ABSTRACT
Microbes and microbial components potentially impact the performance of pigs through immune stimulation and altered metabolism. These immune modulating factors can include endotoxin from gram negative bacterial outer membrane component, commonly referred to as lipopolysaccharide (LPS). In this study, our objective was to examine the relationship between intestinal barrier integrity, endotoxin and inflammation with feed efficiency (FE), using pig lines divergently selected for residual feed intake (RFI) as a model. Twelve gilts (62 ± 3 kg BW) from the low RFI (LRFI, more efficient) and 12 from the high RFI (HRFI, less efficient) were used. Individual performance data was recorded for 5 wk. At the end of the experimental period, ADFI of LRFI pigs was less (P < 0.001), ADG not different between the 2 lines (P = 0.72) but the G:F of LRFI pigs was greater than for HRFI pigs (P = 0.019). Serum endotoxin concentration (P < 0.01) and the acute phase protein haptoglobin (P < 0.05) were greater in HRFI pigs. Transepithelial resistance of the ileum, transport of fluorescein isothiocyanate labeled-Dextran and-LPS in ileum and colon, as well as tight junction protein mRNA expression in ileum, did not differ between the lines, indicating the 2 lines did not differ in transport characteristics at the intestinal level. Ileum inflammatory markers, myeloperoxidase (P < 0.05) and IL-8 (P < 0.10), were found to be greater in HRFI pigs. Alkaline phosphatase (ALP) activity was significantly increased in the LRFI pigs in ileum and liver tissues and negatively correlated with blood endotoxin (P < 0.05). Lysozyme activity in the liver was not different between the lines; however, the LRFI pigs had a twofold greater lysozyme activity in ileum (P < 0.05). Despite the difference in their activity, ALP or lysozyme mRNA expression was not different between the lines in either tissue. Decreased endotoxin and inflammatory markers and the enhanced activities of antimicrobial enzymes in the LRFI line may not fully explain the difference in the FE between the lines, but they have the potential to prevent the growth potential in HRFI pigs. Further studies are needed to identify the other mechanisms that may contribute to the greater endotoxin and acute phase proteins in the HRFI pigs and the greater FE in the LRFI pigs.
Keywords: endotoxin, feed efficiency, intestinal integrity, pig
INTRODUCTION
Improved feed efficiency (FE) has become a major goal in pig research and breeding programs for economic, environmental and food security reasons. Residual feed intake (RFI) has been adopted as a reliable method for measuring, selecting for and studying FE. Pigs with low RFI (LRFI) consume less feed for a given amount of growth and backfat than pigs with greater RFI (HRFI; Gilbert et al., 2007; Cai et al., 2008). Yorkshire pigs selected for LRFI for 5 generations differed by up to 124 g/day in ADFI with no significant reduction in BW gain compared with randomly selected HRFI pigs (Boddicker et al., 2011). However, the physiology that underlies this improved FE has been poorly defined.
Inflammation can have a major impact on growth performance and FE (Schinckel et al., 1995) and could contribute to decreased maintenance nutrient requirements in pigs selected for reduced RFI (Barea et al., 2010; Boddicker et al., 2011). Lipopolysaccharide (LPS), a Gram negative bacterial outer membrane component referred also as endotoxin, is a chronic innate immune stimulator in pigs (Webel et al., 1997; Gabler et al., 2008; Weber and Kerr, 2008). Importantly, Gram negative bacteria are present in the gastrointestinal tract in large amounts and can serve as a major source of systemic endotoxin (Ravin et al., 1960; Cani and Delzenne, 2010). However, intestinal barrier integrity plays critical host defense functions against luminal immunogens such as endotoxin. Once in circulation, endotoxin activates the immune system via toll like receptors (TLR), which result in cytokine and acute phase protein production and the repartitioning of nutrients for immune function, rather than toward anabolism (Kimball et al., 2003; Rakhshandeh and de Lange, 2012). Additionally, detoxification processes can prevent the negative effects of endotoxin (Elsbach, 2000). Therefore, our objective was to examine the relationship between intestinal barrier integrity, endotoxin and inflammation with FE, using pig lines divergently selected for RFI as a model.
MATERIALS AND METHODS
All animal procedures were approved by the Iowa State University Institutional Animal Care and Use Committee and adhered to the ethical and humane use of animals for research. All chemicals used for the experiment were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Animals and Experimental Design
Twelve pigs per line (62 ± 3 kg, BW) were selected and matched across lines for age and weight from the seventh generation of the Iowa State University RFI selection project (Cai et al., 2008). Pigs were individually penned and had free access to water and feed at all times. All pigs were fed a common commercial corn-soybean meal-distillers dry grain with soluble diet formulated to meet or exceed the nutrient requirements for this size pig (NRC, 1998). Feed intake and BW were recorded on a weekly basis and used to calculate ADG and RFI for each pig, as previously described (Cai et al., 2008; Young et al., 2011).
After 5 wk, all pigs were fasted overnight and whole blood (10 mL) was collected via venipuncture and serum separated by centrifugation at 2000 × g for 10 min at 4°C. Thereafter, pigs were euthanized via captive bolt followed by exsanguination. Immediately after euthanasia, segments of ileum and mid colon were collected and flushed with ice cold Krebs-Henseleit buffer (consisting of, in mmol/L: 25 NaHCO3, 120 NaCl, 1 MgSO4, 6.3 KCl, 2 CaCl2, 0.32 NaH2PO4; pH 7.4) to remove any undigested food material. Fresh ileum and colon segments were used for ex vivo integrity measures and for mucosal scrapings. A 20 cm segment of ileum 150 cm from the ileal-cecal junction and a 10 cm segment of proximal colon 60 cm from the rectum were isolated. Ileum and colon segments were flushed with ice cold Krebs-Henseleit buffer. For mucosal scrapings, intestinal segments were then cut open longitudinally along the mesenteric border and the epithelial layer gently scrapped with a glass slide without disturbing the underlying lamina propria. Ileum and colon samples that were not used for ex vivo work were then snap frozen in liquid nitrogen and stored at –80°C until analysis.
Intestinal Integrity
Electrophysiological measurements were taken using modified Ussing chambers as previously described (Albin et al., 2007; Gabler et al., 2009; Moeser et al., 2012). Briefly, fresh segments of the ileum and colon were removed and placed on ice in Krebs-Henseleit buffer for transport to the laboratory, kept under constant aeration until clamped in the modified Ussing chambers. To assess tight junction integrity and the mucosal to serosal endotoxin transport, tissues stripped of outer serosal layers were immediately mounted in a modified Ussing Chamber, with each chamber connected to a pair of dual channel current and voltage electrodes submerged in 3% noble agar bridges and filled with 3M potassium chloride for electrical conductance (Physiologic Instruments Inc., San Diego, CA and World Precision Instruments Inc., New Haven, CT). Each segment (0.71 cm2) was bathed on its mucosal and serosal sides with Krebs buffer and constantly gassed with 95% O2–5% CO2 mixture. The temperature of all tissues and apparatus was constantly maintained at 37°C using circulating warm water. A short circuit current was established and stabilized for 10 min and transepithelial resistance (TER) was measured using the included software (Acquire and Analyze, Physiological instruments).
After recording the basal electrophysiological measurements, the mucosal to serosal macromolecule transport of fluorescein isothiocyanate labeled dextran (4.4 KDa; FITC-Dextran) was assessed to measure the integrity of both ileum and colon, as previously described (Wang et al., 2001). Briefly, the mucosal chambers were treated with 2.2 mg/mL FITC-Dextran, and chamber samples from both sides were collected every 10 to 15 min. The relative fluorescence was then determined using a fluorescent plate reader (Bio-Tek, Winooski, VT), with excitation and emission wavelengths of 485 and 520 nm, respectively. An apparent permeability coefficient (Papp) was then calculated using the area of the membrane and rate of FITC-Dextran transport, where dQ/dt = transport rate (µg/min); C0 = initial concentration in the donor chamber (µg/mL); A = area of the membrane (cm2): Papp = dQ/(dt×A×C0).
The mucosal to serosal transport of LPS was also assessed as previously described by Tomita et al. (2004). Briefly, the mucosal chambers were challenged with 20 µg/mL fluorescein isothiocyanate labeled LPS (FITC-LPS) and chamber samples from both sides were collected every 10 to 15 min. The fluorescence and apparent permeability coefficient was calculated as described for FITC-Dextran.
Circulating Endotoxin
Serum endotoxin concentrations were measured by an end point fluorescent assay using the recombinant factor C (rFC) system (Lonza, Basel, Switzerland). Briefly, the serum samples were diluted 1000X in pyrogen free water and 100 μL of the samples and standards were added to a 96 well round bottom plate and incubated at 37°C for 10 min. After incubation, 100 μL of rFC enzyme, rFC assay buffer and rFC substrate were added at a ratio of 1:4:5 to the plate and an initial reading were taken followed by 1 h incubation at 37°C. Thereafter, the relative fluorescence unit (RFU) for each well was determined (excitation 380 nm and emission 440 nm). The concentration of the endotoxin was interpolated from the standard curve constructed from the standards and corrected for sample dilution.
Alkaline Phosphatase activity
Alkaline phosphatase (ALP) activity was measured using the Quantichrom ALP assay kit (DALP-250, Gentaur, Bioassay systems, Hayward, CA). Protein was extracted using potassium phosphate buffer (PPB), pH 6.0 from liver and ileum and the protein concentration was determined using BCA assay (Pierce, Rockford, IL) and 50 μL of sample was added to a 150 μL working solution containing magnesium acetate, p-nitrophenyl phosphate and assay buffer in a 96 well plate. The optical density at 405 nm was measured at time 0 and after 4 min using a Synergy 4 microplate reader (Bio-Tek) and ALP activity was calculated according to the manufacturer's instructions.
Myeloperoxidase Activity
Myeloperoxidase (MPO) activity was measured in the whole ileal tissue as an indicator of inflammation and neutrophil infiltration using an o-dianasidine assay (Suzuki et al., 1983; de La Serre et al., 2010). Tissue samples were homogenized in PPB pH 6.0, containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and then freeze-thawed on ice and vortexed 3 times. Samples were then centrifuged at 10,000 × g for 15 min at 4°C. The resulting supernatant was transferred to a new tube and the remaining pellet was resuspended in 500 μL of PPB + 0.5% HTAB. The resuspended pellet was freeze-thawed and homogenized twice and 500 μL of this solution was transferred to a new tube. Samples were then centrifuged again at 10,000 × g for 15 min at 4°C and the supernatant was collected. The final supernatant was mixed with o-dianasidine dihydrochloride and 0.005% hydrogen peroxide. One unit of MPO activity was expressed as the amount of MPO needed to degrade 1 µmol of hydrogen peroxide/min/mL. Absorbance was read at 460 nm for 10 min reaction time and absorbance was calculated on mL sample/mg tissue basis.
Lysozyme activity
Whole ileum and liver samples were analyzed for lysozyme activity using the EnzChek fluorescent assay which compares sample lysozyme activity to lysozyme activity on Micrococcus Lysodeikticus cell walls (Invitrogen-Molecular Probes, Carlsbad, California). Samples were diluted and fluorescence was measured using excitation emission wavelengths of 485 and 530 nm and the lysozyme activity were interpolated from the standard curve constructed from the standards and corrected for sample dilution.
Ileal Interleukin-8 and Systemic Acute Phase Protein Assays
Ileal protein (100 μg) was extracted using PPB, pH 6.0 and analyzed for IL-8 concentration using a porcine-specific ELISA (DuoSet Porcine IL8, catalog number DY535, R&D Systems, Minneapolis, MN) per the manufacturer's instructions. Serum haptoglobin, C-reactive protein (CRP; ALPCO Diagnostics, Salem, NH) and LPS binding protein (LBP; Hycult Biotech, Plymouth, PA) were analyzed using commercially available ELISA kits. Briefly, serum samples were added to wells adsorbed with anti-porcine haptoglobin, CRP and LBP antibodies. After washing, horseradish peroxidase (HRP) conjugated anti haptoglobin, CRP and LBP antibodies were added to the plate. After another washing, the HRP was assayed by the addition of the chromogenic substrate 3,3',5,5'-tetramethylbenzidine (TMB) and the absorbance was measured at 450 nm. The quantity of haptoglobin, CRP and LBP in the test samples was interpolated from the standard curve constructed from the standards and corrected for sample dilution.
Quantitative Real-time PCR
Total RNA was isolated from tissue samples using Trizol (Invitrogen Inc.) reagent according to the manufacturer's protocol and the RNA pellets were resuspended in nuclease free water. To eliminate potential genomic DNA contamination, the RNA samples were treated with a DNase I kit (DNA-free, Ambion Inc., Austin, TX) per the manufacturer's instructions. Total RNA was quantified by measuring the absorbance at 260nm using a spectrophotometer (ND-100, NanoDrop Technologies, Rockland, DE) and the purity was assessed by determining the ratio of the absorbance at 260 and 280 nm. All samples had 260/280 nm ratios above 1.8. The integrity of the RNA preparations was verified by visualization of the 18S and 28S ribosomal bands stained with ethidium bromide after electrophoresis on 1.2% agarose gels (E-gel; Invitrogen Inc.). A good preparation was indicated by the presence of 28S and 18S bands that were not smeared and by the 28S band stained with a greater intensity than the 18S band. Total RNA (1 μg) was reverse transcribed using a commercially available cDNA synthesis kit (iScript, BioRad Laboratories, Hercules, CA). The iScript kit used a blend of oligo (dT) and random hexamer primers for cDNA synthesis and the reverse transcriptase is RNase H+ to ensure removal of the RNA template. The primers used for real-time reverse-transcription real time (RT)-PCR are presented in Table 1.
Table 1.
Primer sequences for quantitative real-time PCR
| Target gene | Sense (5'- 3') | Antisense (5'- 3') |
|---|---|---|
| Acyloxyacyl hydrolase (159 bp) | TCAGGGGGACAGAAATATGG | CCAGAATCACGCAGAATCAC |
| Lysozyme (NM_214392.2; 80 bp) | CGGTGCGAGTTCGCCAGAATTC | AAACACACCCAGTTCGCCAGGC |
| Intestinal alkaline phosphatase (XM_003133729; 139 bp) | GTCAGAACGGAGTTCGGAAG | AGGCCCATGAGGTGTGTTAC |
| Claudin 3 (NM_001160075; 95 bp) | CATCGGCAGCAGCATTATC | ACACTTTGCACTGCATCTGG |
| Claudin 4 (NM_001161637.1; 110 bp) | AGGTGATGGGTATCGCCCTGGC | CGACGTGACGATGTTGCTGCC |
| Occludin (NM_00163647; 115 bp) | ATCCTGGGTGTGATGGTGTT | ACTGGTTGCAGAGGGCATAG |
| β-2-Microglobulin | TGGTCTTTCTACCTTCTGGTCC | TGTGATGCCGGTTAGTGGTCTC |
Amplification was performed in a total volume of 25 μL containing 1X iQ SYBR Green Supermix (BioRad Laboratories), forward and reverse primers (0.1 μg/μL) and 1 μL of the 20 μL cDNA reaction. After an initial 5 min denaturation step at 95°C, the reactions were cycled 40 times under these parameters: 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. Optical detection was performed at 72°C. At the end of the PCR, melt curve analysis was conducted to validate the specificity of the primers. A non-template control was run with every assay and all determinations were performed in duplicate. Presence of a single PCR product of the correct size for each primer set was verified by visualizing the PCR products via electrophoresis on 1% agarose gels stained with ethidium bromide. The mRNA abundance values for each sample were normalized to β2 microglobulin according to the ΔCT method (Livak and Schmittgen, 2001). The expression of β2 microglobulin did not differ (P > 0.5) between the RFI lines.
Statistical Analyses
All results were expressed as least squares (LS) means ± SEM. The main effect of line (HRFI vs. LRFI) was determined by the Proc Mixed procedure (SAS Inst. Inc., Cary, NC) with age matched pairs (repetition) as a random effect. However, for Ussing chamber data, tissue (colon versus ileum) was also included as a fixed effect. Statistical significance of differences was determined by Tukey's range test for pair wise comparisons. Differences were deemed significant at P ≤ 0.05 and tendencies at P ≤ 0.10. Phenotypic correlations between serum endotoxin and acute phase proteins, ileum endotoxin permeability, ALP and lysozyme with RFI, ADG, ADFI and G:F were computed based on residuals derived from the above models using the CORR procedure of SAS.
RESULTS
Twelve gilts per line weighing approximately (62 ± 3, kg BW) were used for the study. At the end of the experimental period, growth performance was measured. As expected, the LRFI pigs consumed less feed than their HRFI counterparts (ADFI: 1.90 vs. 2.23 kg/day, P < 0.001). However, the ADG did not differ between the lines (0.67 vs. 0.65, kg/day, P = 0.72). Because the LRFI pigs consumed less feed for the same ADG, their G:F ratio was greater than that of the HRFI pigs (0.35 vs. 0.29, P = 0.019). These results confirm that the effect of selection for RFI was maintained in the gilts used for this study.
Intestinal integrity was assessed by measuring the TER and macromolecule permeability in freshly isolated ileum and colon samples. Transepithelial resistance, an electrophysiological measure of intestinal integrity, was not different in either the ileum or colon of pigs divergently selected for RFI (Table 2). However, irrespective of line, colon TER was significantly less than in the ileum (P < 0.05). Further, these data were supported by additional ex vivo analysis of intestinal integrity using FITC-Dextran, a macromolecule permeability marker. No differences between lines were observed in Papp for either the ileum or colon (P > 0.05, Table 2).
Table 2.
Intestinal integrity is not altered in gilts divergently selected for low (LRFI) and high (HRFI) residual feed intake
| Ileum | Colon | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | LRFI1 | HRFI1 | LRFI1 | HRFI1 | SEM | Line | Tissue | Line*Tissue |
| TER2 (Ω/cm2) | 108 | 103 | 78 | 73 | 13.9 | 0.66 | 0.04 | 0.99 |
| FITC-Dextran3 (Papp) | 0.48 | 0.52 | 0.13 | 0.19 | 0.09 | 0.15 | < 0.001 | 0.50 |
| FITC-LPS3 (Papp) | 2.76 | 3.63 | 3.95 | 3.39 | 0.72 | 0.83 | 0.54 | 0.35 |
1 n = 6 pigs/line.
2TER = transepithelial resistance.
3Papp = Apparent permeability coefficient, µg/mL/min/cm.
Ex vivo intestinal endotoxin transport characteristics were also assessed using FITC-LPS in modified Ussing chambers in the 2 RFI lines. Similar to the TER and FITC-Dextran values, we found no differences between the lines in either ileum and colon FITC-LPS transport (P > 0.05, Table 2). The tight junction proteins claudin 3 and 4, and occludin are 3 important proteins involved in intestinal barrier function and integrity. Gene expression analysis of these tight junction proteins also indicated no difference between the lines (P > 0.05, Table 3).
Table 3.
Ileum tight junction protein gene expression pigs selected for high (HRFI) or low residual feed intake (LRFI)
| Gene | HRFI1,2 | LRFI1,2 | SEM | P-value |
|---|---|---|---|---|
| Claudin 3 | 14.76 | 14.41 | 0.530 | 0.65 |
| Claudin 4 | 8.09 | 8.00 | 0.874 | 0.94 |
| Occludin | 13.75 | 12.01 | 0.936 | 0.22 |
1 n = 6 pigs/line.
2Mean gene expression from β-2-microglobulin housekeeper.
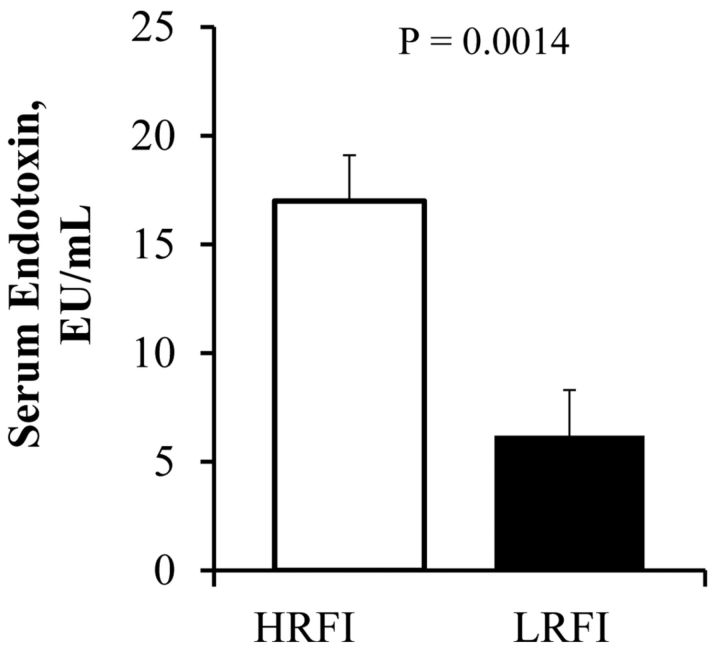
Serum endotoxin concentrations were found to be lower in the LRFI than in the HRFI gilts (P < 0.05, Fig. 1). Furthermore, the serum acute phase protein, haptoglobin, was also less in LRFI pigs than in HRFI gilts (P < 0.05, Table 4). Lipopolysaccharide binding protein concentrations were 25% less in the HRFI gilts, compared with their LRFI counterparts (P = 0.38, Table 4). The presence of a greater endotoxin load and the consequent increase in haptoglobin secretion has the potential to cause a generalized inflammation. There was also a tendency (P = 0.08) for serum CRP to be increased in the HRFI pigs (Table 4). The ileum was also assessed for the presence of general inflammatory markers. Ileum myeloperoxidase activity was lower in the LRFI pigs than in the HRFI pigs (P = 0.047, Table 4). The proinflammatory cytokine, IL-8 protein expression, also tended to be less in the ileum of LRFI versus HRFI pigs (P = 0.062, Table 4).
Fig. 1.
Circulating serum endotoxin concentrations in pigs divergently selected for high (HRFI) or low (LRFI) residual feed intake (n = 12 pigs/line).
Table 4.
Serum and tissue acute phase protein and cytokine concentrations in pigs divergently selected for high (HRFI) and low (LRFI) residual feed intake
| Item | HRFI1 | LRFI1 | SEM | P-Value |
|---|---|---|---|---|
| Serum haptoglobin, mg/mL | 0.51 | 0.23 | 0.129 | 0.043 |
| Serum C-reactive protein, ng/mL | 266 | 174 | 50 | 0.08 |
| Serum lipopolysaccharide binding protein, ng/mL | 7233 | 9019 | 1972 | 0.38 |
| Ileum IL-8, µg/g protein | 1.67 | 1.10 | 0.271 | 0.062 |
| Ileum myeloperoxidase activity, mU/mg protein | 4.14 | 2.90 | 0.586 | 0.047 |
1 n = 12 pigs/Line
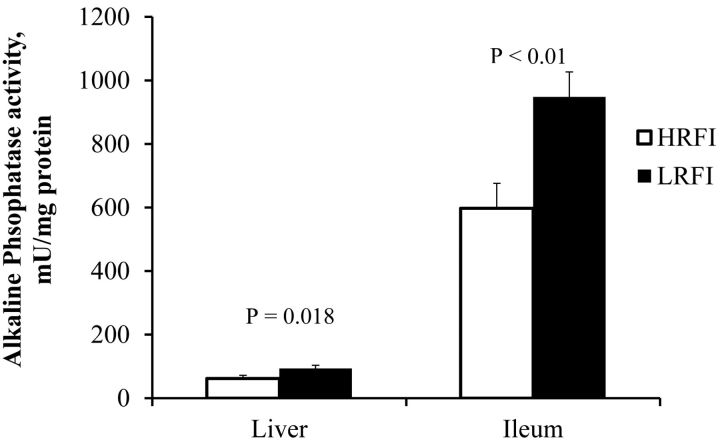
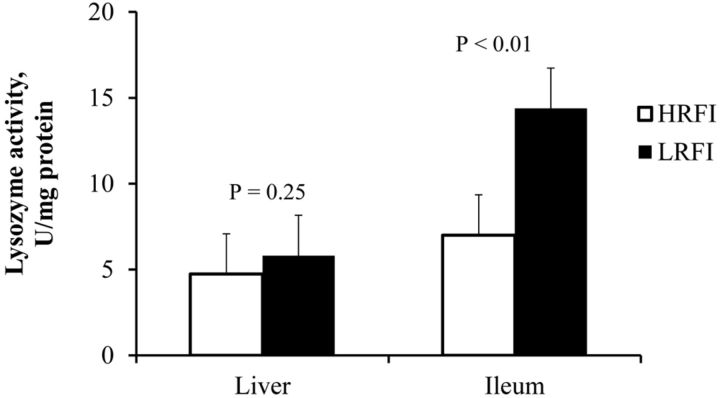
Alkaline phosphatase activity (Fig. 2) was measured and found to be significantly increased in the LRFI pigs in both liver (P < 0.018) and ileum (P < 0.01). Lysozyme activity in the liver was not different between the lines but ileum lysozyme activity in LRFI pigs was almost twice as high as in HRFI pigs (P < 0.01, Fig. 3). Although we saw differences in activity of these enzymes, surprisingly, no difference was found in the mRNA expression in either ileum or liver (Table 5). The mRNA expression of LPS detoxification gene acyloxyacyl hydrolase (AOAH) present in liver and ileum tissues was quantified to assess differences between the 2 lines. The expression profiles of these genes was not different, indicating that at the mRNA level, LPS detoxification enzyme AOAH expression was not altered due to blood endotoxin, selection for RFI, or FE (Table 5).
Fig. 2.
Liver and Ileum alkaline phosphatase activity in pigs divergently selected for high (HRFI) or low (LRFI) residual feed intake (n = 6 pigs/line).
Fig. 3.
Liver and ileum lysozyme activity in pigs divergently selected for high (HRFI) or low (LRFI) residual feed intake (n = 6 pigs/line).
Table 5.
Lipopolysaccharide and Gram positive bacteria detoxification mRNA in pigs divergently selected for high (HRFI) and low (LRFI) residual feed intake
| Gene | HRFI1,2 | LRFI1,2 | SEM | P-value |
|---|---|---|---|---|
| Liver Acyloxyacyl hydrolase | 5.38 | 6.34 | 0.877 | 0.30 |
| Ileum Acyloxyacyl hydrolase | 7.45 | 6.98 | 0.354 | 0.37 |
| Ileum Lysozyme | 8.22 | 7.41 | 0.977 | 0.57 |
| Ileum Alkaline Phosphatase | 12.38 | 11.97 | 0.773 | 0.71 |
1 n = 6 pigs/line.
2Mean gene expression from β-2-microglobulin housekeeper.
Residual correlations of performance traits with endotoxin transport and serum concentrations, ALP and RFI were generally moderate (Table 6). Feed efficiency (G:F) was moderately negatively correlated with RFI index, serum haptoglobin and C-reactive protein (P < 0.05). Furthermore, there tended to be a moderate negative correlation between FE and serum endotoxin (P = 0.052), but not ileum endotoxin permeability (P = 0.13). However, no significant correlations existed with pig RFI index. Interestingly, this is the first data to our knowledge in swine that shows a significant positive correlation between intestinal endotoxin transport and circulating endotoxin (P = 0.019). Furthermore, ileum ALP activity was moderately negatively correlated with endotoxin concentration (P = 0.027). Lysozyme activity and LBP were poorly correlated with all variables.
Table 6.
Residual correlations of performance variables, endotoxin permeability, serum acute phase proteins and alkaline phosphatase in gilts divergently selected for residual feed intake1
| ADFI2 | ADG2 | G:F | RFI Index3 | Serum endotoxin2 | Serum Haptoglobin2 | Serum CRP2 | Serum LBP2 | Ileum Endotoxin permeability2 | Ileum ALP2 | Ileum Lysozyme2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADFI2 | 1.00 | 0.35 | -0.58 | 0.65 | 0.57 | 0.45 | 0.28 | 0.02 | 0.50 | -0.45 | -0.21 |
| 0.003 | 0.003 | 0.0008 | 0.003 | 0.14 | 0.19 | 0.92 | 0.10 | 0.14 | 0.49 | ||
| ADG2 | 1.00 | 0.55 | 0.02 | 0.14 | -0.49 | -0.24 | 0.14 | -0.29 | 0.28 | 0.40 | |
| 0.006 | 0.92 | 0.52 | 0.11 | 0.25 | 0.51 | 0.35 | 0.38 | 0.20 | |||
| G:F | 1.00 | -0.55 | -0.40 | -0.59 | -0.47 | 0.08 | -0.47 | 0.47 | 0.34 | ||
| 0.007 | 0.052 | 0.042 | 0.019 | 0.72 | 0.13 | 0.12 | 0.27 | ||||
| RFI Index3 | 1.00 | 0.34 | 0.34 | 0.20 | -0.01 | 0.57 | -0.43 | -0.26 | |||
| 0.13 | 0.27 | 0.36 | 0.95 | 0.052 | 0.17 | 0.41 | |||||
| Serum endotoxin2 | 1.00 | 0.66 | 0.13 | -0.04 | 0.66 | -0.63 | -0.43 | ||||
| 0.019 | 0.53 | 0.84 | 0.019 | 0.027 | 0.16 | ||||||
| Serum Haptoglobin2 | 1.00 | 0.35 | 0.02 | 0.47 | -0.18 | -0.39 | |||||
| 0.26 | 0.95 | 0.12 | 0.57 | 0.21 | |||||||
| Serum CRP2 | 1.00 | 0.12 | -0.17 | 0.01 | 0.12 | ||||||
| 0.57 | 0.60 | 0.96 | 0.57 | ||||||||
| Serum LBP2 | 1.00 | -0.36 | -0.28 | -0.32 | |||||||
| 0.24 | 0.37 | 0.31 | |||||||||
| Ileum Endotoxin permeability2 | 1.00 | -0.40 | -0.49 | ||||||||
| 0.19 | 0.11 | ||||||||||
| Ileum ALP2 | 1.00 | 0.25 | |||||||||
| 0.43 | |||||||||||
| Ileum Lysozyme2 | 1.00 |
1Upper row = residual correlations. Bottom row = P-values.
2ADFI = kg/d; ADG = kg/d; Serum endotoxin = EU/mL; Serum haptoglobin = mg/mL; Serum C-reactive protein (CRP) = ng/mL; Serum lipopolysaccharide binding protein (LBP) = ng/mL; Ileum endotoxin permeability = Papp; Ileum alkaline phosphatase (ALP) = mU/mg; Ileum lysozyme = U/mg.
3Residual feed intake (RFI) = ADFI-β1(ontest BW deviation)+β2(offtest BW deviation)+β3(metabolic mid-BW)+β4(ADG)+β5(offtest backfat)
DISCUSSION
The current study was conducted to identify if differences in intestinal integrity, circulating endotoxin and its associated inflammatory markers may partially be responsible for difference in FE between pigs that were divergently selected for RFI. The physiological mechanisms underlying RFI or FE in swine are poorly defined. However, differences in nutrients and energy digestibility, metabolic efficiency of nutrient use, basal metabolic rate and energy expenditure are presumed to be contributing to differences in RFI, either alone or in combination (Herd and Arthur, 2009; Barea et al., 2010; Boddicker et al., 2011). Endotoxin and its associated inflammation can reduce digestibility and alter intestinal nutrient transport and post-absorptive metabolism (Albin et al., 2007; Rakhshandeh and de Lange, 2012). The net effect of increased circulating endotoxin or endotoxemia is reduced growth, increased energy expenditure and antagonizing lean tissue accretion (Orellana et al., 2007). Thus, partitioning of energy and other nutrients away from growth and toward immune system requirements may contribute to differences in FE.
In the present study, although acute compared with that typically used in LPS challenge models, endogenous blood endotoxin concentration was increased threefold in HRFI gilts. Although, characterization of intestinal microbial populations was not within the scope of this study, we cannot rule out RFI line specific differences in Enterobacteriaceae populations that may partially explain the increase in circulating endotoxin. Interestingly, the presence of greater circulating endotoxin also has the potential to affect intestinal health and function (Gardiner et al., 1995; Albin et al., 2007). As such, we reported greater ileal MPO activity and IL-8 protein expression in the HRFI pigs with augmented serum endotoxin. Both of these markers are commonly used for assessing intestinal inflammation and neutrophil infiltration (Suzuki et al., 1983). Remarkably, little has been reported with regard to pig FE and “leaky” or porous gut and intestinal integrity. No differences between the lines were observed in intestinal TER. This was corroborated further by no differences in the paracellular transport of 4.4 kDa FITC-Dextran or FITC-LPS. Although decreased expression of tight junction proteins claudin 3 and 4 during inflammation have been implicated in increased intestinal permeability (Pinton et al., 2010), the mRNA expressions of occludin, claudins 3 and 4 were not different between the our pig lines. These data agree with the intestinal integrity LPS challenge data reported by Albin et al. (2007). Altogether, these data indicate that in healthy pigs, intestinal integrity is tightly controlled and may not contribute significantly to differences in FE.
The presence of greater blood endotoxin has the potential to contribute to the development of an inflammatory state and reduce growth potential. In our study, serum endotoxin and the acute phase protein, haptoglobin, were found to be significantly lower in the LRFI pigs. Our values for haptoglobin, CRP and LBP are in agreement with the values reported in non-challenged pigs (Barbe et al., 2011; Diack et al., 2011). However, serum concentrations of acute-phase proteins are known to increase after stress and inflammation (Pineiro et al., 2007; Pineiro et al., 2009) and the magnitude of the response is generally related to the severity of the stress or disease (Hall et al., 1992; Murata et al., 2004; Williams et al., 2009). Even though our pigs were considered healthy, we observed an acute increase in serum haptoglobin and CRP in our HRFI gilts, compared with their LRFI, more FE counterparts. Although difficult to interpret, we speculate that an increase in acute phase proteins is a response to acute tissue damage, inflammation or stress. Studies have reported no straight forward correlations with LPS and acute phase proteins in pig serum (Van Gucht et al., 2006). However, we observed a significant and moderate correlation between serum LPS and haptoglobin, but not CRP.
Interestingly, the high immune response of HRFI pigs doesn't appear to be a result of increased intestinal permeability, but could be a result of differences in detoxification processes. Animals and humans harbor reduced concentrations of circulating endotoxin even in normal and healthy conditions (Erridge et al., 2007). The potential biological process that might account for the decreased endotoxin concentrations in the LRFI pigs may include the greater efficiency in endotoxin clearance, neutralization or detoxification. These processes occur in various tissues, including immune cells, liver, kidney and intestine. Furthermore, various binding proteins and enzymes such as ALP, lysozyme, and AOAH are involved (Munford et al., 2009). Alkaline phosphatase is a hydrolase enzyme present in liver, intestine and kidney tubules and it dephosphorylates bacterial LPS and reduces its toxicity (Poelstra et al., 1997; Bates et al., 2007). Greater ALP activity in both liver and ileum of LRFI pigs indicates that they deactivated or neutralized endotoxin more efficiently. It is speculated that pigs are vulnerable to enteric infections and susceptible diarrhea, and growth-check due to compromised mucosal alkaline phosphatase (Lackeyram et al., 2010). Further, intestinal ALP reduces trans-mucosal passage of bacteria and also protects against LPS-induced inflammation (Lallès, 2010). This may be affirmed by the moderately negative correlation between ALP and serum endotoxin concentration in our study. Further evidence suggests that ALP can also mitigate BW loss after an immune challenge (Bol-Schoenmakers et al., 2010).
To further explore the difference in endotoxin metabolism, we measured AOAH mRNA expression. Acyloxyacyl hydrolase is an important lipase enzyme that selectively removes the secondary fatty acyl chains attached to the primary chains in the lipid A moiety and detoxifies endotoxin (McDermott and Fenwick, 1992). This leads to an LPS molecule which could bind the signaling proteins MD2/TLR4, but does not have the potential to initiate the signal or can only be a partial agonist (Lu et al., 2005). The fact that we did not see evidence of differential AOAH mRNA expression in the intestine or liver was surprising. However, AOAH mRNA and activity appear to be correlated in a tissue or cell specific manner (Feulner et al., 2004). Therefore, liver and intestinal mRNA expression may not be correlated in swine or immune and Kupffer cell specific expression needs to be determined, where activity and expression of AOAH are greater.
Lysozyme is another important antimicrobial peptide secreted by various cells of the body, including cells in the intestine and liver. Lysozyme regulates microbial populations by lysing the bacterial cell wall component peptidoglycan and also binds to and detoxifies LPS (Takada et al., 1994). In our study, although lysozyme activity in the liver was not different between the 2 lines and ileum lysozyme activity was greater in LRFI pigs. This may explain the decreased serum endotoxin and haptoglobin concentrations in the LRFI pigs and greater FE. Supporting this hypothesis, recent studies found that feeding lysozyme to pigs improved their health and FE (May et al., 2012; Nyachoti et al., 2012). However, tissue lysozyme activity was not determined in these studies. Surprisingly, although ALP and lysozyme activity was different between the 2 lines in ileum and liver, mRNA expression of both enzymes were not different, indicating a post-translational mechanism which may act differently in the 2 lines of pigs.
We speculate that the LRFI pigs are exposed to comparatively lower concentrations of endotoxin and stress intermittently, although the 2 lines shared the same environmental conditions (i.e., housing and diets). We hypothesize that the HRFI pigs with reduced endotoxin detoxification would have increased stress and acute tissue damage compared with the LRFI gilts. Together, this would explain the increase in acute phase proteins. Pigs repeatedly exposed to endotoxin have augmented haptoglobin concentrations compared with a single endotoxin exposure (Dritz et al., 1996; Wright et al., 2000). In addition, increased reactive oxygen species production and reduced mitochondrial coupling may also explain our data. Reactive oxygen species production increases protein carbonyl formation in low FE broiler (Bottje et al., 2006) and steer (Sandelin, 2005) tissues. Importantly, these proteins may be more susceptible to degradation and turnover which would contribute to the acute phase protein response we report herein and the reduced FE phenotype.
In conclusion, our results indicate that LRFI pigs seem to have a more robust intestinal and liver endotoxin detoxification and greater active anti-microbial enzymes including ALP, ileum lysozyme and the inflammatory mediator enzyme myeloperoxidase, and that HRFI pigs seem to be undergoing a greater level of basal inflammation. Although decreased serum endotoxin and the associated decreased inflammatory markers and the enhanced activities of antimicrobial enzymes in the LRFI pigs may not explain the line difference in FE wholly, it has the potential to be a significant contributing factor. Further studies are needed to identify other mechanisms that contribute to the reduced endotoxin concentrations in the LRFI pigs and how this is associated with their greater FE.
Footnotes
This research was supported by the Agriculture and Food Research Initiative Competitive Grants 2010-65206-20670 (to N. K. Gabler) and 2011-68004-30336 (to J. F. Patience) from the USDA National Institute of Food and Agriculture.
LITERATURE CITED
- Albin D. M., Wubben J. E., Rowlett J. M., Tappenden K. A., Nowak R. A. 2007. Changes in small intestinal nutrient transport and barrier function after lipopolysaccharide exposure in two pig breeds. J. Anim Sci 85: 2517–2523. [DOI] [PubMed] [Google Scholar]
- Barbe F., Atanasova K., Van Reeth K. 2011. Cytokines and acute phase proteins associated with acute swine influenza infection in pigs. Vet J 187: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea R., et al. 2010. Energy utilization in pigs selected for high and low residual feed intake. J. Anim Sci 88: 2062–2072. [DOI] [PubMed] [Google Scholar]
- Bates J. M., Akerlund J., Mittge E., Guillemin K. 2007. Intestinal Alkaline Phosphatase Detoxifies Lipopolysaccharide and Prevents Inflammation in Zebrafish in Response to the Gut Microbiota. Cell Host & Microbe 2: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddicker N., Gabler N. K., Spurlock M. E., Nettleton D., Dekkers J. C. 2011. Effects of ad libitum and restricted feed intake on growth performance and body composition of Yorkshire pigs selected for reduced residual feed intake. J. Anim Sci 89: 40–51. [DOI] [PubMed] [Google Scholar]
- Bol-Schoenmakers M., et al. 2010. Intestinal alkaline phosphatase contributes to the reduction of severe intestinal epithelial damage. European Journal of Pharmacology 633: 71–77. [DOI] [PubMed] [Google Scholar]
- Bottje W., Pumford N. R., Ojano-Dirain C., Iqbal M., Lassiter K. 2006. Feed efficiency and mitochondrial function. Poultry science 85: 8–14. [DOI] [PubMed] [Google Scholar]
- Cai W., Casey D. S., Dekkers J. C. 2008. Selection response and genetic parameters for residual feed intake in Yorkshire swine. J. Anim Sci 86: 287–298. [DOI] [PubMed] [Google Scholar]
- Cani P. D., Delzenne N. M. 2010. Gut Microbiota, Diet, Endotoxemia, and Diseases. Wiley-VCH Verlag GmbH & Co; KGaA. [Google Scholar]
- de La Serre C. B., et al. 2010. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. American Journal of Physiology- Gastrointestinal and Liver Physiology 299: G440–G448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diack A. B., Gladney C. D., Mellencamp M. A., Stear M. J., Eckersall P. D. 2011. Characterisation of plasma acute phase protein concentrations in a high health boar herd. Veterinary Immunology and Immunopathology 139: 107–112. [DOI] [PubMed] [Google Scholar]
- Dritz S. S., et al. 1996. Influence of lipopolysaccharide-induced immune challenge and diet complexity on growth performance and acute-phase protein production in segregated early-weaned pigs. J. Anim Sci 74: 1620–1628. [DOI] [PubMed] [Google Scholar]
- Elsbach P. 2000. Mechanisms of disposal of bacterial lipopolysaccharides by animal hosts. Microbes and Infection 2: 1171–1180. [DOI] [PubMed] [Google Scholar]
- Erridge C., Attina T., Spickett C. M., Webb D. J. 2007. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 86: 1286–1292. [DOI] [PubMed] [Google Scholar]
- Feulner J. A., et al. 2004. Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infection and immunity 72: 3171–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabler N. K., Radcliffe J. S., Spencer J. D., Webel D. M., Spurlock M. E. 2009. Feeding long-chain n-3 polyunsaturated fatty acids during gestation increases intestinal glucose absorption potentially via the acute activation of AMPK. J. Nutr Biochem 20: 17–25. [DOI] [PubMed] [Google Scholar]
- Gabler N. K., Spencer J. D., Webel D. M., Spurlock M. E. 2008. n-3 PUFA attenuate lipopolysaccharide-induced down-regulation of toll-like receptor 4 expression in porcine adipose tissue but does not alter the expression of other immune modulators. J. Nutr Biochem 19: 8–15. [DOI] [PubMed] [Google Scholar]
- Gardiner K. R., et al. 1995. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut 36: 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H., et al. 2007. Genetic parameters for residual feed intake in growing pigs, with emphasis on genetic relationships with carcass and meat quality traits. J. Anim Sci 85: 3182–3188. [DOI] [PubMed] [Google Scholar]
- Hall W. F., Eurell T. E., Hansen R. D., Herr L. G. 1992. Serum haptoglobin concentration in swine naturally or experimentally infected with Actinobacillus pleuropneumoniae. J. Am Vet Med Assoc 201: 1730–1733. [PubMed] [Google Scholar]
- Herd R. M., Arthur P. F. 2009. Physiological basis for residual feed intake. J. Anim Sci 87: E64–71. [DOI] [PubMed] [Google Scholar]
- Kimball S. R., et al. 2003. Endotoxin induces differential regulation of mTOR-dependent signaling in skeletal muscle and liver of neonatal pigs. Am J Physiol Endocrinol Metab 285: E637–644. [DOI] [PubMed] [Google Scholar]
- Lackeyram D., Yang C., Archbold T., Swanson K. C., Fan M. Z. 2010. Early weaning reduces small intestinal alkaline phosphatase expression in pigs. J. Nutr 140: 461–468. [DOI] [PubMed] [Google Scholar]
- Lallès J.-P. 2010. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutrition Reviews 68: 323–332. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. 2001. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lu M., et al. 2005. Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria. Nat Immunol 6: 989–994. [DOI] [PubMed] [Google Scholar]
- May K. D., Wells J. E., Maxwell C. V., Oliver W. T. 2012. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J. Anim Sci 90: 1118–1125. [DOI] [PubMed] [Google Scholar]
- McDermott C., Fenwick B. 1992. Neutrophil activation associated with increased neutrophil acyloxyacyl hydrolase activity during inflammation in cattle. Am J Vet Res 53: 803–807. [PubMed] [Google Scholar]
- Moeser A. J., Borst L. B., Overman B. L., Pittman J. S. 2012. Defects in small intestinal epithelial barrier function and morphology associated with peri-weaning failure to thrive syndrome (PFTS) in swine. Research in veterinary science 93: 975–982. [DOI] [PubMed] [Google Scholar]
- Munford R., Lu M., Varley A. 2009. Chapter 2 Kill the Bacteria... and Also Their Messengers? In: Frederick W. A. (ed.) Advances in Immunology No. Volume 103. p. 29–48. Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Shimada N., Yoshioka M. 2004. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet J 168: 28–40. [DOI] [PubMed] [Google Scholar]
- Nyachoti C. M., Kiarie E., Bhandari S. K., Zhang G., Krause D. O. 2012. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J. Anim Sci 90: 252–260. [DOI] [PubMed] [Google Scholar]
- Orellana R. A., et al. 2007. Insulin stimulates muscle protein synthesis in neonates during endotoxemia despite repression of translation initiation. Am J Physiol Endocrinol Metab 292: E629–636. [DOI] [PubMed] [Google Scholar]
- Pineiro C., Piñeiro M., Morales J., Andrés M., Lorenzo E., Pozo M. D., Alava M. A., Lampreave F. 2009. Pig-MAP and haptoglobin concentration reference values in swine from commercial farms. Vet J 179:78–84. [DOI] [PubMed] [Google Scholar]
- Pineiro M., Piñeiro C., Carpintero R., Morales J., Campbell F. M., Eckersall P. D., Toussaint M. J. M., Lampreave F. 2007. Characterisation of the pig acute phase protein response to road transport. Vet J 173: 669–674. [DOI] [PubMed] [Google Scholar]
- Pinton P., Braicu C., Nougayrede J.-P., Laffitte J., Taranu I., Oswald I. P. 2010. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 140:1956–1962. [DOI] [PubMed] [Google Scholar]
- Poelstra K., et al. 1997. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol 151: 1163–1169. [PMC free article] [PubMed] [Google Scholar]
- Rakhshandeh A., de Lange C. F. 2012. Evaluation of chronic immune system stimulation models in growing pigs. Animal: An international journal of animal bioscience 6: 305–310. [DOI] [PubMed] [Google Scholar]
- Ravin H. A., Rowley D., Jenkins C., Fine J. 1960. On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. J. Exp Med 112: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin B. A. 2005. Association of mitochondrial biochemistry and electron transport chain protein expression with feed efficiency in Angus cattle. PhD. Dissertation, Univ. Arkansas, Fayetteville [Google Scholar]
- Schinckel A. P., et al. 1995. Effects of antigenic challenge on growth and composition of segregated early-weaned pigs. Swine Health and Production 3: 228–234. [Google Scholar]
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. 1983. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Analytical Biochemistry 132: 345–352. [DOI] [PubMed] [Google Scholar]
- Takada K., Ohno N., Yadomae T. 1994. Detoxification of lipopolysaccharide (LPS) by egg white lysozyme. FEMS Immunology & Medical Microbiology 9: 255–263. [DOI] [PubMed] [Google Scholar]
- Tomita M., Ohkubo R., Hayashi M. 2004. Lipopolysaccharide transport system across colonic epithelial cells in normal and infective rat. Drug Metab Pharmacokinet 19: 33–40. [DOI] [PubMed] [Google Scholar]
- Van Gucht S., et al. 2006. Effect of porcine respiratory coronavirus infection on lipopolysaccharide recognition proteins and haptoglobin levels in the lungs. Microbes Infect 8: 1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Fang C. H., Hasselgren P.-O. 2001. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology 281: R1013–R1023. [DOI] [PubMed] [Google Scholar]
- Webel D. M., Finck B. N., Baker D. H., Johnson R. W. 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 75: 1514–1520. [DOI] [PubMed] [Google Scholar]
- Weber T. E., Kerr B. J. 2008. Effect of sodium butyrate on growth performance and response to lipopolysaccharide in weanling pigs. J. Anim Sci 86: 442–450. [DOI] [PubMed] [Google Scholar]
- Williams P. N., Collier C. T., Carroll J. A., Welsh T. H., Jr., Laurenz J. C. 2009. Temporal pattern and effect of sex on lipopolysaccharide-induced stress hormone and cytokine response in pigs. Domest Anim Endocrinol 37: 139–147. [DOI] [PubMed] [Google Scholar]
- Wright K. J., et al. 2000. Integrated adrenal, somatotropic, and immune responses of growing pigs to treatment with lipopolysaccharide. J. Anim. Sci. 78: 1892–1899. [DOI] [PubMed] [Google Scholar]
- Young J. M., Cai W., Dekkers J. C. 2011. Effect of selection for residual feed intake on feeding behavior and daily feed intake patterns in Yorkshire swine. J. Anim Sci 89: 639–647. [DOI] [PubMed] [Google Scholar]