Abstract
The lesser mealworm, Alphitobius diaperinus (Panzer, 1797) (Coleoptera: Tenebrionidae), is considered the primary insect pest in broiler farms in Brazil. In this study, we characterized the susceptibility of A. diaperinus populations from broiler farms of southern Brazil to cypermethrin and chlorpyrifos. Larvae and adults of A. diaperinus were exposed to these technical insecticides diluted in acetone in residual bioassays. A geographic variation in the susceptibility of larvae and adults of A. diaperinus to both insecticides was detected. The larval LC50 for cypermethrin ranged from 0.43 to 7.33 µg a.i./cm2. Two populations from Santa Catarina state presented higher resistance ratios of 13.6- and 17-fold. When adults were exposed to cypermethrin, the LC50 ranged from 0.46 to 4.93 µg a.i./cm2, with population SC-3 from Santa Catarina having lower susceptibility (resistance ratio of 10.7-fold). When exposed to chlorpyrifos, A. diaperinus larvae present LC50 values ranging from 0.21 to 4.30 µg a.i./cm2. Larvae from Paraná and Santa Catarina (SC-1 population) presented the highest resistance ratios, ranging from 10- to 20-fold. In adults, the LC50 of chlorpyrifos ranged from 0.17 to 5.30 µg a.i./cm2, showing a maximum resistance ratio of 31-fold in a population from Paraná state. Based on LC99 values, candidate diagnostic concentrations of 15 and 12 µg a.i./cm2 of cypermethrin and chlorpyrifos, respectively, were also estimated for the resistance monitoring of A. diaperinus in Brazil. The implications of these results in Insect Resistance Management are discussed.
Keywords: poultry, lesser mealworm, pyrethroid, organophosphate, insecticide resistance
The lesser mealworm, Alphitobius diaperinus (Panzer, 1797) (Coleoptera: Tenebrionidae), is a key pest species of the poultry industry worldwide (Axtell 1999, Lambkin 2011). In broiler farms, species is abundant where food and suitable temperature provide favorable environmental conditions for its development and rapid proliferation (Chernaki-Leffer et al. 2002). This species causes damage to the structure of poultry operations by boring through wood in flooring and building insulation, due to the darkling beetle behavior of building galleries (Despins et al. 1987). However, the main economic losses are the beetles’ effects on the development of the poultry. Poultry ingest the beetles, which replaces the intake of the nutritionally balanced poultry feed, reducing their weight gain (Despins and Axtell 1995). The ingestion of beetles can also transmit diseases, such as avian sarcoma leukosis virus (Eidson et al. 1966), bursal disease virus (McAllister et al. 1995), and Coronavirus (Watson et al. 2000). Beetles can also transmit several bacteria, such as Micrococcus sp., Streptococcus sp., Corynebacterium sp., Staphylococcus aureus, Proteus mirabilis, Paracolobactrum intermedium, Escherichia coli, and Salmonella typhimurium, among others (De Las Casas et al. 1972; McAllister et al. 1994, 1996; Goodwin and Waltman 1996). It is estimated that this insect causes annual losses of more than $9 million to the poultry industry in Georgia alone, the largest broiler producing state in United States (Guillebeau et al. 2008).
The current control tactics used against A. diaperinus in broiler farms normally have limited efficacy. In an attempt to reduce population densities, poultry bedding removal and a period of poultry absence are recommended (Hamm et al. 2006). Another control tactic is to cover the poultry bedding with tarpaulin to promote fermentation, thus raising the temperature above levels critical to beetle survival (Salin et al. 1998). These methods have limited efficacy, since they do not reach all insects present, due to some insects surviving in galleries in the farm structure, favoring new infestations. Alternative control strategies, such as biopesticides and entomopathogenic fungi have some efficacy against A. diaperinus but are rarely used in broiler farms (Chernaki-Leffer et al. 2007, Rezende et al. 2009, Szczepanik et al. 2016). Though some of these management strategies reduce the infestation of this species, the use of insecticides remains the main control strategy (Oliveira et al. 2016). Currently, the insecticides registered for application in poultry facilities in Brazil include pyrethroids and organophosphates that contain the active ingredients cypermethrin and chlorpyrifos, respectively. These insecticides are widely used, isolated or in mixture, in commercial formulated products against A. diaperinus (Silva et al. 2007, Oliveira et al. 2016). This reduced number of insecticides registered for use against this species favors the evolution of resistance.
Resistance to insecticides is a result of the chemical control of A. diaperinus worldwide. Resistance of this species to the insecticides fenitrothion and permethrin has been reported in turkey farms in the United Kingdom (Cogan et al. 1996) and to fenitrothion, deltamethrin, cyfluthrin, and lambda-cyhalothrin in broiler farms in Australia (Lambkin 2005, Lambkin and Rice 2006, 2010; Lambkin and Furlong 2011). In the Americas, since 2006, resistance to carbaryl, methoxychlor, DDT, cyfluthrin, permethrin, cypermethrin, tetrachlorvinphos, and chlorpyrifos has been documented in broiler farms in Texas and Arkansas (Hamm et al. 2006, Steelman 2008, Singh and Johnson 2015). In Brazil, there have been no resistance cases reported; however, low susceptibility to cypermethrin and dichlorvos were detected in A. diaperinus in broiler farms of the Paraná state (Chernaki-Leffer et al. 2011). To test this report further, the objective of the current study was to characterize the susceptibility of A. diaperinus populations from southern Brazil to cypermethrin and chlorpyrifos and to estimate diagnostic concentrations for resistance monitoring programs.
Materials and Methods
Populations
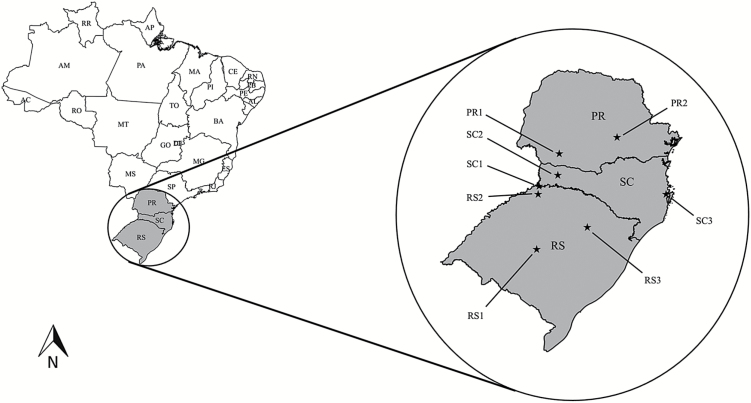
To determine the susceptibility data to insecticides, populations of A. diaperinus were collected in poultry farms in southern Brazil, where there is the highest number of broiler farms in the country (Table 1; Fig. 1). Larvae and adults of A. diaperinus were collected and transported to the laboratory, where they were placed in plastic pots (24 cm × 18 cm × 8 cm [length by width by height]) containing wood shavings, commercial chicken feed, and a piece of apple (water source), according to the methodology proposed by Singh and Johnson (2015). Insects were maintained in a climatic room at 28 ± 1°C, relative humidity 70 ± 10% and a photophase of 14 h. In addition to the field populations, a susceptible reference population (Lab) was also tested. The Lab population has been kept in the laboratory for >3 yr, free of selection pressure by insecticides.
Table 1.
Identification, location, date, and number of A. diaperinus (larvae and adults) collected in broilers farms from southern Brazil used to characterize the susceptibility to insecticides
| Pop. Code | City, state | Farm | Latitude | Longitude | Date | Number |
|---|---|---|---|---|---|---|
| Lab | Santa Maria, RS | UFSM | – | – | – | – |
| RS-1 | Santa Maria, RS | Avesui | 29°41′03″S | 53°48′25”W | October, 2016 | 500 |
| RS-2 | Miraguaí, RS | Canterle | 27º29′39″S | 53º44′51″W | October, 2016 | 800 |
| RS-3 | Vista Alegre do Prata, RS | Ramon | 28º48′31″S | 51º47′25″W | October, 2016 | 900 |
| SC-1 | Itapiranga, SC | Hickmann | 27º10′12″S | 53º42′39″W | October, 2016 | 2,500 |
| SC-2 | Sul Brasil, SC | Bida | 26º44′10″S | 52º57′53″W | October, 2016 | 5,000 |
| SC-3 | Biguaçú, SC | Vieira | 27º29′38″S | 48º39′21″W | October, 2016 | 2,000 |
| PR-1 | Verê, PR | Zanetti | 25º52′51″S | 52º54′28″W | October, 2016 | 800 |
| PR-2 | Imbituva, PR | Jesus | 25º13′45″S | 50º36′19″W | October, 2016 | 2,800 |
Fig. 1.
Locations of collection of A. diaperinus populations in broiler farms from southern Brazil used to characterize the susceptibility to insecticides.
Bioassays
In the bioassays, technical-grade cypermethrin (92.6% purity) and chlorpyrifos (97.5% purity) provided by Ouro Fino Saúde Animal Ltda., Cravinhos, São Paulo, Brazil, were used. Both insecticides were diluted in acetone to prepare concentrations for testing in the residual contact bioassay, as suggested by Hamm et al. (2006). For each population, five to seven concentrations (0.016 to 16 μg a.i./cm2) of each insecticide were applied in glass Petri dishes (9 cm × 1 cm [diameter by height]) using a repetition pipette (1 ml per dish). Petri dishes were rotated manually so that an even layer of insecticide dried on the inner surface. Control dishes were prepared using 1 ml of acetone only. Petri dishes were then placed for 2 h in a fume hood to dry. After this, each dish was infested with 10–20 larvae (8–10 mm length) or 10–20 adults (30–40 d in adult stage) of the F1 or F2 generations in the laboratory. The bioassays were repeated twice for each population and development stage, with each concentration being repeated twice per bioassay (a total of four replicates per concentration). After infestation, all plates were placed in a climatic chamber at 25 ± 1°C, relative humidity 70 ± 10%, and photophase of 12 h. Mortality was assessed after 48 h. Adults and larvae were considered dead if they were unable to move out of a 5-cm circle within 15 min, as proposed by Hamm et al. (2006).
Statistical Analyses
To estimate the LC50 (LC—lethal concentration) and respective confidence intervals (CIs), the concentration-mortality data of each population were submitted to Probit analysis (PROC PROBIT, SAS Institute 2000). A likelihood ratio test was conducted to test the hypothesis that the LCp values (lethal concentration at which a percentage mortality P is attained) were equal. If the hypothesis was rejected, pairwise comparisons were performed and significance was declared if CIs did not overlap (Savin et al. 1977, Robertson et al. 2007). The significance of differences among slopes was determined by likelihood ratio test for parallelism and equality (Savin et al. 1977). Resistance ratios were calculated by dividing the LC50 of the populations from broiler farms by the corresponding parameter for the susceptible reference population (Lab). To estimate diagnostic concentrations for insect resistance monitoring programs, a joint analysis was performed. In this analysis, mortality data were fitted with a binomial model using the log-log complement connection function (gompit) (PROC PROBIT, SAS Institute 2000). In this analysis, LC99 values and respective CIs were estimated. Based on the LC99 values, candidate diagnostic concentrations were designated for the resistance monitoring of A. diaperinus to cypermethrin and chlorpyrifos in Brazil.
Results
Susceptibility to Cypermethrin
There was significant variation in the biological activity of cypermethrin against A. diaperinus larvae (Table 2). For the populations tested, cypermethrin concentrations from 0.16 to 16 µg a.i./cm2 caused larval mortality ranging from 7 to 92%. For larvae from nine geographically distinct populations of A. diaperinus, the LC50 values ranged from 0.43 (Lab population) to 7.33 (SC-1 population) µg a.i./cm2. A similar susceptibility was detected to cypermethrin in A. diaperinus larvae from the Lab population and some populations collected in the Rio Grande do Sul state, RS-1 and RS-2. In contrast, the RS-3 population and those populations from broiler farms of Santa Catarina (SC-1, SC-2 and SC-3) and Paraná state (PR-1 and PR-2) showed a lower susceptibility than previous populations (Table 2). However, the SC-1 and SC-3 populations had lower susceptibility to cypermethrin than the Lab population and all other field populations tested. These two populations presented the highest resistance ratios of 13.6- and 17-fold, respectively (Table 2).
Table 2.
Concentration-mortality response (LC; µg a.i./cm2) of larvae and adults of A. diaperinus from southern Brazil exposed to cypermethrin
| Pop. code | n | Slope ± SEa | LC50 (95% CI)a,b | χ2c | dfd | Resistance ratioe |
|---|---|---|---|---|---|---|
| Larvae | ||||||
| Lab | 186 | 1.10 ± 0.24 a | 0.43 (0.27–0.72) a | 2.28 | 4 | – |
| RS-1 | 182 | 1.36 ± 0.29 ab | 0.50 (0.24–1.13) a | 1.45 | 4 | 1.2 |
| RS-2 | 210 | 1.06 ± 0.12 a | 0.45 (0.21–0.79) a | 6.86 | 4 | 1.0 |
| RS-3 | 183 | 1.80 ± 0.19 b | 1.70 (1.29–3.28) b | 2.83 | 4 | 3.9 |
| SC-1 | 290 | 1.11 ± 0.25 a | 7.33 (4.13–11.87) c | 5.43 | 5 | 17.0 |
| SC-2 | 210 | 1.19 ± 0.19 a | 2.22 (1.95–2.94) b | 3.70 | 4 | 5.2 |
| SC-3 | 187 | 1.09 ± 0.18 a | 5.87 (4.81–8.71) c | 3.67 | 4 | 13.6 |
| PR-1 | 183 | 1.67 ± 0.17 ab | 1.72 (1.20–2.32) b | 1.01 | 4 | 4.0 |
| PR-2 | 180 | 1.47 ± 0.12 ab | 1.84 (1.14–2.45) b | 4.07 | 4 | 4.3 |
| Adults | ||||||
| Lab | 150 | 1.39 ± 0.42 abc | 0.46 (0.27–0.88) a | 5.25 | 4 | – |
| RS-1 | 133 | 1.64 ± 0.22 b | 1.23 (0.40–1.72) ab | 1.05 | 4 | 2.7 |
| RS-2 | 210 | 1.16 ± 0.16 a | 0.77 (0.38–1.02) ab | 3.59 | 4 | 1.7 |
| RS-3 | 184 | 1.85 ± 0.19 c | 0.71 (0.28–1.05) ab | 2.50 | 4 | 1.6 |
| SC-1 | 242 | 1.06 ± 0.17 a | 2.09 (0.92–3.93) bc | 2.72 | 4 | 4.5 |
| SC-2 | 181 | 1.57 ± 0.50 abc | 1.43 (0.99–1.64) b | 6.05 | 4 | 3.1 |
| SC-3 | 187 | 3.18 ± 0.68 d | 4.93 (3.11–6.62) c | 1.09 | 4 | 10.7 |
| PR-1 | 185 | 1.54 ± 0.12 bc | 3.99 (2.88–6.24) c | 5.01 | 4 | 8.7 |
| PR-2 | 214 | 1.02 ± 0.18 a | 2.45 (1.78–3.60) bc | 6.76 | 4 | 5.3 |
aLC50 values designated by different letters within a column are significantly different from each other through nonoverlap of 95% confidential intervals. Significance of differences among slopes determined by likelihood ratio test of equality followed by pairwise comparisons using nonoverlapping fiducial limits.
bLC50: concentration of cypermethrin (µg a.i./cm2) required to kill 50% of insects in the observation period of 48 h.
c P > 0.05 in the goodness-of-fit test.
dDegrees of freedom.
eResistance ratio = (LC50 of indicated population)/(LC50 of Lab population).
In the bioassays with adult A. diaperinus, significant variation was also detected in the susceptibility to cypermethrin (Table 2). The concentrations from 0.025 to 10 µg a.i./cm2 caused mortality ranging from 5 to 94%. In adults from nine distinct populations of A. diaperinus, the LC50 for cypermethrin ranged from 0.46 (Lab population) to 4.93 (SC-3 population) µg a.i./cm2 (Table 2). Based on the LC50 values, similar susceptibility was detected between populations from Rio Grande do Sul state (RS-1, RS-2, and RS-3) and the susceptible reference population (Lab). Similar susceptibility was also observed among the populations RS-1, RS-2, RS-3, SC-1, SC-2, and PR-2, with LC50 values ranging from 0.71 to 2.45 µg a.i./cm2 (Table 2). However, there was significantly lower susceptibility to cypermethrin in adults from the SC-3 and PR-1 populations, compared with Lab, SC-2, and those populations collected in the Rio Grande do Sul state (Table 2). The variation in susceptibility indicated by adult mortality in the PR-1 and SC-3 populations represents the highest resistance ratio observed, ranging from 8.7- to ~11-fold (Table 2).
For estimating LC99, the mortality data of all populations tested were pooled and analyzed jointly. The LC99 of cypermethrin against A. diaperinus larvae was estimated to be 15.14 [CI 95% (9.33–21.08)] µg a.i./cm2 (n = 1811; slope [± SE] = 2.02 [± 0.16]; χ2 = 8.69; df = 4). For the adults, the LC99 was 14.30 [CI 95% (7.27–22.18)] µg a.i./cm2 (n = 1686; slope [± SE] = 1.75 [± 0.12]; χ2 = 10.06; df = 4). From the LC99 values, the candidate diagnostic concentration of 15 µg a.i./cm2 is suggested for resistance monitoring programs of A. diaperinus (larvae and adults) to cypermethrin in Brazil.
Susceptibility to Chlorpyrifos
Significant variation was also detected in the susceptibility of A. diaperinus larvae to chlorpyrifos (Table 3). Concentrations from 0.016 to 30 µg a.i./cm2 caused larval mortality ranging from 2 to 97%. In bioassays with larvae, the LC50 values ranged from 0.21 (Lab population) to 4.30 (PR-1 population) µg a.i./cm2. A similar susceptibility to chlorpyrifos was observed in larvae from the Lab, RS-1, RS-2, and SC-2 populations, with LC50 values ranging from 0.21 to 1.26 µg a.i./cm2. However, the RS-2 population do not differ in terms of susceptibility of the populations RS-3, SC-1, SC-3, and PR-2 (Table 3). In contrast, larvae from the PR-1 population showed significantly lower susceptibility than previous populations, with an LC50 of 4.30 µg a.i./cm2. This population also had the highest resistance ratio to chlorpyrifos of more than 20-fold (Table 3).
Table 3.
Concentration-mortality response (LC; µg a.i./cm2) of larvae and adults of A. diaperinus from southern Brazil exposed to chlorpyrifos
| Pop. code | n | Slope ± SEa | LC50 (95% CI)a,b | χ2c | dfd | Resistance ratioe |
|---|---|---|---|---|---|---|
| Larvae | ||||||
| Lab | 181 | 2.12 ± 0.60 bc | 0.21 (0.09–0.33) a | 1.74 | 4 | – |
| RS-1 | 182 | 1.19 ± 0.27 ab | 0.52 (0.27–0.98) a | 3.20 | 4 | 2.5 |
| RS-2 | 150 | 1.16 ± 0.36 ab | 1.26 (0.29–2.22) ab | 1.58 | 4 | 6.0 |
| RS-3 | 151 | 1.40 ± 0.34 ab | 1.66 (1.23–2.26) b | 3.62 | 4 | 7.9 |
| SC-1 | 240 | 1.09 ± 0.20 a | 2.28 (1.09–4.24) bc | 1.32 | 4 | 10.8 |
| SC-2 | 186 | 1.76 ± 0.24 bc | 0.32 (0.25–0.79) a | 9.84 | 4 | 1.5 |
| SC-3 | 184 | 1.82 ± 0.28 c | 1.30 (0.50–2.02) b | 1.95 | 4 | 6.2 |
| PR-1 | 154 | 1.42 ± 0.29 b | 4.30 (3.22–4.85) c | 2.49 | 4 | 20.5 |
| PR-2 | 185 | 1.04 ± 0.40 a | 2.36 (1.31–4.16) bc | 1.10 | 4 | 11.2 |
| Adults | ||||||
| Lab | 187 | 1.95 ± 0.24 bc | 0.17 (0.07–0.34) a | 4.51 | 4 | – |
| RS-1 | 182 | 2.28 ± 0.55 c | 0.29 (0.18–0.40) a | 2.62 | 4 | 1.7 |
| RS-2 | 156 | 1.87 ± 0.33 bc | 1.13 (0.26–1.59) a | 2.67 | 4 | 6.6 |
| RS-3 | 180 | 1.01 ± 0.27 a | 0.70 (0.23–1.12) a | 5.18 | 4 | 4.1 |
| SC-1 | 139 | 3.00 ± 0.66 c | 0.52 (0.30–0.78) a | 1.14 | 4 | 3.0 |
| SC-2 | 182 | 1.24 ± 0.31 ab | 0.26 (0.21–0.33) a | 11.00 | 4 | 1.5 |
| SC-3 | 177 | 1.50 ± 0.16 b | 0.62 (0.20–0.90) a | 1.32 | 4 | 3.6 |
| PR-1 | 160 | 2.22 ± 0.65 c | 5.30 (3.49–7.37) b | 2.94 | 4 | 31.2 |
| PR-2 | 152 | 1.83 ± 0.27 bc | 2.90 (1.72–3.83) b | 2.15 | 4 | 17.0 |
aLC50 values designated by different letters within a column are significantly different from each other through nonoverlap of 95% confidential intervals. Significance of differences among slopes determined by likelihood ratio test of equality followed by pairwise comparisons using nonoverlapping fiducial limits.
bLC50: concentration of chlorpyrifos (µg a.i./cm2) required to kill 50% of insects in the observation period of 48 h.
c P > 0.05 in the goodness-of-fit test.
dDegrees of freedom.
eResistance ratio = (LC50 of indicated population)/(LC50 of Lab population).
There was also significant variation in the susceptibility to chlorpyrifos in adults from distinct Brazilian populations of A. diaperinus (Table 3). Concentrations from 0.16 to 30 µg a.i./cm2 caused mortality ranging from 6 to 93%. The LC50 for adults from nine populations ranged from 0.17 (Lab population) to 5.30 (PR-1 population) µg a.i./cm2 (Table 3). A similar susceptibility to chlorpyrifos was observed in adults from Lab, populations collected in broiler farms of Rio Grande do Sul (RS-1, RS-2, and RS-3) and Santa Catarina (SC-1, SC-2, and SC-3) states, with LC50 values ranging from 0.17 (population Lab) to 1.13 (population RS-2) µg a.i./cm2 (Table 3). In contrast, adults from Paraná state showed significantly lower susceptibility than previous populations, with LC50 values ranging from 2.90 to 5.30 µg a.i./cm2 (Table 3). This difference in susceptibility represents a resistance ratio of 17- and 31.2-fold, (Table 3).
The estimated LC99 of chlorpyrifos against A. diaperinus larvae was 11.03 [CI 95% (5.23–18.51)] µg a.i./cm2 (n = 1613; slope [± SE] = 1.89 [± 0.14]; χ2 = 5.33; df = 4). For the adults, the LC99 was 12.81 [CI 95% (6.08–19.09)] µg a.i./cm2 (n = 1515; slope [SE]= 2.00 [± 0.14]; χ2 = 7.56; df = 4). Based on these values, the candidate diagnostic concentration of 12 µg a.i./cm2 can be considered for the resistance monitoring of A. diaperinus (larvae and adults) to chlorpyrifos in Brazil.
Discussion
The larvae and adults from southern Brazilian populations of A. diaperinus presented significant interpopulation variation in the observed susceptibility to cypermethrin and chlorpyrifos. The susceptibility of A. diaperinus larvae to these insecticides, based on LC50 values ranging from 0.43 to 7.33 µg a.i./cm2 and 0.21 to 4.30 µg a.i./cm2, represented resistance ratios of 17- and 20-fold, respectively. Lower variation in larval susceptibility was reported for cypermethrin, permethrin, and cyfluthrin in Arkansas, with a resistance ratio inferior to 15-fold (Steelman 2008). In another study, larvae also presented lower than 14-fold resistance to cyfluthrin in Arkansas (Singh and Johnson 2015) and to beta-cyfluthrin (3.5-fold resistance) in Texas (Lyons et al. 2017). In contrast, a higher resistance ratio up to 29-fold, was recorded for cyfluthrin in beetles’ larvae from poultry farms in the eastern United States (Hamm et al. 2006). However, in these same populations, resistance to tetrachlorvinphos, an organophosphate with a similar mode of action of chlorpyrifos, was lower than ninefold. A lower resistance ratio, inferior to threefold, was also verified to chlorpyrifos and tetrachlorvinphos in larvae from broiler house facilities in Arkansas (Steelman 2008).
High variation in the susceptibility to cypermethrin and chlorpyrifos was also observed among adult populations of A. diaperinus from southern Brazil. In this development stage, the LC50 to cypermethrin and chlorpyrifos ranged from 0.46 to 4.93 µg a.i./cm2 and 0.17 to 5.30 µg a.i./cm2, respectively, showing 10- and 31-fold resistance. A similar resistance ratio was also reported to cypermethrin in adult beetles from north Paraná (up to 17-fold resistance) (Chernaki-Leffer et al. 2011). However, populations from western and southwestern Paraná had strong resistance to cypermethrin, ranging from 36- to 92-fold. A similar resistance ratio was also observed to cyfluthrin, of up to 10-fold, in adult beetles from the eastern United States (Hamm et al. 2006) and to beta-cyfluthrin, lambda-cyhalothrin, and deltamethrin, up to 16-fold, in Australia (Lambkin and Furlong 2011). However, a lower resistance ratio, inferior to fivefold, was reported to cypermethrin, permethrin, and cyfluthrin in beetles from broiler houses in Arkansas (Steelman 2008). In contrast, higher resistance ratios, from 19- to 36-fold, were reported for cyfluthrin in adult A. diaperinus from broiler farms in Australia (Lambkin and Rice 2006, Lambkin and Furlong 2011). To organophosphates, a similar resistance ratio, from 1- to 14-fold, was reported in populations of A. diaperinus from Paraná (Lapa, Araucária, and Cascavel); to dichlorvos, an insecticide that act as an acetylcholinesterase inhibitor, resistance ratios were similar to chlorpyrifos (Chernaki-Leffer et al. 2011). However, populations of the same state from Pato Branco, Londrina, and Corbélia showed higher resistance to dichlorvos, of up to 134-fold (Chernaki-Leffer et al. 2011). In another study, a higher resistance ratio up to 79-fold, was also reported to fenitrothion in adult beetles in Australia (Lambkin 2005). The high resistance ratio to chlorpyrifos and dichlorvos in A. diaperinus populations from Paraná reported here and in previous studies indicates a possible cross-resistance among these insecticides, as published in another Coleopteran species (Attia and Frecker 1984, Zettler and Cuperus 1990).
The interpopulation variation in the susceptibility to chemical insecticides is a common phenomenon when bioassays are repeated (Robertson et al. 2007). However, from an Insect Resistance Management (IRM) perspective, variation in susceptibility is also an indication of the ability of the beetles to adapt to the insecticides, especially in populations with the highest LC50 values. In other words, the relative high resistance ratio to cypermethrin and chlorpyrifos in some A. diaperinus populations from Santa Catarina and Paraná seems a probable response of high exposure to selection pressure. Based on this, IRM strategies should be adopted, by associating physical and chemical methods using insecticides with distinct modes of action against this species, to delay or prevent resistance evolution (Wolf et al. 2015).
Understanding the mode of action of each insecticide is also crucial in developing an effective IRM program. Cypermethrin is a sodium channel modulator that causes excitatory paralysis of the insect, whereas chlorpyrifos is an acetylcholinesterase inhibitor (Insecticide Resistance Action Committee [IRAC] 2017). Both active ingredients are widely used against A. diaperinus in broiler farms in Brazil and other countries (Steelman 2008, Tomberlin et al. 2008, Chernaki-Leffer et al. 2011, Oliveira et al. 2016). Specifically, in Brazil, the reduced number of active ingredients registered for use in broiler farms has contributed to the resistance evolution in A. diaperinus. Thus, insecticides from other chemical groups should be considered against this pest, such as spinosad and imidacloprid, which mimic the agonistic action of nicotinic acetylcholine receptors. Additionally, chlorfenapyr, which uncouples oxidative phosphorylation through the disruption of the proton gradient and interferes with metabolic processes and energy production in mitochondria (IRAC 2017). Spinosad, imidacloprid, and chlorfenapyr are not registered for use in broiler farms in Brazil, but they showed high efficacy against A. diaperinus in Australia and the United States (Lambkin and Rice 2007, Singh and Johnson 2015). Spinosad also increased the mortality of resistant beetles to cyfluthrin and fenitrothion, due to the absence of cross-resistance among these insecticides (Lambkin and Furlong 2014). Therefore, the Brazilian pesticide regulatory agencies and companies should register these and other chemical insecticides for use in broiler farms, to improve the management of A. diaperinus.
In the context of IRM, monitoring the susceptibility of A. diaperinus to insecticides is essential to subsidize their management. For susceptibility monitoring, the candidate diagnostic concentrations of 15 and 12 µg a.i./cm2 of cypermethrin and chlorpyrifos, respectively, are suggested. The use of these diagnostic concentrations in residual contact bioassays make this procedure fast, practical, and compatible with large scale bioassays in IRM programs. Future efforts should be concentrated on collecting representative samples of this species, especially in those regions with the highest amount of broiler farms. Then, expose the beetles to the diagnostic concentrations defined here, to identify resistant levels in Brazilian populations of this species. In summary, the compilation of this susceptibility database provides an opportunity for poultry companies and producers to identify possible changes in the susceptibility of A. diaperinus to cypermethrin and chlorpyrifos due to resistance evolution, thus establishing effective IRM strategies.
Acknowledgments
We thank Ouro Fino Saúde Animal Ltda for providing the technical insecticides to perform this study.
References Cited
- Attia F. I., and Frecker T.. 1984. Cross-resistance spectrum and synergism studies in organophosphorus-resistant strains of Oryzaephilus surinamensis (L.) (Coleoptera: Cucugidae) in Australia. J. Econ. Entomol. 77: 1367–1370. [Google Scholar]
- Axtell R. C. 1999. Poultry integrated pest management: status and future. Integr. Pest Manag. Rev. 4: 53–73. [Google Scholar]
- Chernaki-Leffer A., Biesdorf S., Almeida L., Leffer E., and Vigne F.. 2002. Isolamento de enterobactérias em Alphitobius diaperinus e na cama de aviários no oeste do estado do Paraná, Brasil. Rev. Bras. Cienc. Avic. 4: 243–247. [Google Scholar]
- Chernaki-Leffer A. M., Sosa-Gòmez D. R., and Almeida L. M.. 2007. Selection for entomopathogenic fungi and LD50 of Metarhizium anisopliae (Metsch.) Sorok. for the lesser mealworm Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Rev. Bras. Cienc. Avic. 9: 187–191. [Google Scholar]
- Chernaki-Leffer A. M., Sosa-Gómez D. R., Almeida L. M., and Lopes I. O. N.. 2011. Susceptibility of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) to cypermethrin, dichlorvos and triflumuron in Southern Brazil. Rev. Bras. Entomol. 55: 125–128. [Google Scholar]
- Cogan P., Webb D., and Wakefield M.. 1996. A comparison of four residual insecticides for the control of the lesser mealworm beetle (Alphitobius diaperinus Panzer) in turkey broiler houses in the UK. Int. J. Pest. Manag. March/April: 52–55. [Google Scholar]
- De Las Casas E., Harein P. K., and Pomeroy B. S.. 1972. Bacteria and fungi within the lesser mealworm collected from poultry brooder houses. Environ. Entomol. 1: 27–30. [DOI] [PubMed] [Google Scholar]
- Despins J. L., and Axtell R. C.. 1995. Feeding behavior and growth of broiler chicks fed larvae of the darkling beetle, Alphitobius diaperinus. Poult. Sci. 74: 331–336. [DOI] [PubMed] [Google Scholar]
- Despins J. L., Turner E. C. Jr., and Ruszler P. L.. 1987. Construction profiles of high rise caged layer houses in association with insulation damage caused by the lesser mealworm, Alphitobius diaperinus (Panzer) in Virginia. Poult. Sci. 66: 243–250. [Google Scholar]
- Eidson C. S., Schmittle S. C., Goode R. B., and Lal J. B.. 1966. Induction of leukosis tumors with the beetle Alphitobius diaperinus. Am. J. Vet. Res. 27: 1053–1057. [PubMed] [Google Scholar]
- Goodwin M. A., and Waltman W. D.. 1996. Transmission of Eimeria, viruses, and bacteria to chicks: darkling beetles (Alphitobius diaperinus) as vectors of pathogens. J. Appl. Poult. Res. 5: 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillebeau P., Hinkle N., and Roberts P.. 2008. Summary of losses from insect damage and cost of control in Georgia 2006. Department of Entomology Special Committee on Insect Survey and Losses for 2006. Available from (https://athenaeum.libs.uga.edu/bitstream/handle/10724/35246/SurveyLoss06.pdf?sequence=1) (accessed 2 February 2018). [Google Scholar]
- Hamm R. L., Kaufman P. E., Reasor C. A., Rutz D. A., and Scott J. G.. 2006. Resistance to cyfluthrin and tetrachlorvinphos in the lesser mealworm, Alphitobius diaperinus, collected from the eastern United States. Pest Manag. Sci. 62: 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (IRAC) Insecticide Resistance Action Committee 2017. Insecticide resistance action committee mode of action working group IRAC MoA of action classification scheme, version 8.3 Available from http://www.irac-online.org/documents/moa-classification/?ext=pdf) (accessed 2 February 2018).
- Lambkin T. A. 2005. Baseline responses of adult Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) to fenitrothion, and susceptibility status of populations in Queensland and New South Wales, Australia. J. Econ. Entomol. 98: 938–942. [DOI] [PubMed] [Google Scholar]
- Lambkin T. A., and Furlong M. J.. 2011. Metabolic mechanisms only partially explain resistance to pyrethroids in Australian broiler house populations of lesser mealworm (Coleoptera: Tenebrionidae). J. Econ. Entomol. 104: 629–635. [DOI] [PubMed] [Google Scholar]
- Lambkin T. A., and Furlong M. J.. 2014. Application of spinosad increases the susceptibility of insecticide resistant Alphitobius diaperinus (Coleoptera: Tenebrionidae) to pyrethroids. J. Econ. Entomol. 107: 1590–1598. [DOI] [PubMed] [Google Scholar]
- Lambkin T. A., and Rice S.. 2006. Baseline responses of Alphitobius diaperinus (Coleoptera: Tenebrionidae) to cyfluthrin and detection of strong resistance in field populations in eastern Australia. J. Econ. Entomol. 99: 908–913. [DOI] [PubMed] [Google Scholar]
- Lambkin T. A., and Rice S.. 2007. Baseline responses of Alphitobius diaperinus (Coleoptera: Tenebrionidae) to spinosad, and susceptibility of broiler populations in Eastern and Southern Australia. J. Econ. Entomol. 100: 1423–1427. [DOI] [PubMed] [Google Scholar]
- Lambkin T. A., and Rice S.. 2010. Responses of susceptible and cyfluthrin-resistant broiler house populations of lesser mealworm (Coleoptera: Tenebrionidae) to cyhalothrin. J. Econ. Entomol. 103: 2155–2163. [DOI] [PubMed] [Google Scholar]
- Lyons B. N., Crippen T. L., Zheng L., Teel P. D., Swiger S. L., and Tomberlin J. K.. 2017. Susceptibility of Alphitobius diaperinus in Texas to permethrin- and β-cyfluthrin-treated surfaces. Pest Manag. Sci. 73: 562–567. [DOI] [PubMed] [Google Scholar]
- McAllister J. C., Steelman C. D., and Skeeles J. K.. 1994. Reservoir competence of the lesser mealworm (Coleoptera: Tenebrionidae) for Salmonella typhimurium (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 31: 369–372. [DOI] [PubMed] [Google Scholar]
- McAllister J. C., Steelman C. D., Newberry L. A., and Skeeles J. K.. 1995. Isolation of infectious bursal disease virus from the lesser mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 74: 45–49. [DOI] [PubMed] [Google Scholar]
- McAllister J. C., Steelman C. D., Skeeles J. K., Newberry L. A., and Gbur E. E.. 1996. Reservoir competence of Alphitobius diaperinus (Coleoptera: Tenebrionidae) for Escherichia coli (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 33: 983–987. [DOI] [PubMed] [Google Scholar]
- Oliveira D., Cardoso R., Mamprim A., Angeli L., Oliveira D., Cardoso R., Mamprim A., and Angeli L.. 2016. Laboratory and field evaluation of a cypermethrin-based insecticide for the control of Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae) and its in-vitro effects on Baeauveria bassiana bals. vuill. (Hypocreales: Cordycipitaceae). Rev. Bras. Ciência Avíc. 18: 371–380. [Google Scholar]
- Rezende S. R. F., Curvello F. A., Fraga M. E., Reis R. C. S., Castilho A. M. C., and Agostinho T. S. P.. 2009. Control of the Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) with entomopathogenic fungi. Rev. Bras. Cienc. Avic. 11: 121–127. [Google Scholar]
- Robertson J. L., Russell R. M., Preisler H. K., and Savin N. E.. 2007. Bioassays with arthropods. CRC, Boca Raton, FL, p.199. [Google Scholar]
- Salin C., Vernon P., and Vannier G.. 1998. The supercooling and high temperature stupor points of the adult lesser mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 34: 385–394. [Google Scholar]
- SAS Institute 2000. Statistical analysis system: getting started with the SAS learning. SAS Institute, Cary, NC. [Google Scholar]
- Savin N. E., Robertson J. L., and Russell R. M.. 1977. A critical evaluation of bioassay in insecticide research: likelihood ratio tests of dose-mortality regression. Bull. Entomol. Soc. Am. 23: 257–266. [Google Scholar]
- Silva G. S., Michels M. G., Toma S. B., Terra F. E., Soares V. E., and Costa A. J.. 2007. Effectiveness of the compound chlorpyrifos + cypermethrin + citronellal against Alphitobius diaperinus. Laboratory analysis and residue determination in carcasses. Rev. Bras. Cienc. Avic. 9: 157–160. [Google Scholar]
- Singh N., and Johnson D.. 2015. Baseline susceptibility and cross-resistance in adult and larval Alphitobius diaperinus (Coleoptera: Tenebrionidae) collected from poultry farms in Arkansas. J. Econ. Entomol. 108: 1994–1999. [DOI] [PubMed] [Google Scholar]
- Steelman C. D. 2008. Comparative susceptibility of adult and larval lesser mealworms, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae), collected from broiler houses in Arkansas to selected insecticides. J. Agric. Urban Entomol. 25: 111–125. [Google Scholar]
- Szczepanik M., Gliszczyńska A., Hnatejko M., and Zawitowska B.. 2016. Effects of halolactones with strong feeding-deterrent activity on the growth and development of larvae of the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Appl. Entomol. Zool. 51: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomberlin J. K., Richman D., and Myers H. M.. 2008. Susceptibility of Alphitobius diaperinus (Coleoptera: Tenebrionidae) from broiler facilities in Texas to four insecticides. J. Econ. Entomol. 101: 480–483. [DOI] [PubMed] [Google Scholar]
- Watson D. W., Guy J. S., and Stringham S. M.. 2000. Limited transmission of turkey Coronavirus in young turkeys by adult Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Med. Entomol. 37: 480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J., Potrich M., Lozano E. R., Gouvea A., and Pegorini C. S.. 2015. Combined physical and chemical methods to control lesser mealworm beetles under laboratory conditions. Poult Sci. 94: 1145–1149. [DOI] [PubMed] [Google Scholar]
- Zettler L. J., and Cuperus G. W.. 1990. Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) in wheat. J. Econ. Entomol. 83: 1677–1681. [Google Scholar]