Abstract
The chemical recycling of end‐of‐life polymers can add some value to a future circular economy. In this regard, the hydrogenative degradation of end‐of‐life PLA was investigated to produce 1,2‐propanediol as product, which is a useful building block in polymer chemistry. In more detail, the commercially available Ru‐MACHO‐BH complex was applied as catalyst to degrade end‐of‐life PLA efficiently to 1,2‐propanediol under mild conditions. After investigations of the reaction conditions a set of end‐of‐life PLA goods were subjected to degradation.
Keywords: green chemistry, catalysis, polymers, recycling, degradation
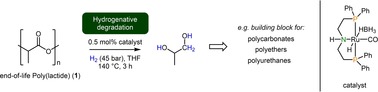
The hydrogenative degradation of end‐of‐life poly(lactide) is described. The transformation is based on the Ruthenium‐catalyzed hydrogenation of the ester function to produce 1,2‐propanediol as a beneficial chemical.
The impact of plastics on mankind or human life is impressively underlined by countless applications and numerous advantages compared to other materials.1, 2 Nevertheless, a significant amount of the plastics is accessed from fossil resources and after the obligations are fulfilled the plastic waste is mainly converted to greenhouse gas carbon dioxide via incineration processes, which creates numerous negative issues.3 A fossil resources‐free and “carbon dioxide‐neutral” alternative is created by the use of polymers based on renewable resources.4 The starting materials are derived from biosynthesis, which converts carbon dioxide, water and energy to useful precursors.5 For instance one important representative is poly(lactide) (PLA, 1) which is based on lactic acid derived from biological processes.6 Lactic acid can either be polymerized to PLA via polycondensation reaction or by initial conversion to lactide, which can be transformed to PLA via ring opening polymerization (ROP).7 A key benefit of poly(lactide) is the near carbon neutral performance, even if the polymer is incinerated, due to the integration of atmospheric carbon dioxide in biosynthesis.8 Furthermore, the polymer is to some extend biodegradable, which causes less environmental problems, but it is not applicable in current industrial composting plants, because of the time needed to accomplish complete degradation.9 However, even if the advantages of plastics from renewable resources are obvious, conflicts can arise from restricted cultivation area and competition with food production and energy production.10 Therefore the recycling of end‐of‐life PLA goods can be useful option to solve some of the issues. In this regard the chemical recycling presents an interesting tool for allowing an efficient recycling.11 In more detail, the polymer is converted to low‐molecular weight chemicals in a depolymerization/ degradation step, which can be either applied as monomer or as monomer precursor to regenerate the polymer or produce other polymers in a polymerization step.12 In consequence a recycling of the chemical functions is feasible. However, currently the implementation of chemical recycling lacks in cost efficiency, high energy demand and toleration of impurities and additives.13
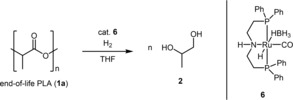
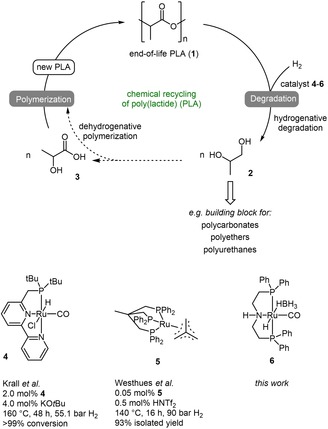
Different approaches for the chemical recycling of end‐of‐life PLA have been accounted so far. For instance alcoholysis, pyrolysis and hydrogenation have been reported.14, 15, 16, 17, 18 Recently Westhues et al. and Krall et al. reported a hydrogenative degradation reaction based on Ruthenium catalysis to yield 1,2‐propanediol (2) as low‐molecular weight chemical, which can be applied as building block for different types of polymers (Scheme 1).17, 18, 19 Both systems require addition of acid or base, high hydrogen pressures and long reaction times. Recently, we have demonstrated the potential of the Ru‐MACHO‐BH complex 6 in the hydrogenative depolymerization of poly(bisphenol A carbonate), which allowed transformations e. g. acid/base‐free conditions, at lower temperatures, low hydrogen pressure and within short times (Scheme 1).20 Based on that initial study we investigated herein the capability of complex 6 in the hydrogenative degradation of end‐of‐life PLA.
Scheme 1.
Synthesis of 1,2‐propanediol from end‐of‐life PLA.
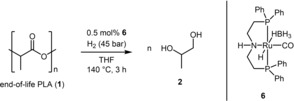
The optimization of the reaction conditions of EoL‐PLA degradation was studied with end‐of‐life transparent cups of PLA. For this purpose, pieces of the sample (68.2 μmol of 1 a based on the monomeric unit) together with the Ruthenium catalyst 6 (0.5 mol% in respect to 1 a) were dissolved in THF under argon atmosphere and the mixture was transferred to an autoclave. Initially, the autoclave was pressurized with 45 bar of H2. Subsequently the autoclave was stirred and heated to 140 °C for 6 hours (Table 1, entry 1). Afterward, the reaction mixture was concentrated under vacuum and an aliquot was used for 1H NMR analysis. The 1H NMR spectrum revealed a signal at 1.09 ppm (CH 3, 3H, d, J=6.4 Hz), 3.33 ppm (CH 2, 1H, dd, J=11.1 Hz, J=7.8 Hz), 3.56 ppm (CH 2, 1H, dd, J=11.1 Hz, J=3.1 Hz) and 3.84 ppm (CH, 1H, m). The obtained data are in accordance with an authentic sample of 1,2‐propanediol; therefore 2 was produced as major compound.21
Table 1.
Ruthenium‐catalyzed hydrogenative degradation of poly(lactide) (1 a) ‐ optimization of the reaction conditions.
|
| |||||
|---|---|---|---|---|---|
|
Entry[a] |
Catalyst loading [mol %] |
T [°C] |
t [h] |
p [bar] |
Yield 2 [%][b] |
|
1 |
0.5 |
140 |
6 |
45 |
>99 |
|
2 |
0.5 |
140 |
3 |
45 |
>99 |
|
3 |
0.5 |
140 |
1 |
45 |
33 |
|
4 |
0.25 |
140 |
3 |
45 |
32 |
|
5 |
0.1 |
140 |
3 |
45 |
<1 |
|
6 |
0 |
140 |
3 |
45 |
<1 |
|
7 |
0.5 |
120 |
3 |
45 |
67 |
|
8 |
0.5 |
100 |
3 |
45 |
4 |
|
9 |
0.5 |
140 |
3 |
30 |
95 |
|
10 |
0.5 |
140 |
3 |
20 |
<1 |
|
11[c] |
0.5 |
140 |
1 |
45 |
<1 |
|
12[d] |
0.5 |
140 |
1 |
45 |
<1 |
[a] Reaction conditions: poly(lactide) (1 a) (68.2 μmol based on the repeating unit of 1 a), 6 (0–0.5 mol%, 0–0.341 μmol based on the repeating unit of 1 a), THF (1.0 mL), 100–140 °C, 1–6 h, 20–45 bar H2. [b] The yield was determined by 1H NMR. [c] Toluene as solvent. [d] Hexane as solvent.
The NMR yield of 2 was determined by relating the integrals of the methyl group at 1.09 ppm from 2 with those of leftover polymer/oligomer at 1.57 ppm. In this regard, an NMR yield of >99 % was calculated (Table 1, entry 1). Comparing the obtained result with the performance of Ruthenium‐based hydrogenation catalysts (Scheme 1, 4 and 5) revealed the formation of 2 within shorter reaction times (4: 48 h; 5: 16 h; 6: 3 h), lower or same temperature (4: 160 °C; 5: 140 °C; 6: 140 °C), and at lower hydrogen pressure (4: 55.1 bar; 5: 90 bar; 6: 45 bar).17, 18 However, compared with complex 5 (0.05 mol%) a higher catalyst loading of 0.5 mol% is required. Subsequently, the reaction time was reduced to 3 h revealing the same yield of 2 (Table 1, entry 2). A further reduction to one hour showed a yield of 33 % (Table 1, entry 3). In the next experiments the catalyst loading was studied by gradually reducing the loading from 0.5 mol% to 0.1 mol% (Table 1, entries 4–6).
At 0.25 mol% loading a diminished yield of 32 % of 2 was noticed, while at lower loading or without any catalyst no product formation was detected. Next the influence of reaction temperature was investigated (Table 1, entries 7–8). A good yield of 67 % of 2 was obtained at 120 °C, while at lower temperatures only minor amounts of product 2 were realized. A reduction of the hydrogen pressure to 20 bar revealed an inactivity of the catalyst (Table 1, entries 9 and 10). Moreover, the solvent THF was replaced by toluene or hexane, but no product formation was observed (Table 1, entries 11 and 12). Based on the results of the optimization study, the following reaction conditions were selected for further experiments: 0.5 mol% of catalyst 6, 140 °C, 45 bar H2 and 3 h reaction time (Table 1, entry 1). Next a set of PLA containing end‐of‐life goods were subjected to hydrogenative degradation (Table 2). First different transparent and colourless products were tested (Table 2, entries 1–6). In most cases excellent conversion of PLA was detected, while NMR yields of 65–73 % were realized (Table 2, entries 2–6). Noteworthy, for calculation of the yield it was assumed that the goods are composed of 100 % PLA, since the number/amount of additives is unknown. Moreover, a transparent cup was tested, which has been used, washed and dried (Table 2, entry 6). Here a good conversion of 78 % of 1 and a good yield of 73 % of 2 was obtained. Moreover, dyed PLA products were hydrogenated (Table 2, entries 7–14). Excellent conversion of PLA was found for most products, while in case of 1 i a low conversion and for 1 m no conversion was detected (Table 2, entries 9 and 13). However, the obtained yields for 2 are to some extend lower compared with transparent products. After successful degradation of the used cups 1 a under the optimized conditions, a scale up was carried out with 0.5 g of 1 a (Scheme 2). Here product 2 was obtained in >99 % NMR yield and in 89 % isolated yield after distillation.22
Table 2.
Ruthenium‐catalyzed hydrogenative degradation of PLA goods.
|
| |||
|---|---|---|---|
|
Entry[a] |
Product |
Conversion of 1[ b] |
Yield of 2 [%][c] |
|
1 |
transparent cup (1 a) |
>99 |
>99 |
|
2 |
transparent disposable food box (1 b) |
>99 |
65 |
|
3 |
transparent Sushi box cover (1 c) |
>99 |
67 |
|
4 |
transparent plastic sheet (1 d) |
>99 |
68 |
|
5 |
transparent bottle (1 e) |
>99 |
66 |
|
6 |
used/washed/dried transparent cups (1 f) |
78 |
73 |
|
7 |
drinking straw with green strips (1 g) |
>99 |
67 |
|
8 |
disposable knife with talcum powder (1 h) |
>99 |
31 |
|
9 |
lid for espresso mugs (contains ∼20–30 % talcum powder) (1 i) |
41 |
<1 |
|
10 |
black lid for coffee mugs (1 j) |
>99 |
59 |
|
11 |
Sushi box (black base) (1 k) |
>99 |
74 |
|
12 |
pink ice cream spoon (1 l) |
>99 |
4 |
|
13 |
coffee paper cup coating (1 m) |
<1 |
<1 |
|
14 |
disposable blue gloves (1 n) |
>99 |
5 |
[a] Conditions: 1 a–1 n (4.9 mg, 68.2 μmol based on the repeating unit), 6 (0.2 mg, 0.5 mol%, 0.341 μmol based on the repeating unit of 1), THF (1.0 mL), 140 °C, 3 h, 45 bar H2 [b] The conversion of PLA was determined by relating the 1H NMR signals of PLA to the signals of the monomer. [c] The yield was determined by 1H NMR with an internal standard of 5‐tert‐butyl‐m‐xylene. The amount of substance of 2 was linked to the amount of substance in the initial PLA‐good (presumption: PLA‐good contains 100 % of PLA).
Scheme 2.
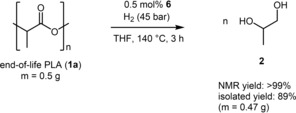
Degradation of PLA cups – scale‐up experiment.
Next the simultaneous hydrogenative depolymerization/degradation was studied (Scheme 3). In this regard, a mixture of end‐of‐life PLA and end‐of‐life poly(bisphenol A carbonate) (7) was reacted with hydrogen in the presence of complex 6.17 Both polymers were converted to the corresponding monomer in good to excellent yields. On the other hand, a mixture of PLA 1 a and poly(propylene carbonate) (9) was subjected to hydrogenative depolymerization/ degradation.23 Noteworthy, both polymers were converted to product 2 in excellent yield.
Scheme 3.
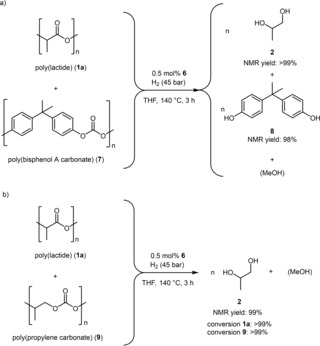
Simultaneous hydrogenative depolymerization/degradation of PLA and a) poly(bisphenol A carbonate) or b) poly(propylene carbonate).
In addition, a consecutive degradation approach of PLA and poly(oxymethylene) (10) was investigated in accordance to Klankermayer and co‐worker (Scheme 4).24 In this regard, end‐of‐life PLA is converted to 2, which is required for the degradation of 10 to produce a cyclic acetal, which can be useful chemical, e. g. as monomer for new polymers.24
Scheme 4.
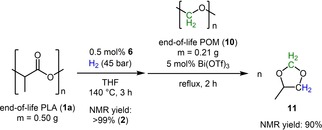
Consecutive degradation of PLA and POM.
In more detail, 1 a was depolymerized with hydrogen in the presence of catalytic amounts of complex 6 to form 2 in >99 % yield. After 3 hours the excess of hydrogen was released and into the reaction mixture poly(oxymethylene) 10 (monomeric ratio 1 a : 10 1 : 1) and catalytic amounts of bismuth(III) triflate (5 mol%) was added.25 After 2 hours at refluxing conditions (oil bath temperature: 90 °C) 4‐methyl‐1,3‐dioxolane (11) was obtained in 90 % yield.
In summary, we have studied a homogenous catalyst system for the hydrogenative degradation of EoL‐poly(lactide). The commercially available Ruthenium‐MACHO‐BH complex was used at low catalyst loadings to yield 1,2‐propanediol as a beneficial chemical in good to excellent yields. Compared to established hydrogenative degradation methods milder conditions were required for the degradation of PLA goods. This might be one step towards achieving the UN's goals for a sustainable development (SDGs 9,11) and a future waste management system (SGD 12).26
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Financial support from the Universität Hamburg (UHH) is gratefully acknowledged. We thank Prof. Dr. Axel Jacobi von Wangelin, Dr. Dieter Schaarschmidt and Bernhard Gregori (all UHH) for general discussions and support. We thank the “Molekülmemory”‐group (UHH) and Kevin Schlütter (UHH) for support.
T.-O. Kindler, C. Alberti, E. Fedorenko, N. Santangelo, S. Enthaler, ChemistryOpen 2020, 9, 401.
References
- 1. Elias H.-G., An Introduction to Plastics., Wiley-VCH Verlag GmbH&Co. KGaA, Weinheim, 2003. [Google Scholar]
- 2.Plastics are typically composed of polymers and often other substances e. g. fillers, plasticizers, colorants.
- 3.
- 3a. Tukker A., Plastics Waste: Feedstock Recycling, Chemical Recycling and Incineration., Smithers Rapra Press, 1997; [Google Scholar]
- 3b. Thompson R. C., Swan S. H., Moore C. J., vom Saal F. S., Philos. Trans. R. Soc. London Ser. B 2009, 364, 1973; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c. Al-Salem S. M., Lettieri P., Baeyens J., Waste Manage. 2009, 29, 2625; [DOI] [PubMed] [Google Scholar]
- 3d. Perlmutter D. P., Rothstein R. L., The Challenge of Climate Change: Which Way Now?, John Wiley & Sons, 2010; [Google Scholar]
- 3e. Gilpin A., Environmental Economics – A Critical Overview, Wiley & Sons Ltd, 2000. [Google Scholar]
- 4. Lu X. B., Liu Y., Zhou H., Chem. Eur. J. 2018, 24, 11255. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Garlotta D., J. Polym. Environ. 2001, 9, 63; [Google Scholar]
- 5b. Zhang X., Fevre M., Jones G. O., Waymouth R. M., Chem. Rev. 2018, 118, 839; [DOI] [PubMed] [Google Scholar]
- 5c. Greene J. P., Sustainable Plastics – Environmental Assessments of Biobased, Biodegradable, and Recycled Plastics, John Wiley & Sons, 2014; [Google Scholar]
- 5d. Mittal V., Renewable Polymers – Synthesis, Processing, and Technology, John Wiley & Sons, 2011. [Google Scholar]
- 6.
- 6a. Groot W., Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, And Application, John Wiley & Sons, 2010; [Google Scholar]
- 6b. Lim L. T., Auras R., Rubino M., Prog. Polym. Sci. 2008, 33, 820; [Google Scholar]
- 6c. Moon S. I., Lee C. W., Miyamoto M., Kimura Y., J. Polym. Sci. Part A 2000, 38, 1673; [Google Scholar]
- 6d. Dechy-Cabaret O., Martin-Vaca B., Bourissou D., Chem. Rev. 2004, 104, 6147; [DOI] [PubMed] [Google Scholar]
- 6e. Datta R., Henry M., J. Chem. Technol. Biotechnol. 2006, 81, 1119. [Google Scholar]
- 7. Vaidya A. N., Pandey R. A., Mudliar S., Kumar M. S., Chakrabarti T., Devotta S., Crit. Rev. Environ. Sci. Technol. 2005, 35, 429. [Google Scholar]
- 8.
- 8a. Hajighasemi M., Nocek B. P., Tchigvintsev A., Brown G., Flick R., Xu X., Cui H., Hai T., Joachimiak A., Golyshin P. N., Biomacromolecules 2016, 17, 2027; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Gil-Castell O., Badia J. D., Ingles-Mascaros S., Teruel-Juanes R., Serra A., Ribes-Greus A., Polym. Degrad. Stab. 2018, 158, 40; [Google Scholar]
- 8c. Badia J. D., Gil-Castell O., Ribes-Greus A., Polym. Degrad. Stab. 2017, 137, 35; [Google Scholar]
- 8d. Badia J. D., Stromberg E., Kittikorn T., Ek M., Karlsson S., Ribes-Greus A., Polym. Degrad. Stab. 2017, 143, 9; [Google Scholar]
- 8e. Yang J., Pan H. W., Li X., Sun S. L., Zhang H. L., Dong L. S., RSC Adv. 2017, 7, 46183; [Google Scholar]
- 8f. Wang D., Yu Y., Ai X., Pan H., Zhang H., Dong L., Polym. Adv. Technol. 2019, 30, 203. [Google Scholar]
- 9.
- 9a. Ghorpade V. M., Gennadios A., Hanna M. A., Bioresour. Technol. 2001, 76, 57; [DOI] [PubMed] [Google Scholar]
- 9b. Satti S. M., Shah A. A., Marsh T. L., Auras R., J. Polym. Environ. 2018, 26, 3848; [Google Scholar]
- 9c. Sedničková M., Pekařová S., Kucharczyk P., Bočkajd J., Janigová I., Kleinová A., Jochec-Mošková D., Omaníková L., Perďochová D., Koutný M., Sedlařík V., Alexy P., Chodák I., Int. J. Biol. Macromol. 2018, 113, 434; [DOI] [PubMed] [Google Scholar]
- 9d. Satti S. M., Shah A. A., Auras R., Marsh T. L., Polym. Degrad. Stab. 2017, 144, 392; [Google Scholar]
- 9e. Karamanlioglu M., Preziosi R., Robson G. D., Polym. Degrad. Stab. 2017, 137, 122; [Google Scholar]
- 9f. Pikon K., Czop M., Pol. J. Environ. Stud. 2014, 23, 969; [Google Scholar]
- 9g. Nampoothiri K. M., Nair N. R., John R. P., Bioresour. Technol. 2010, 101, 8493; [DOI] [PubMed] [Google Scholar]
- 9h. Briassoulis D., Dejean C., Picuno P., J. Polym. Environ. 2010, 18, 364. [Google Scholar]
- 10. Rahimi A. R., Garciá J. M., Nat. Rev. Chem. 2017, 1, 1. [Google Scholar]
- 11.
- 11a. Lu X.-B., Liu Y., Zhou H., Chem. Eur. J. 2018, 24, 11255; [DOI] [PubMed] [Google Scholar]
- 11b. Rahimi A., García J. M., Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar]
- 12.
- 12a. Aguado J., Serrano D. P., Feedstock Recycling of Plastic Wastes., The Royal Society Of Chemistry, 1999; [Google Scholar]
- 12b. Liu Y., Zhou H., Guo J.-Z., Ren W.-M., Lu X.-B., Angew. Chem. Int. Ed. 2017, 56, 4862; [DOI] [PubMed] [Google Scholar]
- 12c. Hong M., Chen E. Y.-X., Nat. Chem. 2016, 8, 42. [DOI] [PubMed] [Google Scholar]
- 13. Ignatyev I. A., Thielemans W., Vander Beke B., ChemSusChem 2014, 7, 1579. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Andersson S. R., Hakkarainen M., Inkinen S., Södergård A., Albertsson A.-C., Biomacromolecules 2010, 11, 1067; [DOI] [PubMed] [Google Scholar]
- 14b. Tsuji H., Ikarashi K., Polym. Degrad. Stab. 2004, 85, 647; [Google Scholar]
- 14c. Makino K., Arakawa M., Kondo T., Chem. Pharm. Bull. 1985, 33, 1195; [DOI] [PubMed] [Google Scholar]
- 14d. Tsuji H., Polymer 2002, 43, 1789; [Google Scholar]
- 14e. Tsuji H., Yamada T., J. Appl. Polym. Sci. 2003, 87, 412; [Google Scholar]
- 14f. Song X., Wang H., Yang X., Liu F., Yu S., Liu S., Polym. Degrad. Stab. 2014, 110, 65; [Google Scholar]
- 14g. Iñiguez-Franco F., Auras R., Dolan K., Selke S., Holmes D., Rubino M., Soto-Valdez H., Polym. Degrad. Stab. 2018, 149, 28; [Google Scholar]
- 14h. Faisal M., Saeki T., Tsuji H., Daimon H., Fujie K., WIT Trans. Ecol. Environ. 2006, 92, 225. [Google Scholar]
- 15.
- 15a. Petrus R., Bykowski D., Sobota P., ACS Catal. 2016, 6, 5222; [Google Scholar]
- 15b. Roman-Ramirez L. A., Mckeown P., Jones M. D., Wood J., ACS Catal. 2019, 9, 409; [Google Scholar]
- 15c. Leibfarth F. A., Moreno N., Hawker A. P., Shand J. D., J. Polym. Sci. Part A 2012, 50, 4814; [Google Scholar]
- 15d. Hirao K., Nakatsuchi Y., Ohara H., Polym. Degrad. Stab. 2010, 95, 925; [Google Scholar]
- 15e. Bykowski D., Grala A., Sobota P., Tetrahedron Lett. 2014, 55, 5286; [Google Scholar]
- 15f. Carné Sánchez A., Collinson S. R., Eur. Polym. J. 2011, 47, 1970; [Google Scholar]
- 15g. Fliedel C., Vila-Vicosa D., Calhorda M. J., Dagorne S., Avilés T., ChemCatChem 2014, 6, 1357; [Google Scholar]
- 15h. Plichta A., Lisowska P., Kundys A., Zychewicz A., Dębowski M., Florjańczyk Z., Polym. Degrad. Stab. 2014, 108, 288; [Google Scholar]
- 15i. Liu M., Guo J., Gu Y., Gao J., Liu F., ACS Sustainable Chem. Eng. 2018, 6, 15127; [Google Scholar]
- 15j. Alberti C., Damps N., Meißner R. R. R., Enthaler S., ChemistrySelect 2019, 4, 6845; [Google Scholar]
- 15k. Song X., Zhang X., Wang H., Liu F., Yu S., Liu S., Polym. Degrad. Stab. 2013, 98, 2760; [Google Scholar]
- 15l. Alberti C., Damps N., Meißner R. R. R., Hofmann M., Rijono D., Enthaler S., Adv. Sus. Sys. 2019, DOI: 10.1002/adsu.201900081; [Google Scholar]
- 15m. McKeown P., Román-Ramírez L. A., Bates S., Wood J., Jones M. D., ChemSusChem 2019, 12, 5233. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Dai L., Liu R., Si C., Green Chem. 2018, 20, 1777; [Google Scholar]
- 16b. Tsuji H., Kondoh F., Polym. Degrad. Stab. 2017, 141, 77; [Google Scholar]
- 16c. Jiang B., Tantai X., Zhang L., Hao L., Sun Y., Deng L., Shi Z., RSC Adv. 2015, 5, 50747; [Google Scholar]
- 16d. Huang W., Qi Y., Cheng N., Zong X., Zhang T., Jiang W., Li H., Zhang Q., Polym. Degrad. Stab. 2014, 101, 18; [Google Scholar]
- 16e. Tsuneizumi Y., Kuwahara M., Okamoto K., Matsumura S., Polym. Degrad. Stab. 2010, 95, 1387. [Google Scholar]
- 17. Westhues S., Idel J., Klankermayer J., Sci. Adv. 2018, 4, eaat9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krall E. M., Klein T. W., Andersen R. J., Nett A. J., Glasgow R. W., Reader D. S., Dauphinais B. C., Mc Ilrath S. P., Fischer A. A., Carney M. J., Hudson D. J., Robertson N. J., Chem. Commun. 2014, 50, 4884–4887. [DOI] [PubMed] [Google Scholar]
- 19.see also
- 19a. Shuklov I. A., Dubrovina N. V., Schulze J., Tietz W., Kühlein K., Börner A., Chem. Eur. J. 2014, 20, 957; [DOI] [PubMed] [Google Scholar]
- 19b. Balaraman E., Fogler E., Milstein D., Chem. Commun. 2012, 48, 1111. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Alberti C., Eckelt S., Enthaler S., ChemistrySelect 2019, 4, 12268–12271; [Google Scholar]
- 20b. Kindler T.-O., Alberti C., Sundermeier J., Enthaler S., ChemistryOpen 2019, DOI: 10.1002/open.201900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The NMR data were compared with the NMR data of an authentic sample of 2.
- 22. 2 was obtained in an optical purity of 10 %; therefore to some extend racemerization took place. [a]D=2.2° (c=1 g/100 mL, CHCl3, 20 °C). [a]max=22.685° (c=1.020 g/100 mL, CHCl3, 20 °C, wavelength: 589.3 nm, 20 °C), Welbes L. L., Scarrow R. C., Borovik A. S., Chem. Commun. 2004, 2544. [Google Scholar]
- 23. 9 was also converted to 2 in the absence of 1 a.
- 24. Beydoun K., Klankermayer J., ChemSusChem 2020, 13, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The consecutive degradation of 1 n and 10 was also performed one-pot in an autoclave under an atmosphere of hydrogen, but no formation of product 11 was observed.
- 26.https://sustainabledevelopment.un.org/ (07. 02. 2020).