Abstract
The marine dinoflagellate genus Alexandrium includes a number of species that produce potent neurotoxins responsible for paralytic shellfish poisoning, which in humans may cause muscular paralysis, neurological symptoms and, in extreme cases, death. Because of the genetic diversity of different genera and species, molecular tools may help to detect the presence of target microorganisms in marine field samples. Here we employed a loop-mediated isothermal amplification (LAMP) method for the rapid and simple detection of toxic Alexandrium species. A set of four primers were designed based upon the conserved region of the 5.8S rRNA gene of members of the genus Alexandrium. Using this detection system, toxic Alexandrium genes were amplified and visualized as a ladder-like pattern of bands on agarose gels under isothermal condition within 60 min. The LAMP amplicons were also directly visualized by eye in the reaction tube by the addition of SYBR Green I. This LAMP assay was 10-fold more sensitive than a conventional PCR method with a detection limit of 5 cells per tube when targeting DNA from Alexandrium minutum. The LAMP assay reported here indicates the potential usefulness of the technique as a valuable simple, rapid alternative procedure for the detection of target toxic Alexandrium species during coastal water monitoring.
Keywords: loop-mediated isothermal amplification (LAMP), shellfish poisoning, rapid detection of algal blooms, neurotoxin, toxic Alexandrium, 5.8S rRNA gene
Introduction
Toxic dinoflagellates of the genus Alexandrium are the primary organisms responsible for harmful algal blooms (HABs) (Du et al., 2002; Usup et al., 2002). Moreover, for some reasons, such HABs appear to be increasing in frequency, intensity and distribution (John et al., 2003). HABs are now recognized worldwide as having serious implications for seafood safety, and environmental and economic concerns. In addition to the formation of red tides, some species of dinoflagellates produce a range of toxins that are poisonous to organisms higher in the food chain (Taroncher-oldenburg & Anderson, 2000). Marine dinoflagellates of the genus Alexandrium include a number of species responsible for paralytic shellfish poisoning (Judge et al., 1993). Paralytic shellfish toxins (PSTs), produced by Alexandria, are potent neurotoxins that can be concentrated by filter-feeding shellfish (Gallacher et al., 1997). Consumption of PST-contaminated shellfish may cause muscular paralysis, neurological symptoms and, in extreme cases, death (Anderson, 1997; Pierce & Kirkpatrick, 2001). HAB events have been reported from the South China Sea, where Hong Kong and other coastal cities have suffered considerable economic losses from frequent occurrences of HABs, some of which have been highly toxic (Anderson et al., 1996; Wang et al., 2003).
It is important to monitor coastal waters for the presence of toxin-producing Alexandrium from the source. Monitoring coastal waters for the presence of HAB species is essential in assessing the potential for bloom formation. Traditionally, this type of monitoring involves morphological identification and bioluminescence capacity, mating compatibility and enumeration of target species by light or electron microscopy in addition to toxicity tests of shellfish (Anderson et al., 1994). However, these identification methods can be difficult for long-term monitoring and the characteristic morphological features are often difficult to determine because they can be influenced by environmental factors and culture conditions (Taylor & Fukuyo, 1998).
Because of the genetic diversity of different genera and species, molecular methods may be useful for the detection of target microorganisms in marine field samples (Leaners et al., 1991; Medlin et al., 1998; LaJeunesse, 2001; Galluzzi et al., 2004). Because of their rapidity, PCR-based methods, molecular probes, restriction fragment length polymorphism (RFLP), and immunological techniques using polyclonal and monoclonal antibodies have been widely studied for the detection of toxic Alexandrium species (Nagasaki et al., 1991; Judge et al., 1993; Penna & Magnani, 1999; Bowers et al., 2000; Coyne et al., 2001; Godhe et al., 2001; Galluzzi et al., 2004; John & Medlin 2005). Although these techniques have significantly increased the ability to detect toxic Alexandrium species, their requirement for a high-precision instrument for amplification is complicated and costly. Immunological techniques require the identification of a phenotypic epitope, which may be influenced by the environment (Hosoi-Tanabe & Sako, 2005). This has prevented their widespread use in field laboratories, for example, as a routine diagnostic tool. Therefore, recent studies of Alexandrium species have focused on the search for better methods of identification.
The invention of loop-mediated isothermal amplification (LAMP) has opened up a new method for molecular detection and identification (Notomi et al., 2000). The principle of LAMP is autocycling strand displacement DNA synthesis in the presence of Bst DNA polymerase with high strand displacement activity under isothermal conditions between 60 and 65 °C within 60 min. The detailed amplification mechanism has been described elsewhere (Notomi et al., 2000; Mori et al., 2001; Enosawa et al., 2003; Parida et al., 2004). The reaction relies on recognition of the DNA target by six independent regions, making this kind of assay highly specific. The LAMP assay is rapid and the amplification efficiency is equivalent to that of PCR-based methods (Nagamine et al., 2002; Poon et al., 2004). More importantly, the approach is less costly, and all reactions can be developed in an isothermal environment. The potential applications of LAMP methodology have been demonstrated in recent years (Maruyama et al., 2003; Maeda et al., 2005; Ohtsuka et al., 2005). Here we demonstrate the feasibility of using the LAMP technique to detect toxic Alexandrium species.
Materials and methods
Algal cultures
Eight algal strains used in this study were isolated from the south coast of China. The six Alexandrium strains, Alexandrium minutum AMTW02, Alexandrium andersoni ADC02, Alexandrium catenella Balech 1985 ACDH03, Alexandrium tamarense ATMJ01, A. catenella L65 and A. tamarense L66, are toxic and their identification was confirmed by the Institute of Hydrobiology of Jinan University, China. Prorocentrum donghaiense PD01 and Karenia mikimotoi KM01 were used to determine the specificity of LAMP detection. All strains were maintained in f/2 medium (Guillard & Ryther, 1962) at 20±1 °C. Cool-white fluorescent bulbs provided light on a standard 12/12-h light–dark cycle. Between 8 and 15 days after inoculation, when cultures were in the exponential phase of growth, algal cell density was accurately determined by enumeration using a 0.1-mL Plankton count box. Algal cells were collected by centrifugation at 10 000 g for 5 min at 4 °C.
DNA preparation from cultures
DNA was extracted using a DNeasy Plant Mini Kit (QIAGEN) according to the manufacturer's instructions. Alexandrium minutum DNA was isolated by two methods. The first used a DNeasy Plant Mini Kit (QIAGEN). In the second method, A. minutum pellets containing 5 × 104 or 1 × 105 cells were incubated at 95 °C for 10 min and quickly placed on ice for 5 min, then centrifuged at 12 000 g for 1 min at 4 °C. The supernatants containing DNA were used in the following tests.
LAMP primer design
rRNA gene sequences have been successfully employed for the detection of various toxic dinoflagellates in seawater samples, because these sequences are highly conserved (Scholin et al., 1995; Hershkovitz & Lewis, 1996; Medina et al., 2001; Moon-van der Staay et al., 2001; Galluzzi et al., 2004; Bolch & de Salas, 2007). The primers used in this study were designed by an alignment of all available ITS1–5.8S–ITS2 rRNA gene sequences for the genus Alexandrium. Sequences were downloaded from GenBank or obtained from the literature. The alignment was constructed by using clustalw. The alignment included sequences of several strains of A. minutum, A. tamarense, A. catenella and A. andersoni. The 5.8S region is very conserved among these species. A set of four primers was designed to target six conserved sequences of the 5.8S region. In order to confirm the sequence specificity, we used the Basic Local Alignment Search Tool (blast) to search the GenBank and DDBJ databases for all published sequences identical to the primers. The primers were selected based on the criteria described by Notomi et al. (2000). In addition to the general criteria of primer design, such as 40–60 mol% G+C content but avoiding terminal dimer formation, 3′ hairpins, and self-complementarity, special care was taken to adjust the melting temperatures (Tm) of the primers in such a way that the Tm values were in the following order: F1C and BIC>F2 and B2>F3 and B3. The inner primers are described as forward inner primer (FIP) and backward inner primer (BIP). The forward inner primer consisted of the complementary sequence of F1C (25 nt), a T-T-T-T linker and F2 (19 nt). The backward inner primer consisted of B1C (24 nt), a T-T-T-T linker and the complementary sequence of B2 (20 nt). The outer primers were F3 and B3, which located outside of the F2 and B2 regions, respectively. The primer sequences and locations are indicated in Table 1 and Fig. 1. The primers were synthesized commercially by Invitrogen biotech (Guangzhou, China).
1.
DNA oligonucleotide primer sequences used for LAMP
| Primer | Primer type | Length | Sequence (5′→3′) |
| FIP | Forward inner (5′F1C-TTTT-F2-3′) | 48 nt F1C, 25 nt; F2, 19 nt | ACMTTCTTCCAACAGCATCTCTTACTTTTGCTGTATCGTATCGTCCTG |
| BIP | Backward inner (5′B1C-TTTT-B2-3′) | 48 nt B1C, 24 nt; B2, 20 nt | TCGAACAGAACAAGGTTGATTACCTTTTGGAYATTCAGATCATTCGCG |
| F3 | Forward outer | 22 nt | CACCRGATACCAACCTCACAGG |
| B3 | Backward outer | 23 nt | CAAGCAHACCTTCAAGMATATCC |
M, A or C; Y, T or C; H, A or C or T.
See Galluzzi et al. (2004) for sequence details.
1.
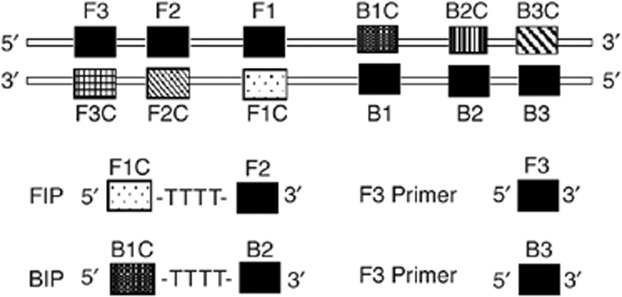
Schematic representation of primers used in this study. The LAMP inner (FIP and BIP) and outer (F3 and B3) primer pairs are shown.
LAMP reaction
The LAMP was carried out in a total reaction mixture of 25 μL containing 1.6 μM (each) of the primers FIP and BIP, 0.2 μM (each) of the primers F3 and B3, 1.6 mM of dNTPs, 6 mM MgSO4, 1 M betaine (Sigma), 1 × thermopol buffer (New England Biolabs), 1 μL (8 U) Bst DNA polymerase (New England Biolabs), and the specified amounts of target genomic DNA, which were incubated at 65 °C for 60 min. Positive and negative controls were included in each run, and all precautions to prevent cross-contamination were observed.
Monitoring of amplification by LAMP assay
Following incubation at 65 °C for 60 min, a 5-μL sample of the LAMP assay products was separated by electrophoresis on 2% agarose gels, stained with ethidium bromide and visualized on a UV transilluminator. In order to facilitate the field application of the LAMP assay, monitoring of amplification by the LAMP assay was also checked by eye. Following amplification, the tubes were inspected through observation of a colour change following addition of 1 μL (1 : 100) of SYBR Green I dye to the tube. In the case of a positive amplification, the original orange colour of the dye changes to green, which can be judged by eye under natural light.
PCR reaction
In order to compare the sensitivity of the LAMP assay, PCR was performed with the two outer primers F3 and B3. The amplification was carried out in a total reaction volume of 50 with 5 μL of the buffer solution (100 mM Tris-HCl, 500 mM KCl, 15 mM MgCl2, pH 8.3), 5 μL (10 pmol μL−1) of a pair of appropriate primers, 4 μL dNTPs mixture (2.5 mM of each dNTP) and 0.25 μL (5 U uL−1) Taq DNA polymerase were mixed. The thermal profile for PCR was 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, and a final extension cycle at 72 °C for 7 min. The amplified products were then analysed through a 1.5% agarose gel by electrophoresis in Tris-borate buffer, and the target bands were visualized by staining with ethidium bromide.
Specificity of the LAMP assay
To evaluate the specificity of the LAMP, five cells of A. minutum were employed in the LAMP reaction at 65 °C for 60 min in the absence or presence of 100 ng of non-Alexandrium DNA. The LAMP assay was also used to amplify the DNAs of different species of toxic Alexandrium and other non-Alexandrium cultures (10 ng per reaction, respectively). The DNAs of all strains were obtained via a simple boiling method. The products were separated by 2% agarose gels electrophoresis.
Results
A successful LAMP reaction with specific primers produced many bands of different sizes. The LAMP assay used here was standardized with the toxic A. minutum. When the sample tube did not contain target DNA, no amplification was seen. The LAMP yields extremely large amounts of DNA, and this enabled inspection by eye (Mori et al., 2001). The LAMP reaction mixture, which contained amplified fragments, turned green after the addition of SYBR Green I, whereas a solution with no amplicons retained the original orange colour of SYBR Green I. Inspection by eye with SYBR Green I demonstrated equivalent sensitivity to agarose gel electrophoresis under natural light (Fig. 2b). Inspection by eye was simple and rapid. Therefore, this method facilitates the application of LAMP, especially in field laboratory settings.
2.
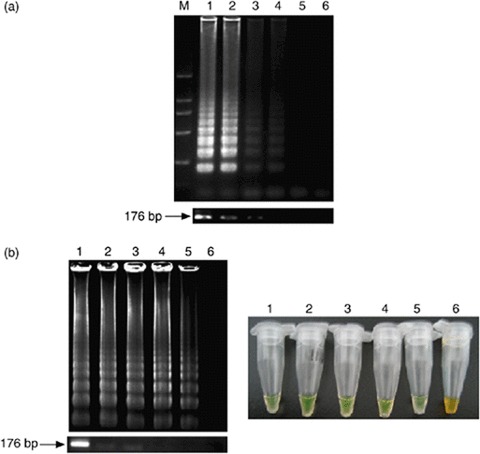
Comparative sensitivities of visual inspection and electrophoretic analyses of LAMP and PCR for the detection of Alexandrium minutum. (a) The number above each lane represents the dilution of the purified A. minutum DNA: lane M, 2-kb ladder used as a size marker; lanes 1-6, DNA of A. minutum at 1, 100, 10, 1 pg per tube, 100 and 10 fg per tube, respectively. The lower figures are electrophoretic data from the PCR analysis. PCR shows a 176-bp amplification product. (b) Lane M, 2-kb ladder used as a size marker; lanes 1–6, dilution of the A. minutum cells (DNA extracted by boiling) at 500, 100, 50, 10, 5 and 1 cells/tube, respectively. The right figure is the visual inspection of the LAMP products following the addition of SYBR Green I. The lower figure is the sensitivity of PCR for the detection of A. minutum cells as observed by agarose gel analysis.
Sensitivity of the LAMP and PCR assays for the detection of A. minutum
To ascertain the detection limit of the LAMP assay for the detection of A. minutum, serial 10-fold dilutions of the extracted DNA were used and compared with the results of conventional PCR. A serial dilution of A. minutum cells was also used to evaluate the detection limits of LAMP and PCR. The detection limits of the LAMP assay and PCR for purified DNA were found to be 1 and 10 pg per tube, respectively (Fig. 2a). The comparative sensitivity of LAMP and PCR indicated that LAMP was 10-fold more sensitive than PCR. Amplification by LAMP revealed a ladder-like pattern, whereas the PCR showed a 176-bp amplicon.
The detection limits of the LAMP assay and PCR for A. minutum cells were found to be 5 and 50 cells per tube, respectively (Fig. 2b); LAMP was again 10-fold more sensitive than PCR. The LAMP products turned green after the addition of SYBR Green I. Thus, the sensitivity of the LAMP methods can simply be judged by eye, and the results were in agreement with detection via electrophoresis. The boiling method shortened the time for DNA extraction and facilitated the usefulness of the LAMP assay for rapid detection of Alexandrium species, especially for offshore operations.
Specificity of the LAMP reaction for Alexandrium
Five cells of A. minutum were amplified in the LAMP reaction at 65 °C for 60 min in the absence or presence of 100 ng of non-Alexandrium DNA. The products were separated by agarose gel electrophoresis, as shown in Fig. 3. We can clearly see that the LAMP reaction was not influenced by the presence of large amounts of non-Alexandrium genomic DNA. Notomi et al. (2000) reported that the presence of 100 ng of human genomic DNA in a LAMP reaction mixture to detect six copies of hepatitis B virus target did not adversely affect the amplification efficiency and produced insignificant background. Our results were consistent with their results.
3.
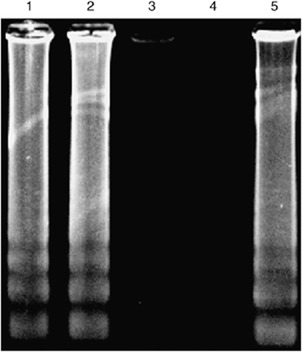
Specificity of the LAMP reaction for detection of Alexandrium (standardized with the toxic Alexandrium minutum): lane 1, 5 cells of A. minutum were amplified in the presence of 100 ng of Prorocentrum donghaiense 01 DNA; lane 2, 5 cells of A. minutum were amplified in the presence of 100 ng of Karenia mikimotoi 01 DNA; lane 3, LAMP reaction in the presence of 100 ng of P. donghaiense 01 DNA; lane 4, LAMP reaction in the presence of 100 ng of K. mikimotoi 01 DNA; lane 5, positive control (five cells of A. minutum).
The specificity of the LAMP was also established by checking the reactivity with other algal strains, as discussed in the ‘Materials and methods’. Significant amplification of DNAs isolated from the toxic Alexandrium species was observed after 60 min of incubation. Reaction products were detected only when DNA of cells of members of the genus Alexandrium was present, giving rise to a typical ladder-like pattern. In contrast, the DNAs of non-Alexandrium strains were not amplified even after 90 min of incubation (Fig. 4).
4.
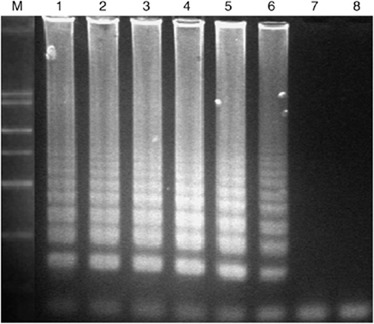
Electrophoretic analysis of LAMP amplified products. Lane M, 2-kb ladder used as a size marker; lanes 1–8, LAMP carried out in the presence of genomic DNA (10 ng per tube) from Alexandrium minutum AMTW02, Alexandrium andersoni ADC02, Alexandrium catenella Balech 1985 ACDH03, Alexandrium tamarense ATMJ01, A. catenella L65, A. tamarense L66, Prorocentrum donghaiense PD01 and Karenia mikimotoi KM01, respectively.
Discussion
In recent years, it has been shown that the geographical range of toxic Alexandrium species has been increasing (Anderson et al., 1996; Lilly et al., 2002; Choong & Yoshihiko 2005). The best approach to minimize the risk to humans should involve the continuous monitoring of activity of toxic Alexandrium species to track their presence and provide advance warning of a risk of a large-scale harmful bloom.
To our knowledge, this is the first report of the application of the LAMP assay technique for the rapid and specific detection of toxic Alexandrium species. Compared with conventional PCR, the LAMP assay reported here is advantageous owing to its simple operation, rapid reaction and ease of detection. The LAMP assay is a simple detection tool in which the reaction is carried out in a single tube by mixing the thermopol buffer, primers and Bst DNA polymerase, and incubation of the mixture at 65 °C for 1 h. There is no need for a thermal cycler because there is no heat denaturation step of the template DNAs with this method. The only equipment needed for the LAMP reaction is a regular laboratory water bath or a heating block that can provide a constant temperature of 65 °C. Although there is no need for a thermal cycler, some of the double-stranded DNA seems to become single-stranded at high temperatures in the presence of high concentrations of betaine, a reagent that facilitates DNA strand separation because it stabilizes DNA (Nagamine et al., 2001).
It is known that PCR inhibitors in samples reduce the sensitivity of PCR when attempting to detect a target gene (Wilson, 1997; Horisaka et al., 2004). However, the LAMP method is able to detect 1 pg of the target gene even in the presence of 100 ng of other bacterial genomic DNAs (Notomi et al., 2000). The sensitivity of LAMP was less affected by various components of the clinical samples than was PCR; therefore, DNA purification from samples could be omitted (Kaneko et al., 2007). As such, the sensitivity level of the LAMP method will allow detection of Alexandrium species not only at bloom concentrations but also in field samples containing only a small number of cells, which will be extremely useful for long-term monitoring programmes.
DNA extraction by the boiling technique prior to the LAMP test and visualization of reaction products using SYBR Green I DNA stain were employed to reduce the time required to perform the electrophoretic test and to simplify the procedure. We believe that the inexpensive running costs of the method make this technology very applicable to monitoring harmful algae in developing countries.
Although the present study provides only preliminary results, it does suggest that the LAMP assay will prove to be useful for the rapid monitoring of toxic and harmful Alexandrium algae.
Acknowledgements
This study was supported by the Key Agricultural Program of Guangdong Provincial Department of Science and Technology (2005A11601101), the Science Foundation of the Ministry of Education of China, 706046 and the National Natural Science Foundation of China (20436020).
Footnotes
Editor: Jorge Crosa
References
- Anderson DM. (1997) Turning back the harmful red tide. Nature 388: 513. [Google Scholar]
- Anderson DM, Kulis DM, Doucette GJ, Gallagher JC, Balech E. (1994) Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeastern United States and Canada. Mar Biol 120: 467–478. [Google Scholar]
- Anderson DM, Kulis DM, Qi YZ, Zheng L, Lu S, Lin YT. (1996) Paralytic shellfish poisoning in Southern China. Toxicon 34: 579–590. [DOI] [PubMed] [Google Scholar]
- Bolch CJS, De Salas MF. (2007) A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium“tamarensis complex” to Australasia. Harmful Algae 6: 465–485. [Google Scholar]
- Bowers HA, Tengs T, Glasgow HB, Jr, Burkholder JM, Rublee PA, Oldach DW. (2000) Development of real-time PCR assays for rapid detection of Pfiesteria piscicida and related dinoflagellates. Appl Environ Microbiol 66: 4641–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong JK, Yoshihiko S. (2005) Molecular identification of toxic Alexandrium tamiyavanichii (Dinophyceae) using two DNA probes. Harmful Algae 4: 984–991. [Google Scholar]
- Coyne KJ, Hutchins DA, Hare CE, Cary SC. (2001) Assessing temporal and spatial variability in Pfiesteria piscicida distributions using molecular probing techniques. Aquat Microb Ecol 24: 275–285. [Google Scholar]
- Du JL, Erdner D, Dyhrman S, Anderson D. (2002) Molecular approaches to understanding population dynamics of the toxic dinoflagellate Alexandrium fundyense. Biol Bull 203: 244–245. [DOI] [PubMed] [Google Scholar]
- Enosawa M, Kageyama S, Sawai K, Watanabe K, Notomi T, Onoe S, Mori Y, Yokomizo Y. (2003) Use of loop-mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis. J Clin Microbiol 41: 4359–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher S, Flynn KJ, Franco JM, Brueggemann EE, Hines HB. (1997) Evidence for production of paralytic shellfish toxins by bacteria associated with Alexandrium spp. (Dinophyta) in culture. Appl Environ Microbiol 63: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Penna A, Bertozzini E, Vila M, Garces E, Magnani M. (2004) Development of a real-time PCR assay for rapid detection and quantification of Alexandrium minutum (a Dinoflagellate). Appl Environ Microbiol 70: 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godhe A, Otta SK, Rehnstam-Holm AS, Karunasagar I, Karunasagar I. (2001) Polymerase chain reaction in detection of Gymnodium mikimotoi and Alexandrium minutum in field samples from southwest India. Mar Biotechnol 3: 152–162. [DOI] [PubMed] [Google Scholar]
- Guillard RR, Ryther JH. (1962) Studies of marine phytoplanktonic diatoms. Cyclolella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8: 229–239. [DOI] [PubMed] [Google Scholar]
- Hershkovitz MA, Lewis LA. (1996) Deep-level diagnostic value of the rDNA-ITS region. Mol Biol Evol 13: 1276–1295. [DOI] [PubMed] [Google Scholar]
- Horisaka T, Fujita K, Iwata T, Nakadai A, Okatani T, Horikita T, Taniguchi T, Honda E, Yokomizo Y, Hayashidani H. (2004) Sensitive and specific detection of Yersinia pseudotuberculosis by loop-mediated isothermal amplification. J Clin Microbiol 42: 5349–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi-Tanabe S, Sako Y. (2005) Rapid detection of natural cells of Alexandrium tamarense and A. catenella (Dinophyceae) by fluorescence in situ hybridization. Harmful Algae 4: 319–328. [Google Scholar]
- John U, Medlin LK. (2005) Development of specific rRNA probes to distinguish between geographic clades of the Alexandrium tamarense species complex. J Plank Res 27: 199–204. [Google Scholar]
- John U, Fensome RA, Medlin LK. (2003) The application of a molecular clock based on molecular sequences and the fossil record to explain biogeographic distributions within the Alexandrium tamarense“species complex” (Dinophyceae). Mol Biol Evol 20: 1015–1027. [DOI] [PubMed] [Google Scholar]
- Judge BS, Scholin CA, Anderson DM. (1993) RFLP analysis of a fragment of the large-subunit ribosomal RNA gene of globally distributed populations of the toxic dinoflagellate Alexandrium. Est Ecol 185: 329–330. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Kawana T, Fukushima E, Suzutani T. (2007) Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J Bioch Bio 70: 499–501. [DOI] [PubMed] [Google Scholar]
- LaJeunesse TC. (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinum using the ITS region: in search of a “species” level marker. J Phycol 37: 866–880. [Google Scholar]
- Leaners G, Scholin C, Bhaud Y, Saint-Hilaire D, Herzog M. (1991) A molecular phylogeny of dinoflagellate protists (Pyrrhophyta) inferred from the sequence of 24S rRNA divergent domains D1 and D8. J Mol Evol 32: 53–63. [DOI] [PubMed] [Google Scholar]
- Lilly EL, Kulis DM, Gentien P, Anderson DM. (2002) Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from the western Pacific: evidence from DNA. J Plank Res 24: 443–452. [Google Scholar]
- Maeda H, Kokeguchi S, Fujimoto C, Tanimoto I, Yoshizumi W, Nishimura F, Takashiba S. (2005) Detection of periodontal pathogen Porphyromonas gingivalis by loop-mediated isothermal amplification method. FEMS Immunol Med Microbiol 43: 233–239. [DOI] [PubMed] [Google Scholar]
- Maruyama F, Kenzaka T, Yamaguchi N, Tani K, Nasu M. (2003) Detection of bacteria carrying the stx2 gene by in situ loop-mediated isothermal amplification. Appl Environ Microbiol 69: 5023–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M, Collins AG, Silberman JD, Sogin ML. (2001) Evaluating hypotheses of basal animal phylogeny using complete sequences of large and small subunit rRNA. Proc Natl Acad Sci USA 98: 9707–9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlin L, Lange M, Wellbrock U, Donner G, Elbrächter M, Hummert C, Luckas B. (1998) Sequence comparisons link toxic European isolates of Alexandrium tamarense from the Orkney Islands to toxic North American stocks. Eur J Protistol 34: 329–335. [Google Scholar]
- Moon-van der Staay SY, De Wachter R, Vaulot D. (2001) Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 409: 607–610. [DOI] [PubMed] [Google Scholar]
- Mori Y, Nagamine K, Tomita N, Notomi T. (2001) Detection of Loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 289: 150–154. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Watanabe K, Ohtsuka K, Hase T, Notomi T. (2001) Loop-mediated isothermal amplification reaction using a nondenatured template. Clin Chem 47: 1742–1743. [PubMed] [Google Scholar]
- Nagamine K, Hase T, Notomi T. (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16: 223–229. [DOI] [PubMed] [Google Scholar]
- Nagasaki K, Uchida U, Ishida Y. (1991) A monoclonal antibody which recognizes the cell surface of red tide alga Gymnodinium nagasakiense. Nippon Suisan Gakkaishi 57: 1211–1214. [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K, Yanagawa K, Takatori K, Hara-kudo Y. (2005) Detection of Salmonellaenterica in naturally contaminated liquid eggs by loop-mediated isothermal amplification, and characterization of Salmonella isolates. Appl Environ Microbiol 71: 6730–6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida M, Posadas G, Inoue S, Hasebe F, Morita K. (2004) Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol 42: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Magnani M. (1999) Identification of Alexandrium (Dinophyceae) species using PCR and rDNA-targeted probes. J Phycol 35: 615–621. [Google Scholar]
- Pierce RH, Kirkpatrick GJ. (2001) Innovative techniques for harmful algal toxin analysis. Environ Toxicol Chem 20: 107–114. [DOI] [PubMed] [Google Scholar]
- Poon LLM, Leung CSW, Tashiro M, Chan KH, Wong BWY, Yuen KY, Guan Y, Peiris JSM. (2004) Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay. Clin Chem 50: 1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholin CA, Hallegraeff GM, Anderson DM. (1995) Molecular evolution of the Alexandrium tamarense“species complex” (Dinophyceae): dispersal in the North American and West Pacific regions. Phycologia 34: 472–485. [Google Scholar]
- Taroncher-oldenburg G, Anderson DM. (2000) Identification and characterization of three differentially expressed gene, encoding S-adenosylhomocysteine hydrolase, methionine aminopeptidase, and a histone-like protein, in the toxic dinoflagellate Alexandrium fundyense. Appl Environ Microbiol 66: 2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FJR, Fukuyo Y. (1998) The neurotoxigenic dinoflagellate genus Alexandrium Halim: general introduction. Physiological Ecology of Harmful Algal Blooms (Anderson DM, Cembella AD, Hallegraeff GM, eds), pp. 381–404. Springer-Verlag, Heidelberg. [Google Scholar]
- Usup G, Pin LC, Ahmad A, Teen LP. (2002) Phylogenetic relationship of Alexandrium tamiyavanichii (Dinophyceae) to other Alexandrium species based on ribosomal RNA gene sequences. Harmful Algae 1: 59–68. [Google Scholar]
- Wang CH, Wang YY, Sun YY, Xie XT. (2003) Effect of antibiotic treatment on toxic production by Alexandrium tamarense. Biom Env Sc 16: 340–347. [PubMed] [Google Scholar]
- Wilson IG. (1997) Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol 63: 3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]


