ABSTRACT
Exposure to animals persistently infected (PI) with bovine viral diarrhea virus (BVDV) results in immunomodulation of cohorts that may have health and growth consequences; however, effects may differ in low-risk, preconditioned (PC) vs. high-risk, auction market (AM) beef cattle. Our objective was to compare health and performance of PC or AM management systems with (PI) or without (CON) presence of a PI-BVDV pen mate using a 2 × 2 factorial arrangement. Four shipment blocks of crossbred PC steers (n = 236) from 3 ranch-origins were weaned, dewormed, vaccinated, tested for PI-BVDV, and kept on the ranch for ≥42 d. Subsequently, PC steers were transported to a stocker receiving unit (RU), weighed (251 ± 2 kg), blood sampled, stratified by d −1 BW, and assigned randomly to treatment (PCPI or PCCON) with no additional processing. Simultaneously, 4 blocks of crossbred AM calves (n = 292) were assembled from regional auction markets and transported to the RU ± 36 h from PC arrival. The AM calves were weighed (245 ± 1.3 kg), stratified by gender and d −1 BW, processed under the same regimen used for PC steers at their origin ranch except bull calves were castrated, and then assigned randomly to treatment (AMPI or AMCON). Treatment pens (0.45 ha) were arranged spatially such that PI did not have fence-line or water source contact with CON. Calves were fed identically and followed the same antibiotic treatment protocol. Daily BW gain for the entire 42-d receiving trial was greater (P < 0.001) for PC (1.2 kg) compared with AM (0.85 kg). There was an exposure effect (P = 0.002) on ADG from d 28 to 42; CON gained 1.12 kg vs. 0.90 kg BW for PI cohort. Morbidity was markedly greater (P < 0.001) in AM (70%) vs. PC (7%), resulting in (P < 0.001) an antibiotic treatment cost of $20.52 and $2.48/animal, respectively. Treatment with a third antibiotic occurred more often (P = 0.04) for PI cohort, and the percentage of chronically ill cattle was greatest (P = 0.06) for AMPI. Upon arrival, BVDV type 1a, 1b, and 2a titers were greater for PC (treatment × day, P < 0.001), and the percentage seropositive to BVDV type 1a on d 0 was 100% for PC vs. 23% in AM. Platelets increased transiently (P < 0.001) with greater platelets observed in AM (P < 0.001). Results indicate that PC calves gain faster and require fewer antibiotic treatments during the receiving period. Exposure to PI reduced BW gain from d 28 to 42, increased the number of calves treated thrice, and increased chronically ill cattle for AM.
Keywords: bovine respiratory disease, bovine viral diarrhea virus, preconditioning
INTRODUCTION
Bovine respiratory disease (BRD) is a multifaceted disease involving a combination of stress, immunocompromise, and viral and bacterial pathogens. Controlling BRD among newly received cattle is the greatest challenge facing the stocker and feedlot segments of beef cattle production (Edwards, 2010). Preconditioning is a management practice to prepare immature calves for stocker or feedlot entry by reducing stress and enhancing disease protection through pre-arrival vaccination (Cole, 1985; Duff and Galyean, 2007). Preconditioned calves have improved health and performance compared with high-risk calves originating from auction markets (Clark et al., 2006; Seeger et al., 2008). However, the adoption rate of management factors including weaning (50.2%) or respiratory vaccination (39.4%) in U.S. beef cow-calf operations remains low (USDA, 2010).
Bovine viral diarrhea virus (BVDV) may contribute to BRD pathogenesis through indirect effects of immunosuppression (Welsh et al., 1995). Calves born persistently infected (PI) with BVDV are a primary source of BVDV transmission (Fulton et al., 2005). Although prevalence of PI-BVDV animals in the feedlot is estimated to be relatively low (0.3%; Loneragan et al., 2005), a single PI-BVDV animal has the potential to continuously expose cohorts in an entire pen and adjacent pens to the virus. Because research observations are conflicting, the decision to invest in PI-BVDV control programs for newly received cattle remains controversial. One inconsistency among the literature is the use of experimental animals with varied management and health history. It is hypothesized that health and performance are altered by exposure to a PI-BVDV pen mate in high-risk, commingled cohorts; however, the effects of PI-BVDV exposure are less extensive in single-source, preconditioned calves. Our objective was to evaluate effects of PI-BVDV exposure and determine if health and performance outcomes differ for these divergent management systems.
MATERIALS AND METHODS
Animal methods and experimental procedures were approved by the University of Arkansas Animal Care and Use Committee.
Cattle
Experimental Cattle
A total of 528 crossbred, male beef calves were used to determine effects of weaning management and PI-BVDV exposure. Calves from 2 different weaning management systems were used for the receiving trial: 1) low-risk, single-source, preconditioned (PC) crossbred steer calves (n = 236; initial BW = 251 ± 2 kg) arrived in 4 shipment blocks from 3 Arkansas cow-calf ranches and 2) high-risk, commingled, auction market (AM) crossbred bull (n = 210) and steer (n = 82) calves (initial BW = 245 ± 1.3 kg) that arrived in 4 shipment blocks assembled from multiple Arkansas auction markets. The 4 shipment blocks of PC calves originated from cow-calf herds located in Izard (block 1 and 4), Hempstead (block 2), and Pulaski (block 3) County, AR. The PC calves arrived at the University of Arkansas Agricultural Experiment Station located near Savoy (receiving unit; RU) on October 27, 2008, January 19, August 11, or December 6, 2009, respectively. The PC cattle were considered to be low-risk for developing signs of BRD because they were weaned and vaccinated against BRD pathogens ≥42 d before shipment and maintained as a single source without commingling.
Each shipment block of AM calves was assembled by an order buyer from 2 to 3 public auction markets located in Northwest or North Central, AR and arrived at the RU on October 25, 2008, January 20, August 10, or December 5, 2009 for the 4 shipment blocks, respectively. Order buyers were instructed to purchase AM cattle of similar BW and phenotype as the accompanying PC steers. The AM cattle were considered to be high-risk for developing signs of BRD because they did not have known health or vaccination history and were commingled extensively resulting in greater probability of physiological stress and exposure to BRD pathogens.
The main effects of management (AM or PC) and PI-BVDV exposure (exposed = PI or not exposed = CON) resulted in 4 treatments arranged as a 2 × 2 factorial. For PC treatments, 42 to 91 d before trial initiation depending on block, randomly selected steers were weaned and ear-notched to test for PI-BVDV status at a commercial laboratory (Cattle Stats, LLC, Oklahoma City, OK) using the antigen-capture ELISA (ACE) method (Idexx Laboratories, Inc., Westbrook, ME). Also on the day of weaning, PC calves were administered 1) a pentavalent modified-live virus (MLV) respiratory vaccine containing infectious bovine rhinotracheitis virus, BVDV type 1a and 2a, parainfluenza-3 virus, and bovine respiratory syncytial virus isolates [Express 5, Boehringer Ingelheim Vetmedica, Inc. (BIVI), St. Joseph, MO], 2) Manheimia haemolytica-Pastuerella multocida bacterin-toxoid (Pulmo-guard PHM-1, BIVI), and 3) pour-on or injectable anthelmintic depending on block (Cydectin, BIVI). Approximately 14 d later, PC calves were administered a clostridial bacterin-toxoid (Alpha 7, BIVI) and revaccinated with a pentavalent MLV respiratory vaccine. During the 42- to 91-d preconditioning phase, PC steers were isolated from other cattle on the ranch, fed hay or pasture along with a supplement, and remained on their origin ranch until approximately d −2 when they were shipped to the RU. Upon arrival to the RU, PC calves were held in a single, isolated unloading pen with ad libitum access to hay and water until trial initiation. On d −1, PC calves were weighed and returned to the isolated unloading pen. The following d (d 0), PC calves were weighed, blood samples were collected via jugular venipunture into evacuated tubes (Vacutainer; BD Inc, Franklin Lakes, NJ) to determine total and differential peripheral blood leukocytes (PBL; 10.8 mg K2-EDTA, 6-mL tube, Ref 367863) and BVDV antibody titers (SST, 10-mL tube, Ref 367985), stratified by d −1 BW, and then assigned randomly to treatment (PCCON or PCPI).
To coincide with PC groups, AM calves were assembled from regional auction markets and delivered to the RU ± 36 h from PC arrival. Upon arrival to the RU, AM calves were held in a single, isolated unloading pen with ad libitum access to hay and water until trial initiation. On d −1, AM calves were weighed, identified with a unique ear identification tag, ear notched to test for PI-BVDV status at a commercial laboratory (Cattle Stats) using the ACE method (Idexx Laboratories), and returned to their isolated unloading pen. One AM animal in block 3 was identified as PI-BVDV and removed from the study and quarantined on d 0. On d 0, AM cattle received the same vaccinations and processing regimen as described for PC on their origin ranch; therefore, the first known vaccination and processing for AM occurred on d 0 rather than 42 to 91 d previously on an origin ranch for PC. For AM calves, revaccination of the pentavalent MLV respiratory vaccine (Express 5, BIVI) occurred on d 14 in addition to administration of a clostridial bacterin-toxoid (Alpha 7, BIVI). Additionally, for AM on d 0, bull calves were castrated surgically, stratified by gender and d −1 BW, then AM calves were assigned randomly to treatment (AMCON or AMPI). Upon trial initiation, cattle were moved to their randomly assigned 0.45-ha pens and provided 0.91 kg/d (as-fed basis) of a receiving supplement (Table 1; 15.3% CP, DM basis) and ad libitum access to bermudagrass hay (13.1% CP, 64% NDF, 42% ADF, DM basis) and water. Supplement offered was step-wise increased to a maximum of 2.73 kg/d as each pen completely consumed the supplement offered for 2 consecutive days.
Table 1.
Ingredient composition of supplement (%, as-fed basis) for newly received cattle
| Maximum feeding rate | ||
|---|---|---|
| Item | 1.82 kg/d | 2.73 kg/d |
| Ingredient | ||
| Corn, cracked | 68.36 | 68.59 |
| Dried distillers grain with solubles | 26 | 26 |
| Salt, white | 1 | 1 |
| Limestone | 2 | 2 |
| Molasses | 2 | 2 |
| Vitamin A, D, E, premix1 | 0.15 | 0.09 |
| Trace mineral premix2 | 0.085 | 0.055 |
| Corn/Rumensin premix3 | 0.4 | 0.26 |
1Contained 5,896,000 IU vitamin A, 1,179,200 IU vitamin D, and 15,257 IU vitamin E/kg.
2Trace mineral premix contained 12% Zn, 8% Mn, 4% Cu, 500 mg of Co, 2,000 mg of I, and 600 mg Se/kg.
3Provided 88 mg of monensin (Elanco Animal Health, Indianapolis, IN)/kg of supplement.
All BW measurements were obtained individually without withholding feed or water on 2 consecutive days at the beginning (d −1 and 0) and end (d 42 and 43) of the receiving trial using a stanchion equipped with electronic load cells to determine overall differences in gain performance. To determine interim differences in BW gain performance, individual BW was recorded at 14-d intervals during the trial (d 14 and 28). Furthermore, calves were blood sampled from the right jugular vein on d 14 and 28 to determine interim total and differential PBL and BVDV type 1a, 1b, and 2a antibody titers. Anticoagulated whole blood was collected from each animal into EDTA tubes, kept refrigerated (5°C), and analyzed within 24 h to determine percentage and concentrations (n cells/μL) of total and differential (lymphocytes, neutrophils, monocytes, eosinophils, and basophils) PBL, platelets, red blood cells, hemoglobin, and hematocrit with an automated hemacytometer (Cell-Dyn 3500 system, Abbott Laboratories, Abbott Park, IL) standardized for analysis of bovine blood.
Blood collected from each animal in the plain evacuated tubes was centrifuged at 2,100 × g for 20 min at 20°C, and serum was decanted into duplicate aliquots and stored frozen at −20° C. Subsequently, 5 individual sample aliquots were selected randomly from each treatment pen from d 0 and 28 and shipped on ice via overnight parcel service to the Iowa State University Veterinary Diagnostic Laboratory (ISUVDL, Ames, IA) for determination of serum neutralizing antibody titer concentration and seroconversion against BVDV type 1a (Singer strain) using the virus neutralization (VN) assay. The second set of serum aliquot samples were pooled within day (d 0 and 28 only) and treatment pen and shipped on ice via overnight parcel service to the Texas Veterinary Medical Diagnostic Laboratory – Amarillo (TVMDL, Amarillo, TX) for determination of serum neutralizing antibody titer concentrations against BVDV type 1a (NADL strain), 1b (TGAC strain), and 2a (125 strain) using the VN assay. All titers were reported as the reciprocal of the greatest dilution of serum to provide complete protection of cells. For the individual titer analysis (ISUVDL), the least dilution of serum tested was 1:2, whereas the greatest dilution tested was 1:4096. Serum that did not provide complete protection at the 1:2 level was reported as <2 and was considered seronegative to BVDV type 1a. Samples with a reported serum neutralization value of ≥2 were considered seropositive to BVDV type 1a. For the pooled titer analysis (TVMDL), the least dilution of serum tested was 1:4, whereas the greatest dilution tested was 1:1024. For both titer analyses, the reported values were log2 transformed before statistical analysis. Differences among BVDV subgenotype 1a, 1b, and 2a titer responses were evaluated to provide field-study insight of antigenic differences for the 3 BVDV genotype strains tested after MLV respiratory vaccination and exposure to PI-BVDV type 1b pen mate.
Persistently Infected Cattle
Two groups of animals that had been previously ear-notched, tested at a commercial laboratory (Cattle Stats), and identified as positive for PI-BVDV according to ACE method were acquired from a stocker cattle operation in Washington County, OK, to be used as PI-BVDV exposure sources. Upon arrival to the RU, each PI-BVDV animal was ear-notched a second time, and samples were shipped via overnight parcel service to a different laboratory [Oklahoma Animal Disease Diagnostic Lab, Stillwater, OK or USDA, ARS, National Animal Disease Center (NADC), Ames, IA] for rapid affirmation of positive PI-BVDV status using the same ACE procedure. Group 1 (n = 10) was assembled before trial initiation and used for shipment block 1 and 2, whereas group 2 (n = 9) was assembled before beginning block 3 and used for block 3 and 4 of the trial. Depending on block, 4 to 8 PI-BVDV challenge calves were assigned randomly to 1 of 4 or 1 of 8 PI-designated pens by drawing pen assignment number. An appropriate number of PI-BVDV calves were assembled for each group to allow for available alternates if an originally designated PI-BVDV animal died. Each PI-BVDV calf that died during the trial (Group 1, n = 2; Group 2, n = 1) was replaced immediately with a confirmed PI-positive alternate. Additionally, anticoagulated blood was collected from each PI-BVDV challenge animal and shipped on ice via overnight parcel service to NADC for subsequent differentiation of BVDV subgenotype strain using a method of virus isolation of buffy coat cells and phylogenetic analysis previously described by Ridpath et al. (2011). The subgenotype strain of the 2 PI-positive alternates from Group 1 was not determined and therefore unknown. Of the PI-BVDV calves acquired for use as exposure sources in the current study and subgenotype confirmed, 94.7% (18 of 19) were identified as subgenotype 1b. One PI-BVDV animal from Group 2 was identified as subgenotype 1a, and the pen in which this animal was assigned was removed from all statistical analyses. It is important to note that BVDV type 1b, the subgenotype strain in which the PI-BVDV calves used in this experiment were identified, is the predominant BVDV subgenotype strain isolated from cattle in the US (Fulton et al., 2002; Ridpath et al., 2010); however, the pentavalent MLV respiratory vaccine used in this study contained only BVDV type 1a and 2a strains because currently no commercially licensed MLV respiratory vaccine in the US contains a BVDV 1b isolate.
Pen Assignment and Arrangement
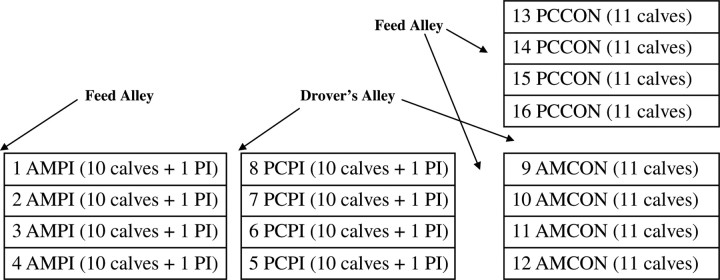
To avoid unwanted PI-BVDV fence-line or water source contact with CON, 0.45-ha mixed-grass receiving pens were arranged spatially before treatment allocation (Figure 1). The spatial pen arrangement used in the current study may have contributed to confounding effects of location within the RU facility; however, we determined the potential confounding effects of fence-line and water source contact between PI and CON to be more significant. Pens measured 30.5 × 152 m, contained 5 m of feed bunk-line, and a fence-line water source shared with an adjacent pen of the same treatment. Furthermore, the treatment pens were configured so that unlike treatment pens were separated by either a 3-m drovers or 5-m feed alley. Within management group (AM or PC), calves were stratified by gender (AM only) and d −1 BW, then assigned randomly to 1 of 2 or 1 of 4 PI or CON pens depending on block (8 to 11 calves/pen) resulting in total experimental unit (pen) replication of 14, 14, 12, and 11 for AMCON, AMPI, PCCON, and PCPI, respectively. For PI treatments, a PI-BVDV type 1b challenge animal was assigned randomly to each PI-designated treatment pen such that pens within block had an equivalent number of cattle. During all weighing and BRD evaluation procedures, CON treatments were evaluated first, followed by PI treatments, to avoid unwanted CON contact with PI challenge animals or experimental cattle in the PI treatments and to reduce potential exposure to fomites contaminated with body fluids or fecal material containing BVDV. Because of spatial treatment arrangement and the necessity to evaluate CON followed by PI, morbidity investigators were not blinded to experimental treatment.
Figure 1.
Illustration (not to scale) of spatial treatment arrangement of newly received calves located at UA Experiment Station (RU). AMCON = auction market, control; AMPI = auction market, exposed to a persistently infected (PI) bovine viral diarrhea virus (PI-BVDV) pen mate; PCCON = preconditioned, control; PCPI = preconditioned, exposed to a PI-BVDV pen mate.
Evaluation and Treatment of BRD
Calves were observed daily for clinical signs of BRD (depression, nasal discharge, ocular discharge, cough, gaunt appearance, inappetance) by 2 experiment station personnel with a combined 35-yr experience evaluating cattle with BRD. If ≥2 visual signs existed, calves were brought to the restraining chute, weighed, and rectal temperature was recorded via a digital thermometer (GLA Agricultural Electronics; San Luis Obispo, CA; readability = ± 0.1°C). If rectal temperature was ≥40°C, cattle were considered morbid, administered antibiotic therapy with enrofloxacin (Baytril, Bayer Animal Health, Shawnee Mission, KS) at a dosage rate of 10 mg/kg of BW, and immediately returned to their study pen. A 48-h post-treatment interval (PTI) was implemented after administration of enrofloxacin, and a second temperature was recorded upon expiration of the initial antibiotic PTI. If the second temperature was ≥40°C, a second antibiotic treatment with florfenicol (Nuflor, Schering-Plough Animal Health, Summit, NJ) was administered at a dosage rate of 40 mg/kg of BW. A 48-h PTI was also implemented for cattle administered florfenicol and rectal temperature was evaluated upon expiration of the second antibiotic PTI. If the temperature was ≥40°C, a third and final antibiotic treatment with ceftiofur HCl (Excenel RTU, Pfizer Animal Health, New York, NY) was administered at a dosage rate of 2.2 mg/kg of BW and repeated for 2 consecutive days after the initial injection of ceftiofur HCl. If at any time rectal temperature was <40°C, the animal was left untreated and returned immediately to the study pen until ≥2 subsequent signs warranted re-examination. Treatment data were recorded for individual animal including treatment date, rectal temperature and the amount (mL) of each antibiotic administered.
CALCULATIONS AND STATISTICAL ANALYSES
Calculations
Average antibiotic treatment cost was calculated using a fixed cost of $0.65/mL for enrofloxacin, $0.47/mL for florfenicol, and $0.65/mL for ceftiofur HCl. An animal was classified as chronically ill if 3 antibiotic treatments were administered coupled with ≤0.45 kg ADG for the entire 42-d trial. The BRD relapse rate was determined by dividing the number of calves in each experimental treatment treated with a second antibiotic by the total number of calves in each experimental treatment treated for BRD.
Statistical Analyses
Performance and morbidity data were analyzed as a randomized complete block design using the MIXED procedure (SAS Inst., Inc., Cary, NC). Pen was considered the experimental unit. The class statement included block (date of arrival), treatment, and replicate. Block was considered a random effect in the statistical model. Single degree of freedom orthogonal contrasts evaluating effects of management (PC or AM), exposure (PI or CON), and their interaction were used. If the interaction was significant (P ≤ 0.10), treatment means were separated with a t-test using the PDIFF option in SAS. For main effects of management and exposure, a P-value ≤ 0.05 was considered statistically significant. For BVDV titer and leukocyte repeated measures data, the model included treatment, day, and treatment × day. Block, treatment, day, and pen were used in the class statement. Block was the random variable, pen within block was the subject, and the repeated statement was day. The covariance model structure used was AR(1). Contrasts for the repeated measures data included management, exposure, and management × exposure interaction. The Pearson correlation coefficient between BRD morbidity and d 0 BVDV titer concentration was generated using the CORR procedure of SAS.
RESULTS AND DISCUSSION
Animal Performance
No treatment interactions (P ≥ 0.10) were observed for animal performance (Table 2). Main effects of management were frequently observed for growth performance; PC steers had greater (P < 0.001) ADG compared with AM calves from d 0 to 14 (1.65 vs. 0.66 kg/d), d 0 to 28 (1.28 vs. 0.77 kg/d), and d 0 to 42 (1.20 vs. 0.85 kg/d). This indicates that weaning management intended to improve disease resistance and mitigate physiological stress of beef calves results in greater BW gain performance during the stocker receiving period; however, commingling and additional stressors resulting from the marketing process experienced by AM, but not PC, should be considered. Our observation of improved performance for preconditioned calves is in agreement with other reports in the literature. Step et al. (2008) compared health and performance of beef calves from a single-source ranch using a preconditioning management strategy (Ranch), to calves procured and assembled through multiple auction markets (Market), or a commingled group (COMM) containing a portion of Ranch and Market origin cattle. In that study, regardless of weaning or vaccination status, calves originating from Ranch tended to have greater ADG than COMM or Market calves for the 42-d receiving period. In a trial conducted by Clark et al. (2006), low-risk preconditioned calves had greater ADG during the finishing period than high-risk auction market calves of unknown history; however, no differences in performance were observed during the 28-d receiving period. Seeger et al. (2008) observed that auction market steers of unknown origin or health history gained 0.18 kg/d less during a finishing period than weaned beef calves receiving a health protocol on their origin ranch before marketing. Pritchard and Mendez (1990) suggested that non-preconditioned calves may fully compensate for initial performance loss by the end of the feeding period; however, the non-preconditioned cattle used in that experiment were maintained as a single-source and were not subjected to stressors associated with the auction market system.
Table 2.
Effects of weaning management and persistently infected bovine viral diarrhea virus exposure on performance of newly received beef calves
| Auction market | Preconditioned | Contrast,1P = | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Control | PI-exposed | Control | PI-exposed | SEM | Management | Exposure | Interaction |
| No. pens2 | 14 | 14 | 12 | 11 | ||||
| BW, kg | ||||||||
| Initial | 249 | 248 | 250 | 249 | 9.26 | 0.36 | 0.43 | 0.58 |
| d 14 | 257 | 258 | 273 | 273 | 9.75 | < 0.001 | 0.60 | 0.72 |
| d 28 | 270 | 270 | 285 | 286 | 9.09 | < 0.001 | 0.57 | 0.73 |
| d 42 | 286 | 283 | 301 | 299 | 7.51 | < 0.001 | 0.19 | 0.71 |
| ADG, kg | ||||||||
| d 0 to 14 | 0.54 | 0.77 | 1.64 | 1.66 | 0.19 | < 0.001 | 0.16 | 0.20 |
| d 0 to 28 | 0.74 | 0.81 | 1.25 | 1.32 | 0.15 | < 0.001 | 0.10 | 0.97 |
| d 28 to 42 | 1.10 | 0.89 | 1.13 | 0.90 | 0.15 | 0.77 | 0.003 | 0.87 |
| d 0 to 42 | 0.86 | 0.84 | 1.21 | 1.18 | 0.10 | < 0.001 | 0.29 | 0.99 |
1Management = main effect of weaning management (auction market vs. preconditioned); Exposure = main effect of PI-BVDV exposure (control vs. persistently infected bovine viral diarrhea virus exposure); Interaction = management × exposure.
2Each pen consisted of 8 to 11 animals depending on block.
Overall ADG from d 0 to 42 was not affected (P = 0.29) by the presence of a PI-BVDV pen mate; however, during the final interim period (d 28 to 42), PI-BVDV exposure resulted in a 0.22 kg/d decrease (P = 0.003) in ADG. The performance loss observed from d 28 to 42 agrees with the findings of Hessman et al. (2009), in which cattle directly exposed to a PI-BVDV animal gained 0.15 kg/d less BW than non-exposed cattle during a 66 d feedlot starter period. Elam et al. (2008) observed a tendency for PI-BVDV-exposed cattle to gain less BW from d 0 to 28, but overall ADG was not affected by PI-BVDV exposure in that study. Performance from d 0 to 28 was not affected (P = 0.10) by PI-BVDV exposure in our trial; nevertheless, during the initial weeks of our study, PI-BVDV animals may have unintentionally served as a trainer animal because they had been previously acclimated to the RU. In a series of 4 trials, Loerch and Fluharty (2000) observed increases in eating behavior and variable ADG for newly received calves with a trainer cow present in the pen during the initial 14 d, but overall (28-d) differences in growth were undetectable in 3 of 4 trials. Another consideration is that short-term exposure to a low-virulent BVDV strain from a PI pen mate could imitate an unattenuated autogenous vaccination resulting in undetectable or small performance differences during the initial weeks of PI-BVDV exposure. However, as PI-BVDV exposure continued, a progressive effect on host immune activity or inflammation may have resulted in the performance loss observed from d 28 to 42 because repeated immune stimulation results in nutrients being preferentially used for immune and homeostatic pathways rather than tissue deposition (Klasing and Korver, 1997; Spurlock, 1997). Furthermore, the catabolic effects of proinflammatory cytokine stimulation from continuous PI-BVDV exposure may have inhibited growth (Johnson, 1997) from d 28 to 42 in our study. Therefore, performance consequences in newly received cohorts exposed to a PI-BVDV pen mate may be delayed several weeks because immunomodulation resulting from PI-BVDV exposure and these potential effects on growth is progressive. Alternatively, the immunomodulatory effects of PI-BVDV exposure may have a more indirect effect on growth via increased susceptibility of cohorts to complicated infections with other viruses or bacteria involved in BRD, thereby, decreasing health and performance (Gardner et al., 1999). Nevertheless, our ADG results suggest there may be a significant metabolic cost associated with continuous exposure to a PI animal shedding a low-virulence BVDV strain. The performance loss may not be immediate but rather several weeks subsequent when nutrients used for humoral and cell-mediated immunity or when catabolic effects of proinflammatory cytokine stimulation associated with repeated BVDV exposure presumably reach a threshold effect on growth performance. Because BW measurements were not recorded beyond the 42-d receiving period, the duration of performance loss and whether there was compensatory gain during the finishing phase is unknown and warrants further investigation. Published research evaluating PI-BVDV exposure effects on performance is both limited and conflicting; therefore, additional studies with careful consideration of management and health history of experimental cattle, differences in the virulence and subgenotype strain of PI-BVDV challenge animals, and epidemiological factors are required to determine effects of PI-BVDV exposure on performance throughout the various stages of the beef production system. Furthermore, a better understanding of metabolic responses to physiological alteration and immunomodulation from PI-BVDV exposure is needed.
Animal Health
During the pretrial preconditioning phase, 2 of 236 (0.8%) PC steers required antibiotic treatment on their origin ranch (data not shown). Total BRD morbidity rate was markedly greater (P < 0.001) for AM compared with PC with 70.4 and 6.7% of calves, respectively requiring treatment for clinical BRD (Table 3). Furthermore, a greater percentage of AM calves required treatment with a second (P < 0.001) or third (P = 0.001) antibiotic. Our observations of a management effect on BRD morbidity were similar to Clark et al. (2006) in which BRD morbidity rates were 64.4 and 2.0% in steers considered either high- or low-risk for developing signs of BRD, respectively. Other studies (Macartney et al., 2003; Seeger et al., 2008) reported that calves of unknown health history had greater morbidity compared with those administered health protocols before marketing. Similarly, Roeber et al. (2001) observed that cattle originating from a preconditioning program had fewer hospital visits than cattle originating from auction markets. Step et al. (2008) reported morbidity being 11.1% for ranch-origin and 41.9% for auction-market calves. Within the ranch-origin calves, those weaned and shipped directly to the feedlot had greater morbidity compared with those kept on the ranch 45 d; however, vaccination with a MLV respiratory vaccine at weaning did not reduce morbidity during the receiving period among the 2 treatments kept on the ranch 45 d. This observation may suggest that weaning management had a more profound effect on reducing subsequent morbidity than did administration of a MLV respiratory vaccine.
Table 3.
Effects of weaning management and persistently infected bovine viral diarrhea virus (PI-BVDV) exposure on health of newly received beef calves
| Auction market | Preconditioned | Contrasts,1P = | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Control | PI-exposed | Control | PI-exposed | SEM | Management | Exposure | Interaction |
| No. pens2 | 14 | 14 | 12 | 11 | ||||
| Total BRD morbidity3, % | 67.2 | 73.7 | 7.7 | 5.7 | 4.30 | < 0.001 | 0.50 | 0.22 |
| Treated once, % | 33.1 | 30.2 | 3.2 | 0 | 4.00 | < 0.001 | 0.40 | 0.96 |
| Day of first treatment | 3.0 | 2.7 | 2.5 | 0.9 | 1.47 | 0.23 | 0.33 | 0.49 |
| Treated twice, % | 26.0 | 25.9 | 1.1 | 0.6 | 3.78 | < 0.001 | 0.94 | 0.95 |
| Day of second treatment | 7.3 | 6.2 | 4.0 | 2.6 | 1.65 | 0.007 | 0.30 | 0.92 |
| Treated thrice, % | 8.0 | 17.5 | 3.2 | 4.9 | 3.08 | 0.002 | 0.04 | 0.15 |
| Day of third treatment | 10.7 | 11.1 | 7.1 | 6.1 | 3.07 | 0.08 | 0.90 | 0.76 |
| Relapse4, % | 50.6 | 58.5 | 60.3 | 100 | 20.78 | 0.07 | 0.09 | 0.26 |
| Chronically ill5, % | 1.1b | 7.6a | 0.4b | 0.3b | 2.26 | 0.03 | 0.07 | 0.06 |
| Antibiotic cost, $/calf | 18.49 | 22.55 | 2.31 | 2.65 | 2.01 | < 0.001 | 0.10 | 0.16 |
a,bMeans within a row without a common superscript are different (P < 0.05).
1Management = main effect of weaning management (auction market vs. preconditioned); Exposure = main effect of PI-BVDV exposure (control vs. PI-BVDV exposure); Interaction = management × exposure.
2Each pen consisted of 8 to 11 animals depending on block.
3Percentage of calves clinically diagnosed with bovine respiratory disease (BRD) and administered antibiotic 1 or more times.
4Relapse percentage calculated by dividing the number of calves within each experimental treatment treated with a second antibiotic by the total number of calves in each experimental treatment treated for BRD.
5Cattle were classified as chronically ill if 3 antibiotic treatments were administered and ADG ≤ 0.45 kg for the entire 42-d trial.
Antibiotic treatment cost during the 42-d receiving period was increased (P < 0.001) for AM ($20.52/animal) compared with PC ($2.48/animal). Moreover, the percentage of chronically ill cattle was greater (P = 0.03) for AM. The differences observed for health variables among the 2 cattle management sources in the current study with supporting evidence from recent research would suggest clearly that single-source PC calves have fewer BRD-related health problems, improved animal well-being, and reduced antibiotic usage compared with commingled AM calves of unknown management and health history. Nevertheless, the improved health parameters observed for PC vs. AM in the current study cannot be attributed solely to preconditioning management because PC calves were not commingled or subjected to the auction market system (Taylor et al., 2010).
The total BRD morbidity rate was not affected (P = 0.50) by PI-BVDV exposure; however, treatment with a third antibiotic occurred more often (P = 0.04) for PI-BVDV-exposed cohorts. Although a treatment interaction was not significant (P = 0.15) for the number of cattle treated thrice, the numerical difference was more pronounced within AM calves. The AMPI had 9.5% more calves treated thrice than AMCON, whereas PCPI had only 1.7% more calves treated thrice than PCCON. A treatment interaction (P = 0.06) was observed for the percentage of chronically ill animals; AMPI had the greatest number of chronically ill calves (7.6%), AMCON was intermediate (1.1%), and PCCON and PCPI were least (0.4 and 0.3%, respectively). This observation suggests that weaning management and exposure to a PI-BVDV pen mate affect health additively because percentage of chronically ill was greatest for AMPI, with no difference among PCCON and PCPI. A numerical, but not statistical difference (P = 0.10), was observed for PI-BVDV-exposed cohorts having an increased antibiotic treatment cost which averaged $12.59 and $10.40/animal for PI and CON, respectively. Within AM cohorts, AMPI had an antibiotic treatment cost of $4.06/animal more than AMCON; this numerical increase was similar to the estimated PI-BVDV testing cost ($4.25/sample including next day parcel service fees). Our observations among AMPI and AMCON agree with another study using high-risk calves of unknown history (Hessman et al., 2009) in that overall morbidity was not different for direct PI-exposed or unexposed groups; however, percentage of first relapse, chronic illness, and fatalities were greater for the direct PI-exposed groups. In that trial, treatment cost/animal was not statistically different (P = 0.24), but a numerical increase of $1.19/animal was observed for direct PI-exposed groups. Loneragan et al. (2005) determined that cattle exposed to a PI-BVDV animal had a 43% greater risk of initial BRD treatment and clinically ill cattle with a PI-BVDV animal in the pen were administered more antibiotic treatments than non-exposed pens (1.76 vs. 1.46). Conversely, Booker et al. (2008) observed no differences in BRD health among PI- and non-PI-exposed pens, whereas O'Conner et al. (2005) observed that disease prevalence was reduced in pens containing low-risk, single-source cattle with a PI-BVDV calf present. Elam et al. (2008) used low-risk, preconditioned heifers that also received antibiotic metaphylaxis on-arrival and reported no difference in health with either short- (60 h) or long-term (duration of study) exposure to a PI-BVDV pen mate. Their observation of no health differences for PI-exposed cohorts using low-risk, preconditioned heifers would agree with our observation of few health differences between PCCON and PCPI treatments. In a feedlot study using auction-derived cattle (Stevens et al., 2007), morbidity was increased for pens with short-term (13 to 18 d) exposure to PI-BVDV. Conversely, a subsequent study conducted in the same feedlot facility (Stevens et al., 2009) in which the PI animal was removed more rapidly (≤d 2) and overall morbidity and mortality were considerably less, initial BRD treatment was reduced for PI-exposed pens.
Rationale for conflicting animal health observations in PI-BVDV-exposed cohorts reported in the literature is explained 3-fold: 1) previous management and health history of cattle used within or among scientific experiments is varied; thus, susceptibility of experimental calves to PI-BVDV exposure differ, 2) the virulence of BVDV strain transmitted by PI challenge animals, in addition to the exposure rate and duration of PI-BVDV challenge differs among experiments, and 3) variation of the complex population dynamics of disease transmission and protection of a given experimental pen or treatment group exists (Stokka, 2010). Our observation of chronically ill cattle being most prevalent for AMPI, with less pronounced animal health differences observed among PCPI and PCCON treatments, suggests that previous management and vaccination against BVDV and other respiratory pathogens may afford some degree of cohort tolerance to a PI-BVDV pen mate. The extent that PI-BVDV exposure affected economically important traits related to BRD morbidity in the current model was affected by previous management and health history in newly received calves, suggesting that the 2 factors are additive. Therefore, the decision to invest in a PI-BVDV control program for stocker or feedlot production systems should be based partly upon the perceived BRD risk level of each group of newly received calves.
BVDV Antibody Titers
BVDV type 1a Individual Titer Analysis
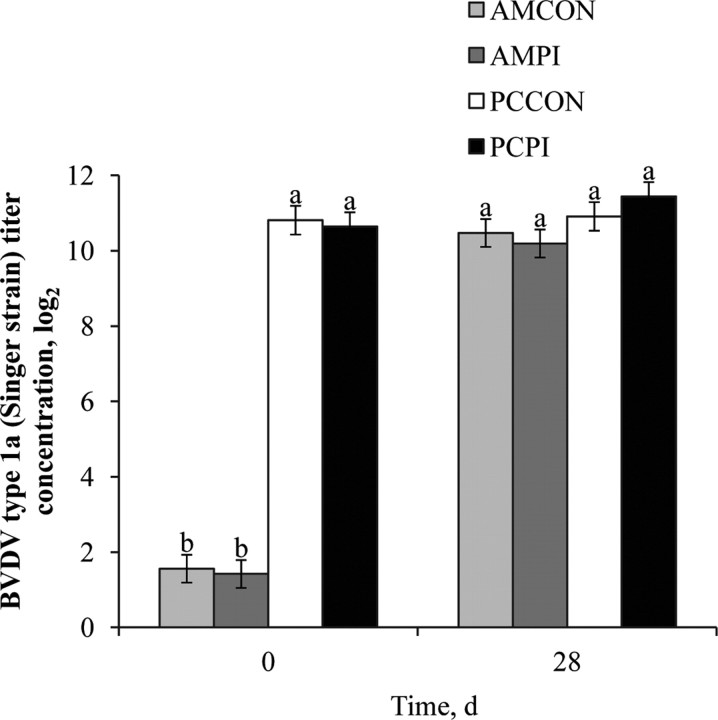
Upon arrival at the RU, BVDV type 1a (Singer strain) antibody titers were markedly greater (treatment × day, P < 0.001; Figure 2) for PC, and percentage seropositive on d 0 was 100% for PC vs. 23% for AM. The difference in antibody titers against BVDV type 1a on d 0 suggests that AM calves were relatively naïve, and PC calves had greater humoral immune protection against BVDV challenge. Furthermore, greater serum antibody titers against BVDV type 1a on d 0 negatively correlated (P < 0.001; correlation coefficient = 0.52) with subsequent clinical BRD morbidity in our trial. Previous serological data (Martin et al., 1999; O'Connor et al., 2001) also indicate that greater serum antibody titers against BVDV upon arrival at the feedlot correspond with a subsequent reduction in risk for clinical BRD. Bolin and Ridpath (1995) observed that calves with greater titer levels of passively acquired viral neutralizing antibody in serum had reduced duration and severity of clinical disease after intranasal BVDV challenge. In another trial (Fulton et al., 2006), vaccination with 2 doses of MLV respiratory vaccine containing both BVDV type 1a and 2a isolates provided protection against viremia in calves exposed to PI-BVDV type 2a challenge animals during a 35-d observation period. However, it has also been suggested that decreased serum concentrations of pre-existing antibodies, particularly those heterologous to the subgenotype strain shed by PI challenge animals, may not be sufficient to provide complete protection (Fulton et al., 2005).
Figure 2.
Effects of weaning management and persistently infected bovine viral diarrhea virus (PI-BVDV) exposure on individual serum antibody concentrations against BVDV type 1a (Singer strain). Effect of treatment × day (P < 0.001), day (P < 0.001), and management (P < 0.001). Means within a day without a common superscript differ (P ≤ 0.05). AMCON = auction market, control; AMPI = auction market, exposed to a PI-BVDV pen mate; PCCON = preconditioned, control; PCPI = preconditioned, exposed to a PI-BVDV pen mate.
By d 28, BVDV type 1a titer concentrations were similar for all treatments; however, the average incidence of first, second, and third BRD treatment episode was d 2.3, 5, and 8.8, respectively, with fewer BRD episodes occurring after d 28. It should be considered that AM received identical vaccinations and processing procedures as PC, only the timing of administration differed, suggesting that MLV respiratory vaccination before market-associated stress and pathogen exposure is critical to achieve sufficient antibody titer concentrations before the initial 14 d of receiving when BRD is most frequent. Furthermore, our observations lend evidence that on-arrival MLV respiratory vaccination in recently vaccinated PC calves may be redundant because the PC steers in our study were not revaccinated on d 0, yet 100% were seropositive for BVDV type 1a on d 0 and BRD morbidity for PC calves during the 42-d receiving period was low (7.2 %).
BVDV type 1a, 1b, and 2a Pooled Titer Analysis
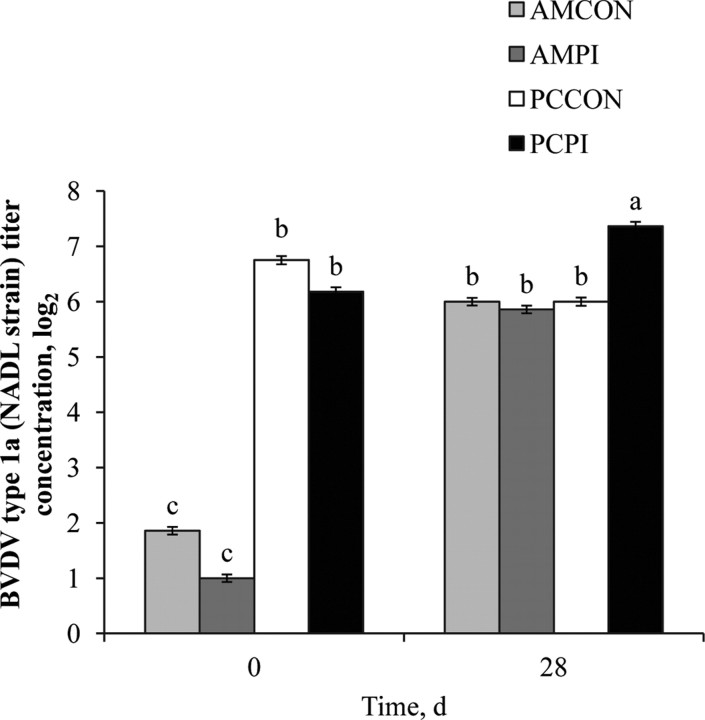
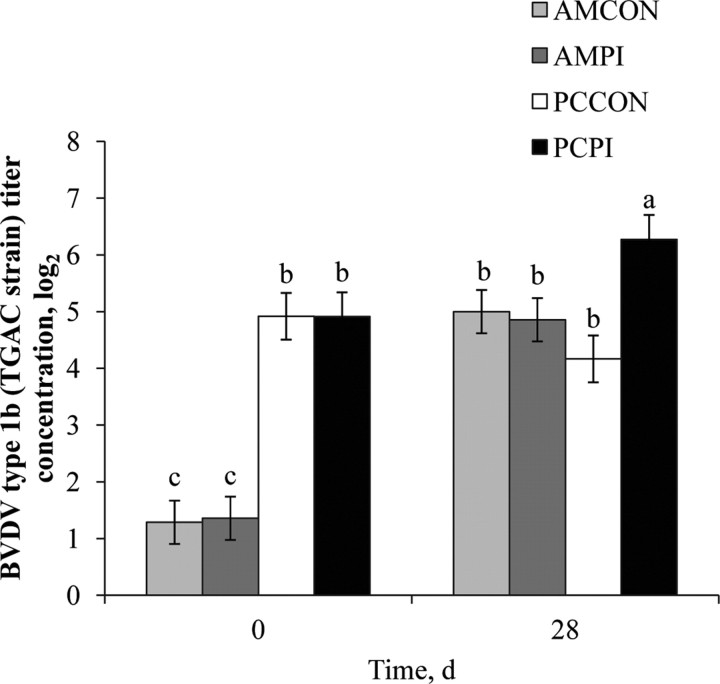
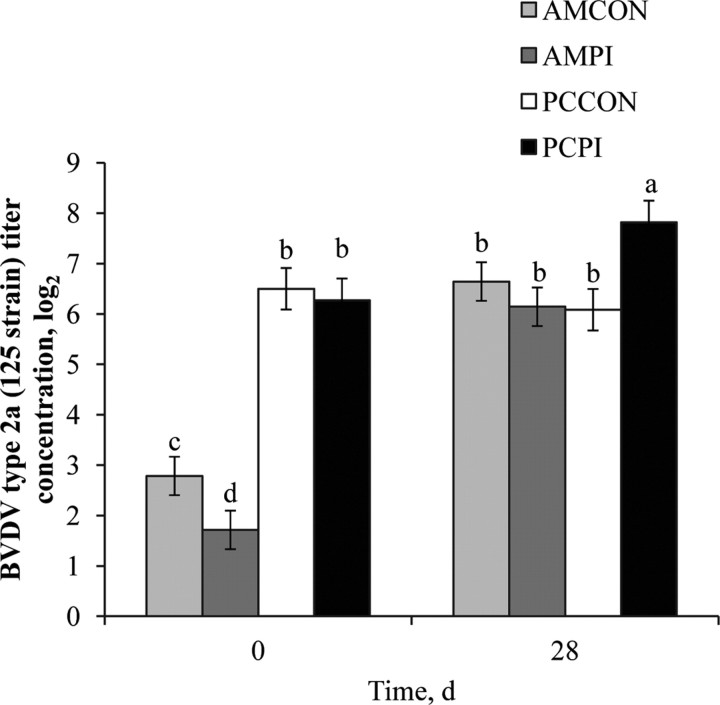
Similar to results of the individual titer analysis, a management effect (P < 0.001) was observed for pooled BVDV type 1a (NADL strain; Figure 3), BVDV type 1b (TGAC strain; Figure 4), and BVDV type 2a (125 strain; Figure 5) titers; each antibody titer concentration was greater for PC compared with AM. After 28-d continuous exposure to a PI-BVDV type 1b pen mate, a main effect of exposure was not observed for BVDV type 1a (P = 0.88), 1b (P = 0.11), or 2a (P = 0.96) titer concentrations. However, a treatment × day interaction was observed (P < 0.001) for each of the 3 BVDV subgenotype titers analyzed. On d 28, log titer concentrations for BVDV type 1a, 1b, and 2a were increased 1.4, 2.1, and 1.7 logs, respectively, for PCPI vs. PCCON. Therefore, continuous exposure to a PI-BVDV pen mate stimulated the humoral immune response in PC cohorts, and the magnitude of increase was greatest for the homologous BVDV strain (type 1b). Burciaga-Robles et al. (2010) observed increased antibody titers to BVDV type 1b (TGAC strain) but not type 1a (Singer strain) or 2a (125 strain) when steers were exposed to 2 PI-BVDV type 1b challenge animals for 72 h. In a study comparing virus neutralization results between BVDV strains belonging to different subgenotypes, Ridpath et al. (2010) reported that the ratio of virus cross-neutralization among BVDV type 1a and 1b is 36%. Similar increases in titer concentrations after 28-d PI-exposure were not evident (P ≥ 0.05) for AMPI; however, the MLV respiratory vaccine administered on d 0 and 14 to AM calves, which contained BVDV type 1a and 2a isolates, likely confounded antibody titer response to PI-BVDV type 1b exposure. Nevertheless, increases in serum antibody titers associated with PI-BVDV-exposed cohorts may be greater for the homogenous subgenotype strain shed by a PI-BVDV pen mate.
Figure 3.
Effects of weaning management and persistently infected bovine viral diarrhea virus (PI-BVDV) exposure on pooled serum antibody concentrations against BVDV type 1a (NADL strain). Effect of treatment × day (P < 0.001), day (P < 0.001), and management (P < 0.001). Means without a common superscript differ (P ≤ 0.05). AMCON = auction market, control; AMPI = auction market, exposed to a PI-BVDV pen mate; PCCON = preconditioned, control; PCPI = preconditioned, exposed to a PI-BVDV pen mate.
Figure 4.
Effects of weaning management and persistently infected bovine viral diarrhea virus (PI-BVDV) exposure on pooled serum antibody concentrations against BVDV type 1b. Effect of treatment × day (P < 0.001), day (P < 0.001), and management (P < 0.001). Means without a common superscript differ (P ≤ 0.05). AMCON = auction market, control; AMPI = auction market, exposed to continuous PI-BVDV challenge; PCCON = preconditioned, control; PCPI = preconditioned, exposed to a PI-BVDV pen mate.
Figure 5.
Effects of weaning management and persistently infected bovine viral diarrhea virus (PI-BVDV) exposure on pooled serum antibody concentrations against BVDV type 2. Effect of treatment × day (P < 0.001), day (P < 0.001), and management (P < 0.001). Means without a common superscript differ (P ≤ 0.05). AMCON = auction market, control; AMPI = auction market, exposed to a PI-BVDV pen mate; PCCON = preconditioned, control; PCPI = preconditioned, exposed to a PI-BVDV pen mate.
Hematology
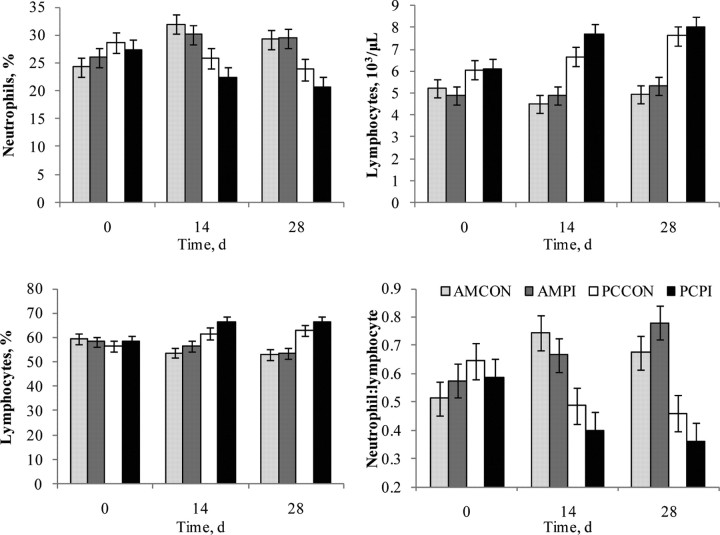
Effects of management (P < 0.001; Table 4) and day (P < 0.001) were observed for total PBL concentration. Total PBL averaged 10.9 and 8.6 × 103/μL for PC and AM treatments, respectively, and total PBL concentration increased overall with time. Our observation of total PBL being greater, and BRD morbidity being least for PC, suggests total PBL concentration may correspond with stress, clinical BRD, or both; however, clarification of potential stress/disease interactions on total PBL concentration is needed. Treatment × day interactions were observed for percentage neutrophils (P = 0.002), percentage lymphocytes (P = 0.004), lymphocyte count (P = 0.02), and neutrophil:lymphocyte ratio (P < 0.001; Figure 6). Neutrophils increased for AM but not for PC calves (management effect, P < 0.001), whereas lymphocyte count and percentage increased for PC but not AM as the study progressed (management effect, P < 0.001). This resulted in the overall neutrophil:lymphocyte ratio (N:L) being greater (P < 0.001) for AM calves; as the trial progressed, N:L increased for AM but decreased for PC (treatment × day, P < 0.001). Other studies (Kegley et al., 1997; Ishizaki and Kariya, 2010) report transient increases in N:L for cattle experiencing transport stress; however, an additive effect from additional stressors also shown to increase N:L such as weaning (Hickey et al., 2003) and commingling (McGlone et al., 1993) may explain the extended duration of increased N:L observed for the AM calves in our study. Respiratory pathogens can also modulate total and differential PBL in cattle; challenge with BVDV, Manheimia haemolytica, or both were reported to affect several PBL concentrations (Burciaga-Robles et al., 2010).
Table 4.
Overall effects of weaning management and persistently infected bovine viral diarrhea virus exposure on hematology of newly received beef calves sampled on d 0, 14, and 28
| Auction market | Preconditioned | Contrasts,1P = | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Control | PI-exposed | Control | PI-exposed | SEM | Management | Exposure | Interaction |
| No. pens2 | 14 | 14 | 12 | 11 | ||||
| Total PBL3, n × 103/μL | 8.53 | 8.68 | 10.77 | 10.98 | 0.39 | < 0.001 | 0.62 | 0.94 |
| Neutrophils, n/μL | 2,344 | 2,407 | 2,599 | 2,403 | 161 | 0.41 | 0.66 | 0.39 |
| Neutrophils, % | 28.4 | 28.5 | 26.1 | 23.4 | 1.22 | 0.002 | 0.27 | 0.24 |
| Lymphocytes, n/μL | 4,886 | 5,040 | 6,784 | 7,255 | 358 | < 0.001 | 0.35 | 0.64 |
| Lymphocytes, % | 55.3 | 56.1 | 60.2 | 63.6 | 1.60 | < 0.001 | 0.18 | 0.38 |
| Neutrophil:lymphocyte | 0.64 | 0.67 | 0.53 | 0.45 | 0.04 | < 0.001 | 0.47 | 0.13 |
| Monocytes, n/μL | 1,071 | 1,021 | 1,100 | 1,030 | 64.2 | 0.75 | 0.32 | 0.87 |
| Monocytes, % | 13.3 | 12.8 | 10.8 | 10.1 | 0.57 | < 0.001 | 0.28 | 0.89 |
| Eosinophils, n/μL | 141 | 120 | 178 | 185 | 24.0 | 0.03 | 0.75 | 0.52 |
| Eosinophils, % | 1.7 | 1.4 | 1.7 | 1.8 | 0.23 | 0.38 | 0.58 | 0.39 |
| Red blood cells, n × 106/μL | 9.60 | 9.86 | 9.75 | 9.92 | 0.14 | 0.40 | 0.10 | 0.71 |
| Hemoglobin, g/100 mL | 11.64 | 11.95 | 11.88 | 12.08 | 0.15 | 0.18 | 0.07 | 0.69 |
| Hematocrit, % | 34.9 | 35.9 | 35.5 | 36.0 | 0.44 | 0.36 | 0.09 | 0.59 |
| Platelets, n × 103/μL | 555.6 | 542.1 | 459.3 | 461.6 | 29.0 | 0.002 | 0.84 | 0.77 |
1Management = main effect of weaning management (auction market vs. preconditioned); Exposure = main effect of PI (persistently infected) -BVDV (bovine viral diarrhea virus) exposure (control vs. PI-BVDV exposure); Interaction = management × exposure.
2Each pen consisted of 8 to 11 animals depending on block.
3Total number of peripheral blood leukocytes (PBL; includes neutrophils, lymphocytes, monocytes, eosinophils, and basophils)
Figure 6.
Effects of weaning management and persistently infected bovine viral diarrhea virus (PI-BVDV) exposure on hematology of newly received beef calves. AMCON = auction market, control; AMPI = auction market, exposed to a PI-BVDV pen mate; PCCON = preconditioned, control; PCPI = preconditioned, exposed to a PI-BVDV pen mate. Neutrophils, %: effect of treatment × day (P = 0.002) and management (P = 0.002). Lymphocytes, %: effect of treatment × day (P = 0.004) and management (P < 0.001). Lymphocytes: effect of day (P = 0.005), treatment × day (P = 0.02), and management (P < 0.001). Neutrophil:lymphocyte: effect of treatment × day (P = 0.001) and management (P < 0.001).
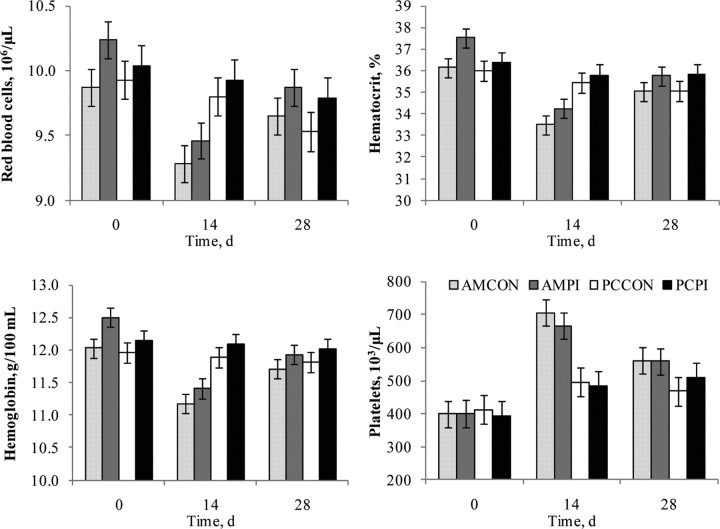
A treatment × day interaction was observed for concentrations of red blood cells (P < 0.001), hemoglobin (P < 0.001), platelets (P = 0.03), and percentage hematocrit (P < 0.001; Figure 7). A tendency for an exposure effect occurred for hemoglobin (P = 0.07) and hematocrit (P = 0.09); mean values were greater for PI-exposed cohorts. A management effect (P < 0.001) was observed for platelet concentration; AM had greater platelets on d 14 and 28, which may indicate the presence of increased inflammation or a more stimulated cell-mediated immune response because platelets are an abundant source of CD154, a signaling molecule for T- and B-cell activation (Sowa et al., 2009). Our observations of greater clinical BRD for AM, and the timeframe that clinical morbidity occurred supports the association among platelet level and cell-mediated immune stimulation because the majority of clinical BRD occurred by d 14, which correlates with peak platelet concentrations observed on d 14. Further research is needed to understand the effects of stress and PI-BVDV exposure on PBL and platelet concentrations and to determine the validity of either as a biomarker for stress and disease in cattle.
Figure 7.
Effects of weaning management and persistently infected bovine viral diarrhea virus (PI-BVDV) exposure on hematology of newly received beef calves. AMCON = auction market, control; AMPI = auction market, exposed to a PI-BVDV pen mate; PCCON = preconditioned, control; PCPI = preconditioned, exposed to a PI-BVDV pen mate. Red blood cells: effect of day (P < 0.001), treatment × day (P < 0.001), and exposure (P = 0.10). Hemoglobin: effect of day (P < 0.001), treatment × day (P < 0.001), and exposure (P = 0.07). Hematocrit, %: effect of day (P < 0.001), treatment × day (P < 0.001), and exposure (P = 0.09). Platelets: effect of day (P < 0.001), treatment × day (P = 0.03), and management (P = 0.002).
In conclusion, the health management history of a particular pen or group of cattle and whether they are exposed to a PI-BVDV pen mate may be important aspects that affect the duration and severity of clinical BRD because chronically ill cattle were greatest for AMPI. Variation of the health management history of experimental animals among, or within, studies may explain conflicting health and performance results of PI-BVDV exposure in the published literature and may confound results if unaccounted. Additional research considering these issues is needed to determine if PI-BVDV testing and removal in newly received calves is economically justified for different beef production systems. Furthermore, BW gain performance was reduced from d 28 to 42 for PI-exposed cohorts suggesting a metabolic cost associated with immunomodulation from exposure to a PI-BVDV pen mate; however, the duration of performance loss and whether compensatory BW gain occurs during the finishing phase was unknown because the study ended on d 42. Our results suggest that minimal effects on health of single-source, PC steers exposed to a PI-BVDV pen mate during a 42-d receiving period do not justify the costs incurred for labor, shipping, and laboratory testing of ear-notch samples and removal of PI-positive animals. Nevertheless, high-risk, commingled, AM cattle that are naïve to various BRD pathogens and experience greater clinical BRD morbidity may warrant testing and removal of PI-BVDV animals because an additive effect of management and PI-exposure was evident for percentage chronically ill. Supportive research from randomized controlled trials is needed to better understand the health, performance, and economic consequences of PI-BVDV exposure in cohorts from different weaning management systems.
Footnotes
Appreciation is expressed to Miller Poultry & Cattle, Gallery Ranch, Pendergrass Cattle Co., Dr. Paul Beck, and Arkansas Dept. of Correction for their cooperation and providing cattle used in the study. The authors also acknowledge Boehringer Ingelheim Vetmedica, Inc., St. Joseph MO, for product donation, USDA-ARS Poultry Production and Product Safety Research Unit, Fayetteville AR, for the use of laboratory equipment, Patricia Federico and Kathryn Fulk, USDA-ARS, National Animal Disease Center, Ames IA, for assistance with laboratory analyses and Doug Galloway, Pete Hornsby and Carlee Jamison, University of Arkansas, Fayetteville for assistance with sample collection and animal care.
LITERATURE CITED
- Bolin S.R., Ridpath J.F. 1995. Assessment of protection from systemic infection or disease afforded by low to intermediate titers of passively acquired neutralizing antibody against bovine viral diarrhea virus in calves. Am. J. Vet. Res. 56:755–759. [PubMed] [Google Scholar]
- Booker C.W., Abutarbush S.M., Morley P.S., Guichon P.T., Wildman B.K., Jim G.K., Schunicht O.C., Pittman T.J., Perrett T., Ellis J.A., Appleyard G., Haines D.M. 2008. The effect of bovine viral diarrhea virus infections on health and performance of feedlot cattle. Can. Vet. J. 49:253–260. [PMC free article] [PubMed] [Google Scholar]
- Burciaga-Robles L.O., Step D.L., Krehbiel C.R., Holland B.P., Richards C.J., Montelongo M.A., Confer A.W., Fulton R.W. 2010. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Manheimia haemolytica on clinical signs and immune variables: Model for bovine respiratory disease via viral and bacterial interaction. J. Anim. Sci. 88:2166–2178. [DOI] [PubMed] [Google Scholar]
- Clark J.H., Olson K.C., Schmidt T.B., Larson R.L., Ellersieck M.R., Alkire D.O., Meyer D.L., Rentfrow G.K., Carr C.C. 2006. Effects of respiratory disease risk and a bolus injection of trace minerals at receiving on growing and finishing performance by beef steers. Prof. Anim. Sci. 22:245–251. [Google Scholar]
- Cole N.A. 1985. Preconditioning steers for the feedlot. Vet. Clin. North Am. Food Anim. Prac. 1:401–411. [DOI] [PubMed] [Google Scholar]
- Duff G.C., Galyean M.L. 2007. Board-Invited Review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T.A. 2010. Control methods for bovine respiratory disease for feedlot cattle. Vet. Clin. Food Anim. 26:273–284. [DOI] [PubMed] [Google Scholar]
- Elam N.A., Thomson D.U., Gleghorn J.F. 2008. Effects of long- or short-term exposure to a calf identified as persistently infected with bovine viral diarrhea virus on feedlot performance of freshly weaned, transport-stressed beef heifers. J. Anim. Sci. 86:1917–1924. [DOI] [PubMed] [Google Scholar]
- Fulton R.W., Briggs R.E., Ridpath J.F., Saliki J.T., Confer A.W., Payton M.E., Duff G.C., Step D.L., Walker D.A. 2005. Transmission of bovine viral diarrhea virus 1b to susceptible and vaccinated calves by exposure to persistently infected calves. Can. J. Vet. Res. 69:161–169. [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Johnson B.J., Briggs R.E., Ridpath J.F., Saliki J.T., Confer A.W., Burge L.J., Step D.L., Walker D.A., Payton M.E. 2006. Challenge with Bovine viral diarrhea virus by exposure to persistently infected calves: protection by vaccination and negative results of antigen testing in nonvaccinated acutely infected calves. Can. J. Vet. Res. 70:121–127. [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Ridpath J.F., Saliki J.T., Briggs R.E., Confer A.W., Burge L.J., Purdy C.W., Loan R.W., Duff G.C., Payton M.E. 2002. Bovine viral diarrhea virus (BVDV) 1b: Predominant BVDV subtype in calves with respiratory disease. Can. J. Vet. Res. 66:181–190. [PMC free article] [PubMed] [Google Scholar]
- Gardner B.A., Dolezal H.G., Bryant L.K., Owens F.N., Smith R.A. 1999. Health of finishing steers: Effects on performance, carcass traits, and meat tenderness. J. Anim. Sci. 77:3168–3175. [DOI] [PubMed] [Google Scholar]
- Hessman B.E., Fulton R.W., Sjeklocha D.B., Murphy T.A., Ridpath J.F., Payton M.E. 2009. Evaluation of economic effects and the health and performance of the general cattle population after exposure to cattle persistently infected with bovine viral diarrhea virus in a starter feedlot. Am. J. Vet. Res. 70:73–85. [DOI] [PubMed] [Google Scholar]
- Hickey M.C., Drennan M., Earley B. 2003. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J. Anim. Sci. 81:2847–2855. [DOI] [PubMed] [Google Scholar]
- Ishizaki H., Kariya Y. 2010. Road transportation stress promptly increases bovine peripheral blood absolute NK cell counts and cortisol levels. J. Vet. Med. Sci. 72:747–753. [DOI] [PubMed] [Google Scholar]
- Johnson R.W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. [DOI] [PubMed] [Google Scholar]
- Kegley E.B., Spears J.W., Brown T.T., Jr 1997. Effect of shipping and chromium supplementation on performance, immune response, and disease resistance of steers. J. Anim. Sci. 75:1956–1964. [DOI] [PubMed] [Google Scholar]
- Klasing K.C., Korver D.R. 1997. Leukocytic cytokines regulate growth rate and composition following activation of the immune system. J. Anim. Sci. 75:58–67. [Google Scholar]
- Loerch S.C., Fluharty F.L. 2000. Use of trainer animals to improve performance and health of newly arrived feedlot calves. J. Anim. Sci. 78:539–545. [DOI] [PubMed] [Google Scholar]
- Loneragan G.H., Thomson D.U., Montgomery D.L., Mason G.L., Larson R.L. 2005. Prevalence, outcome, and health consequences associated with persistent infection with bovine viral diarrhea virus in feedlot cattle. J. Am. Vet. Med. Assoc. 226:595–601. [DOI] [PubMed] [Google Scholar]
- Macartney J.E., Bateman K.G., Ribble C.S. 2003. Health performance of feeder calves sold at conventional auctions versus special auctions of vaccinated or conditioned calves in Ontario. J. Am. Vet. Med. Assoc. 223:677–683. [DOI] [PubMed] [Google Scholar]
- Martin S.W., Nagy E., Armstrong D., Rosendal S. 1999. The associations of viral and mycoplasmal antibody titers with respiratory disease and weight gain in feedlot calves. Can. Vet. J. 40:560–567. [PMC free article] [PubMed] [Google Scholar]
- McGlone J.J., Salak J.L., Lumpkin E.A., Nicholson R.I., Gibson M., Norman R.L. 1993. Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J. Anim. Sci. 71:888–896. [DOI] [PubMed] [Google Scholar]
- O'Connor A.M., Martin S.W., Nagy E., Menzies P., Harland R. 2001. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in 3 Ontario feedlots. Can. Vet. J. 65:137–142. [PMC free article] [PubMed] [Google Scholar]
- O'Connor A.M., Sorden S.D., Apley M.D. 2005. Association between the existence of calves persistently infected with bovine viral diarrhea virus and commingling on pen morbidity in feedlot cattle. Am. J. Vet. Res. 66:2130–2134. [DOI] [PubMed] [Google Scholar]
- Pritchard R.H., Mendez J.K. 1990. Effects of preconditioning on pre- and post-shipment performance of feeder calves. J. Anim. Sci. 68:28–34. [DOI] [PubMed] [Google Scholar]
- Ridpath J.F., Fulton R.W., Kirkland P.D., Neill J.D. 2010. Prevalence and antigenic differences observed between bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern United States. J. Vet. Diag. Invest. 22:184–191. [DOI] [PubMed] [Google Scholar]
- Ridpath J.F., Lovell G., Neill J.D., Hairgrove T.B., Velayudhan B., Mock R. 2011. Change in predominance of Bovine viral diarrhea virus subgenotypes among samples submitted to a diagnostic laboratory over a 20-year time span. J. Vet. Diagn. Invest. 23: 185–193. [DOI] [PubMed] [Google Scholar]
- Roeber D.L., Speer N.C., Gentry J.G., Tatum J.D., Smith C.D., Whittier J.C., Jones G.F., Belk K.E., Smith G.C. 2001. Feeder cattle health management: Effects on morbidity rates, feedlot performance, carcass characteristics, and beef palatability. Prof. Anim. Sci. 17:39–44. [Google Scholar]
- Seeger J.T., Grotelueschen D.M., Stokka G.L., Sides G.E. 2008. Comparison of feedlot health, nutritional performance, carcass characteristics, and economic value of unweaned beef calves with an unknown health history and weaned beef calves receiving various herd-of-origin health protocols. Bov. Pract. 42:27–39. [Google Scholar]
- Sowa J.M., Crist S.A., Ratcliff T.L., Elzey B.D. 2009. Platelet influence on T- and B-cell responses. Arch. Immunol. Ther. Exp. 57:235–241. [DOI] [PubMed] [Google Scholar]
- Spurlock M.E. 1997. Regulation of metabolism and growth during immune challenge: An overview of cytokine function. J. Anim. Sci. 75:1773–1783. [DOI] [PubMed] [Google Scholar]
- Step D.L., Krehbiel C.R., DePra H.A., Cranston J.J., Fulton R.W., Kirkpatrick J.G., Gill D.R., Payton M.E., Montelongo M.A., Confer A.W. 2008. Effects of commingling beef calves from different sources and weaning protocols during a forty-two-day receiving period on performance and bovine respiratory disease. J. Anim. Sci. 86:3146–3158. [DOI] [PubMed] [Google Scholar]
- Stevens E.T., Thomson D.U., Loneragan G.H., Lindberg N. 2007. Effects of short-term exposure of feeder cattle to calves persistently infected with bovine viral diarrhea virus. Bov. Pract. 41:151–155. [Google Scholar]
- Stevens E.T., Thomson D.U., Reinhardt C.D., Lindberg N. 2009. Effect of testing and removal of feeder calves persistently infected with bovine viral diarrhea virus at the time of feedlot arrival and outcome on health, performance, and carcass characteristics. Bov. Pract. 43:117–121. [Google Scholar]
- Stokka G.L. 2010. Prevention of respiratory disease in cow/calf operations. Vet. Clin. Food Anim. 26:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.D., Fulton R.W., Lehenbauer T.W., Step D.L., Confer A.W. 2010. The epidemiology of bovine respiratory disease: What is the evidence for preventative measures? Can. Vet. J. 51:1351–1359. [PMC free article] [PubMed] [Google Scholar]
- USDA 2010. Beef 2007-08, Part IV: Reference of beef cow-calf management practices in the United States, 2007–08. USDA: APHIS:VS, CEAH; Fort Collins, CO: #523.0210. [Google Scholar]
- Welsh M.D., Adair B.M., Foster J.C. 1995. Effect of BVD virus infection on alveolar macrophage functions. Vet. Immunol. Immunopathol. 46:195–210. [DOI] [PubMed] [Google Scholar]