Abstract
Upper respiratory tract infections are common and important. Although rarely fatal, they are a source of significant morbidity and carry a considerable economic burden. Numerous therapies for the common cold have no effect on symptoms or outcome. Complications such as cough are not improved by over-the-counter preparations, while labelling cough alone as a symptom of asthma may result in unnecessary use of inhaled steroid treatment. Clinical presentation of sore throat does not accurately predict whether the infection is viral or bacterial, while throat culture and rapid antigen tests do not significantly change prescribing practice. Antibiotics have only a limited place in the management of recurrent sore throat due to group A β-haemolytic streptococcal infection. Routine use of antibiotics in upper respiratory infection enhances parent belief in their effectiveness and increases the likelihood of future consultation in primary care for minor self-limiting illness. Respiratory viruses play a major role in the aetiology of acute otitis media (AOM); prevention includes the use of influenza or RSV vaccination, in addition to reducing other risk factors such as early exposure to respiratory viruses in day-care settings and to environmental tobacco smoke. The use of ventilation tubes (grommets) in secretory otitis media (SOM) remains controversial with conflicting data on developmental outcome and quality of life in young children. New conjugate pneumococcal vaccines appear safe in young children and prevent 6–7% of clinically diagnosed AOM.
Upper respiratory tract infections are common and important. Although rarely fatal, they are a source of significant morbidity and carry a considerable economic burden. This review will focus on clinical controversies in the diagnosis and management of upper respiratory tract infections and their complications.
The common cold
The common cold, although usually a minor, self-limiting illness, results in significant school absence and complications such as secondary bacterial infection. Additionally, coryzal illnesses play a significant part in exacerbations of asthma and result in considerable morbidity in children. Each year in the US, $2 billion is spent on over-the-counter (OTC) preparations to relieve cold symptoms, predominantly in children.
Children experience 3–8 colds per year and 10–15% have at least 12 per year, usually associated with attendance at day-care centres or nurseries. The symptoms of rhinorrhoea, sore throat, cough, fever, malaise and myalgia are well-known and last up to 7 days, although a lingering mucopurulent nasal discharge may persist for at least 2 weeks. In infants, onset is more likely to be associated with a high fever, irritability and nasal obstruction affecting feeding and sleep.
The common cold is caused by one of more than 200 viral types and occasionally by other infectious agents (Table 1). The most common agent, rhinovirus, responsible for 25–40% of cases of the common cold is an non-enveloped 30 nm RNA virus belonging to the Picornaviridae family.
Table 1.
Prevalence of viruses associated with the common cold (modified from Treanor and Hayden79).
| Virus |
Estimated % cases of colds annually |
|---|---|
| Rhinovirus | 40 |
| Coronavirus | 10 |
| RSV | 10–15 |
| Parainfluenza virus | 10–15 |
| Influenza virus | 10–15 |
| Others | 5 |
| Unspecified | 20–30 |
Controversies in management of the common cold
Few conditions in medicine have so many ineffective treatment options! Studies evaluating treatments for the common cold include symptomatic treatments, pharmacological agents and antiviral agents performed in naturally occurring or experimentally induced infection. The majority of these studies have been carried out in adults and relied on subjective assessment of symptom severity or objective measures that require considerable co-operation from the subject. In children where assessing symptoms and obtaining objective measurement is more problematic, this may lead to an inability to demonstrate treatment differences.
Decongestant and antihistamine preparations for the common cold
Decongestants and antihistamines either alone or in combination are widely used in children with common cold symptoms. Kogan et al showed that almost 40% of preschool children received OTC cold medications within the previous month, the majority of which were antihistamine/decongestant combinations1. Several studies of combined antihistamine/decongestant preparations have failed to demonstrate any effect on symptoms of the common cold in the preschool age group, although their sedative effect may be perceived as desirable by parents coping with a young child with a common cold2. First generation antihistamines alone have shown favourable effects upon nasal symptoms in adult studies, but this has not been replicated in children. Nasal decongestants improve cold symptoms in adults and increase nasal patency in children, but their potential side-effects such as rebound obstruction and nasal epithelial drying mean that they are not recommended in children3. The use of such medications should be questioned as they appear to offer no protection against the development of otitis media, side-effects are not uncommon and accidental ingestion can occur.
Antibiotic use for the common cold
There is no evidence that antimicrobial agents alter the course or outcome of the common cold and their use is not an effective strategy to prevent complications such as lower respiratory tract infection4. Antibiotic use is potentially harmful causing a significant increase in side-effects and, by increasing colonisation with resistant organisms, with the risk that any subsequent invasive infection will be unresponsive to standard antibiotic regimens5. Several cross-sectional studies have shown the likelihood of culturing a resistant strain of Pneumococcus from the nasopharynx is increased if a patient has recently completed a course of antibiotics5. Mucopurulent rhinitis frequently accompanies the common cold and may persist for at least 2 weeks, but is unaffected by antibiotics6. Despite these consistent findings, up to 44% of children with common cold symptoms are given antimicrobial agents accounting for 6.5 million prescriptions annually in the US7. Characteristics of parents who request antibiotics for their child's cold symptoms are that they are more likely to report severe symptoms, to believe that symptomatic therapy helps cold symptoms, or to be confident that they knew how to treat a cold. Such beliefs may be amenable to parent education in order to reduce antibiotic use in primary care where opportunities also exist to discuss prevention, including immunisation against influenza and the avoidance of environmental tobacco smoke8.
Other therapies for the common cold
Numerous articles have assessed the role of ascorbic acid (vitamin C) in the treatment and prevention of the common cold. While there is no evidence that daily supplementation with large doses of vitamin C prevents colds, for individuals with established cold symptoms, there appears to be modest (8–9%) reduction in symptom days with larger doses producing greater benefits9.
Zinc has been shown to possess anti-viral properties in vitro and zinc lozenges have been proposed for treatment of the common cold. While two studies in adults have reported a positive effect on the duration and severity of common cold symptoms, heterogeneity of data in these and 5 further studies which showed no evidence of benefit has limited the conclusions that can be drawn10. A further community-based study in school-aged children showed zinc lozenges to be ineffective in reducing symptoms or hastening resolution of coryzal symptoms and their lack of palatability and side-effects were significant in the treated group11.
Extracts of the plant Echinacea are widely used in some parts of Europe and the US to treat upper respiratory infections. The majority of available studies have been conducted in adults and report positive results both in the prevention and treatment of upper respiratory infection, but there are significant variations both in Echinacea preparations and in methodological quality of trial data and therefore evidence for efficacy of Echinacea is inconclusive12,13.
Cough in children
Cough is very common in children, occurring acutely in up to 83% of individuals within the first 48 h of cold symptoms and is self-limiting. There is no evidence that the wide-spread use of OTC preparations for acute cough in association with URI is beneficial14. Similarly, antibiotics are no more effective than placebo in resolving acute cough in children15. Families of children with recurrent episodes of cough frequently seek medical attention from primary care physicians and paediatricians because the symptom is distressing and adversely affects the quality of life of both child and their parents. While a persistent moist or productive cough requires investigation, isolated non-productive cough in the absence of evidence of airway obstruction or other evidence of systemic disease may not be abnormal. However, there is a tendency for clinicians to label non-specific cough as due to post-nasal drip, gastro-oesophageal reflux and, increasingly, due to cough-variant asthma. There is evidence to suggest that persistent cough in children is more likely to be related to exposure to indoor or outdoor atmospheric pollution than to atopy16.
Cough and asthma
McKenzie17 and Chang18 have highlighted the difficulties in diagnosing asthma on the basis of cough alone. Whereas cough has previously been under-recognised as a symptom of asthma, asthma in children is increasingly now diagnosed on the basis of cough in the absence of wheeze, and this has contributed to the recently observed increased prevalence of asthma. Such observations are based on adult data suggesting that individuals with asthma may present with cough alone19, while in hospital-based studies, cough has been reported to be the commonest presenting feature of asthma in children20. There are a number of difficulties inherent in using the symptom of cough as an outcome measure in epidemiological studies of respiratory illness in children. The repeatability of reporting of cough is poor, and several studies demonstrate that parental reports of cough frequency in their child, particularly nocturnal cough, are inaccurate when compared to objective measures12. While night cough in children disturbs their parent's sleep, it seems to cause little reduction in quality of children's sleep22. Similarly, the use of diary cards to report nocturnal cough frequency are inaccurate21.
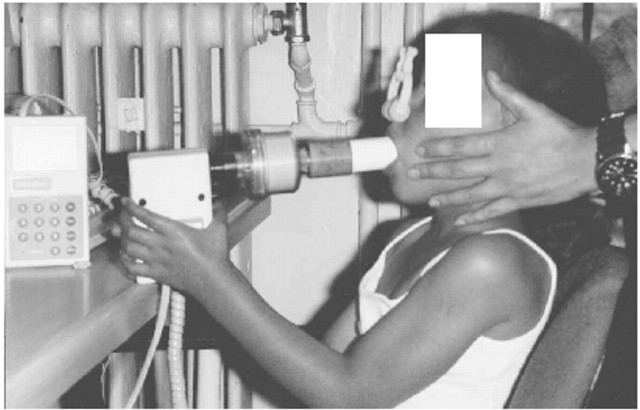
Several epidemiological studies have suggested that children with recurrent cough more closely resemble asymptomatic children than asthmatics in terms of atopic status, lung function, airway responsiveness and family history of allergy23–25. The majority of such children show spontaneous resolution in cough over time24,26. Airways resistance measured by the interrupter technique in preschool children did not differ significantly between coughers and control subjects27 (Fig. 1), while Brooke and colleagues demonstrated that 56% of children identified as having recurrent cough in the pre-school period were symptom-free at follow-up after 2–4 years with 37% reporting continued cough. Importantly, while 7% of children with recurrent cough reported wheeze at follow-up, a similar proportion to a previously symptom-free control group also reported symptoms suggesting asthma28. Therefore, previous clinical studies of children with persistent cough which have suggested that a significant number progress to develop asthma, who have not included an asymptomatic group, may have incorrectly assumed that such children represent cough-variant asthma29.
Fig 1.
Photograph of the interrupter technique (RINT) in use to measure airway resistance in a young child. Picture courtesy of Dr SA McKenzie of the Great Ormond Street Hospital for Children, London, UK, with permission of the child's mother.
While children with persistent or recurrent cough are more likely to use asthma medications as a result of an increased doctor diagnosis of asthma23,28, previous studies that have promoted asthma medication for cough did not use placebo or include objective measures of cough20,31. Chang and colleagues have shown that inhaled salbutamol or beclomethasone in moderate dose is not superior to placebo in reducing subjective or objective measures of cough in the absence of airway obstruction32. Short-course, high-dose, inhaled steroids may be indicated in some children with persistent nocturnal cough. Davies et al demonstrated that while children with persistent cough improve in 2 weeks on placebo, 17 of 24 subjects on inhaled corticosteroid and 8 of 23 on placebo improved objective measures of cough frequency by 75% indicating modest benefit33. Previous studies on cough and asthma suggest that cough associated with asthma should significantly reduce within 1 week of commencing treatment20,31, while suggestions that inhaled steroids should be used for prolonged periods in cough are anecdotal. It is important to stop such treatment if not beneficial and not to increase the dose18.
The relationship between cough, cough receptor sensitivity and airways hyper-reactivity
Although it seems clear that a proportion of children with asthma can present with cough, and cough may be a significant symptom in exacerbations of asthma, recurrent cough in the absence of wheeze differs in important respects from asthma. Although there may be a common trigger, there is good evidence to show that the mechanisms of cough and bronchoconstriction are different and can be separately inhibited21. These findings are supported by clinical studies demonstrating no correlation between cough severity and airway calibre28,34, while in children with asthma there appears to be no relationship between cough receptor sensitivity (CRS) and lung function in the acute and recovery phase18.
In children, there is a temporal relationship between heightened CRS and cough18. Coughers may have either increased CRS or airways hyper-reactivity (AHR) which return to normal values when asymptomatic. Asthmatics with cough as a major symptom may also have increased CRS during exacerbations while CRS during the stable phase of asthma is not increased. Therefore, some children without diagnosed asthma may present with cough as a consequence of heightened CRS, but do not have evidence of airway obstruction. A subset of these children may also have increased AHR while coughing20, but are no more likely to respond to inhaled bronchodilator or corticosteroid treatment than children without AHR19,21. Most children with cough alone are more likely to have heightened cough receptor sensitivity than asthma.
Management of pharyngitis and tonsillitis
The diagnosis and optimum management of pharyngitis or tonsillitis, usually described as sore throat, is controversial and results in a significant use of health service resources. Although largely self-limiting, sore throat is a significant cause of unacceptable morbidity, school absenteeism and loss of earnings in parents and results in a high consultation rate in general practice. While no population studies have been recently undertaken in the UK to assess prevalence or natural history of the condition, in Scotland, acute tonsillitis was the eighth most common presentation in primary care in 1996, a rate of almost 1 in 30. Based upon a cost of £10.00 per consultation, this equates to a cost to the NHS of approximately £60 million per annum. A guideline document produced by the Scottish Intercollegiate Guidelines Network on the management of acute and recurring sore throat in December 2000 and endorsed by the Royal College of Paediatrics and Child Health forms the basis to the following review35,36.
Diagnosis of sore throat
The mode of clinical presentation of sore throat does not reliably predict the aetiologic agent. While a number of scoring systems have been developed which combine clinical and epidemiological findings to differentiate between a bacterial and viral aetiology, the results are conflicting and none accurately identify children with sore throat who require treatment37–39.
A number of studies have suggested that diagnosis of group A β-haemolytic Streptococcus (GABHS) pharyngitis should be based upon the results of throat culture or an antigen-detection test with culture back-up40. However, culture of a throat swab specimen is neither sensitive nor specific for serologically confirmed infection. Results of throat culture correlate poorly with clinical symptoms while a high asymptomatic carriage rate of up to 40% in the general population makes interpretation difficult. Rapid antigen tests for GAHBS are commonly used in the US to aid clinical management of sore throat. While some studies have reported the sensitivity of antigen-detection tests to be around 90%, such tests have proved less sensitive in routine clinical practice although specificity may be as high as 88–100%41,42. In a UK study, the rapid antigen test showed little difference in sensitivity when compared to clinical assessment of sore throat and did not significantly change prescribing practice in primary care43.
Management of sore throat
The majority of children with acute sore throat will only require symptomatic treatment with simple analgesia. Most will have a viral aetiology, particularly in children under the age of 3 years; it is, therefore, not rational to treat all acute sore throats with antibiotics40,44. It has been suggested that the rationale for antibiotic treatment of sore throat is based upon the premise that prompt elimination of GABHS is effective in preventing acute rheumatic fever and in addition will prevent suppurative complications, lead to more rapid resolution of symptoms and prevent spread of infection. While outbreaks of rheumatic fever have been reported in children in the US, the incidence is extremely low in the UK and there is no evidence to support routine antibiotic treatment of acute sore throat to prevent rheumatic fever or to prevent suppurative complications40. Penicillin has been shown to have a significant, though relatively small, benefit in providing symptomatic improvement in children with severe symptoms of sore throat, but is not recommended for routine symptomatic relief in all cases of acute childhood sore throat35,36. Routine use of antibiotics enhances patient belief in antibiotics and increases re-attendance for future minor and self-limiting illness44,45.
There is no evidence to support the use of antibiotics for recurrent sore throat not due to GABHS. In cases of recurrent sore throat associated with isolation of GABHS, there is some limited evidence to suggest that a 10-day course of an antibiotic may reduce the number and frequency of episodes. While a 10-day course of oral penicillin V given 4 times daily is regarded as standard treatment40, several studies have demonstrated a superior effect in eradicating GABHS using a cephalosporin, macrolides or clindamycin35. Advantages of such antibiotics include the use of a shorter course and a more convenient once or twice daily dose schedule, although this needs to be weighed against the increased cost of such antibiotics and the risk of development of GABHS resistance40.
Indications for tonsillectomy in children
Tonsillectomy has a significant peri-operative morbidity with a complication rate of approximately 1–2%. However, a number of studies suggest benefit in childhood, not only in reducing frequency of sore throats but also in general health, including growth46. Therefore, an observation period of 6 months is recommended prior to tonsillectomy to establish the pattern of symptoms and allow the patient and parents to appreciate fully the implication of surgery. If a child with confirmed sore throats due to tonsillitis differentiated from generalised pharyngitis on the basis of history and clinical examination, experiences 6–7 recurrences over 1–2 years despite antibiotic treatment, and symptoms are causing significant morbidity, tonsillectomy should be considered35,36,47. An alternative for individuals who are unwilling or unable to consider surgery is the use of clindamycin48.
Controversies in otitis media (OM)
There are a number of controversial areas in the management of acute otitis media (AOM), recurrent otitis media (recurrent OM) and otitis media with effusion (OME), including antibiotic use in the light of emerging pneumococcal resistance, and indications for surgical management of OME. New developments include the use of pneumococcal conjugate vaccine in the prevention of OM. It is also important to consider the aetiology of OM in order to inform educational strategies for parents in the prevention of OM.
The impact of OM on child health is considerable. While there is little published data on the epidemiology of AOM in the UK, it is estimated that approximately 31 children per 1000 under 5 years are seen annually by primary care practitioners with the condition. In the US, OM is the most frequently diagnosed childhood disease constituting 18% of physician visits. Peak incidence occurs in the under 2 years age group, typically between 6–18 months of age, with a prevalence of 17–20%. A further peak in OM incidence occurs at 5 years, co-inciding with school entry49.
Update on aetiology
Child day-care attendance and exposure to other young children including siblings has been established as one of the major risk factors for AOM, recurrent OM and OME49,50, presumably as a result of increased exposure to upper respiratory tract infections. Breast-feeding appears to reduce OM incidence in several studies with a recent meta-analysis reporting a 13% reduction in OM for exclusive breast-feeding lasting for 3–6 months50. However, some studies have failed to control adequately for exposure to cigarette smoke and social class, which are both associated with increased OM incidence. While breast-feeding confers immunological benefits including the reduction of infant nasopharyngeal colonisation with Haemophilus influenzae and Streptococcus pneumoniae, and the presence of breast milk pneumococcal antibodies, these findings are not correlated with the prevention of OM. An alternative explanation for the benefit of breast-feeding in reducing OM incidence may be a mechanical one. It is known that supine bottle feeding is associated with reflux of milk into the middle ear and increased incidence of abnormal tympanograms when compared to upright feeding. Further support to the contribution of mechanical factors in accounting for differing OM prevalence is suggested by a study reporting a 33% lower incidence in OM in infant who did not use a pacifier after the age of 6 months51.
Conflicting data have been published concerning exposure to environmental tobacco smoke and childhood OM, but studies which have used objective markers of environmental tobacco smoke exposure have demonstrated a significant relationship49. A recent systematic review of evidence relating to parental smoking and prevalence of OM and OME demonstrates a causal relationship with an odds ratio of 1.48 for recurrent OM, 1.38 for middle ear effusion while odds ratios for AOM range from 1.0 to 1.652. A further study shows a significant association between in utero exposure to tobacco smoke, AOM and ear surgery by 5 years of age53.
The role of viruses in the aetiology of otitis media
Acute OM is generally considered a bacterial infection, with Strep. pneumoniae, non-typable H. influenzae and Moraxella catarrhalis being the main pathogens. There is now convincing evidence that respiratory viruses play a significant role in the development of this condition (Table 2)54,55. While earlier studies using viral culture or rapid antigen detection methods identified virus infection in 30–50% of nasopharyngeal specimens from children with OM, the development of polymerase chain reaction methodology has increased the detection rate to between 42–90%56. Several studies have documented viruses in middle ear fluid of children with AOM including respiratory syncytial virus (RSV), influenza and parainfluenza, rhinovirus and, less commonly, adenovirus. RSV appears to be the principal agent invading the middle ear, occurring in the middle ear fluid of 74% of children infected by this virus57. Therefore, viral vaccines become potentially important in the prevention of OM in children. The use of inactivated influenza vaccine or live-attenuated intranasal influenza vaccine reduced the incidence of OM by 36% of children, but its benefit is confined to the peak influenza season58,59. The aim of RSV vaccine is to prevent lower respiratory tract illness in infants, but it could also, potentially, have a major effect on the incidence of OM in young children58.
Table 2.
Prevalence of respiratory viruses in middle-ear fluid of 456 children with acute otitis media (modified from Heikkinen et al57).
| Virus | Number of cases | |
|---|---|---|
| Viral infection | Virus in middle-ear fluid | |
| RSV | 65 | 48 (74 %) |
| Parainfluenza virus | 29 | 15 (52%) |
| Influenza virus | 24 | 10 (42%) |
| Enterovirus | 27 | 3 (11%) |
| Adenovirus | 23 | 1 (4%) |
Antibiotic treatment in acute otitis media
Antibiotic use in AOM varies considerably from 31% in The Netherlands to between 86–98% in the UK, Australia and the US, but complication rates do not differ significantly60. Antibiotics appear to play only a small role in resolving symptoms of AOM and their use is associated with increasing resistance in Strep. pneumoniae and H. influenzae61. A recent Cochrane Review, which included 2202 children, showed only limited benefit of antibiotics in AOM with 17 children needing to be treated to prevent one child experiencing some pain after 2 days62. There was no effect of antibiotics on complications or recurrence of AOM62. Antibiotic use in acute OM appeared to confer no significant improvement in the prevalence of hearing deficit or on recurrence rates of acute OM in a recent meta-analysis, but may have been insufficiently powered to demonstrate clinically significant outcomes62–64. The majority of cases of acute OM resolve spontaneously; however, where antibiotics are prescribed in populations thought to be at increased risk of complications, amoxycillin remains the drug of choice65. Uncomplicated AOM may be treated with a shortened 5–7 day antibiotic course, a practice supported by a number of randomised controlled trials66, although short-course therapy may not be appropriate for young children less than 2 years who are at higher risk of treatment failure67. It has been suggested that children under 2 years of age, who are at particular risk of poor outcome from AOM, may be a group who would benefit from aggressive antibiotic treatment. However, current high prescription rates of antibiotics to treat AOM in this age group are not supported by a recent study by Damoiseaux et al, who demonstrated that, while after 4 days of treatment, antibiotics led to resolution of more symptoms, there were no differences between treatment and placebo groups at 11 days and prevalence of middle ear effusion at 6 weeks68,69.
Management of recurrent otitis media
Recurrent OM is defined as more than three episodes of OM in a 12-month period with interval clearing of any middle ear effusion. While prophylactic antibiotics are frequently recommended, supported by studies showing a reduction in the frequency of episodes of AOM, more recent data have shown that antibiotic prophylaxis has only a modest effect on prevention of AOM episodes, resulting in a reduction of slightly more than one episode of AOM per year67,70. These data, together with concerns about increasing pneumococcal resistance, have resulted in a need for the more judicious use of antibiotics in recurrent OM, perhaps confined to infants under 2 years of age and those attending out-of-home child-care. Ventilation tube placement (tympanostomy) reduces the frequency of OM episodes in children with a history of recurrent OM and may be a valid alternative to prophylactic antibiotic therapy or failed prophylaxis, particularly for children with language delay or hearing deficit71.
Management of secretory otitis media including the use of ventilation tube placement (tympanostomy)
Surgical intervention for OM has generated strong debate and there remains no consensus on the indications for tympanostomy. While insertion of tympanostomy tubes has the potential to reduce frequency of infection, to improve hearing and associated speech development and to prevent long-term complications of OM, there remains a possibility of complications including persistent otorrhoea and damage to the tympanic membrane. There are conflicting data on developmental outcome and quality of life measures in children following ventilation tube placement for OME. In a recent population-based study, ventilation tubes did not improve quality of life in young children with persistent OME72. Current indications for tympanostomy tube insertion include OME, recurrent OM and occasionally Eustachian tube dysfunction and for other subgroups of children such as children with Down's syndrome71.
When OME occurs in an asymptomatic child, observation without therapy is recommended, as studies of the natural history of OME have shown that spontaneous resolution occurs in 50% of children by 4 weeks after an episode of acute OM with up to 10% showing persistent effusion at 3 months60. For bilateral effusion which persists for longer than 3 months, intervention with tympanostomy is advised for a hearing loss of greater than 20 dB following a trial of antibiotic therapy with or without oral corticosteroids73.
Use of pneumococcal conjugate vaccines in otitis media
Infection with Strep. pneumoniae is responsible for 28–55% of all acute bacterial OM74, although epidemiological data are lacking from the UK where tympanocentesis is not routinely performed. The current 23-valent pneumococcal polysaccharide vaccine, 23vPS was licensed in the UK in 1983 and is recommended for children greater than 2 years old with asplenia, immunodeficiency and chronic cardiorespiratory or renal disease. However, polysaccharide antigens do not elicit an immune response in young children but, by introducing a protein conjugate coupled to capsular polysaccharide, T-cell help induces an enhanced antibody response and the induction of immunological memory75.
Candidate pneumococcal conjugate vaccines consist of 7, 9 or 11 capsular polysaccharides conjugated to either a non-toxic variant of diphtheria toxin or an outer membrane protein of non-typable H. influenzae. A recent US multicentre study found that serotypes present in the 7-valent vaccine accounted for only 65% of pneumococcal AOM and suggest that the 9- and 11-valent vaccines would be preferable75. Several recent trials from the US and Finland have assessed the efficacy of the 7-valent pneumococcal vaccine in OM when given according to primary immunisation schedules in infants. In a US study, the vaccine was 7%, 9% and 20% effective in preventing clinically diagnosed AOM episodes, recurrent OM and ventilation tube placement, respectively76. Two Finnish trials showed the efficacy of 7vCRM was similar to 7vOMP pneumococcal vaccine. Although efficacy was 56–57% for culture-confirmed vaccine type AOM and 25–34% for all culture-confirmed pneumococcal AOM, effects on clinically diagnosed AOM was either similar or showed a 6% decrease in AOM for vaccine and control groups. Additionally, there was a 34% increase in AOM episodes caused by non-vaccine serotypes in 7vCRM recipients75,77. While pneumococcal vaccines appear safe and immunogenic in infants, they only prevent perhaps 6–7% of clinically diagnosed AOM74. However, this equates to prevention of up to 1.2 million of the 20 million yearly episodes of AOM in the US74. The American Academy of Pediatrics do not recommend routine vaccination of infants with 7PCV vaccine. However, they suggest the vaccine may be beneficial in children 24–59 months old who have not received pneumococcal vaccine previously and who have a history either of recurrent OM or who have AOM complicated by ventilation tube placement78.
Key points for clinical practice
• Antibiotic use in childhood upper respiratory tract infection, pharyngitis and acute otitis media is not routinely indicated
• Cough, in the absence of wheeze, is more likely to be due to increased cough receptor sensitivity during respiratory tract infection than asthma
• The effects of ventilation tube placement (grommets) on developmental outcome and quality of life in young children with persistent secretory otitis media remains controversial and should be considered only after a period of observation of at least 3 months
• New conjugate pneumococcal vaccines show promise in reducing pneumococcal otitis media in young children. However, they currently appear to have only a modest effect on the incidence of clinically diagnosed acute otitis media
Footnotes
Correspondence to:Dr J V West, Children's Services, Leicestershire and Rutland Healthcare Trust, Bridge Park Plaza, Bridge Park Road, Thurmaston, Leicester LE4 8PQ, UK
References
- 1.Kogan MD, Pappas G, Yu SM, Kotelchuck M. Over-the-counter medication use among US pre-school-age children. JAMA 1994; 272: 1025–30 [PubMed] [Google Scholar]
- 2.Clemens CJ, Taylor JA, Almquist JR, Quinn HC, Metha A, Naylor GS. Is an antihistamine-decongestant combination effective in temporarily relieving symptoms of the common cold in pre-school children? J Pediatr 1997: 130: 463–6 [DOI] [PubMed] [Google Scholar]
- 3.Taverner D, Bickford L, Draper M. Nasal decongestants for the common cold (Cochrane Review). In: Oxford: The Cochrane Library, Issue 2, 2000 [DOI] [PubMed]
- 4.Arroll B, Kenealy T. Antibiotics for the common cold (Cochrane Review). In: Oxford: The Cochrane Library, Issue 2, 2000 [DOI] [PubMed]
- 5.Dowell SF, Marcy SM, Phillips WR, Gerber MA, Schwartz B. Principles of judicious use of antimicrobial agents for pediatric upper respiratory tract infections. Pediatrics 1998; 101: 163–5 [Google Scholar]
- 6.Rosenstein N, Phillips WR, Gerber MA, Marcy SM, Schwartz B, Dowell SF. The common cold. Judicious use of antimicrobial agents. Pediatrics 1998; 101: 181–4 [Google Scholar]
- 7.Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections and bronchitis. JAMA 1998; 11: 875–7 [DOI] [PubMed] [Google Scholar]
- 8.Braun BL, Fowles JB. Characteristics and experiences of parents and adults who want antibiotics for cold symptoms. Arch Fam Med 2000; 9: 596–7 [DOI] [PubMed] [Google Scholar]
- 9.Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold (Cochrane Review). In: Oxford: The Cochrane Library, Issue 2, 2000 [DOI] [PubMed]
- 10.Marshall I. Zinc for the common cold (Cochrane Review). In: Oxford: The Cochrane Library, Issue 2, 2000 [DOI] [PubMed]
- 11.Janosky J, Wald E. Zinc lozenges for treating the common cold in children: a randomised controlled trial. JAMA 1998; 279: 1962–7 [DOI] [PubMed] [Google Scholar]
- 12.Giles JT, Palat CT, Chein SH, Chang ZG, Kennedy DT. Evaluation of Echinacea for treatment of the common cold. Pharmacotherapy 2000; 20: 690–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold (Cochrane Review). In: Oxford: The Cochrane Library, Issue 2, 2000 [DOI] [PubMed]
- 14.Anonymous. Cough medications in children. Drug Ther Bull 1999; 37: 19–21 [DOI] [PubMed] [Google Scholar]
- 15.O'Brien, Dowell SF, Schwartz B, Marcy M, Phillips WR, Gerber MA. Cough illness/bronchitis – principles of judicious use of antimicrobial agents. Pediatrics 1998; 101: 178–81 [Google Scholar]
- 16.Chen Y, Rennie DC, Lockinger LA, Dosman JA. Effect of environmental tobacco smoke on cough in children with a history of tonsillectomy or adenoidectomy. Eur Respir J 1998; 11: 1319–23 [DOI] [PubMed] [Google Scholar]
- 17.McKenzie S. Cough – but is it asthma? Arch Dis Child 1994; 70: 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang AB. Cough, cough receptors and asthma in children. Pediatr Pulmonol 1999; 28: 59–70 [DOI] [PubMed] [Google Scholar]
- 19.Thiadens HA, de Bock GH, Dekker FW et al Identifying asthma and chronic obstructive pulmonary disease in patients with persistent cough presenting to general practitioners: descriptive study. BMJ 1998; 316: 1286–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cloutier MM, Loughlin GM. Chronic cough in children: a manifestation of airway hyperreactivity. Pediatrics 1981; 67: 6–12 [PubMed] [Google Scholar]
- 21.Chang AB. Isolated cough: probably not asthma. Arch Dis Child 1999; 80: 210–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller P, Picciotto A, Davies M, McKenzie SA. Cough and sleep in inner-city children. Eur Respir J 1998; 12: 426–31 [DOI] [PubMed] [Google Scholar]
- 23.Faniran AO, Peat JK, Woolcock AJ. Persistent cough: is it asthma? Arch Dis Child 1998; 79: 411–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright AL, Holberg CJ, Morgan WJ, Taussig LM, Halonen M, Martinez FD. Recurrent cough in childhood and its relation to asthma. Am J Respir Crit Care Med 1996; 153: 1259–65 [DOI] [PubMed] [Google Scholar]
- 25.Ninan TK, Macdonald L, Russell G. Persistent nocturnal cough in childhood: a population based study. Arch Dis Child 1995; 73: 403–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis HM, Haeney M, Jeacock J, Thomas H. Chronic cough in a hospital population; its relationship to atopy and defects in host defence. Arch Dis Child 1989; 64: 1593–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenzie SA, Bridge PD, Healy MJ. Airways resistance and atopy in pre-school children with wheeze and cough. Eur Respir J 2000; 15: 833–8 [DOI] [PubMed] [Google Scholar]
- 28.Brooke AM, Lambert PC, Burton PR, Clarke C, Luyt DK, Simpson H. Recurrent cough; natural history and significance in infancy and early childhood. Pediatr Pulmonol 1998; 26: 256–61 [DOI] [PubMed] [Google Scholar]
- 29.Spelman R. Two-year follow up of the management of chronic or recurrent cough in children according to an asthma protocol. Br J Gen Pract 1991; 41: 406–9 [PMC free article] [PubMed] [Google Scholar]
- 30.Picciotto A, Hubbard M, Sturdy P, Naish J, McKenzie SA. Prescribing for persistent cough in children. Respir Med 1998; 92: 638–41 [DOI] [PubMed] [Google Scholar]
- 31.Hannaway PJ, Hopper GD. Cough variant asthma in children. JAMA 1982; 247: 206–8 [PubMed] [Google Scholar]
- 32.Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Arch Dis Child 1998; 79: 6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies MJ, Fuller P, Picciotto A, McKenzie SA. Persistent nocturnal cough: randomised controlled trial of high dose inhaled corticosteroid. Arch Dis Child 1999; 81: 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoskyns EW, Beardsmore CS, Simpson H. Chronic night cough and asthma severity in children with stable asthma. Eur J Pediatr 1995; 154: 320–5 [DOI] [PubMed] [Google Scholar]
- 35.Scottish Intercollegiate Guidelines Network. Management of acute and recurring sore throat and indications for tonsillectomy. Edinburgh: SIGN, 1999; www.sign.ac.uk
- 36.Royal College of Paediatrics and Child Health. Guidelines for good practice: management of acute and recurring sore throat and indications for tonsillectomy. London: RCPH, 2000
- 37.Woods WA, Carter CT, Schager TA. Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care 1999; 15: 338–40 [DOI] [PubMed] [Google Scholar]
- 38.Wald ER, Green MD, Schwartz B, Barbadora K. A streptococcal score card revisited. Pediatr Emerg Care 1998; 14: 109–11 [DOI] [PubMed] [Google Scholar]
- 39.McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ 1998; 158: 75–83 [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz B, Marcy SM, Phillips WR, Gerber MA, Dowell SF. Pharyngitis – principles of judicious use of antibiotics. Pediatrics 1998; 101: 171–4 [Google Scholar]
- 41.Pitetti RD, Drenning SD, Wald ER. Evaluation of a new rapid antigen detection kit for group A beta-haemolytic streptococci. Pediatr Emerg Care 1998; 14: 396–8 [DOI] [PubMed] [Google Scholar]
- 42.Dagnelie CF, Bartelink ML, van der Graaf Y, Goessens W, de Melker RA. Towards a better diagnosis of throat infections (with group A beta-haemolytic streptococcus) in general practice. Br J Gen Pract 1998; 48: 959–62 [PMC free article] [PubMed] [Google Scholar]
- 43.Burke P, Bain J, Lowes A, Athersuch R. Rational decisions in managing sore throat: evaluation of a rapid test. BMJ 1998; 296: 1646–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997; 314: 722–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warner G, Gantley M, Kinmonth AL. Re-attendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997; 15: 350–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paradise JL, Bluestone CD, Bachman RZ et al Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomised and non-randomised clinical trials. N Engl J Med 1984; 310: 674–83 [DOI] [PubMed] [Google Scholar]
- 47.Pichichero ME. Sore throat after sore throat after sore throat. Are you askng the critical questions? Postgrad Med 1997; 101: 205–6, 209,–12, 215–8 [DOI] [PubMed] [Google Scholar]
- 48.Orrling A, Stjernquist-Desatnik A, Schalen C. Clindamycin in recurrent group A streptococcal pharyngotonsillitis – an alternative to tonsillectomy? Acta Oto-Laryngol 1997; 117: 618–22 [DOI] [PubMed] [Google Scholar]
- 49.Daley KA, Geibank GS. Clinical epidemiology of otitis media. Pediatr Infect Dis 2000; 19: S31–6 [DOI] [PubMed] [Google Scholar]
- 50.Uhari M, Mantysaari K, Neimela M. A meta-analytic review of the risk factors for acute otitis media. Clin Infect Dis 1996; 22: 1079–83 [DOI] [PubMed] [Google Scholar]
- 51.Niemela M, Pihakari O, Pokka T, Uhari M, Uhari M. Pacifier as a risk factor for acute otitis media: a randomised, controlled trial of parental counselling. Pediatrics 2000; 106: 483–8 [DOI] [PubMed] [Google Scholar]
- 52.Strachan DP, Cook DG. Health effects of passive smoking. 4. Parental smoking, middle ear disease and adenotonsillectomy in children. Thorax 1998; 53: 50–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stathis SL, O'Callaghan DM, Williams GM, Najman JM, Andersen MJ, Bor W. Maternal cigarette smoking during pregnancy is an independent predictor for symptoms of middle ear disease at five years post delivery. Pediatrics 1999; 104: e16. [DOI] [PubMed] [Google Scholar]
- 54.Ramilo O. Role of respiratory viruses in acute otitis media: implications for management. Pediatr Infect Dis J 1999; 18: 1125–9 [DOI] [PubMed] [Google Scholar]
- 55.Heikkinen T, Chonmaitree T. Increasing importance of viruses in acute otitis media. Ann Med 2000; 32: 157–63 [DOI] [PubMed] [Google Scholar]
- 56.Heikkinen T. Role of viruses in the pathogenesis of acute otitis media. Pediatr Infect Dis J 2000; 19: 817–23 [DOI] [PubMed] [Google Scholar]
- 57.Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med 1999; 340: 260–4 [DOI] [PubMed] [Google Scholar]
- 58.Eskola J, Hovi T. Respiratory viruses in acute otitis media. N Engl J Med 1999; 340: 312–3 [DOI] [PubMed] [Google Scholar]
- 59.Belshe RB, Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr Infect Dis J 2000; 19: S66–71 [DOI] [PubMed] [Google Scholar]
- 60.Bitnun A, Allen UD. Medical therapy of otitis media: use, abuse, efficacy and morbidity. J Otolaryngol 1998; 27 (Suppl 2): 26–36 [PubMed] [Google Scholar]
- 61.Jacobs MR. Increasing antibiotic resistance among otitis media pathogens and their susceptibility to oral agents based on pharmacodynamic parameters. Pediatr Infect Dis J 2000; 19: S47–56 [DOI] [PubMed] [Google Scholar]
- 62.Glasziou PP, Del Mar CB, Sanders SL. Antibiotics for acute otitis media in children (Cochrane Review). In: Oxford: The Cochrane Library, Issue 3, 2001 [DOI] [PubMed]
- 63.Rosenfeld RM, Vertrees JE, Carr J et al Clinical efficacy of antimicrobial drugs for acute otitis media – meta-analysis of 5400 children from thirty-three randomised trials. J Pediatr 1994; 124: 355–66 [DOI] [PubMed] [Google Scholar]
- 64.Del Mar CB, Glasziou PP, Hayem M. Are antibiotics indicated as initial treatment for acute otitis media? BMJ 1997; 314: 1526–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aronovitz GH. Antimicrobial therapy of acute otitis media: review of treatment recommendations. Clin Ther 2000; 22: 29–39 [DOI] [PubMed] [Google Scholar]
- 66.Kozyrskyj AL, Hildes-Ripstein GE, Longstaffe SE et al Short course antibiotics for acute otitis media (Cochrane Review). In: Oxford: The Cochrane Library, Issue 2, 2000 [DOI] [PubMed]
- 67.Dowell SF, Marcy SM, Phillips WR, Gerber MA, Schwartz B. Otitis media – principles of judicious use of antimicrobial agents. Pediatrics 1998; 101: 165–71 [Google Scholar]
- 68.Damoiseaux RA, van Balen FA, Hoes AW, De Melker RA. Antibiotic treatment of acute otitis media in children under two years of age: evidence based? Br J Gen Pract 1998; 48: 1861–4 [PMC free article] [PubMed] [Google Scholar]
- 69.Damoiseaux RA, van Balen FA, Hoes AW, Verheij TJ, de Melker RA. Primary care based randomised, double blind trial of amoxycillin versus placebo for acute otitis media in children under 2 years. BMJ 2000; 320: 350–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein JO. Nonimmune strategies for prevention of otitis media. Pediatr Infect Dis J 2000; 19: S89–92 [DOI] [PubMed] [Google Scholar]
- 71.Pransky SM. Surgical strategies for otitis media. J Otolaryngol 1998; 27 (Suppl 2): 37–42 [PubMed] [Google Scholar]
- 72.Rovers MM, Krabbe PFM, Straatman H, Ingels K, van der Wilt G-J, Zeilhuis GA. Randomised controlled trial of the effect of ventilation tubes (grommets) on quality of life at age 1–2 years. Arch Dis Child 2001; 84: 45–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stool SE, Berg AO, Berman S et al Managing otitis media with effusion in young children, Quick reference guide for clinicians. AHCPR Publication No. 94-0623. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, 1994
- 74.Eskola J, Kilpi T. Potential of bacterial vaccines in the prevention of acute otitis media. Pediatr Infect Dis J 2000; 19: 72–8 [DOI] [PubMed] [Google Scholar]
- 75.Choo S, Finn A. New pneumococcal vaccines for children. Arch Dis Child 2001; 84: 289–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black S, Shinefield H, Fireman B et al Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J 2000; 19: 187–95 [DOI] [PubMed] [Google Scholar]
- 77.Eskola J, Kilpi T, Palmu A et al Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001; 344: 403–9 [DOI] [PubMed] [Google Scholar]
- 78.American Academy of Pediatrics. Committee on Infectious Diseases. Policy Statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine, pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 2000; 106: 362–6 [DOI] [PubMed] [Google Scholar]
- 79.Treanor JJ, Hayden FG. Viral infections. In: Murray JF, Nadel JA, Mason R, Boushey HA. (eds) Textbook of Respiratory Medicine, 3rd edn. Philadelphia, PA: WB Saunders, 2000