazolium (NBT) assay
The NBT assay was done in fibroblasts grown to confluence in 96-well flatbottom plates. The cells were washed with 2% EMEM and then preincubated with various inhibitors or control medium for 30 min at 37°C. The cells were then challenged with rhinovirus (MOI of 10 TCID50/cell), and NBT solution was added to each well at a final concentration of 1 mg/mL. After incubation for 6 h, the cells were washed 3 times with methanol, and the reduced NBT was extracted by adding a mixture of 92 μL of 10 M KOH and 108 μL of dimethyl sulfoxide. Optical density was measured at 620 nm on an automated optical reader. All data presented represent the mean ± SD of at least 3 separate experiments.
Quantitative hydrogen peroxide assay
The concentration of hydrogen peroxide was determined in cell culture supernatants with a commercially available colorimetric assay (Bioxytech H2O2-560; OXIS International, Portland, OR). For this assay, cell culture supernatants were collected 1 h after challenge of cells with virus. All assays were done in triplicate on an automatic spectrophotometric plate reader (Anthos HTII). Sample concentrations were determined from optical density values by use of a standard curve based on a linear regression. The data presented represent the mean ± SD of 3 separate experiments.
Quantitation of virus
Virus titrations were done in 96-well microtiter plates (Falcon Labware, Oxnard, CA). Serial 10-fold dilutions of each specimen were made, and 2 × 104 MRC-5 cells were added to each well. The plates were incubated at 33°C for 7 days and then examined for viral cytopathic effects. The virus titers were calculated by standard methods [13]. All data presented represent the mean ± SD of at least 3 separate experiments except as noted.
Results
Oxidative stress and IL-8 elaboration are independent of rhinovirus replication
RV39 is a major-group rhinovirus that requires attachment to ICAM-1 to produce infection. As expected, incubation of MRC-5 cells with anti-ICAM inhibited viral replication, as assessed by development of cytopathic effect and quantitative viral cultures of supernatant medium (figure 1). In contrast, incubation with anti-ICAM before viral challenge had no effect either on NBT dye reduction (data not shown) or on the concentration of IL-8 elaborated into the supernatant medium 6 h after challenge (figure 1). In similar experiments with unpurified virus, UV inactivation of virus and preincubation of virus with soluble ICAM-1 (provided by Boehringer Ingelheim Pharmaceuticals) both inhibited viral replication but had no effect on IL-8 elaboration (data not shown).
Figure 1.
Effect of antibody to intercellular adhesion molecule (ICAM)-1 on rhinovirus type 39 (RV39) replication and rhinovirusinduced interleukin-8 (IL-8) elaboration. Data are mean ± SD.
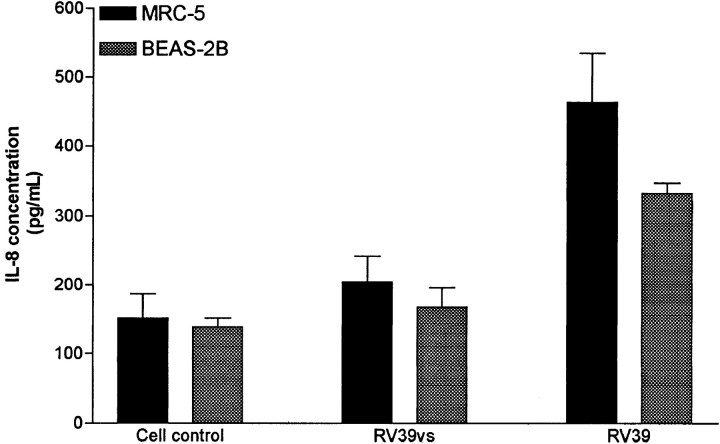
Figure 2.
Interleukin-8 (IL-8) elaboration induced by challenge of human embryonic lung fibroblast cells (MRC-5) and human bronchial epithelial cells (BEAS-2B) with wild-type rhinovirus (RV39) and variant strain rhinovirus (RV39vs). Data are mean ± SD.
A variant strain of RV39 (RV39vs), identified during the course of these studies, did not induce IL-8 elaboration. This variant strain replicated normally, as assessed by cytopathic effect and quantitative viral cultures, compared with the wildtype virus. After 48 h of incubation, virus titers in the supernatant medium from MRC-5 cells infected with wild-type virus were 2.8 ± 0.4 TCID50/mL, compared with 2.4 ± 0.5 TCID50/mL in medium from cells infected with the variant strain. The variant strain was neutralized by type-specific antiserum to RV39, and replication was inhibited by pretreatment of cells with anti-ICAM antibody (data not shown). The variant strain of rhinovirus did not induce oxidative stress in MRC-5 cells, as determined by hydrogen peroxide production. Hydrogen peroxide concentrations in supernatant medium from control and rhinovirus-challenged MRC-5 cells were 1.18 ± 0.43 and 5.3 ±1.4 μM, respectively, whereas the supernatant from MRC-5 cells challenged with RV39vs contained 0.89 ± 0.18 μM H2O2. Similarly, the RV39vs did not produce oxidative stress in BEAS-2B cells, as determined by NBT dye reduction or by fluorescence staining of carbonyl groups (data not shown). In contrast to wild-type RV39, challenge of cells with this virus in vitro was not associated with activation of NF-μB (data not shown) or with IL-8 elaboration in either MRC-5 cells or BEAS-2B cells (figure 2). Challenge of MRC-5 cells with wildtype RV39 and RV39vs simultaneously resulted in IL-8 levels comparable to those seen after challenge with RV39 alone, suggesting that the RV39vs was not inhibiting cellular elaboration of IL-8. The observations that inhibition of viral replication with anti-ICAM antibody does not affect IL-8 elaboration and that the variant strain replicates normally but does not produce an IL-8 response suggest that virus-induced oxidative stress and IL-8 elaboration are independent of attachment to ICAM-1 or viral replication.
Pretreatment of cells with DPI but not ibuprofen, allopurinol, or rotenone inhibits rhinovirus-induced IL-8 elaboration and viral replication
DPI is a flavoprotein inhibitor with potent activity against NADPH oxidase. Pretreatment of fibroblasts with DPI (40 μM) reduced Superoxide anion production and virusinduced IL-8 elaboration (figure 3). The reduction in oxidative stress was also demonstrated by decreased H2O2 production. Hydrogen peroxide concentrations in supernatant medium from control and rhinovirus-challenged MRC-5 cells were 1.18 ± 0.43 and 5.3 ± 1.4μM, respectively. The supernatant from MRC-5 cells challenged with rhinovirus in the presence of DPI (40 μM) contained 0.48 ± 0.18 μM H2O2. A similar inhibitory effect of DPI was seen in BEAS-2B cells. The IL-8 concentration in medium from BEAS-2B cells challenged with rhinovirus was reduced from 246 ± 25 to 121 ± 20 pg/mL by pretreatment with 40 μM DPI. The IL-8 concentrations in medium from BEAS-2B cells without viral challenge were 144 ± 15 pg/mL without DPI and 97 ± 11 pg/mL with DPI. The reduction in IL-8 elaboration after treatment with DPI was associated with a reduction in viral replication, as measured by quantitative viral cultures of supernatant medium collected 48 h after challenge (figure 4). Treatment of the cells with Mn-TBAP, which mimics the activity of Superoxide dismutase, in hibited oxidative stress and IL-8 elaboration in response to viral challenge but had no effect on viral replication. These observations suggest that the inhibition of viral replication by DPI was not a result of the inhibition of oxidative stress. Similar effects of DPI on virus-induced IL-8 elaboration and viral replication were seen after challenge of fibroblasts with either RSV or coronavirus 229E. RSV challenge in the presence of DPI (40 μM) resulted in an IL-8 concentration and virus titer in supernatant medium of 74 ± 10 pg/mL and 0.85 ± 0.09 TCID50/mL, respectively, compared with 1139 ± 118 pg/mL and 3.83 ± 0.12 TCID50/mL in control cells. Similarly, coronavirus challenge in the presence of DPI resulted in an IL-8 concentration and virus titer of 69 ± 20 pg/mL and 0.5 ± 0.001 TCID50/mL, respectively, compared with 1053 ± 51 pg/mL and 3.9 ± 0.13 TCID50/mL in control cells. Pretreatment of cells with DPI also inhibited replication of the variant strain virus.
Figure 3.
Effect of diphenylene iodonium (DPI; 40 μM) on nitroblue tetrazolium (NBT) dye reduction (as shown by optical density at 620 nm) and interleukin-8 (IL-8) elaboration in response to challenge of human embryonic lung fibroblast cells with rhinovirus type 39. Data are mean ± SD.
Figure 4.
Effect of diphenylene iodonium (DPI; 40 μM), ibuprofen (100 μM), allopurinol (500 μM), and rotenone (10 μM) on rhinovirus replication and rhinovirus-induced interleukin-8 (IL-8) elaboration in human embryonic lung fibroblast cells. Data are mean ± SD. RV39, rhinovirus type 39.
In contrast to the effect of pretreatment of cells with DPI, inhibitors of other cellular sources of reactive oxygen species had no effect. Treatment of cells with ibuprofen, a cyclooxygenase inhibitor, allopurinol, an inhibitor of xanthine oxidase, or rotenone, an inhibitor of mitochondrial Superoxide generation, had no effect on either IL-8 elaboration or viral replication (figure 4).
Fibroblasts from a subject with absent p47-phox have reduced production of Superoxide onion and elaboration of IL-8 after rhinovirus challenge, compared with normal skin fibroblasts
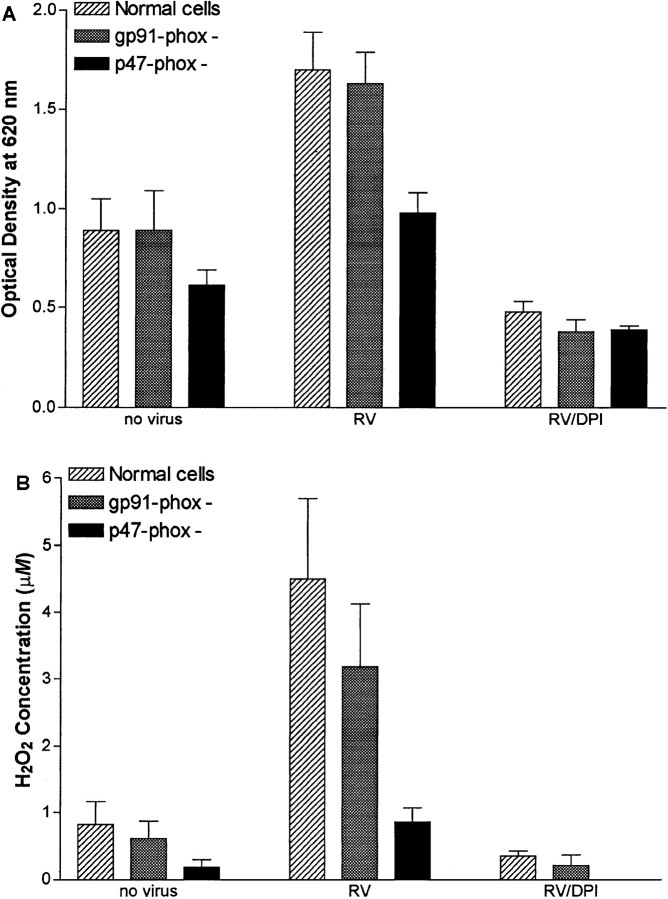
NBT dye reduction and H2O2 production were decreased in fibroblasts obtained from a p47-phox-deficient subject, compared with either cells from the normal control or fibroblasts from a subject with gp91-phox deficiency both at baseline and after challenge with rhinovirus (figure 5). Similarly, IL-8 concentrations in supernatant medium from these fibroblasts were ∼33% of the concentrations in supernatant from normal fibroblasts both before and 6 h after challenge with rhinovirus, respiratory syncytial virus, or coronavirus 229E (figure 6). In contrast, IL-8 elaboration by fibroblasts from a subject with gp91-phox deficiency was comparable to that by fibroblasts from the normal control after challenge with each of the viral pathogens.
Figure 5.
Superoxide anion production as measured by nitroblue tetrazolium dye reduction (A) and H2O2 production (B) in response to rhinovirus (RV) challenge in normal skin fibroblasts, skin fibroblasts from patient deficient in gp91-phox, and skin fibroblasts from patient deficient in p47-phox both with and without preincubation of cells with diphenylene iodonium (DPI; 40 μM). Data are mean ± SD.
Figure 6.
Interleukin-8 (IL-8) elaboration induced by rhinovirus, coronavirus, and respiratory syncytial virus (RSV) in normal skin fibroblasts, skin fibroblasts from patient deficient in gp91-phox, and skin fibroblasts from patient deficient in p47-phox. Data are mean ± SD.
Fibroblasts from patients with chronic granulomatous disease supported viral replication, although the quantity of virus present in supernatant medium after 48 h was slightly decreased, compared with that seen with normal skin fibroblast controls. The quantitative virus titers were 3.6 ± 0.1 (mean ± SD) TCID50/mL in fibroblasts from normal skin, compared with 2.5 ± 0.3 and 2.7 ± 0.3 TCID50/mL in skin fibroblasts from p47-phox- and gp91-phox-deficient patients, respectively.
Although the fibroblasts from the p47-phox-deficient patient had a reduced oxidative response and decreased production of IL-8 after rhinovirus challenge, compared with normal fibroblasts, the p47-phox-deficient cells did respond to rhinovirus challenge. Pretreatment of cells with DPI completely blocked the oxidative response to rhinovirus challenge as measured by NBT dye reduction (figure 5). Similarly, DPI pretreatment inhibited rhinovirus-induced elaboration of IL-8. IL-8 concentrations in the p47-phox-deficient cells without viral challenge were 40 ± 14 pg/mL, compared with 158 ± 54 pg/mL after rhinovirus challenge. Rhinovirus challenge of these cells after pretreatment with DPI (40 μM), allopurinol (500 μM), or rotenone (10 μM) resulted in IL-8 concentrations of 13.4 ± 2.2, 323 ± 100, and 186 ± 104 pg/mL, respectively. DPI pretreatment also inhibited viral replication in the fibroblasts from the p47-phox-deficient patient.
Discussion
The data presented here allow 3 conclusions. First, production of reactive oxygen species and oxidative stress in cells in response to rhinovirus challenge is not a direct result of viral attachment to ICAM-1 or of viral replication. Second, virusinduced production of reactive oxygen species is inhibited by pretreatment of cells with DPI but not by inhibitors of xanthine oxidase, cyclooxygenase, or mitochondrial metabolism. Finally, p47-phox, a cytoplasmic component of NADPH oxidase, may be involved in virus-induced Superoxide production and IL-8 elaboration.
The first goal of these studies was to determine whether rhinovirus attachment and replication were required for the production of cellular oxidative stress and subsequent elaboration of IL-8. Previous studies had suggested that prevention of rhinovirus replication by blockade of attachment to ICAM-1 or UV inactivation of virus prevented virus-associated IL-8 elaboration [14]. These previous studies differed from our studies in that IL-8 elaboration was measured 24 h after challenge of the cell monolayer with a relatively low inoculum of virus. Under these conditions, virus-cell interaction by progeny virus produced as a result of viral replication presumably makes a substantial contribution to the IL-8 detected in the supernatant. Thus, inhibition of viral replication that eliminates this amplification would cause a corresponding reduction in IL-8 concentration. In our study, the effect of this amplification was eliminated by measurement of IL-8 in supernatants collected only 6 h after challenge of the monolayer with a large inoculum of virus. Our observations that a variant strain of RV39 replicates normally in fibroblast and BEAS-2B cells but does not cause oxidative stress or IL-8 elaboration is further evidence that viral attachment to ICAM-1 and subsequent replication events are not required for these processes.
A second objective of these studies was to determine the source of the oxidative stress that appears to be a key component of virus-induced IL-8 elaboration. Pretreatment of either fibroblast or BEAS-2B cells with DPI, a flavoprotein inhibitor, inhibited both viral replication and virus-induced IL-8 elaboration. In contrast, inhibition of xanthine oxidase, cyclooxygenase, or mitochondrial metabolism had no effect on rhinovirus-induced IL-8 elaboration. These observations sug gested that an NADPH oxidase-like enzyme might be the source of the virus-induced oxidative stress. Further evaluation making use of skin fibroblasts from subjects with known defects in NADPH oxidase suggested that p47-phox may be involved in this process.
The NADPH oxidase of neutrophils generates reactive oxygen species as a part of the host-defense mechanism of these phagocytic cells. NADPH oxidase is a complex enzyme consisting of a membrane-bound cytochrome and several cytoplasmic components. The cytochrome is composed of two proteins, gp91-phox and p22-phox. Although these two proteins have been detected in nonmyeloid cells [15–17], recent evidence suggests that the oxidase responsible for production of reactive oxygen species in signal transduction pathways of nonmyeloid cells may in fact be part of a family of enzymes closely related to NADPH oxidase [18, 19].
The cytoplasmic components of NADPH oxidase have also been detected in nonmyeloid cell lines. Both p47-phox and p67-phox have been identified in rat and rabbit aortic fibroblasts and in guinea pig gastric mucosal cells [15, 17, 20]. Furthermore, depletion of p67-phox from rabbit aortic fibroblasts by immunoprecipitation resulted in a loss of NADPH oxidase activity in a cell-free assay [15]. Viral challenge of skin fibroblasts from a patient with deficient p47-phox was associated with a substantial decrease in the production of reactive oxygen species and in IL-8 elaboration, compared with normal controls. However, comparison of virus-challenged cells with unchallenged control cells revealed that the p47-phox-deficient cells did respond to the viral challenge and this response was inhibited by pretreatment with DPI but not by allopurinol or rotenone. These observations suggest either that another flavoprotein enzyme system contributes to the response to viral challenge or that the need for p47-phox is not an absolute requirement for partial functioning of the fibroblast NADPH oxidase system [21, 22].
These studies suggest that the stimulation of reactive oxygen species by rhinovirus challenge of fibroblasts occurs at the cell surface even in the absence of viral replication and involves a flavoprotein that may act in concert with p47-phox. The studies with RSV and coronavirus suggest that elaboration of IL-8 after challenge with these respiratory pathogens may be a result of a similar mechanism. In light of the evidence that reactive oxygen species-mediated host responses to rhinovirus may play an important role in the pathogenesis of illness, elucidation of the source of the reactive oxygen species may provide new targets for potential treatments for viral respiratory disease.
Acknowledgments
Matilda Latham and Jerrod Roussel provided technical support for these studies.
Footnotes
Presented in part: 36th annual meeting of the Infectious Diseases Society of America, Denver, November 1998 (abstract 308); Pediatric Academic Societies annual meeting, San Francisco, May 1999 (abstract 1032).
The protocol for collection of specimens from human volunteers was approved by the Medical University of South Carolina Institutional Review Board for Human Subjects, and written informed consent was obtained from all participants.
References
- 1.Grunberg K, Timmers MC, Smits HH, et al. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner RB, Weingand KW, Yeh CH, Leedy D. Association between nasal secretion interleukin-8 concentration and symptom severity in experimental rhinovirus colds. Clin Infect Dis. 1998;26:840–6. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z, Tang W, Ray A, et al. Rhinovirus stimulation of interleukin-6 in vivo and in vitro: evidence for nuclear factor κB-dependent transcriptional activation. J Clin Invest. 1996;97:421–30. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Z, Tang W, Gwaltney JM, Jr, Wu Y, Elias JA. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol. 1997;273:L814–24. doi: 10.1152/ajplung.1997.273.4.L814. [DOI] [PubMed] [Google Scholar]
- 5.Douglass JA, Dhami D, Gurr CE. Influence of interleukin-8 challenge in the nasal mucosa in atopic and nonatopic subjects. Am J Respir Crit Care Med. 1994;150:1108–13. doi: 10.1164/ajrccm.150.4.7921444. [DOI] [PubMed] [Google Scholar]
- 6.Biagioli MC, Kaul P, Singh I, Turner RB. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic Biol Med. 1999;26:454–62. doi: 10.1016/s0891-5849(98)00233-0. [DOI] [PubMed] [Google Scholar]
- 7.Mastronarde JG, Monick MM, Hunninghake GW. Oxidant tone regulates IL-8 production in epithelium infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:237–44. doi: 10.1165/ajrcmb.13.2.7626291. [DOI] [PubMed] [Google Scholar]
- 8.Kaul P, Biagioli MC, Turner RB, Singh I. Association of alterations in cellular redox pathways with rhinovirus-induced interleukin-8 elaboration [abstract 722] Pediatr Res. 1997;41:123. [Google Scholar]
- 9.Sanders SP, Siekierski ES, Porter JD, Richards SM, Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72:934–42. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul P, Singh I, Turner RB. Effect of nitric oxide on rhinovirus replication and virus-induced interleukin-8 elaboration. Am J Respir Crit Care Med. 1999;159:1193–8. doi: 10.1164/ajrccm.159.4.9808043. [DOI] [PubMed] [Google Scholar]
- 11.Greve JM, Davis G, Meyer AM, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–47. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 12.Staunton DE, Merluzzi VJ, Rothlein R, Barton R, Marlin SD, Springer TA. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–53. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 13.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 14.Subauste MC, Jacoby DB, Richards SM, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus: induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest. 1995;96:549–57. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA. 1997;94:14483–8. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radeke HH, Cross AR, Hancock JT, et al. Functional expression of NADPH oxidase components (alpha- and beta-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991;266:21025–9. [PubMed] [Google Scholar]
- 17.Wang HD, Pagano PJ, Du Y, et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–8. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 18.Banfi B, Maturana A, Jaconi S, et al. A mammalian H(+) channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–42. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 19.Suh YA, Arnold RS, Lassegue B, et al. Cell transformation by the superoxide-generating oxidase Moxl. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 20.Teshima S, Rokutan K, Nikawa T, Kishi K. Guinea pig gastric mucosal cells produce abundant Superoxide anion through an NADPH oxidase-like system. Gastroenterology. 1998;115:1186–96. doi: 10.1016/s0016-5085(98)70090-3. [DOI] [PubMed] [Google Scholar]
- 21.Koshkin V, Lotan O, Pick E. The cytosolic component p47(phox) is not a sine qua non participant in the activation of NADPH oxidase but is required for optimal Superoxide production. J Biol Chem. 1996;271:30326–9. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- 22.Cross AR, Yarchover JL, Curnutte JT. The superoxide-generating system of human neutrophils possesses a novel diaphorase activity. Evidence for distinct regulation of electron flow within NADPH oxidase by p67-phox and p47-phox. J Biol Chem. 1994;269:21448–54. [PubMed] [Google Scholar]