ABSTRACT
Bovine respiratory disease complex (i.e., shipping fever and bacterial bronchopneumonia) is a multifaceted respiratory illness influenced by numerous environmental factors and microorganisms. Bovine respiratory disease (BRD) is just one component of BRD complex. Because BRD is moderately heritable, it may be possible to reduce the incidence of BRD through genetic selection. The objectives of this study were to determine the heritability and associative genetic relationships among immune system traits (i.e., cortisol, total IgG, IgG isotypes, and IL-8) in cattle monitored for BRD incidence. At an average of 83 d after weaning (219 d age and mean = 221.7 kg [SD 4.34]), crossbred Bos taurus steer calves (n = 2,869) were received at a commercial feedlot in southeastern Colorado over a 2-yr period. At receiving, jugular blood samples were collected at 212 (yr 1) and 226 d (yr 2) of age for immune trait analyses. The BRD phenotype was defined as a binomial variable (0 = no and 1 = yes) and compared with immune system traits measured at receiving (prior to illness onset). An animal identified as BRD positive exhibited ≥ 2 clinical signs (i.e., eye or nasal discharge, cough, lethargy, rapid breathing, acute interstitial pneumonia, or acute upper respiratory syndrome and/or a rectal temperature > 39.7°C). Heritability and genetic correlation estimates for categorical variable BRD, cortisol, IgG, IgG1, IgG2, and IL-8 were estimated from a sire model using ASREML. Heritability estimates were low to moderate for BRD (0.17 ± 0.08), cortisol (0.13 ± 0.05), IgG (0.15 ± 0.05), IgG1 (0.11 ± 0.05), IgG2 (0.24 ± 0.06), and IL-8 (0.30 ± 0.06). A moderate negative genetic correlation was determined between BRD and cortisol (rg = −0.19 ± 0.32). Moderate positive correlations were found between BRD with IgG (0.42 ± 0.28), IgG1 (0.36 ± 0.32), and IL-8 (rg = 0.26 ± 0.26). Variation in the BRD phenotype and immune system traits suggested herd health improvement may be achieved through genetic selection.
Keywords: bovine respiratory disease, cattle, immune system
INTRODUCTION
Prevention and treatment of bovine respiratory disease (BRD) cost the cattle industry over US$700 million annually (NASS, 2011). Although more than 85% of feedlots in the United States use a vaccination protocol for viruses that contribute to the development of BRD, the number of cases has not decreased (Gifford et al., 2012; APHIS, 2013). Therefore, effective prevention and/or early detection strategies for BRD must be developed to reduce its incidence in cattle. Prevention and diagnosis of BRD is further complicated by the complexity of this disease. Stress, inadequate nutrition, poor management, and/or genetics predispose cattle to polymicrobial respiratory infections (Ellis, 2001; Duff and Galyean, 2007), increasing an animal's susceptibility to contracting BRD.
Because BRD is a complex trait that is heritable, economically relevant, and difficult to measure, genomic selection could be used to reduce its incidence. However, to improve accuracy of selection, the genetic component of BRD must be delineated. Immune system traits such as cortisol, IgG and its subclasses (IgG1 and IgG2), and IL-8 all influence an individual's response to stress and immune system function (Stanley et al., 1984; Hull et al., 2001; Salak-Johnson and McGlone, 2007). Deciphering the genetic relationships among immune system traits will aid in predicting an individual's likelihood to develop BRD. We hypothesized that immune system traits measured at receiving were genetically correlated with BRD. The long-term aim of this research is to elucidate both genotypic and phenotypic relationships of BRD with physiological, behavioral, and performance traits. Specifically, the objectives of this study were to 1) estimate the genetic variation of BRD and immune system traits (i.e., cortisol, IgG, and IL-8), 2) evaluate the genetic relationships with BRD and immune traits measured prior to the onset of illness, and 3) assess the genetic relationships within immune system traits.
MATERIALS AND METHODS
Animal Procedures
The Colorado State University Institutional Animal Care and Use Committee approved all animal procedures. Crossbred Bos taurus steer calves (n = 2,869) were received at a commercial feedlot located in southeastern Colorado from a ranch in western Nebraska over 2 consecutive years. All cattle were shipped approximately 536 km and were received over a 3-d period for each year. Descriptive statistics by year of steers studied are provided in Table 1. To minimize the effects of shipping stress, cattle were kept overnight in receiving pens, provided hay and water, and processed the following day.
Table 1.
Descriptive statistics of calf ages, weights, and background days on ranch and at receiving in Bos taurus cattle weaned in central Nebraska and received at the South Eastern Colorado Research Center (Fort Collins, CO)1
| Trait | n | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Weaning age, d | 1,376 | 135 | 21.27 | 72 | 191 |
| Year 1 | 791 | 134 | 22.83 | 91 | 191 |
| Year 2 | 585 | 137 | 18.86 | 72 | 173 |
| Weaning weight, kg | 1,395 | 158.0 | 26.48 | 81.7 | 240.4 |
| Year 1 | 802 | 161.1 | 25.49 | 90.7 | 236.8 |
| Year 2 | 593 | 153.7 | 27.21 | 81.7 | 240.4 |
| Background,2 d | 1,394 | 83 | 22.27 | 51 | 113 |
| Year 1 | 802 | 78 | 22.89 | 51 | 110 |
| Year 2 | 593 | 89 | 19.57 | 56 | 113 |
| Receiving weight, kg | 2,864 | 221.7 | 24.34 | 150.6 | 335.7 |
| Year 1 | 1,551 | 223.9 | 23.46 | 156.0 | 324.8 |
| Year 2 | 1,313 | 219.1 | 25.11 | 150.6 | 335.7 |
| Days after receiving BRD treated,3 d | 792 | 25 | 29.60 | 5 | 215 |
| Year 1 | 698 | 21 | 20.47 | 7 | 215 |
| Year 2 | 94 | 57 | 56.40 | 5 | 215 |
1Modified from McAllister (2010).
2Time spent after weaning but before shipping to feedlot.
3BRD = bovine respiratory disease. Year 1 steers were not vaccinated on receiving, whereas yr 2 steers were vaccinated for bovine viral diarrhea, Pasteurella multocida, and Mannheimia haemolytica.
During processing, steers received a radio frequency identification tag, a visual identification tag, and a lot tag. Steers were also vaccinated, dewormed, implanted with a growth promotant, and measured for BW and behavior; additionally, blood samples were collected. Prior to shipping, cattle were vaccinated in Nebraska against bovine viral diarrhea, Pasteurella multocida, and Mannheimia haemolytica (Pyramid 2 + Type II BVD and Presponse SQ; Boehringer Ingelheim Vetmedica Inc., St. Joseph, MO) before weaning and again at weaning. Year 1 cattle were not vaccinated on arrival at the feedlot. However, at the request of the commercial feedlot owners in yr 2, cattle were vaccinated on feedlot entry for common viral and bacterial agents associated with BRD: bovine viral diarrhea, Pasteurella multocida, and Mannheimia haemolytica (Pyramid 2 + Type II BVD and Presponse SQ). This difference in vaccination protocols resulted in 45% of steers developing BRD in yr 1 and 7% in yr 2 (Table 1), which more accurately reflects the range of vaccination procedures in the beef industry (i.e., no vaccinations versus multiple vaccinations). All steers received oral and pour-on parasiticides, oxfendazole (Synanthic Suspension 9.06%; Boehringer Ingelheim Vetmedica Inc.) and ivermectin (ProMectin B Pour-on; Vedco, Inc., St. Joseph, MO) on arrival to the feedlot. In yr 1, growth promotants containing 80 mg trenbolone acetate and 16 mg estradiol (Revalor-IS; Intervet/Schering-Plough, Millsboro, DE) were administered per feedlot recommendation with a reimplant after 72 d on feed. In yr 2, cattle were implanted with a 200 d delayed releasing growth promotant of 200 mg trenbolone acetate and 40 mg estradiol (Revalor XS; Intervet/Schering-Plough). Collection of repeated phenotypic performance data (i.e., chute score, flight time, processing time, weight, and growth measurements via ultrasound) occurred on arrival and on d 73 and 130 in yr 1 and d 85 and 148 in yr 2. Approximately 10 mL of blood was collected via jugular venipuncture using vacutainers containing EDTA for whole-genome genotyping. Whole blood was shipped to Zoetis (Kalamazoo, MI) for DNA extraction and genotyping using the Bovine SNP50 BeadChip (Illumina Inc., San Diego, CA). Plasma was isolated from whole blood samples and frozen at −20°C until plasma immune trait analyses.
Health Management
Commercial feedlot personnel monitored steer health on a daily basis. Animals identified as either sick or lame were removed and relocated to the Colorado State University South Eastern Colorado Research Center (SECRC; Fort Collins, CO), adjacent to the commercial feedlot, for further evaluation. A dirt lane of 6.10 m separated these 2 facilities. Steers identified as BRD positive had to exhibit ≥ 2 clinical signs and/or rectal temperature > 39.7°C. Clinical signs included eye discharge, nasal discharge, cough, lethargy, rapid breathing, and atypical interstitial pneumonia or acute upper respiratory syndrome. Rectal temperature was regarded to be an overall determining factor because it is less subjective than visual appraisal alone. Based on these criteria, steers were binomially classified as treated (1; n = 792) versus not treated (0; n = 2,077) for BRD.
Treatment protocols were administered in accordance with the commercial feedlot and SECRC standard operating procedures. Steers with a rectal temperature below 39.7°C and displaying no clinical signs between d 5 and 7 after treatment of tulathromycin (Draxxin; Pfizer Animal Health, Zoetis, Kalamazoo, MI) were considered recovered and returned to their respective pens at the commercial feedlot. Steers with rectal temperatures above 39.7°C or exhibiting multiple clinical signs of BRD within 7 d of the initial treatment received a second BRD treatment of fluoroquinolone (Baytril; Bayer Animal Health, Leverkusen, Germany). Steers were monitored for 5 d, reevaluated, and treated a third time using oxytetracycline (Bio-Mycin 200; Boehringer Ingelheim Vetmedica Inc.) if exhibiting BRD signs. Animals that were previously treated and recovered from BRD but again developed clinical signs of BRD continued on a treatment protocol based on the last treatment record. Steers requiring >3 treatments were removed from the study with further treatment protocols made at the discretion of the commercial feedlot. All other illnesses lacking sufficient evidence of BRD were treated as diagnosed (e.g., bloat, lameness, and pinkeye). Similar to the feedlot, while at the SECRC, steers with clinical signs of BRD were fed a commercial diet (66 Mcal/hundredweight DM). Steers were also fed long-stemmed hay to acclimate them to the bunk feeding.
Laboratory Analyses
Plasma isolated from jugular blood collected on receiving was used to determine circulating cortisol, IL-8, IgG, IgG1, and IgG2 concentrations. Plasma samples were measured for cortisol and IL-8 at the University of Illinois (Urbana-Champaign, IL). Plasma cortisol was measured using a commercially available RIA kit (Coat-A-Count; Siemens Healthcare, Malvern, PA) following manufacturer procedures. Intra- and interassay CV were 4.5 and 8.5%, respectively, and the minimum detectable concentration was 100 ng/dL. Interleukin 8 was measured in duplicate using a commercially available ELISA (R&D Systems, Inc., Minneapolis, MN). Circulating IgG, IgG1, and IgG2 were measured using an ELISA-based method at South Dakota State University according to the manufacturer's instructions (Bethyl Laboratories, Inc., Montgomery, TX). Immunoglobulin levels greater than the standard curve were subsequently remeasured.
Statistical Analyses
Descriptive statistics were generated using the boot and pastecs packages in R (R Core Team, 2013). Observation numbers, means, SE, SD, and minimum and maximum values are reported. Values outside the normal range were determined for IgG, IgG1, and IgG2 using Cook's distance > 4/n. Measures for IgG > 100 mg/mL and IgG1 and IgG2 > 75 mg/mL were removed, as values were outside the normal range and were biologically irrelevant according to estimates reported in literature (Butler, 1983; Gilbert et al., 1988). All values < 0 mg/mL were adjusted to 0 to account for nonspecific binding from the ELISA kit. After values outside the normal range were removed, plasma measurements for cortisol, IgG, IgG1, IgG2, and IL-8 were within the expected range.
A single-trait sire model presented below was used to determine heritability for BRD, cortisol, IgG, IgG1, IgG2, and IL-8 was
Specifically, y was a vector of observations, X was a known incidence matrix relating contemporary group and process order effects in b to observations in y, Z was a known incidence matrix relating random sire effects in s to observations in y, and e was a vector of random residual errors. Random effects in the model were assumed to have 0 means and variances represented as follows:
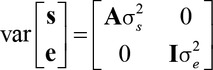 |
in which A was Wright's numerator relationship matrix and I was an identity matrix whose order was equal to the number of observations in y. For BRD, a binary trait, a probit link function was used, thereby implicitly defining a residual variance of 1. Genetic correlations were estimated for each of the 15 trait pairs using the bivariate form of the sire model presented above with covariances fitted between traits.
The genetic parameters for all traits and their SE were estimated using ASREML (version 3.0; VSN International, Ltd., Hemel Hempstead, UK), which uses an average information REML algorithm. Additive genetic variance was calculated as 4 times the sire variance estimated through ASREML.
Contemporary group was included as a fixed effect in the above models with process order within day used as a covariate. For dependent variables measured on receiving (i.e., cortisol, immunoglobulins, and IL-8), contemporary group consisted of ranch unit of origin (n = 3) and entry process date (n = 6). The contemporary group for BRD, a postreceiving trait, included ranch unit of origin (n = 3), feedlot pen (n = 11), and entry process date (n = 6). Half of the steers were commercial (n = 1,395) and the other half was seed stock (n = 1,469); therefore, only the commercial steers were backgrounded before shipping. Commercial versus seed stock steers were not commingled; therefore, the fixed effect pen would have accounted for a steer's backgrounding status.
Calves in the study were the result of multiple sire breeding pastures. Sire identification was performed via DNA sampling of both sire and progeny using a commercial DNA laboratory. A sparse historical pedigree was obtained from the ranch of origin and used to construct a 5-generation pedigree starting with the sires of animals in the study. There were 308 unique sires with progeny that had data and 548 total sires in the full pedigree. In this 5-generation pedigree, dam information was extremely scarce. Therefore, for the purpose of estimating heritability and genetic correlation, a sire model was used to best describe the flow of genetic material from one generation to next.
RESULTS
Descriptive Statistics and Heritability
The average weight for steers (n = 2,864) entering the feedlot was 221.75 kg (SD 24.34). In yr 1, 1,551 calves (223.90 kg [SD 23.46]) were received at an average age of 212 d, and in yr 2, 1,318 calves (219.10 kg [SD 25.11]) were received at an average age of 226 d (Table 1). Steers were treated for BRD an average of 36 d earlier in yr 1 (SD 20.47) compared with yr 2 (SD 56.40). Additionally, a total of 698 steers were treated for BRD in yr 1 and 94 in yr 2. Descriptive statistics with corresponding SE and SD for the immune system traits determined prior to steers developing BRD is provided in Table 2. Total IgG was greater than IgG1, which was greater than IgG2. Values outside the normal range for IgG (measure > 100 mg/mL), IgG1 (measure > 75 mg/mL), and IgG2 (measure > 75 mg/mL) did not overlap among immunoglobulins. Biologically irrelevant immunoglobulin samples removed from analyses were attributed to sample contamination. Only 5, 6, and 6 samples for IgG, IgG1, and IgG2, respectively, were removed from analysis. Both IgG2 (0.24 ± 0.06) and IL-8 (0.30 ± 0.06; Table 2) had moderate heritability estimates and low heritability estimates were determined for BRD, cortisol, IgG, and IgG1 (h2 ≤ 0.17 ± 0.08).
Table 2.
Descriptive statistics of plasma immune response traits of Bos taurus calves weaned in central Nebraska and received at the South Eastern Colorado Research Center (Fort Collins, CO) measured at receiving 1
| Item | n | Mean | SE | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Cortisol, ng/mL | 2,861 | 35.46 | 30.71 | 16.42 | 3.60 | 105.80 |
| IgG, mg/mL | 2,864 | 18.92 | 24.92 | 13.33 | 0.00 | 98.80 |
| IgG1, mg/mL | 2,863 | 13.89 | 12.57 | 6.73 | 0.00 | 73.50 |
| IgG2, mg/mL | 2,863 | 11.25 | 16.42 | 8.78 | 0.00 | 75.00 |
| IL-8, pg/mL | 2,823 | 447.85 | 4.79 | 254.44 | 223.00 | 1,366.23 |
1IgG = Total Immunoglobulin, IgG1 = Immunoglobulin 1, IgG2 = Immunoglobulin 2, IL-8 = Interleukin 8.
Genetic Relationships with Bovine Respiratory Disease
Moderate positive genetic correlations (rg ≥ 0.36 ± 0.32) were detected between BRD with IgG and IgG1 (Table 3). Also, there was a nonsignificant weak positive correlation between IL-8 and BRD. Conversely, BRD status was negatively genetically correlated with plasma cortisol (rg = −0.19 ± 0.32; Table 3).
Table 3.
Heritability estimates (h2; diagonal) and genetic correlations (rg; above diagonal) with SE for bovine respiratory disease (BRD), cortisol, immunoglobulins (IgG, IgG2, IgG1, and IgG2), and IL-8 measured at receiving in Bos taurus cattle weaned in central Nebraska and received at the South Eastern Colorado Research Center (Fort Collins, CO)1,2,3,4
| Item | BRD | Cortisol | Total IgG | IgG1 | IgG2 | IL-8 |
|---|---|---|---|---|---|---|
| BRD | 0.17 (0.08) | −0.19 (0.32) | 0.42 (0.28) | 0.36 (0.32) | 0.07 (0.25) | 0.26 (0.26) |
| Cortisol | 0.13 (0.05) | −0.19 (0.26) | −0.12 (0.29) | 0.06 (0.22) | −0.08 (0.29) | |
| Total immunoglobulin | 0.15 (0.05) | 0.85 (0.12) | 0.83 (0.09) | 0.05 (0.20) | ||
| IgG1 | 0.11 (0.05) | 0.55 (0.16) | 0.01 (0.22) | |||
| IgG2 | 0.24 (0.06) | −0.11 (0.17) | ||||
| IL-8 | 0.30 (0.06) |
1Contemporary group for cortisol, IgG, IgG1, IgG2, and IL8 defined as ranch and entry process date, and contemporary group for BRD defined as ranch, lot, and process date. All analyses were fit with covariate process order within day.
2Genetic correlations estimated in ASREML (VSN International, Ltd., Hemel Hempstead, UK) using the sire model.
3Beef cattle (n = 2,869) measured over 2 yr.
4IgG = Total Immunoglobulin, IgG1 = Immunoglobulin 1, IgG2 = Immunoglobulin 2, IL-8 = Interleukin 8.
Immune Response Parameter Genetic Relationships
A low genetic correlation between both IgG and IgG1 with cortisol (rg ≤ −0.19 ± 0.26) as well as a weak relationship with IL-8 (rg = −0.08 ± 0.29) and IgG2 (rg = 0.06 ± 0.22) with cortisol was detected. There was a weak negative genetic correlation between IL-8 and IgG2 (rg = −0.11 ± 0.17). As expected, a strong positive genetic correlation was observed among IgG, IgG1, and IgG2 (rg ≥ 0.83 ± 0.09). Greater than 50% of total IgG is composed of IgG1 and 20 to 40% is composed of IgG2 (Hashira et al., 2000).
DISCUSSION
Applicability of Results to U.S. Beef Industry
The average steer in the United States weighs approximately 200 to 320 kg at weaning (6 to 10 mo age). Calves used in the current study were slightly smaller than average at weaning (158 ± 26.48), which was attributed to calves being only 4.5 mo of age. Because calves were younger and lighter, calves were backgrounded until weights were adequate for finishing. Only half of the steers used in this study had weight and backgrounding information because half of the calves were seed stock and the other half were commercial. Upon receiving, calves were approximately 7 mo of age and had weights were similar to that of the national average at that age (Table 1). The majority of steers remained healthy throughout the trial, with a little more than one-third (38%) treated for BRD. In yr 1 (not vaccinated), 45% of steers developed BRD compared with 7% in yr 2 (vaccinated). Generally, 16.2% of U.S. cattle entering a feedlot develop respiratory disease (APHIS, 2013). Rates of BRD in yr 1 were much higher than average; however, none of the steers were vaccinated for the BRD complex. Year 2 BRD rates were well below the average 87.5% of cattle vaccinated for BRD that still develop BRD (APHIS, 2013).
In Table 2, more variation was observed in cortisol levels in the current study than in previous research (7.00 to 29.50 ng/mL; Bristow and Holmes, 2007). Immunoglobulin G and isotype levels were lower in these steers than in beef calves from previous research (Waldner and Rosengren, 2009). Similar to our findings, Kimura et al. (2002) determined plasma IL-8 levels in dairy cattle with retained placentas ranged from 134 to 300 pg/mL. Number of observations, calf age, breed, and environmental conditions of the stressor (i.e., shipping versus a squeeze chute) all differed with each study making it difficult to compare immune trait levels across studies. Limited information is available on immune system traits in receiving beef steers; therefore, it was difficult to assess if plasma levels were typical of this age or if differing levels were attributed to environmental conditions. Immune system trait plasma levels were within the expected range of the kits used, but it should be noted that levels varied greatly among steers.
Presented in Table 4 are previously reported heritability estimates for BRD, cortisol, total IgG, IgG1, and IgG2 for comparison with those reported in this study. Previous heritability estimates for BRD confirm the estimate (h2 = 0.17 ± 0.08) in the current research (Snowder et al., 2005, 2006, 2007; Schneider et al., 2010). It is possible that heritability estimates reported in this study were inflated due to dominance; however, given the structure of the data and the unknown breed composition of the dam, these effects were unable to be accounted for. These results provide evidence to suggest that improvement on BRD is possible through genetic selection. However, accuracy of heritability estimates may be improved for BRD through a more accurate phenotype, increased incidence reporting for individuals, development and increased use of genomic markers, and/or incorporating genetic relationships through multiple trait evaluation into estimates of genetic merit.
Table 4.
Literature heritability (h2) estimates for bovine respiratory disease (BRD), cortisol, and immune response measures in livestock
| Variable | Breed | n | h 2 d 1 | Source |
|---|---|---|---|---|
| BRD | Beef cattle | 10,142 | 0.10 ± 0.02 | Muggli-Cockett et al. (1992) |
| Beef cattle | 110,412 | 0.20 ± 0.012 | Snowder et al. (2005) | |
| 0.48 ± 0.013 | ||||
| Beef cattle | 18,112 | 0.08 ± 0.01 | Snowder et al. (2006) | |
| 0.18 ± 0.013 | ||||
| Beef cattle | 18,112 | 0.08 ± 0.01 | Snowder et al. (2007) | |
| Angus × | 1,519 | 0.07 ± 0.04 | Schneider et al. (2010) | |
| Cortisol | Swiss Landrace | 417 | 0.40 ± 0.02 | Kadarmideen and Janss (2007) |
| IgG | Holstein | 1,293 | 0.12 ± 0.06 | Mazengera et al. (1985) |
| IgG1 | Beef cattle | – | 0.05 ± 0.17 | Gilbert et al. (1988) |
| IgG2 | Holstein | 190 | 0.28 ± 0.22 | Mallard et al. (1983) |
| Holstein | 137 | 0.02 ± 0.31 | Detilleux et al. (1994) | |
| Holstein | 1,293 | 0.10 ± 0.07 | Mazengera et al. (1985) | |
| Red Danish | 758 | 0.12 ± 0.08 | Thode Jensen and Christensen (1975) | |
| IL-8 | – | – | – | – |
1 h 2 d = direct genetic effect.
2Preweaning heritability estimate.
3Calculated on an underlying continuous scale.
Given the moderate heritability and genetic correlation estimates between BRD and cortisol, plasma concentration of cortisol may serve as an index variable that can be used for indirect selection for BRD or used to create an index for BRD susceptibility. Limited research was previously published describing the heritability of cortisol; however, stress research in pigs demonstrated cortisol is moderately heritable (h2 = 0.40; Kadarmideen and Janss, 2007). Although steers in the current study were not intentionally stressed, the act of shipping creates a stressed phenotype in some animals. A low to moderate heritability for cortisol suggested that steers varied in their innate response to environmental stressors, potentially leaving them more or less susceptible to illness. Based on the negative genetic correlation with BRD and the moderate heritability, cortisol levels measured after shipping may serve as a potential candidate for selection for BRD susceptibility.
Low to moderate heritability estimates were observed for IgG and isotypes in the current study. Previous reports of heritability for IgG and its subclasses ranged from 0.00 to 0.28 in dairy calves, supporting the current research estimates in beef cattle (Mallard et al., 1983; Detilleux et al., 1994). Interleukin 8 had a moderate heritability estimate of 0.30 ± 0.06. No known research is available that described the genetic variability of IL-8. However, these results provide evidence to suggest that development of a BRD susceptibility index through the incorporation of IgG, corresponding isoforms, and IL-8 may decrease the incidence of BRD in cattle. Future research must be conducted to better characterize the phenotype for BRD susceptibility and identify genomic regions associated with BRD.
Genetic Relationships Involving Bovine Respiratory Disease
Results suggested that there was a genetic relationship between circulating cortisol measured after shipping and the development of BRD. Initially, the negative genetic relationship between cortisol and BRD was surprising, as prior reports indicated that cortisol levels increase after transportation, thereby increasing an animal's susceptibility to disease (Jericho, 1979; Slocombe et al., 1984). However, when exposed to acute stressors, the immune system will react in an immunoprotective manner (Salak-Johnson and McGlone, 2007; Carroll and Forsberg, 2007; Dhabhar, 2009). In our study, cortisol was measured after shipping, which would constitute an acute stressor. Therefore, this may, in part, explain why there was a negative genetic correlation between cortisol and BRD. Additionally, McGlone et al. (1993) determined that there was a moderate phenotypic correlation (rp = 0.35, P = 0.036) between natural killer cell cytotoxicity and plasma cortisol concentrations. It is possible that cortisol secretion after an acute stressor versus a chronic stressor is a different trait from a genetic standpoint; therefore, phenotypic relationships observed in previous research may not translate to a genetic observation. Therefore, more research must be conducted to elucidate these effects.
Total IgG, IgG1, and IgG2 are major components of the humoral immune system that are part of the adaptive immune response that protects cattle against bacterial and viral challenges (Stanley et al., 1984). These data suggested that selection for total IgG and/or IgG1 could impact whether a steer developed BRD. Interestingly, there was no apparent genetic relationship between IgG2 and BRD. In cattle, IgG levels have been reported to decrease after shipping (Kegley et al., 1997). Immunoglobulin G1 has been shown to increase in allergy-type reactions and indicate a T helper 2 response (Kemeny et al., 1986), whereas IgG2 increases suggest a stronger T helper 1 response and T cell memory (Estes and Brown, 2002). Also, in the presence of encapsulated bacteria such as Mannheimia haemolytica, a key bacterium involved in BRD, IgG2 was increased. Immunoglobulin G2 antibodies have also been shown to be associated with reduced incidence of cattle becoming infected with respiratory bovine coronaviruses (Lin et. al., 2000, 2001). As IgG1 makes up the majority of total IgG and has a differing mode of regulation compared with IgG2, it is plausible that the genetic relationship between IgG1 and IgG2 with BRD would differ. To date, genetic relationships between BRD status and total IgG or isotypes of IgG have not been reported in the literature. These results suggested that negative selection pressure for IgG and IgG1 and positive selection pressure for plasma cortisol may result in progeny less susceptible to developing BRD.
This is the first description of a genetic relationship between BRD and IL-8. Interleukin 8 is a cytokine that serves as a chemoattractant signal, recruiting neutrophils to the sight of infection (Hull et al., 2001). Phenotypically, Caswell et al. (1998) demonstrated that IL-8 expression was upregulated in lesions of pneumonic pasteurellosis in beef calves. It was concluded that IL-8 is integral to the recruitment of neutrophils to establish lesions in cattle with pneumonia. In vitro, low levels of Pasteurella haemolytica, a Gram-negative bacterium associated with BRD, induced high concentrations of IL-8 (Morsey et al., 1996). Similar to previous phenotypic reports, there appears to be a moderate positive genetic correlation between IL-8 and BRD. Therefore, genetic selection against IL-8 may decrease a steer's susceptibility to developing BRD. Although a moderate genetic correlation existed between BRD and IL-8, further investigation is warranted to determine if IL-8 is useful as a predictor of BRD.
Genetic Relationships of Immune Responses
To our knowledge, this is the first study to evaluate the genetic relationships among cortisol, IgG, IgG2, and IL-8. Human maternal total IgG is composed of 52% IgG1, 40% IgG2, 2% IgG3, and 2% IgG4 (Hashira et al., 2000). Similar ratios would be expected for cattle, but immunoglobulins 3 and 4 were not measured; therefore, specific ratios could not be determined. However, as expected, concentrations of total IgG > IgG1 > IgG2. Because of this relationship, strong genetic correlations were expected between total IgG and isoforms, which was confirmed given the ≥0.83 ± 0.09 genetic correlation. Also, there was a strong genetic correlation between IgG1 and IgG2 (0.55 ± 0.16). Although total IgG and isoforms differ in phenotypic function, the strong genetic correlation between total IgG and isoforms suggests that they are under similar genetic control. However, the genetic correlation between IgG1 and IgG2 suggested they differ genetically. Selection for total IgG will subsequently impact both IgG1 and IgG2. Selection for IgG1 or IgG2 will influence the other isoform, but not as strongly.
Limited reports were available describing the interrelationships among immunoglobulins, cortisol, and IL-8. Kimata et al. (1992) determined that IL-8 had little influence on in vitro secretion of IgG1, IgG2, IgG3, or IgG4. Previous phenotypic relationships between IL-8 and IgG and isoforms confirm weak genetic correlation estimates determined in the current study. Results suggested that selection for IL-8 will have little impact on total IgG or isoforms and vice versa. Interleukin 8 is a chemoattractant that impacts neutrophil production, influences innate immune response through angiogenesis (Murphy, 2008), and is inhibited by cortisol (Dovio et al., 2004; Sorenson et al., 2012). Therefore, a decrease in cortisol levels should increase IL-8 production, potentially leading to increased pain and inflammation. However, because only a low genetic correlation was observed, the genetic relationship between cortisol with IL-8 appeared to be minimal at best in these animals. Overall, IgG, IgG1, IgG2, and cortisol appeared to be largely independent of IL-8. Therefore, it is possible to develop a selection index for BRD susceptibility that includes cortisol, total IgG, and IL-8 without confounding genetic relationships among component traits.
Overall, reported low to moderate genetic correlations support our hypothesis that there is a genetic relationship between BRD and immune system traits. Specifically, heritability estimates and genetic relationships of cortisol and IgG with BRD suggest herd health may be improved through 1) genetic selection with breeding values and selection indices and/or 2) improvement of the BRD phenotype through incorporation of immune traits in receiving management strategies. Admittedly, measurement of these parameters for use in management may be difficult given sample collection and time needed to deliver results from these diagnostic assays. This study described genetic relationships between treatment for BRD, stress hormones, and immune system traits. Several parameters had a favorable genetic relationship with BRD and may be useful to direct future research to reduce the incidence of BRD through genetic improvement. This is especially useful establishing a genetic selection model, because establishing a pedigree and relevant environmental factors to BRD is difficult; therefore, genetic improvement must be primarily driven through advancements in application and development of genomic prediction technologies.
LITERATURE CITED
- Animal and Plant Health Inspection Service (APHIS) Vaccine usage in U.S. feedlots. 2013 https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_is_VaccineUsage.pdf (Accessed March 1, 2016.)
- Bristow D. J., Holmes D. S. 2007. Cortisol levels and anxiety-related behaviors in cattle. Physiol. Behav. 90:626–628. doi: 10.1016/j.physbeh.2006.11.015 [DOI] [PubMed] [Google Scholar]
- Butler J. E. 1983. Bovine immunoglobulins: An augmented review. Vet. Immunol. Immunopathol. 4:43–152. doi: 10.1016/0165-2427(83)90056-9 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Forsberg N. E. 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. North Am.: Food Anim. Pract. 23:105–149. doi: 10.1016/j.cvfa.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Caswell J. L., Middleton D. M., Sorden S. D., Gordon J. R. 1998. Expression of the neutrophil chemoattractant interleukin-8 in the lesions of bovine pneumonic pasteurellosis. Vet. Pathol. 35:124–131. doi: 10.1177/0300985898035 [DOI] [PubMed] [Google Scholar]
- Detilleux J. C., Koehler K. J., Freeman A. E., Kehrli M. E., Jr, Kelley D. H. 1994. Immunological parameters of periparturient Holstein cattle: Genetic variation. J. Dairy Sci. 77:2640–2650. doi: 10.3168/jds.S0022-0302(94)77205-2 [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S. 2009. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 16:300–317. doi: 10.1159/000216188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovio A., Sartori M. L., Masera R. G., Peretti L., Perotti L., Angeli A. 2004. Effects of physiological concentrations of steroid hormones and interleukin-11 on basal and stimulated production of interleukin-8 by human osteoblast-like cells with different functional profiles. Clin. Exp. Rheumatol. 22:79–84. [PubMed] [Google Scholar]
- Duff G. C., Galyean M. L. 2007. Board-invited review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A. 2001. The immunology of the bovine respiratory disease complex. Vet. Clin. North Am.: Food Anim. Pract. 17:535–550. doi: 10.1016/S0749-0720(15)30005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes D. M., Brown W. C. 2002. Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet. Immunol. Immunopathol. 90:1–10. doi: 10.1016/S0165-2427(02)00201-5 [DOI] [PubMed] [Google Scholar]
- Gifford C. A., Holland B. P., Mills R. L., Maxwell C. L., Farney J. K., Terrill S. J., Step D. L., Richards C. J., Burciaga Robles L. O., Krehbiel C. R. 2012. Growth and development symposium: Impacts of inflammation on cattle growth and carcass merit. J. Anim. Sci. 90:1438–1451. doi: 10.2527/jas.2011-4846 [DOI] [PubMed] [Google Scholar]
- Gilbert R. P., Gaskins C. T., Hillers J. K., Brinks J. S., Denham A. H. 1988. Inbreeding and immunoglobulin G1 concentrations in cattle. J. Anim. Sci. 66:2490–2497. [DOI] [PubMed] [Google Scholar]
- Hashira S., Okitsu-Negishi S., Yoshino K. 2000. Placental transfer of IgG subclasses in a Japanese population. Pediatr. Int. 42:337–342. doi: 10.1046/j.1442-200x.2000.01245.x [DOI] [PubMed] [Google Scholar]
- Hull J., Ackerman H., Isles K., Usen S., Pinder M., Thomson A., Kwiatkowski D. 2001. Unusual haplotypic structure of IL-8, a susceptibility locus for a common respiratory virus. Am. J. Hum. Genet. 69:413–419. doi: 10.1086/321291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jericho K. W. F. 1979. Update on pasteurellosis in young cattle. Can. Vet. J. 20:333–335. [PMC free article] [PubMed] [Google Scholar]
- Kadarmideen H. N., Janss L. L. G. 2007. Population and systems genetics analyses of cortisol in pigs divergently selected for stress. Physiol. Genomics 29:57–65. doi: 10.1152/physiolgenomics.00144.2006 [DOI] [PubMed] [Google Scholar]
- Kegley E. B., Spears J. W., Brown T. T., Jr 1997. Effect of shipping and chromium supplementation on performance, immune response, and disease resistance of steers. J. Anim. Sci. 75:1956–1964. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Urbanek R., Amlot P. L., Ciclitira P. J., Richards D., Lessof M. H. 1986. Sub-class of IgG in allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergy. Clin. Allergy 16:571–581. doi: 10.1111/j.1365-2222.1986.tb01996.x [DOI] [PubMed] [Google Scholar]
- Kimata H., Yoshida A., Ishioka C., Lindley I., Mikawa H. 1992. Interleukin 8 (IL-8) selectively inhibits immunoglobulin E production induced by IL-4 in human B cells. J. Exp. Med. 176:1227–1231. doi: 10.1084/jem.176.4.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Goff J. P., Kehrli M. E., Jr, Reinhardt T. A. 2002. Decreased neutrophil function as a cause of retained placenta in dairy cattle. J. Dairy Sci. 85:544–550. [DOI] [PubMed] [Google Scholar]
- Lin X. Q., O'Reilly K. L., Storz J., Purdy C. W., Loan R. W. 2000. Antibody responses to respiratory coronavirus infections of cattle during shipping fever pathogenesis. Arch. Virol. 145:2335–2349. doi: 10.1007/s007050070024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., O'Reilly K. L., Burrell M. L., Storz J. 2001. Infectivity-neutralizing and hemaglutinin-inhibiting antibody responses to respiratory coronavirus infections of cattle in pathogenesis of shipping fever pneumonia. Clin. Vaccine Immunol. 8:357–362. doi: 10.1128/CDLI.8.2.357-362.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard B. A., Burnside E. B., Burton J. H., Wilkie B. N. 1983. Variation in serum immunoglobulins in Canadian Holstein-Friesians. J. Dairy Sci. 66:862–866. doi: 10.3168/jds.S0022-0302(83)81868-2 [DOI] [PubMed] [Google Scholar]
- Mazengera K. E., Kennedy B. W., Burnside E. B., Wilkie B. N., Burton J. H. 1985. Genetic parameters of bovine serum immunoglobulins. J. Dairy Sci. 68:2309–2314. doi: 10.3168/jds.S0022-0302(85)81104-8 [DOI] [PubMed] [Google Scholar]
- McAllister C. M. 2010. Genetics of bovine respiratory disease in feedlot cattle. MS Thesis, Colorado State University, Fort Collins, CO. [Google Scholar]
- McGlone J. J., Salak J. L., Lumpkin E. A., Nicholson R. I., Gibson M., Norman R. L. 1993. Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J. Anim. Sci. 71:888–896. [DOI] [PubMed] [Google Scholar]
- Morsey M. A., Popwych Y., Kowalski J., Gerlach G., Godson D., Campos M., Babiuk L. A. 1996. Molecular cloning and expression of bovine interleukin-8. Microb. Pathog. 20:203–212. doi: 10.1006/mpat.1996.0019 [DOI] [PubMed] [Google Scholar]
- Muggli-Cockett N. E., Cundiff L. V., Gregory K. E. 1992. Genetic analysis of bovine respiratory disease in beef calves during the first year of life. J. Anim. Sci. 70:2013–2019. [DOI] [PubMed] [Google Scholar]
- Murphy P. 2008. Chemokines. In: Paul W. E. editor, Fundamental immunology. Lippincott Williams & Wilkins, Philadelphia, PA: p. 681–707. [Google Scholar]
- National Agricultural Statistics Service (NASS) 2011. Cattle death losses. http://usda.mannlib.cornell.edu/usda/current/CattDeath/CattDeath-05-12-2011.pdf. (Accessed March 1, 2016.)
- R Core Team 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. (Accessed Nov. 1, 2016.) [Google Scholar]
- Salak-Johnson J. L., McGlone J. J. 2007. Making sense of apparently conflicting data: Stress and immunity in swine and cattle. J. Anim. Sci. 85:E81–E88. doi: 10.2527/jas.2006-538 [DOI] [PubMed] [Google Scholar]
- Schneider M. J., Trait R. G., Jr, Ruble M. V., Busby W. D., Reecy J. M. 2010. Evaluation of fixed sources of variation and estimation of genetic parameters for incidence of bovine respiratory disease in preweaned calves and feedlot cattle. J. Anim. Sci. 88:1220–1228. doi: 10.2527/jas.2008-1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe R. F., Derksen F. J., Robinson N. E., Trapp A., Gupta A., Newman J. P. 1984. Interactions of cold stress and Pasteurella haemolytica in the pathogenesis of pneumonic pasteurellosis in calves: Method of induction and hematologic and pathologic changes. Am. J. Vet. Res. 45:1757–1763. [PubMed] [Google Scholar]
- Snowder G. D., Van Vleck L. D., Cundiff L. V., Bennet G. L. 2005. Influence of breed, heterozygosity, and disease incidence on estimates of variance components of respiratory disease in preweaned beef calves. J. Anim. Sci. 83:1247–1261. [DOI] [PubMed] [Google Scholar]
- Snowder G. D., Van Vleck L. D., Cundiff L. V., Bennett G. L. 2006. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 84:1999–2008. doi: 10.2527/jas.2006-046 [DOI] [PubMed] [Google Scholar]
- Snowder G. D., Van Vleck L. D., Cundiff L. V., Bennett G. L., Koohmaraie M., Dikeman M. E. 2007. Bovine respiratory disease in feedlot cattle: Phenotypic, environmental, and genetic correlations with growth, carcass, and longissimus muscle palatability traits. J. Anim. Sci. 85:1885–1892. doi: 10.2527/jas.2007-0008 [DOI] [PubMed] [Google Scholar]
- Sorenson M., Jason L., Lerch A., Porter N., Peterson J., Mathews H. 2012. The production of interleukin-8 is increased in plasma and peripheral blood mononuclear cells of patients with fatigue. Neurosci. Med. 3:47–53. doi: 10.4236/nm.2012.31007 [DOI] [Google Scholar]
- Stanley P. J., Corbo G., Cole P. J. 1984. Serum IgG subclasses in chronic and recurrent respiratory infections. Clin. Exp. Immunol. 58:703–708. [PMC free article] [PubMed] [Google Scholar]
- Thode Jensen P., Christensen K. 1975. Genetic analysis of the serum level of IgG2 and total protein in Red Danish cattle. J. Anim. Sci. 40:392–396. [DOI] [PubMed] [Google Scholar]
- Waldner C. L., Rosengren L. B. 2009. Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes. Can. Vet. J. 50:275–281. [PMC free article] [PubMed] [Google Scholar]


