ABSTRACT
Mannan-containing products are capable of modulating immune responses in animals. However, different products may have diverse immunomodulation. The experiment was conducted to examine effects of mannan oligosaccharide (Actigen; ACT) on growth performance and serum concentrations of antibodies and inflammatory mediators in weanling pigs (Sus scrofa) experimentally infected with porcine reproductive and respiratory syndrome virus (PRRSV). A total of 32 PRRSV-negative pigs (3 wk old) were randomly assigned from within blocks to 1 of 4 treatments in a 2 by 2 factorial arrangement [2 types of diet: control (0%) and ACT addition (0.04%); and with and without PRRSV] in a randomized complete block design. Pigs were blocked by initial BW within sex. Ancestry was equalized across treatments. Pigs (8/treatment) were kept individually in each pen. After 2 wk of an 8-wk period of feeding the treatments, pigs received an intranasal inoculation of PRRSV or sham medium at 5 wk of age. Infection by PRRSV decreased ADG, ADFI, and G:F throughout the experiment (P < 0.01). Actigen did not affect ADG (P = 0.450), but decreased (P = 0.047) ADFI from 28 to 42 days postinoculation (DPI). During that time, ACT improved G:F in infected pigs but not in sham controls (interaction, P = 0.009). Dietary ACT did not affect viremia in infected pigs (P > 0.05), but increased PRRSV-specific antibody titer at 35 DPI (P = 0.042). Infection with PRRSV induced the febrile responses of pigs from 3 to 10 DPI (P < 0.001) with return to normal at 14 DPI. During the experimental period, the rectal temperature of pigs was found slightly elevated by ACT (P = 0.045). Infected pigs had greater serum concentrations of IL-1β, tumor necrosis factor (TNF)-α, IL-12, interferon (IFN)-γ, IL-10, and haptoglobin (Hp) than sham controls (P < 0.001). These results indicate that PRRSV stimulated secretion of cytokines involved in innate, T-helper 1, and T-regulatory immune responses. Actigen tended to decrease the serum TNF-α concentration regardless of PRRSV (P = 0.058). The ACT × PRRSV interaction was significant for IL-1β (P = 0.016), IL-12 (P = 0.026), and Hp (P = 0.047), suggesting that infected pigs fed ACT had greater serum concentrations of these mediators than those fed the control. The increases in IL-1β and IL-12 may favorably promote innate and T-cell immune functions in infected pigs fed ACT. Feeding ACT may be useful as ACT is related to increased PRRSV antibody titers and G:F in infected pigs at certain times during infection.
Keywords: actigen, feed efficiency, immune response, mannan, pigs, porcine reproductive and respiratory syndrome virus
INTRODUCTION
Porcine reproductive and respiratory syndrome (PRRS) is an infectious disease caused by PRRS virus (PRRSV) and characterized by reproductive disorders in pregnant sows and respiratory problems in pigs of various ages. The disease is presently a serious concern for the swine industry worldwide and causes a significant loss to swine producers (Neumann et al., 2005; Dietze et al., 2011). Weak initial innate immune response and inefficiency of acquired immunity greatly contribute to persistent or repeated infections in susceptible pigs and herds, and to a some extent, these weakened immune responses may predispose for secondary bacterial co-infections (Mateu and Diaz, 2008; Jung et al., 2009). Thus, apart from application of other methods to heighten the overall health status of the herd, use of feed ingredients or feed additives including spray-dried animal plasma, direct-fed microbials, plant extracts, and mannan oligosaccharide (MOS) has been suggested (Turner et al., 2001; Pettigrew, 2006).
Products of MOS have been demonstrated to be capable of positively modulating immune responses in animals (Davis et al., 2004; Che et al., 2011). However, different products extracted from the yeast cell wall may have diverse immune-related properties because each fraction differs in polymerization degree of mannan, types of terminal linkages of mannan sequences, structure, and proportion of mannan and β-glucan (Young et al., 1998; Bland et al., 2004; Sheng et al., 2006). Therefore, evaluation of effect of each specific MOS product on the immune responses of the host to certain pathogens is necessary because outcome responses, such as performance and disease resistance, may be altered because of alteration of immunomodulation.
The objective of this experiment was to examine the effects of MOS (Actigen; ACT) on growth performance and serum concentrations of antibodies and inflammatory mediators in weanling pigs experimentally infected with PRRSV.
MATERIALS AND METHODS
The experimental protocol was approved by the University of Illinois Institutional Animal Care and Use Committee and the Institutional Biosafety Committee.
Experimental Design, Housing, and PRRSV Challenge
Before commencement of the experiment and PRRSV inoculation, serum samples were collected from pigs at 1 and 5 wk of age to verify if pigs were PRRSV-negative by serological and quantitative real-time reverse-transcription-PCR (qRT-PCR) tests. No PRRSV-specific antibodies or viruses were detected. Also, pigs were confirmed to be negative for Mycoplasma hyopneumoniae and swine influenza virus. A total of 32 weaned pigs [3-wk-old; 6.3 ± 0.6 kg BW; Pig Improvement Company (PIC) line C-22 female × PIC line 337 male], free of PRRSV, were transported from a University research farm to the experimental site, and upon arrival they were placed in disease-containment chambers. Each pig received a daily intramuscular injection of Lincomycin (11 mg/kg of BW; Pharmacia and Upjohn Co., Kalamazoo, MI) for 3 consecutive days after arrival to prevent infections. Pigs were blocked on the basis of initial BW within sex into 4 BW blocks, resulting in a total of 8 blocks. They were randomly assigned from within the same BW block to 1 of 4 treatments in a 2 by 2 factorial arrangement [2 types of diet: control (0%) and ACT supplementation (0.04%); and with and without PRRSV] in a randomized complete block design. Actigen (Alltech, Inc., Nicholasville, KY), a concentrated mannose-rich oligosaccharide fraction, was derived from the cell wall of yeast Saccharomyces cerevisiae. Ancestry was equalized across treatments for all measurements throughout the experimental period.
Pigs inoculated with PRRSV were housed in 1 room and those not inoculated with PRRSV were reared in the other room to avoid cross-contamination. Pigs were penned individually in disease-containment chambers with controlled temperature and a lighting regimen of 18-h light/6-h dark. Chamber temperature was maintained at 32°C for the first 2 wk after pigs arrived, then reduced 2°C each week until the temperature reached 24°C. Containment chambers were separately ventilated with negatively pressurized HEPA-filtered air. Each room contained 8 disease-containment chambers, each of which had 2 pens. A pen measured 0.6 × 1.4 m in floor area and had a plastic-coated expanded-metal floor. There was a self-feeder and nipple waterer in each pen, and pigs had access to feed and water at all times. The basal diets (Table 1) were formulated to contain all of the essential nutrients, which met or exceeded nutritional requirements of pigs (NRC, 1998). Treatment diets were formulated by supplementing the basal diets with 0.04% ACT throughout the 8-wk experimental period. This supplemental amount of ACT was recommended by the manufacturer (Alltech, Inc., Nicholasville, KY).
Table 1.
Composition of basal diets fed to weanling pigs during the experiment (as-fed basis)1
| Phase2 | ||||
|---|---|---|---|---|
| Item | I | II | III | IV |
| Ingredients, % | ||||
| Corn | 38.46 | 43.61 | 58.08 | 68.66 |
| Dried whey | 16.00 | 14.00 | 10.00 | 0.00 |
| Soybean meal, 48% | 10.00 | 18.00 | 26.00 | 27.05 |
| Spray-dried animal plasma | 6.00 | 3.00 | 0.00 | 0.00 |
| Soy protein concentrate3 | 5.00 | 3.00 | 0.00 | 0.00 |
| Select menhaden fish meal | 8.58 | 7.04 | 3.12 | 0.00 |
| Soybean oil | 3.00 | 3.00 | 0.00 | 0.00 |
| Fat, choice white grease | 0.00 | 0.00 | 0.00 | 0.94 |
| Lactose | 9.80 | 5.46 | 0.00 | 0.00 |
| Limestone | 0.26 | 0.19 | 0.91 | 1.12 |
| Dicalcium phosphate | 0.93 | 1.40 | 0.00 | 0.00 |
| Monocalcium phosphate | 0.00 | 0.00 | 0.82 | 1.17 |
| Zinc oxide | 0.42 | 0.42 | 0.00 | 0.00 |
| Mineral premix4 | 0.35 | 0.35 | 0.35 | 0.35 |
| Vitamin premix5 | 0.20 | 0.20 | 0.20 | 0.20 |
| Lysine-HCl | 0.52 | 0.18 | 0.32 | 0.31 |
| DL-Met | 0.48 | 0.08 | 0.07 | 0.06 |
| L-Thr | 0.00 | 0.07 | 0.14 | 0.14 |
| Calculated composition | ||||
| ME, Mcal/kg | 3.45 | 3.45 | 3.45 | 3.45 |
| SID lysine, % | 1.50 | 1.45 | 1.30 | 1.15 |
| Ca, % | 0.90 | 0.90 | 0.80 | 0.80 |
| Available P, % | 0.55 | 0.55 | 0.40 | 0.40 |
| Lactose, % | 21.00 | 14.00 | 7.00 | 0.00 |
| Analyzed composition, % | ||||
| Moisture | 11.14 | 10.59 | 12.22 | 14.67 |
| CP | 22.69 | 21.92 | 19.30 | 16.96 |
| Crude fat | 5.07 | 4.92 | 2.47 | 2.88 |
| Total dietary fiber | 8.06 | 8.53 | 9.71 | 11.24 |
| NDF | 6.10 | 6.50 | 7.39 | 7.09 |
| ADF | 2.21 | 1.98 | 2.62 | 2.73 |
| Total lysine | 1.89 | 1.63 | 1.40 | 1.12 |
1Diets were not supplemented with antibiotics.
2Phase I, II, III, and IV diets were fed to pigs for 7, 7, 14, and 28 d postweaning, respectively.
3Soycomil, Archer Daniels Midland Company, Decatur, IL.
4Provided as milligrams per kilogram of diet: sodium chloride, 3,000; zinc, 100 from zinc oxide; iron, 90 from iron sulfate; manganese, 20 from manganese oxide; copper, 8 from copper sulfate; iodine, 0.35 from calcium iodide; selenium, 0.30 from sodium selenite.
5Provided per kilogram of diet: retinyl acetate, 2,273 μg; cholecalciferol, 17 μg; DL-α-tocopheryl acetate, 88 mg; menadione sodium bisulfate complex, 4 mg; niacin, 33 mg; D-Ca-pantothenate, 24 mg; riboflavin, 9 mg; vitamin B12, 35 μg; choline chloride, 324 mg.
After 2 wk of an 8-wk period of feeding the experimental diets, one-half of pigs were intranasally inoculated with 2 mL of a PRRSV medium containing 1 × 105 50% tissue culture infective dose. The viral strain, Purdue isolate P-129, was obtained from Indiana Animal Disease Diagnostic Laboratory (Purdue University, West Lafayette, IN). The other one-half of pigs received 2 mL of a sham medium (sterile Dulbecco's modified Eagle medium; Sigma-Aldrich Co., St Louis, MO). Frozen inoculums containing PRRSV were thawed and then diluted with Dulbecco's modified Eagle medium to contain the above challenge dose. The inoculums were kept on ice until used to challenge pigs. After PRRSV inoculation, 1 pig from the infected control treatment was culled at 18 d postinoculation (DPI) due to difficult breathing and severe BW loss.
Measurement of Pig Performance and Rectal Temperature
Pigs were weighed at the beginning of the experiment and subsequently once every 2 wk until the end of the experiment. Feeding was manually handled and feeders were refilled with preweighed amounts of feed. Feed disappearance from each pen was determined every 2 wk from −14 to 42 DPI. The ADG, ADFI, and G:F were calculated for each pen. Rectal temperature (RT) was measured at 0, 3, 7, 10, and 14 DPI and subsequently weekly until 42 DPI.
Blood Sampling and Processing
Pigs were sampled via venipuncture from the jugular vein to obtain blood samples at 0 (right before PRRSV inoculation), 3, and 7 DPI and subsequently weekly until 42 DPI. Ten milliliters of blood from each pig were collected into a vacutainer glass blood collection tube containing no anticoagulant. Blood was allowed to clot at room temperature for 45 min and stored overnight at 4°C before serum was harvested at room temperature by centrifugation for 10 min at 1,800 × g. The collected serum was aliquoted and frozen at −80°C, and later analyzed for viremia, antibody titer, IL-1β, tumor necrosis factor (TNF)-α, IL-12, interferon (IFN)-γ, IL-10, and haptoglobin (Hp).
Measurement of Viremia and PRRSV-Specific Antibody
Serum samples from pigs were tested by qRT-PCR method for measurement of viremia as previously described (Che et al., 2011). The viral concentrations were measured in triplicate in the serum samples collected before PRRSV inoculation (−28 and 0 DPI) and after PRRSV inoculation (7, 21, and 35 DPI). Quantification of the sample viral concentrations was calculated and expressed as numbers of cycle threshold.
Serum antibodies against PRRSV were measured in duplicate by a commercial ELISA kit following the procedures described by the manufacturer (IDEXX, Westbrook, ME). The ELISA sample to positive (S/P) ratio was calculated from each serum sample of the infected pigs. Pigs with an S/P ratio of 0.4 or greater were classified as PRRSV-positive.
Analyses of Cytokines and Haptoglobin in Serum
Serum samples were assayed in duplicate with commercial porcine ELISA kits following the protocols provided by the manufacturers. Standards of known recombinant porcine cytokine and Hp concentrations were used to make standard curves. The ELISA kits used for quantification of cytokines and Hp were specific for IL-1β, IFN-γ (Invitrogen, Grand Island, NY), TNF-α, IL-12, IL-10 (R & D Systems, Minneapolis, MN), and Hp (GenWay Biotech, Inc., San Diego, CA). The serum samples were analyzed at 1:2 and 1:10,000 dilutions for cytokines and Hp, respectively. The intra-assay coefficients of variation for IL-1β, TNF-α, IL-12, IFN-γ, IL-10, and Hp were 4.5, 5.2, 3.4, 4.4, 3.3, and 2.7%, respectively. The inter-assay CV for TNF-α, IL-1β, IFN-γ, IL-12, IL-10, and Hp were 7.1, 6.4, 6.8, 6.0, 5.8, and 6.2%, respectively. The results were expressed in picograms or micrograms per milliliter based on a standard curve for cytokines and Hp, respectively.
Statistical Analysis
Data were analyzed as an RCBD with a 2 × 2 factorial treatment arrangement by ANOVA using the MIXED procedure (SAS Inst. Inc., Cary, NC). A pig was considered an experimental unit for all measurements. For pig performance, the model included effects of ACT, PRRSV, and ACT × PRRSV interaction. Fixed effects were ACT and PRRSV, and random effects were initial-weight block. For viremia and antibody titers, data were analyzed within the infected pigs only because no PRRSV-specific antibodies and viruses were detected in sham-inoculated pigs. For RT, cytokines, and Hp, data were analyzed as repeated measures on each individual pig. The model included effects of ACT, PRRSV, day, and their interactions. Treatment differences were compared using the least squares means with a Tukey adjustment. Treatment effects were considered significant at P < 0.05, whereas a trend for a treatment effect was noted when P < 0.10.
RESULTS
Growth Performance
Before PRRSV inoculation, pigs fed ACT had the same growth rate (280 vs. 267 ± 18) as those fed the control (P = 0.589). Similarly, dietary ACT did not affect ADFI (388 vs. 420 ± 27) as compared with the control (P = 0.399). There was no difference (P = 0.127) in G:F between the ACT diet and the control (732 vs. 663 ± 40).
After PRRSV inoculation, infection by PRRSV decreased (P < 0.001) ADG, ADFI, and G:F during 0 to 14 DPI as compared with the sham control (Table 2). The ADG and ADFI of infected pigs were also lower from 14 to 28 DPI than those uninfected (P < 0.001), but there were no effects of ACT (P = 0.547), PRRSV (P = 0.950), or their interaction (P = 0.259) on G:F during this period. From 28 to 42 DPI, infected pigs still grew slower (P = 0.002) and tended to have a decreased G:F (P = 0.052) than uninfected pigs. During the same period, the ACT × PRRSV interaction was significant for G:F (P = 0.042), suggesting that dietary ACT increased G:F in challenged pigs (P = 0.009). In addition, the diet with ACT did not affect ADG (P = 0.450), but decreased ADFI in pigs from 28 to 42 DPI (P = 0.047) as compared with the diet without ACT. Over a 6-wk challenge, infection with PRRSV reduced ADG (P < 0.001), ADFI (P < 0.001), and G:F (P = 0.004) in inoculated pigs as compared with the sham control. Diets supplemented with ACT tended to reduce ADFI (P = 0.072) and to increase G:F (P = 0.073) in pigs as compared with those without ACT.
Table 2.
Effect of mannan oligosaccharide (Actigen; ACT)1 and porcine reproductive and respiratory syndrome virus (PRRSV)2 on pig performance after PRRSV infection
| Treatment4 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | n3 | CON | ACT | ICON | IACT | SEM | ACT | PRRSV | ACT × PRRSV |
| 0 to 14 d after inoculation | |||||||||
| ADG, g | 8 | 674 | 693 | 258 | 288 | 33 | 0.491 | <0.001 | 0.852 |
| ADFI, g | 8 | 1035 | 954 | 563 | 616 | 54 | 0.789 | <0.001 | 0.217 |
| G:F, g/kg | 8 | 663 | 730 | 454 | 453 | 43 | 0.445 | <0.001 | 0.433 |
| d 14 to 28 after inoculation | |||||||||
| ADG, g | 8 | 713 | 709 | 495 | 477 | 30 | 0.650 | <0.001 | 0.708 |
| ADFI, g | 8 | 1281 | 1317 | 957 | 828 | 75 | 0.550 | <0.001 | 0.312 |
| G:F, g/kg | 8 | 567 | 549 | 529 | 583 | 31 | 0.547 | 0.950 | 0.259 |
| d 28 to 42 after inoculation | |||||||||
| ADG, g | 8 | 950 | 925 | 661 | 756 | 44 | 0.450 | 0.002 | 0.216 |
| ADFI, g | 8 | 1963 | 1775 | 1855 | 1538 | 125 | 0.047 | 0.266 | 0.570 |
| G:F, g/kg | 8 | 486 | 526 | 357 | 508 | 26 | 0.003 | 0.052 | 0.042 |
| d 0 to 42 after inoculation | |||||||||
| ADG, g | 8 | 779 | 776 | 479 | 509 | 24 | 0.588 | <0.001 | 0.488 |
| ADFI, g | 8 | 1427 | 1349 | 1127 | 994 | 52 | 0.072 | <0.001 | 0.602 |
| G:F, g/kg | 8 | 572 | 602 | 460 | 515 | 22 | 0.073 | 0.004 | 0.580 |
1Pigs were fed ACT (Actigen, Alltech, Inc., Nicholasville, KY) diets for 8 wk starting at weaning; after 2 wk of 8-wk feeding, pigs were challenged with PRRSV.
2Pigs were challenged with PRRSV at 5 wk of age.
3A pig was an experimental unit; each treatment had 8 pigs except ICON (7 pigs, 1 pig euthanized at 18 d postinfection).
4CON: uninfected control-fed pigs; ACT: uninfected ACT-fed pigs; ICON: infected control-fed pigs; IACT: infected ACT-fed pigs.
Clinical Signs
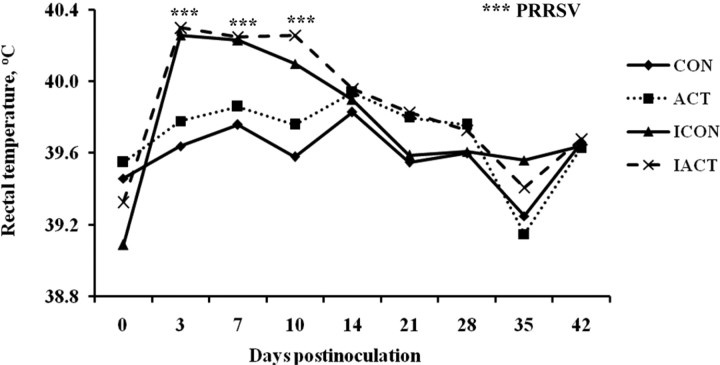
After inoculation, infected pigs first showed signs of sluggishness and loss of appetite beginning 2 to 3 DPI followed by increased RT. Respiratory symptoms such as coughing were not detected, but 1 pig from the infected control treatment was culled at 18 DPI due to difficult breathing and severe BW loss. The RT of infected pigs peaked at 3 DPI (40.3 vs. 39.7 ± 0.08°C), remained high until 10 DPI (40.2 vs. 39.7 ± 0.08°C), and returned to normal at 14 DPI (39.9 vs. 39.9 ± 0.06°C) as compared with sham controls (Figure 1). There was no ACT × PRRSV interaction for the febrile responses in pigs (P = 0.879). The PRRSV infection increased RT in inoculated pigs at 3, 7, and 10 DPI as compared with the sham control (P < 0.001). The ACT × day interaction was not significant for RT. During the experimental period, pigs fed ACT also had a greater RT than those fed the control regardless of PRRSV (P = 0.045). All sham-inoculated pigs showed no clinical signs and remained healthy during the course of the experiment.
Figure 1.
Rectal temperature (RT) of pigs fed control or mannan oligosaccharide (Actigen; ACT, Alltech, Inc., Nicholasville, KY) diets with or without infection of porcine reproductive and respiratory syndrome virus (PRRSV). There was no ACT × PRRSV interaction for the febrile response in pigs. The PRRSV infection increased the RT in inoculated pigs at Days 3, 7, and 10 postinoculation as compared with the sham control (P < 0.001). Pigs fed ACT also had a greater RT than those fed the control regardless of PRRSV (P = 0.045). Values were means; pooled SEM = 0.046. A pig was an experimental unit; each treatment had 8 pigs except ICON (7 pigs, 1 pig euthanized at 18 d postinfection). CON: uninfected control-fed pigs; ACT: uninfected ACT-fed pigs; ICON: infected control-fed pigs; IACT: infected ACT-fed pigs. ***P < 0.001.
Viremia and Antibody Titer
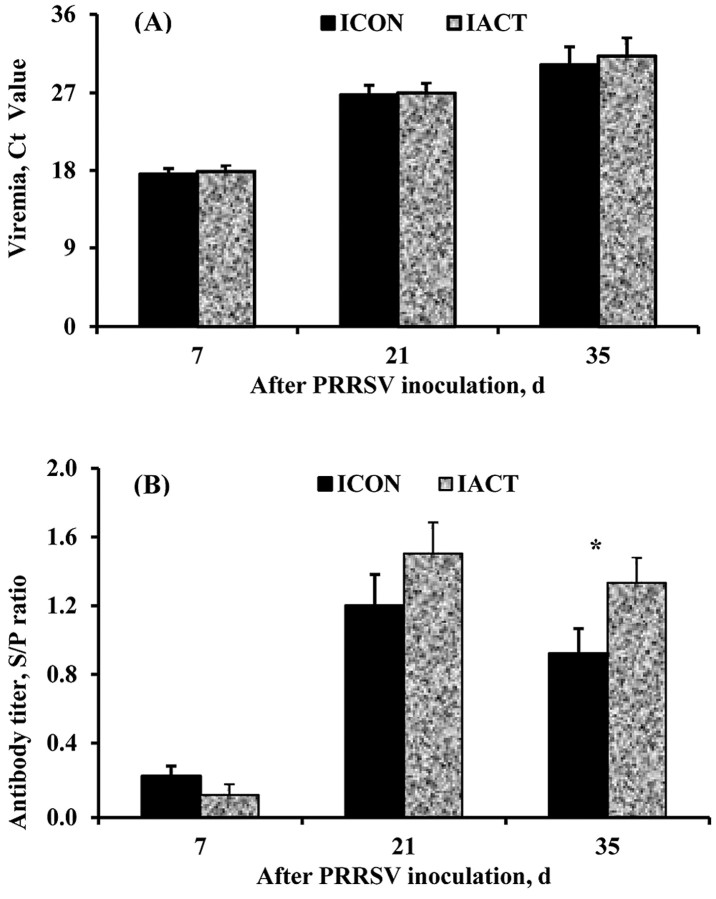
The serological and qRT-PCR tests were used to verify that our PRRSV challenge model was effective. Both tests confirmed that all serum samples collected from pigs at −28 DPI and 0 DPI (right before PRRSV inoculation) were PRRSV-negative. After PRRSV challenge, all pigs inoculated with PRRSV were PRRSV-positive and all those not inoculated with PRRSV were PRRSV-negative. All PRRSV-inoculated pigs remained viremic throughout the experiment, except that there were 3 PRRSV-negative pigs detected at 35 DPI (1 from the infected control treatment and 2 from the infected ACT treatment). The average viremic concentrations of pigs at 7, 21, and 35 DPI are presented as cycle threshold, which is inverse to the viremic concentration (Figure 2A). The cycle threshold values of infected pigs ranged from 14.4 to 22.4 at 7 DPI, 22.4 to 31.5 at 21 DPI, and 22.1 to 37.9 at 35 DPI suggesting gradual clearance of the virus from serum. Dietary ACT did not affect the viremic concentration in infected pigs at 7 (P = 0.768), 21 (P = 0.548), and 35 (P = 0.789) DPI. All pigs that were not inoculated with PRRSV remained PRRSV-free throughout the experiment.
Figure 2.
(A) Viremic concentration and (B) antibody titer in control- or mannan oligosaccharide [Actigen, ACT (Alltech, Inc., Nicholasville, KY)]-fed pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV). The antibody titer is presented as sample to positive control (S/P) ratios and viremic concentration as cycle threshold (Ct) values of all infected pigs. The Ct values presented are inversely related to virus concentrations. The antibody response of pigs to PRRSV was found negative (S/P < 0.4) at 7 d postinoculation and positive at 21 and 35 DPI. Dietary ACT did not affect the antibody titer at 21 DPI (P = 0.139), but increased it at 35 DPI (P = 0.042). Dietary ACT did not affect the viremic concentrations of infected pigs throughout the experimental period. Values were means ± pooled SEM. A pig was an experimental unit; each treatment had 8 pigs except ICON (7 pigs, 1 pig euthanized at 18 d postinfection). ICON: infected control-fed pigs; IACT: infected ACT-fed pigs. *P < 0.05.
The antibody titer presented as S/P ratios was measured at 7, 21, and 35 DPI (Figure 2B). An S/P ratio < 0.4 was considered negative. The antibody response to PRRSV was found negative at 7 DPI and positive at 21 and 35 DPI. Dietary ACT did not affect (P = 0.139) the antibody titer of infected pigs at 21 DPI as compared with the control (S/P ratio: 1.5 vs. 1.2 ± 0.15). However, infected pigs fed the ACT diet had a greater antibody titer (P = 0.042) than those fed the control diet at 35 DPI (S/P ratio: 1.3 vs. 0.9 ± 0.12).
Serum Cytokines and Acute-Phase Protein
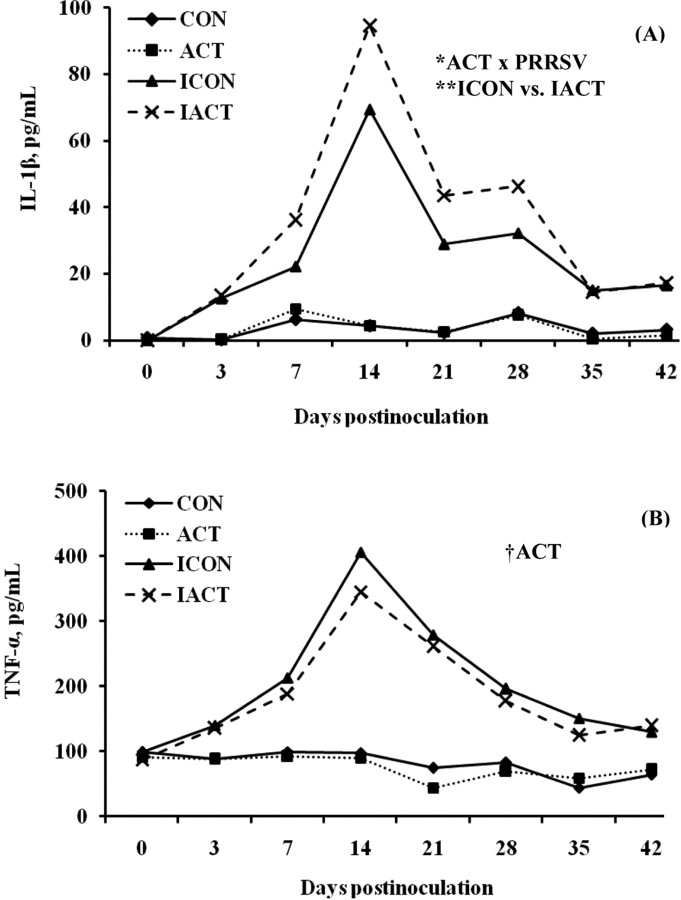
IL-1β and TNF-α. The infection with PRRSV increased (P < 0.001) serum concentrations of IL-1β and TNF-α in infected pigs as compared with the sham control, but this pattern did not hold in the noninfected pigs (Figure 3). Diets supplemented with ACT increased IL-1β concentrations (P = 0.019) and tended to decrease TNF-α concentrations (P = 0.058) in pigs as compared with those not supplemented with ACT. The ACT × PRRSV interaction was found significant for IL-1β only (P = 0.016), indicating that infected pigs fed ACT had a greater IL-1β concentration than those fed the control (P = 0.006). Serum concentrations of IL-1β and TNF-α changed over time (P < 0.001). Concentrations of IL-1β and TNF-α increased to a sharp peak at 14 DPI and then declined, but remained elevated until 42 or 28 DPI, respectively.
Figure 3.
(A) Serum IL-1β and (B) tumor necrosis factor (TNF)-α concentrations in pigs fed control or mannan oligosaccharide (Actigen; ACT, Alltech, Inc., Nicholasville, KY) diets with or without porcine reproductive and respiratory syndrome virus (PRRSV) infection. The concentrations of IL-1β and TNF-α of infected pigs were greater than those of uninfected ones (P < 0.001). Dietary ACT increased the IL-1β concentration (P = 0.019), but tended to decrease the TNF-α concentration (P = 0.058) in pigs as compared with the control diet. There was an ACT × PRRSV interaction for IL-1β (P = 0.016), indicating that infected pigs fed the diet with ACT had a greater IL-1β concentration than those fed the diet without ACT (P = 0.006). There were also significant effects of day or interaction of day × PRRSV on IL-1β and TNF-α (P < 0.001). Values were means; pooled SEM were 1.8 and 6.6 pg/mL for IL-1β and TNF-α, respectively. A pig was an experimental unit; each treatment had 8 pigs except ICON (7 pigs, 1 pig euthanized at 18 d postinfection). CON: uninfected control-fed pigs; ACT: uninfected ACT-fed pigs; ICON: infected control-fed pigs; IACT: infected ACT-fed pigs. †P < 0.1; *P < 0.05; **P < 0.01.
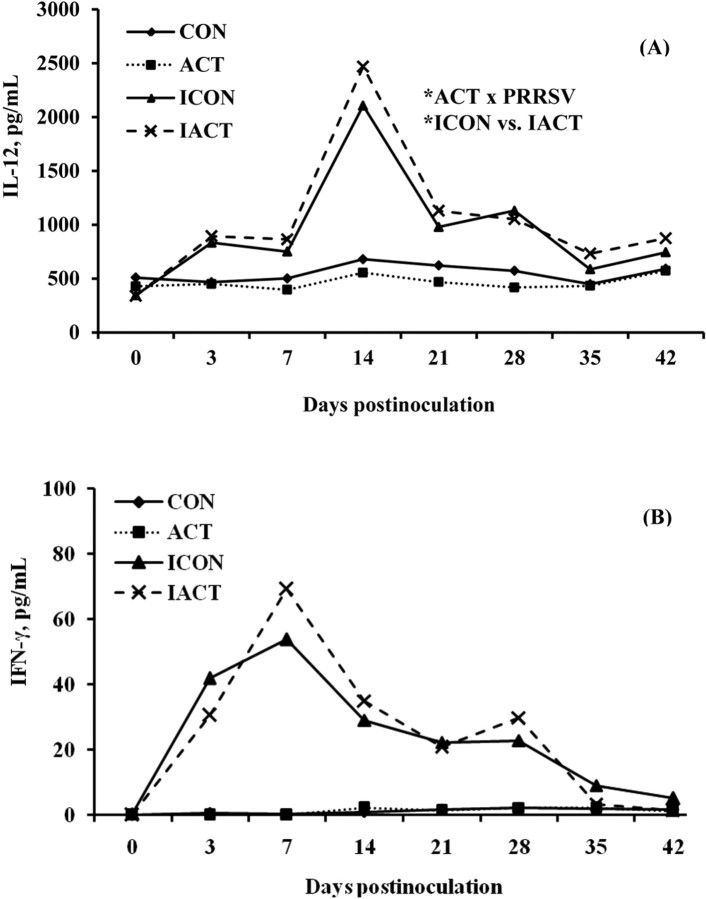
IL-12 and IFN-γ. The PRRSV infection increased (P < 0.001) serum concentrations of IL-12 and IFN-γ in pigs as compared with the sham control (Figure 4). There were no main effects of ACT on IL-12 (P = 0.766) and IFN-γ (P = 0.928). The ACT × PRRSV interaction was significant for IL-12 only (P = 0.026), suggesting that ACT increased the IL-12 concentration in infected pigs as compared with the control (P = 0.048). Serum concentrations of IL-12 and IFN-γ changed over time (P < 0.001). These cytokines sharply reached their greatest concentrations at 7 DPI (IFN-γ) and 14 DPI (IL-12) and declined afterward, but remained increased until 42 DPI.
Figure 4.
(A) Serum IL-12 and (B) interferon (IFN)-γ concentrations in pigs fed control or mannan oligosaccharide (Actigen; ACT, Alltech, Inc., Nicholasville, KY) diets with or without porcine reproductive and respiratory syndrome virus (PRRSV) infection. The IFN-γ and IL-12 concentrations of infected pigs were greater than those of uninfected ones (P < 0.001). There were no effects of diet on either cytokine. There was an ACT × PRRSV interaction (P = 0.026) for IL-12 only, indicating that infected pigs fed ACT had a greater concentration of IL-12 than those fed the control (P = 0.048). There were also significant effects of day or interaction of day x PRRSV on IFN-γ and IL-12 (P < 0.001). Values were means; pooled SEM were 33.4 and 1.6 pg/mL for IL-12 and IFN-γ, respectively. A pig was an experimental unit; each treatment had 8 pigs except ICON (7 pigs, 1 pig euthanized at 18 d postinfection). CON: uninfected control-fed pigs; ACT: uninfected ACT-fed pigs; ICON: infected control-fed pigs; IACT: infected ACT-fed pigs. *P < 0.05.
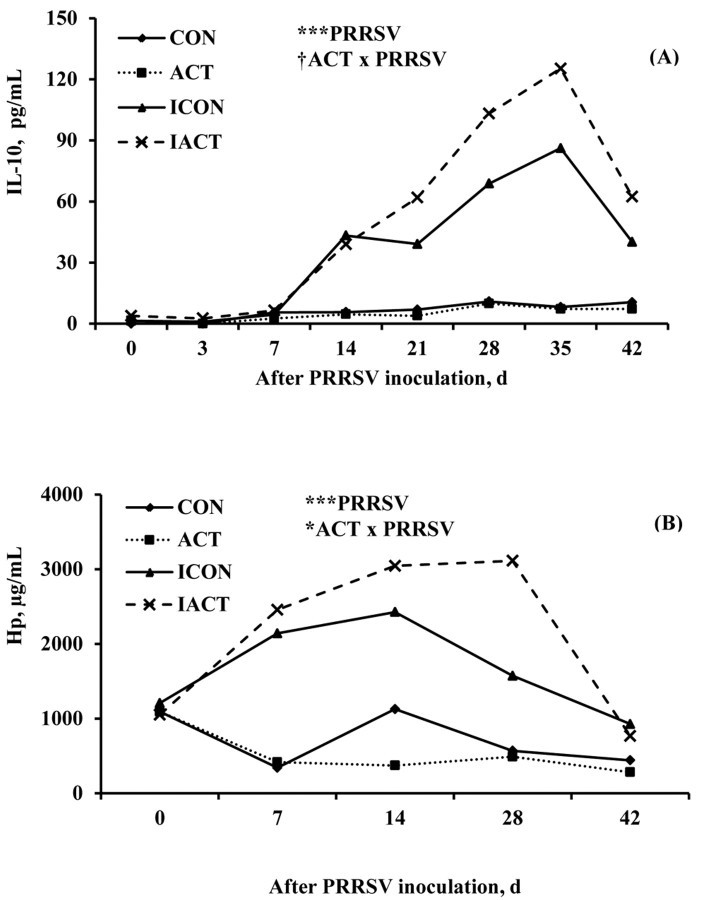
IL-10 and Hp. Serum concentrations of IL-10 and Hp were greater (P < 0.001) in infected pigs than in sham controls (Figure 5). There were no main effects of ACT on IL-10 (P = 0.147) and Hp (P = 0.479). The ACT ×PRRSV interaction tended to be significant for IL-10 (P = 0.088) and was significant for Hp (P = 0.047). Infected pigs fed ACT tended to have a greater concentration of Hp than those fed the control (P = 0.058). Serum concentrations of IL-10 and Hp changed over time (P < 0.001). The IL-10 and Hp increased more slowly before declining, and the peaks were less sharp as compared with the other inflammatory mediators.
Figure 5.
(A) Serum IL-10 and (B) Haptoglobin (Hp) concentrations in pigs fed control or mannan oligosaccharide (Actigen; ACT, Alltech, Inc., Nicholasville, KY) diets with or without porcine reproductive and respiratory syndrome virus (PRRSV) infection. Infection with PRRSV increased the serum concentrations of IL-10 and Hp in infected pigs (P < 0.001). The ACT × PRRSV interaction tended to be significant for IL-10 (P = 0.088), but was significant for Hp (P = 0.047). There were also significant effects of day or interaction of day × PRRSV on IL-10 and Hp (P < 0.001). Values were means; pooled SEM were 4.7 pg/mL and 142.5 μg/mL for IL-10 and Hp, respectively. A pig was an experimental unit; each treatment had 8 pigs except ICON (7 pigs, 1 pig euthanized at 18 d postinfection). CON: uninfected control-fed pigs; ACT: uninfected ACT-fed pigs; ICON: infected control-fed pigs; IACT: infected ACT-fed pigs. †P < 0.1; *P < 0.05; ***P < 0.001.
DISCUSSION
Porcine reproductive and respiratory syndrome virus imposes a substantial loss to swine producers not only in the United States, but also other parts of the world (Neumann et al., 2005; Dietze et al., 2011). It weakens innate immune response at the early stage of infection and reduces the ineffective adaptive immunity during the persistent phase of PRRSV (Murtaugh et al., 2002; Van Reeth et al., 2002). These impacts of PRRSV bring about decreased productivity and possibly increase in susceptibility to other infections (Thanawongnuwech et al., 2000; Escobar et al., 2004). Thus, use of multiple strategies to prevent or alleviate the adverse effects of PRRSV on pig performance and health is definitely needed.
Diets supplemented with MOS have been shown to modulate innate and adaptive immune responses in animals. They altered serum cytokine secretions and increased phagocytic activity of porcine macrophages, blood leukocytes, and serum immunoglobulin concentrations (Shashidhara and Devegowda, 2003; Davis et al., 2004; Che et al., 2011). However, different MOS products extracted from the yeast cell wall may possess diverse immunoregulatory properties because each fraction differs in structural characteristics and proportions of functional carbohydrates (Young et al., 1998; Bland et al., 2004; Sheng et al., 2006). Therefore, we examined the possibility of feeding ACT aimed at alleviating the negative effects of PRRSV infection on pig performance and improving the immune status of the pigs.
Infection with PRRSV reduced loss of appetite and depressed performance. In our experiment, anorexia occurred as early as 2 to 3 DPI and infected pigs showed signs of sluggishness. Escobar et al. (2007) demonstrated that feeding time and activity of PRRSV-infected pigs started to decrease at 1 DPI and remained decreased until the end of the experiment (14 DPI). Toepfer-Berg et al. (2004) showed that PRRSV decreased ADFI beginning 3 DPI and throughout a 12-d experimental period. We found that the ADFI and ADG of infected pigs were greatly reduced during 0 to 28 DPI. However, from 28 to 42 DPI, the ADFI of infected pigs recovered to near normal (91% of the control), whereas the ADG of infected pigs was still substantially affected, with a reduction of 24%. These data suggest that retarded growth of pigs due to PRRSV infection results from not only the reduced feed intake, but also increased nutrient needs for the immune defense processes of the host. It was further observed that feeding ACT ameliorated the adverse effect of PRRSV on feed use. Infected pigs fed ACT had a better G:F during 28 to 42 DPI than those fed the control. In the present experiment, ACT also enhanced PRRSV-specific antibodies in infected pigs at 35 DPI. These combined improvements may be an indicator of efficient redirection of nutrient use for both growth and host defense processes during the chronic phase of infection. Improved feed efficiency in PRRSV-infected pigs consuming MOS was also documented previously (Che et al., 2011). It should also be noted that the ACT-reduced ADFI contributed to the improved G:F in pigs consuming ACT. Our result was consistent with the previous meta-analysis of MOS (Alltech, Inc., Nicholasville, KY), which showed that the improvements in G:F of pigs in most studies were resulted from the MOS-induced reductions of ADFI (Miguel et al., 2004). Together, these data suggest that ACT fed to pigs may alter metabolic changes leading to the reduced ADFI without compromising ADG.
Further, PRRSV caused a febrile response peaking at 3 DPI and returning to normal at 14 DPI. Other researchers also observed a similar fever response pattern (Shibata et al., 2003; Doeschl-Wilson et al., 2009). Activation of the immune system by PRRSV triggers secretions of inflammatory cytokines such as IL-1β, IL-6, and TNF-α that are most responsible for fever. Mild but prolonged fever is often a typical response to infections of a North American PRRSV strain. Depending on PRRSV strain and challenge dose, body temperature of infected pigs may remain sporadically elevated up to 14 or 28 DPI (Thanawongnuwech et al., 2000; Loving et al., 2008). It is worthy of note that during the experimental period, ACT slightly increased the RT of the pigs from 39.7 to 39.8°C regardless of PRRSV. This implies that feeding ACT might have induced changes in physiological and metabolic processes, which would contribute to the adjustment of body temperature. The effect of ACT on body temperature may, to a certain extent, be associated with ACT-induced activity of immune cells which perhaps helps the immune system stay alert, but not over-stimulated. Thus, further research on this aspect is required.
The diet with ACT boosted PRRSV-specific antibodies in infected pigs as compared with the diet without ACT. After exposure to PRRSV, specific antibodies may be detectable as early as 5 to 10 DPI (Murtaugh et al., 2002; Diaz et al., 2005). However, antibodies which appear during the early postinfection period are not efficient at neutralizing PRRSV, and therefore do not provide protection against the infection (Murtaugh et al., 2002; Lopez and Osorio, 2004). Serum antibodies that neutralize PRRSV have been reported to arise about 21 to 28 DPI or later time points (Loemba et al., 1996; Meier et al., 2003). In our experiment, the increased anti-PRRSV antibody response induced by ACT at 35 DPI may importantly contribute to the protection against PRRSV infection. The appearance of neutralizing antibodies is slow and lasts for only a few months, but good correlations between the appearance of neutralizing antibodies and clearance or prevention of viremia have been reported (Yoon et al., 1996; Osorio et al., 2002; Lopez and Osorio, 2004). Although no clear effect of ACT on viremia was observed, ACT enhanced the adaptive immune response at the time which neutralizing antibodies are expected to appear in the circulation and consequently may confer a certain degree of protection. During this period, the improved G:F observed in infected pigs consuming ACT is likely to be associated with the increased antibody titer and numerically less ADFI. Therefore, the effect of ACT on total and neutralizing antibody concentrations and viremia should be examined for longer postinfection periods to further explore this potential benefit of ACT.
Increased concentrations of IL-1β were observed in pigs fed ACT. The PRRSV-induced IL-1β started to rise as early as 3 DPI and remained greater than that of uninfected pigs until 42 DPI. Interleukin-1β is one of the innate cytokines produced earliest after pigs are challenged with PRRSV, and its serum concentration is observed elevated up to 42 DPI (Escobar et al., 2004; Liu et al., 2010; Lunney et al., 2010). It was assumed that IL-1β, together with other cytokines, could have an effect on viral persistence (Lunney et al., 2010). These data imply that IL-1β may play an important role during the early as well as subsequent stages of infection. Indeed, IL-1β is capable of promoting T-cell proliferation and B-cell maturation and multiplication. Infection with PRRSV elicits a weak production of pro-inflammatory cytokines which diminishes the ability of the host to efficiently mount subsequent immune responses (Murtaugh et al., 2002; Mateu and Diaz, 2008). Hence, ACT-mediated upregulation of IL-1β protein in infected pigs may favorably raise the innate immunity against PRRSV.
Infection with PRRSV enhanced production of circulating T helper 1 (Th1)-associated cytokines including IL-12, IFN-γ, and TNF-α. Among these cytokines, infected pigs fed ACT had a greater IL-12 concentration than those fed the control. During infection, IL-12 enhances the killing activity of natural killer cells and induces IFN-γ production in natural killer and T cells (Watford et al., 2003). Also, IL-12 plays a critical role in development of naive T cells to IFN-γ-secreting Th1 cells, which participate in cellular immunity (Holscher, 2004). Although ACT enhanced IL-12 in infected pigs, it did not affect the serum IFN-γ concentration. This suggests that there are multiple components of the immune system, apart from IL-12, influencing the activation of IFN-γ-secreting cells as well as production and release of IFN-γ into peripheral blood. Indeed, IFN-γ production is mediated via IL-18 and produced by different types of immune cells involved in not only early innate response but also the subsequent antigen-specific adaptive immunity (Nakanishi et al., 2001; Schroder et al., 2004). Consistent with earlier reports (Wesley et al., 2006; Loving et al., 2008), our results indicated that IFN-γ was detectable soon after PRRSV infection and its serum concentration was low, but continued to be elevated for a couple of weeks postinfection. In regards to TNF-α, its serum concentration began to increase at 3 DPI and decreased to normal after 28 DPI. These data indicate the involvement of this cytokine in regulation of inflammation during acute and chronic phase responses. Other researchers also found increased concentrations of serum TNF-α as early as 3 to 7 DPI (Liu et al., 2010; Miguel et al., 2010; Che et al., 2011). In general, feeding ACT to infected pigs may fortify the cell-mediated immunity as ACT is associated with the increased concentrations of IL-12 in infected pigs throughout the experimental period.
Release of serum anti-inflammatory mediators, IL-10 and Hp appears to be responsive to PRRSV-induced inflammation. Increased concentrations of IL-10 and Hp occurred later than the innate and Th1-associated cytokines. The IL-10 and Hp concentrations declined toward the end of the experiment, implying that the inflammatory process seems to be repressed. The increase in IL-10 of infected pigs may be dependent on the inflammation intensity caused by PRRSV. Che et al. (2011) found elevated concentrations of IL-10 at 7 DPI. In another study, serum IL-10 increased at 33 DPI, peaked at 38 DPI, and diminished to the pre-infection concentrations (Wang et al., 2011). Interleukin-10 is secreted to suppress inflammation through blocking activation of immune cells and synthesis of pro-inflammatory cytokines (Ouyang et al., 2011). It is also capable of stimulating B cell maturation and antibody production. Consequently, production of IL-10 and other anti-inflammatory molecules is essential to regulate a harmony between protection and immunopathology. Increased IL-10 and PRRSV-specific antibodies, accompanied by decreased IL-12, after acute phase suggest that ACT aids in shifting from Th1 to T helper 2 (Th2) immune responses toward the end of the experiment. In addition, Hp, a type-2 acute phase protein has a strong response to PRRSV and often remains increased for several weeks postinfection before returning to normal (Parra et al., 2006; Gnanandarajah et al., 2008). Cytokines such as TNF-α, IL-1β, and IL-6 induce hepatocyte-produced Hp. The rise of Hp appears to coincide with the increased IL-10. Acute phase proteins including Hp may provide a feedback mechanism by suppressing pro-inflammatory cytokine production (Petersen et al., 2004). Therefore, ACT-mediated increases of IL-10 and Hp in infected pigs during the chronic phase of PRRSV are likely to be crucial in enhancement of acquired immunity and suppression of inflammatory responses.
In summary, feeding ACT to weanling pigs alters the pattern of the immune responses of the pigs to a PRRSV infection during the course of study. Dietary ACT increases serum concentrations of inflammatory mediators that are important in boosting innate and cell-mediated immunity. Further, diets supplemented with ACT potentially mitigate negative impacts of PRRSV through enhanced PRRSV-specific antibodies. Feeding ACT is also efficacious in improving nutrient utilization in pigs infected with PRRSV. However, it should be noted that although not causing any adverse effects, feeding ACT slightly increases the body temperature of the pigs. To maximize use of ACT in diets, its effects on growth and immune response in pigs subjected to other pathogens require further examination, as commercially-reared pigs are frequently exposed to multiple pathogens including PRRSV.
Footnotes
This project was funded by Alltech, Inc., 3031 Catnip Hill Pike, Nicholasville, KY.
LITERATURE CITED
- Bland E. J., Keshavars T., Bucke C. 2004. The influence of small oligosaccharides on the immune system. Carbohydr. Res. 339:1673–1678. [DOI] [PubMed] [Google Scholar]
- Che T. M., Johnson R. W., Kelley K. W., Van Alstine W. G., Dawson K. A., Moran C. A., Pettigrew J. E. 2011. Mannan oligosaccharide improves immune responses and growth efficiency of nursery pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 89:2592–2602. [DOI] [PubMed] [Google Scholar]
- Davis M. E., Maxwell C. V., Erf G. F., Brown D. C., Wistuba T. J. 2004. Dietary supplementation with phosphorylated mannans improves growth response and modulates immune function of weanling pigs. J. Anim. Sci. 82:1882–1891. [DOI] [PubMed] [Google Scholar]
- Diaz I., Darwich L., Pappaterra G., Pujols J., Mateu E. 2005. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 86:1943–1951. [DOI] [PubMed] [Google Scholar]
- Dietze K., Pinto J., Wainwright S., Hamilton C. 2011. Porcine reproductive and respiratory syndrome (PRRS): Virulence jumps and persistent circulation in Southeast Asia. Focus on. No. 5. FAO, Rome, Italy. [Google Scholar]
- Doeschl-Wilson A. B., Kyriazakis I., Vincent A., Rothschild M. F., Thacker E., Galina-Pantoja L. 2009. Clinical and pathological responses of pigs from 2 genetically diverse commercial lines to porcine reproductive and respiratory syndrome virus infection. J. Anim. Sci. 87:1638–1647. [DOI] [PubMed] [Google Scholar]
- Escobar J., Alstine W. G. V., Baker D. H., Johnson R. W. 2004. Decreased protein accretion in pigs with viral and bacterial pneumonia is associated with increased myostatin expression in muscle. J. Nutr. 134:3047–3053. [DOI] [PubMed] [Google Scholar]
- Escobar J., Alstine W. G. V., Baker D. H., Johnson R. W. 2007. Behaviour of pigs with viral and bacterial pneumonia. Appl. Anim. Behav. Sci. 105:42–50. [Google Scholar]
- Gnanandarajah J. S., Dvorak C. M. T., Johnson C. R., Murtaugh M. P. 2008. Presence of free haptoglobin alpha 1S-subunit in acute porcine reproductive and respiratory syndrome virus infection. J. Gen. Virol. 89:2746–2753. [DOI] [PubMed] [Google Scholar]
- Holscher C. 2004. The power of combinational immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases. Med. Microbiol. Immunol. 193:1–17. [DOI] [PubMed] [Google Scholar]
- Jung K., Renukaradhya G. J., Alekseev K. P., Fang Y., Tang Y., Saif L. J. 2009. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: Implications for respiratory viral co-infections. J. Gen. Virol. 90:2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Shi W., Zhou E., Wang S., Hu S., Cai X., Rong F., Wu J., Xu M., Xu M., Li L. 2010. Dynamic changes in inflammatory cytokines in pigs infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Clin. Vaccine Immunol. 17:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loemba H. D., Mounir S., Mardassi H., Archambault D., Dea S. 1996. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch. Virol. 141:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez O. J., Osorio F. A. 2004. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 102:155–163. [DOI] [PubMed] [Google Scholar]
- Loving C. L., Brockmeier S. L., Vincent A. L., Lager K. M., Sacco R. E. 2008. Differences in clinical disease and immune response of pigs challenged with high-dose versus low-dose inoculums of porcine reproductive and respiratory syndrome virus. Viral Immunol. 21:315–325. [DOI] [PubMed] [Google Scholar]
- Lunney J. K., Fritz E. R., Reecy J. M., Kuhar D., Prucnal E., Molina R., Christopher-Hennings J., Zimmerman J., Rowland R. R. R. 2010. Interleukin-8, Interleukin-1β, and Interferon-γ levels are linked to PRRS virus clearance. Viral Immunol. 23:127–134. [DOI] [PubMed] [Google Scholar]
- Mateu E., Diaz I. 2008. The challenge of PRRS immunology. Vet. J. 177:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier W. A., Galeota J., Osorio F. A., Husmann R. J., Schnitzlein W. M., Zuckermann F. A. 2003. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 309:18–31. [DOI] [PubMed] [Google Scholar]
- Miguel J. C., Rodriguez-Zas S. L., Pettigrew J. E. 2004. Efficacy of Bio-Mos for improving nursery pig performance. J. Swine Health Prod. 12:296–307. [Google Scholar]
- Miguel J. C., Chen J., Van Alstine W. G., Johnson R. W. 2010. Expression of inflammatory cytokines and Toll-like receptors in the brain and respiratory tract of pigs infected with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 135:314–319. [DOI] [PubMed] [Google Scholar]
- Murtaugh M. P., Xiao Z., Zuckermann F. 2002. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral. Immunol. 15:533–547. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Yoshimoto T., Tsutsui H., Okamura H. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423–474. [DOI] [PubMed] [Google Scholar]
- Neumann E. J., Kliebenstein J. B., Johnson C. D., Mabry J. W., Bush E. J., Seitzinger A. H., Green A. L., Zimmerman J. J. 2005. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 227:385–392. [DOI] [PubMed] [Google Scholar]
- NRC 1998. Nutrient requirements of swine. 10th ed.Natl. Acad. Press, Washington, DC. [Google Scholar]
- Osorio F. A., Galeota J. A., Nelson E., Brodersen B., Doster A., Wills R., Zuckermann F., Laegreid W. W. 2002. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 302:9–20. [DOI] [PubMed] [Google Scholar]
- Ouyang W., Rutz S., Crellin N. K., Valdez P. A., Hymowitz S. G. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29:71–109. [DOI] [PubMed] [Google Scholar]
- Parra M. D., Fuentes P., Tecles F., Martinez-Subiela S., Martinez J. S., Munoz A., Ceron J. J. 2006. Porcine acute phase protein concentrations in different diseases in field conditions. J. Vet. Med. B Infect. Dis. Vet. Public Health 53:488–493. [DOI] [PubMed] [Google Scholar]
- Petersen H. H., Nielsen J. P., Heegaard P. M. H. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 35:163–187. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. E. 2006. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: Dietary tools, Part 1. Anim. Biotechnol. 17:207–215. [DOI] [PubMed] [Google Scholar]
- Schroder K., Hertzog P. J., Ravasi T. 2004. Interferon-γ: an overview of signals, mechanisms, and functions. J. Leukoc. Biol. 75:163–189. [DOI] [PubMed] [Google Scholar]
- Shashidhara R. G., Devegowda B. 2003. Effect of dietary mannan oligosaccharide on broiler breeder production traits and immunity. Poul. Sci. 82:1319–1325. [DOI] [PubMed] [Google Scholar]
- Sheng K. C., Pouniotis D. S., Wright M. D., Tang C. K., Lazoura E., Pietersz G. A., Apostolopoulos V. 2006. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 118:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata I., Yazawa S., Ono M., Okuda Y. 2003. Experimental dual infection of specific pathogen-free pigs with porcine reproductive and respiratory syndrome virus and pseudorabies virus. J. Vet. Med. B Infect. Dis. Vet. Public Health 50:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawongnuwech R., Brown G. B., Halbur P. G., Roth J.A., Royer R. L., Thacker B. J. 2000. Pathogenesis of porcine reproductive and respiratory syndrome virus-induced increase in susceptibility to streptococcus suis infection. Vet. Pathol. 37:143–152. [DOI] [PubMed] [Google Scholar]
- Toepfer-Berg T. L., Escobar J., Van Alstine W. G., Baker D. H., Salak-Johnson J., Johnson R. W. 2004. Vitamin E supplementation does not mitigate the acute morbidity effects of porcine reproductive and respiratory syndrome virus in nursery pigs. J. Anim. Sci. 82:1942–1951. [DOI] [PubMed] [Google Scholar]
- Turner J. L., Dritz S. S., Minton J. E. 2001. Review: Alternatives to conventional antimicrobials in swine diets. Prof. Anim. Scientist. 17:217–226. [Google Scholar]
- Van Reeth K., Van Gucht S., Pensaert M. 2002. In vivo studies on cytokine involvement during acute viral respiratory disease of swine: Troublesome but rewarding. Vet. Immunol. Immunopathol. 87:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Song T., Yu Y., Liu Y., Shi W., Wang S., Rong F., Dong J., Liu H., Cai X., Zhou E. M. 2011. Immune responses in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 142:170–178. [DOI] [PubMed] [Google Scholar]
- Watford W. T., Moriguchi M., Morinobu A., O'Shea J. J. 2003. The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14:361–368. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Lager K. M., Kehrli M. E., Jr 2006. Infection with porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs. Can. J. Vet. Res. 70:176–182. [PMC free article] [PubMed] [Google Scholar]
- Yoon K. J., Wu L. L., Zimmerman J. J., Hill H. T., Platt K. B. 1996. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral. Immunol. 9:51–63. [DOI] [PubMed] [Google Scholar]
- Young M., Davies M. J., Bailey D., Gradwell M. J., Smestad-Paulsen B., Wold J. K., Barnes R. M. R., Hounsell E. F. 1998. Characterization of oligosaccharides from an antigenic mannan of Saccharomyces cerevisiae. Glycoconj. J. 15:815–822. [DOI] [PubMed] [Google Scholar]