Abstract
The peer-reviewed marine pharmacology literature in 2007–8 is covered in this review, which follows a similar format to the previous 1998–2006 reviews of this series. The preclinical pharmacology of structurally characterized marine compounds isolated from marine animals, algae, fungi and bacteria is discussed in a comprehensive manner. Antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis and antiviral activities were reported for 74 marine natural products. Additionally, 59 marine compounds were reported to affect the cardiovascular, immune and nervous systems as well as to possess anti-inflammatory effects. Finally, 65 marine metabolites were shown to bind to a variety of receptors and miscellaneous molecular targets, and thus upon further completion of mechanism of action studies, will contribute to several pharmacological classes. Marine pharmacology research during 2007–8 remained a global enterprise, with researchers from 26 countries, and the United States, contributing to the preclinical pharmacology of 197 marine compounds which are part of the preclinical marine pharmaceuticals pipeline. Sustained preclinical research with marine natural products demonstrating novel pharmacological activities, will probably result in the expansion of the current marine pharmaceutical clinical pipeline, which currently consists of 13 marine natural products, analogs or derivatives targeting a limited number of disease categories.
Keywords: Drugs, Marine, Chemicals, Metabolites, Natural, Products, Pharmacology, Pharmaceutical, Review, Toxicology
1. Introduction
The current article presents the preclinical pharmacology of marine natural products during 2007–8 maintaining the format used in the previous reviews (Mayer and Lehmann, 2000, Mayer and Hamann, 2002, Mayer and Hamann, 2004, Mayer and Hamann, 2005, Mayer et al., 2007, Mayer et al., 2009). The preclinical pharmacology of antitumor and cytotoxic marine compounds has been reported in separate reviews (Mayer, 1999, Mayer and Lehmann, 2001, Mayer and Gustafson, 2003, Mayer and Gustafson, 2004, Mayer and Gustafson, 2006, Mayer and Gustafson, 2008). We have restricted the current as well as previous reviews to peer-reviewed articles reporting the bioactivity or pharmacology of structurally characterized marine compounds. As we have done previously, we have continued to use a modification of Schmitz's chemical classification (Schmitz et al., 1993) to assign marine natural product structures to six major chemical classes, namely, polyketides, terpenes, peptides, alkaloids, shikimates, and sugars. Novel antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis and antiviral preclinical pharmacology of marine metabolites are presented in Table 1 with the corresponding structures shown in Fig. 1 . Marine compounds that affect the immune and nervous systems, as well as those with anti-inflammatory effects are grouped in Table 2 , with their corresponding structures presented in Fig. 2 . Finally, marine compounds that have been demonstrated to affect a variety of cellular and molecular targets are exhibited in Table 3 , and their structures depicted in Fig. 3 . Several articles were published during 2007–8 reporting the preclinical pharmacology of extracts or structurally uncharacterized marine compounds, and although these have not been included in the present review, they probably may warrant further investigation: antimicrobial effects of the Turkish red alga Jania rubens (Karabay-Yavasoglu et al., 2007); antimicrobial activity in Portuguese marine cyanobacteria Synechocystis sp. and Synechococcus sp. extracts towards Gram-positive bacteria (Martins et al., 2008); antibacterial activity against human and fish bacteria from the sub-Arctic colonial ascidian Synoicum pulmonaria from northern Norway (Tadesse et al., 2008); strong antibiotic-producing potential in actinomycetes from sediments in the Trondheim fjord in Norway (Bredholt et al., 2008); antibacterial activity of deep-sea bacteria from sediments of the West Pacific Ocean (Xu et al., 2007); potent antimicrobial activity in the green alga Enteromorpha intestinalis collected in Gujarat, India (Nair et al., 2007); a nonhemolytic antimicrobial lipopeptide derived from the marine bacterium Bacillus circulans (Das et al., 2008); a T-antigen binding lectin with bioactivity against a broad spectrum of Gram-positive and Gram-negative bacteria from the sea cucumber Holothuria scabra (Gowda et al., 2008); anticoagulant activity of a 100–500 kDa polysaccharide isolated from the fermented red seaweed Lomentaria catenata which was greater than the clinical anticoagulant heparin (Pushpamali et al., 2008); antimycobacterial activity in long-chain fatty acids isolated from the red alga Polysiphonia virgata (Saravanakumar et al., 2008); antileishmanial and anti-trichomonal activity in organic extracts of several Mexican red and brown algae (Freile-Pelegrin et al., 2008, Moo-Puc et al., 2008); anti-HIV-1 activity of novel sulfated galactans isolated from the Chinese red algae Grateloupia longifolia and Grateloupia filicina (Wang et al., 2007a, Wang et al., 2007b); significant anti-herpes simplex virus activity in a 30 kDa polysaccharide isolated from the red alga Gracilaria corticata (Chattopadhyay et al., 2008); immunomodulatory effects of an enzymatic extract from the South Korean marine brown alga Ecklonia cava on murine splenocytes (Ahn et al., 2008); immunostimulant properties of a sulfated polysaccharide isolated from the Brazilian red alga Champia feldmannii (Assreuy et al., 2008); antioxidant and antimicrobial activity of the Indian red and brown seaweed methanolic extracts with high phenolic contents (Devi et al., 2008); and decreased expression of key regulatory genes involved in cholesterol and fatty acid biosynthetic pathways by the lipid extract of the cyanobacterium Nostoc commune var. sphaeroides Kützing (Rasmussen et al., 2008).
Table 1.
Marine pharmacology in 2007–8: marine compounds with antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis, and antiviral activities.
| Drug class | Compound/organisma | Chemistry | Pharmacologic activity | IC50b | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|---|
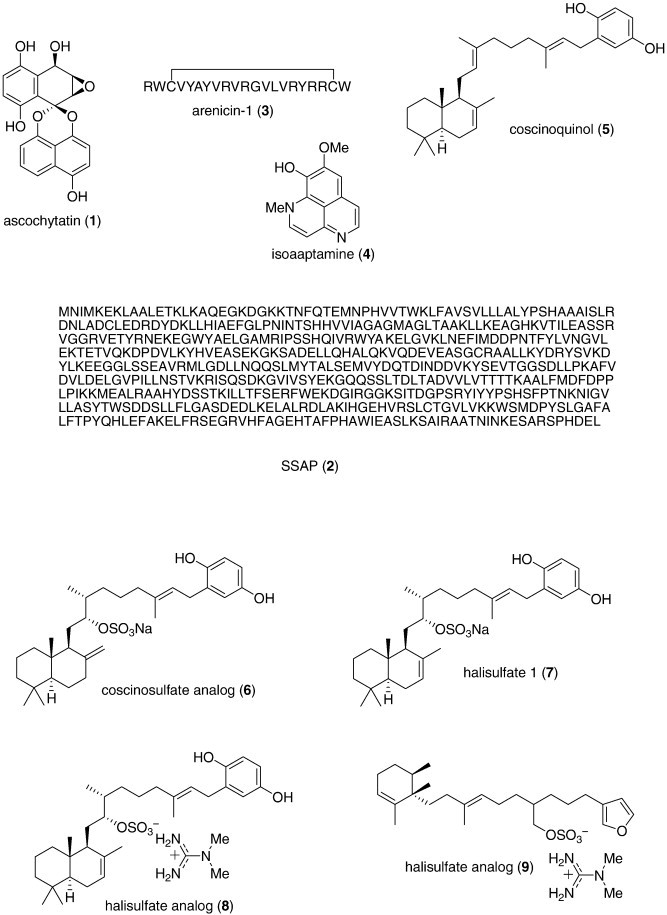
| Antibacterial | Ascochytatin (1)/fungus | Polyketided | B. subtilis inhibition | 0.3 μg++ | TCS (YycG and YycF) regulatory system | JPN | (Kanoh et al., 2008b) |
| Antibacterial | L-Amino acid oxidase SSAP (2)/rockfish | Proteinf | A. salmonicida, P. damselae subsp. piscida and V. parahaemolyticus inhibition | 0.078–0.63 μg/mL+ | H2O2 mediates the antibacterial action | JPN | (Kitani et al., 2008) |
| Antibacterial | Arenicin-1 (3)/polychaete | Peptidef | P. aeruginosa and S. aureus inhibition | 2 μg/mL+ | Binding and disruption of cell membrane | S. KOR | (Lee et al., 2007a, Lee et al., 2007b) |
| Antibacterial | Isoaaptamine (4)/sponge | Alkaloidf | S. aureus inhibition | 3.7 μg/mL | Sortase A inhibition and fibronectin binding | S. KOR | (Jang et al., 2007c) |
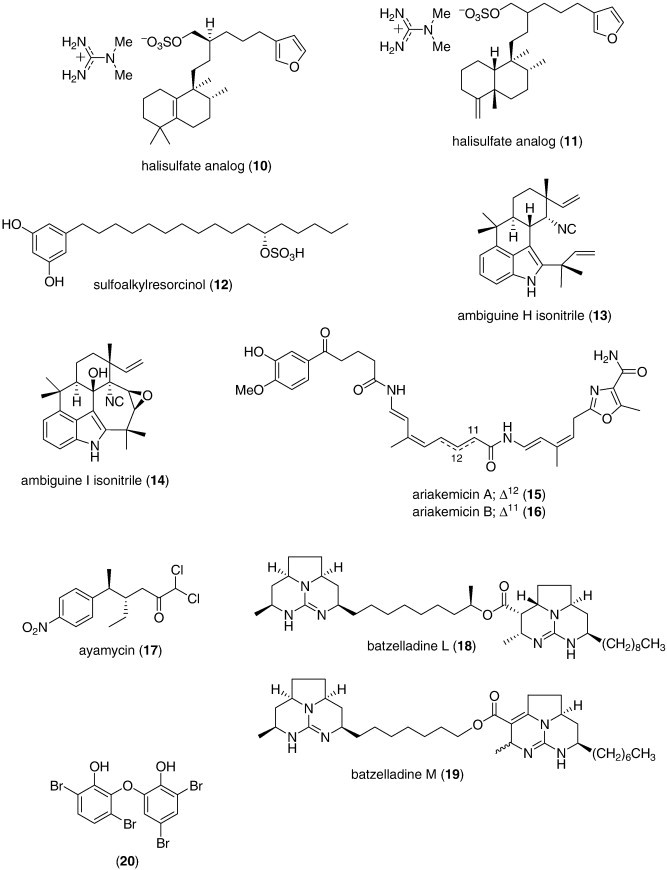
| Antibacterial | Dysidea sp. Sesterterpenes (5, 6, 7, 8, 9, 10, 11) /sponge | Terpenoide | B. subtilis inhibition | 1.56–12.5 μg/mL+ | Isocitrate lyase inhibition | S. KOR | (Lee et al., 2008) |
| Antibacterial | Sulfoalkylresorcinol (12)/fungus | Polyketided | Methicillin-resistant S. aureus inhibition | 12.5 μg/mL+ | FtsZ polymerization inhibition | JPN | (Kanoh et al., 2008a) |
| Antibacterial | Ambiguines H and I (13, 14)/bacterium | Alkaloidf | S. albus and B. subtilis inhibition | 0.08–1.25 μg/mL+ | Undetermined | ISR | (Raveh and Carmeli, 2007) |
| Antibacterial | Ariakemicins A and B (15, 16)/bacterium | Polyketided | S. aureus inhibition | 0.46 μg++ | Undetermined | JPN | (Oku et al., 2008) |
| Antibacterial | Ayamycin (17)/bacterium | Polyketided | Gram-positive and -negative bacteria inhibition | 0.1 μg/mL+ | Undetermined | EGY, GBR | (El-Gendy et al., 2008a) |
| Antibacterial | Batzelladine L and M (18, 19)/sponge | Alkaloidf | S. aureus and methicillin-resistant S. aureus inhibition | 0.25–5.0 μg/mL+ | Undetermined | USA, CHN, ESP, NZL | (Hua et al., 2007) |
| Antibacterial | L. herbacea diphenyl ether (20)/sponge | Polyketided | B. subtilis inhibition | 0.1 μg++ | Undetermined | JPN, IDN, NLD | (Hanif et al., 2007) |
| Antibacterial | Essramycin (21)/bacterium | Alkaloidf | B. subtilis, S. aureus and M. luteus inhibition | 1–85 μg/mL+ | Undetermined | EGY, DEU | (El-Gendy et al., 2008b) |
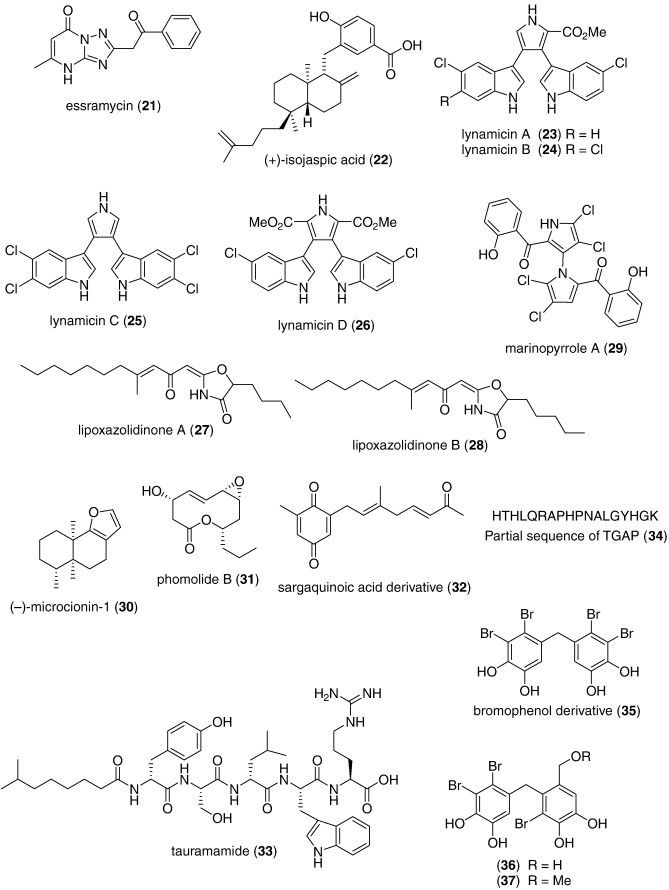
| Antibacterial | (+)-Isojaspic acid (22)/sponge | Terpenoide | S. epidermis inhibition | 2.5 μg/mL+ | Undetermined | USA, NLD | (Rubio et al., 2007) |
| Antibacterial | Lynamicins A–D (23, 24, 25, 26)/bacterium | Alkaloidf | Methicillin-resistant S. aureus inhibition | 1.8–9.5 μg/mL+ | Undetermined | USA | (McArthur et al., 2008) |
| Antibacterial | Lipoxazolidinones A and B (27, 28)/bacterium | Polyketided | Staphylococcus sp., S. pneumoniae, E. faecalis inhibition | 0.5–16 μg/mL+ | Undetermined | USA | (Macherla et al., 2007) |
| Antibacterial | Marinopyrrole A (29)/bacterium | Alkaloidf | Methicillin-resistant S. aureus inhibition | 0.31 μM+++ | Undetermined | USA | (Hughes et al., 2008) |
| Antibacterial | (–)-Microcionin-1 (30)/sponge | Terpenoide | M. luteus inhibition | 6 μg/mL+ | Undetermined | PRT, ESP | (Gaspar et al., 2008) |
| Antibacterial | Phomolide B (31)/fungus | Polyketided | E. coli inhibition | 5–10 μg/mL+ | Undetermined | CHN | (Du et al., 2008) |
| Antibacterial | Sargaquinoic acid derivative (32)/alga | Terpenoide | S. aureus inhibition | 2 μg/mL+ | Undetermined | JPN | (Horie et al., 2008) |
| Antibacterial | Tauramamide (33)/bacterium | Peptidef | Enterococcus sp. inhibition | 0.1 μg/mL+ | Undetermined | CAN, PAP, USA | (Desjardine et al., 2007) |
| Anticoagulant | Anticoagulant polypeptide (TGAP) (34)/bivalve | Proteinf | Inhibition of factor II- to IIa conversion | 77.9 nM | Specific binding to factor Va and factor II | S. KOR | (Jung et al., 2007b) |
| Antifungal | Odonthalia corymbifera bromophenols (35, 36, 37)/alga | Polyketided | Magnaporthe grisea inhibition | 2.0–2.8 μM | Isocitrate lyase inhibition | S. KOR | (Lee et al., 2007a, Lee et al., 2007b) |
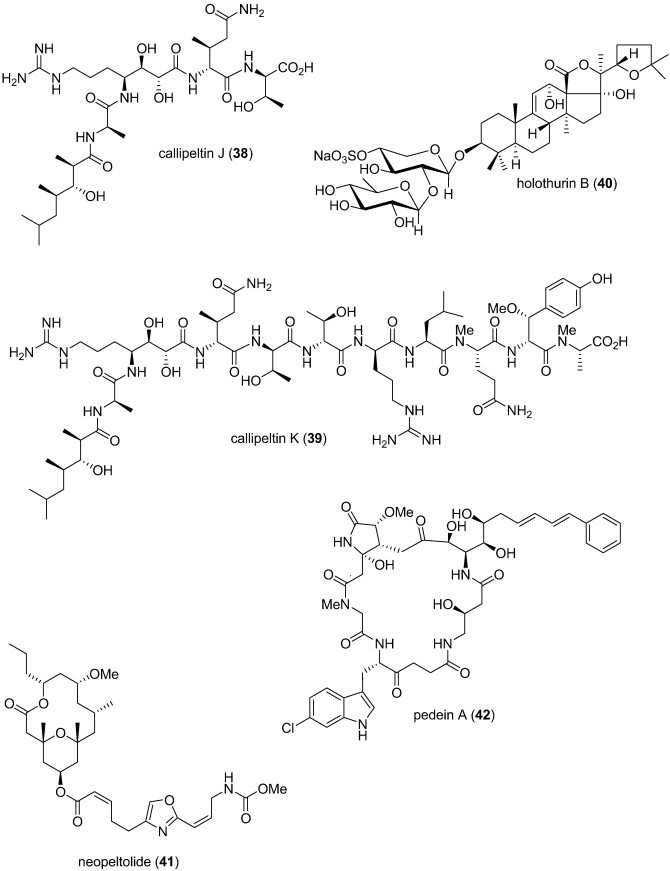
| Antifungal | Callipeltins J and K (38, 39)/sponge | Peptidef | C. albicans inhibition | 1 μM+ | Undetermined | ITA, FRA | (D'Auria et al., 2007) |
| Antifungal | Holothurin B (40)/sea cucumber | Triterpenoid glycosidee | T. mentagrophytes and S. schenckii inhibition | 1.56 μg/mL+ | Undetermined | IND | (Kumar et al., 2007) |
| Antifungal | Neopeltolide (41)/sponge | Polyketided | C. albicans inhibition | 0.62 μg/mL+ | Undetermined | USA | (Wright et al., 2007) |
| Antifungal | Pedein A (42)/bacterium | Peptidef | R. glutinis, S. cerevisae and C. albicans | 0.6–1.6 μg/mL+ | Undetermined | DEU | (Kunze et al., 2008) |
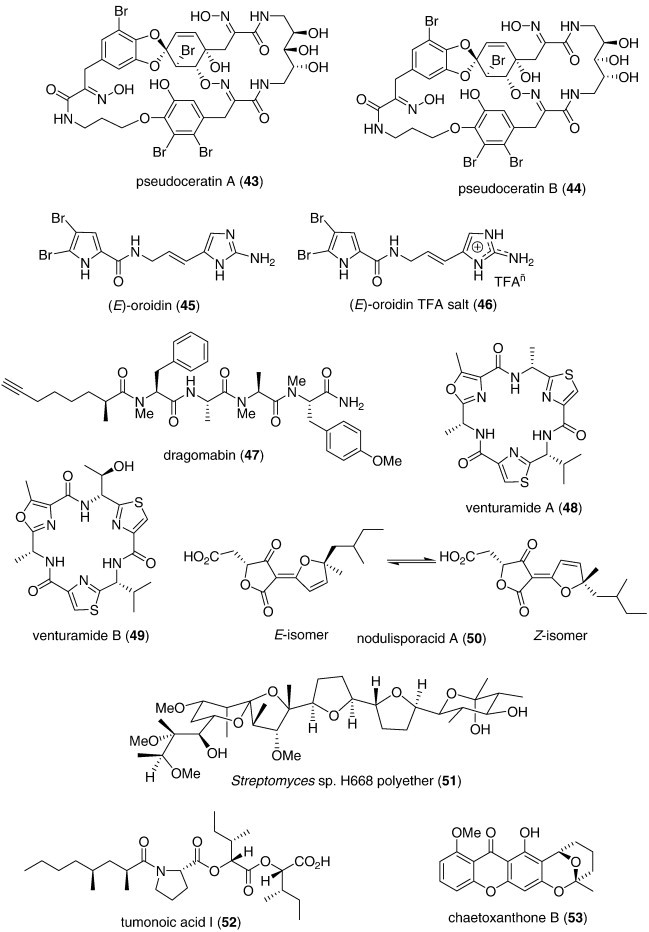
| Antifungal | Pseudoceratins A and B (43, 44)/sponge | PKS/NRPSf | C. albicans and mutant S. cerevisae inhibition | 6.5–8.0 μg++ | Undetermined | JPN | (Jang et al., 2007b) |
| Antimalarial | (E)-Oroidin (45) and (E)-oroidin TFA salt (46)/sponge | Alkaloidf | P. falciparum K1 strain inhibition | 3.9–7.9 μg/mL | FabI inhibition | CHE, GBR, USA, TUR | (Tasdemir et al., 2007) |
| Antimalarial | Dragomabin (47)/bacterium | Peptidef | P. falciparum W2 strain inhibition | 6.0 μM | Undetermined | PAN, USA | (McPhail et al., 2007) |
| Antimalarial | Venturamides A and B (48, 49)/bacterium | Peptidef | P. falciparum W2 strain inhibition | 5.6–8.2 μM | Undetermined | PAN, USA | (Linington et al., 2007) |
| Antimalarial | Nodulisporacid A (50)/fungus | Polyketided | P. falciparum 94 strain inhibition | 1–10 μM | Undetermined | THAI | (Kasettrathat et al., 2008) |
| Antimalarial | Streptomyces sp. H668 polyether (51)/bacterium | Polyketided | P. falciparum D6 and W2 strain inhibition | 0.1–0.2 μg/mL | Undetermined | S. KOR, USA | (Na et al., 2008) |
| Antimalarial | Tumonoic acid I (52)/bacterium | Polyketided | P. falciparum D6 and W2 strain inhibition | 2 μM | Undetermined | PAP, USA | (Clark et al., 2008) |
| Antimalarial | Chaetoxanthone B (53)/fungus | Polyketided | P. falciparum K1 strain inhibition | 0.5 μg/mL | Undetermined | CHE | (Pontius et al., 2008a) |
| Antiprotozoal | Plakortide P (54)/sponge | Polyketidee | Inhibition of L. chagasi and T. cruzi | 0.5–2.3 μg/mL | Undetermined, though not involving nitric oxide | BRA | (Kossuga et al., 2008) |
| Antiprotozoal | Viridamides A and B (55, 56)/bacterium | Peptidef | Inhibition of L. mexicana and T. cruzi | 1.1–1.5 μM | Undetermined | PAN, USA | (Simmons et al., 2008) |
| Antiprotozoal | Chaetoxanthone B (53)/fungus | Polyketided | T. cruzi Tulahuen C4 strain inhibition | 1.5 μg/mL | Undetermined | CHE | (Pontius et al., 2008a) |
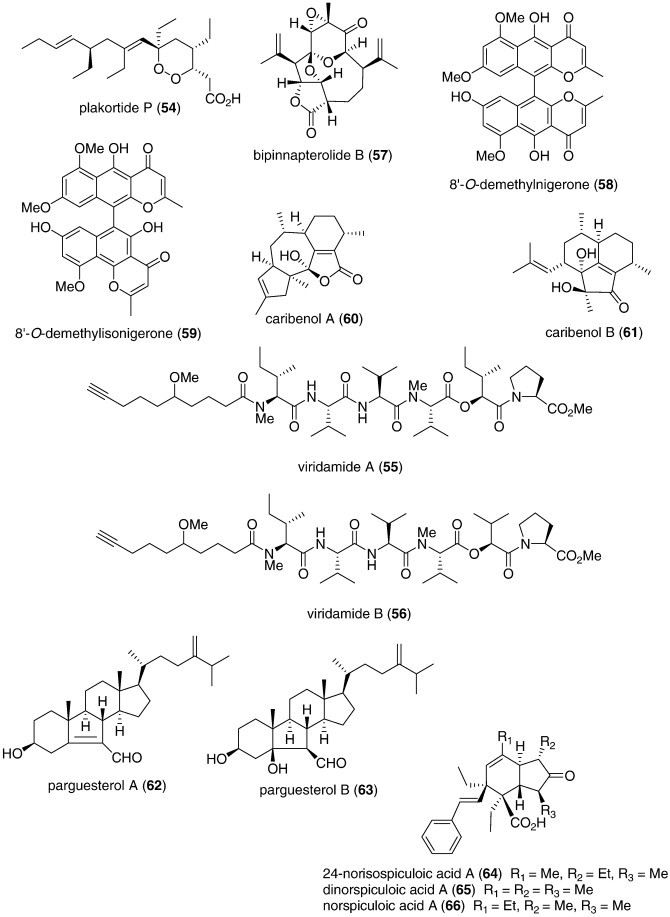
| Antituberculosis | Bipinnapterolide B (57)/coral | Terpenoide | M. tuberculosis inhibition | 128 μg/mL⁎ | Undetermined | USA | (Ospina et al., 2007) |
| Antituberculosis | 8′-O-Demethylnigerone (58) and 8′-O-demethylisonigerone (59)/fungus | Polyketided | M. tuberculosis inhibition | 21.5 and 43.0 μM | Undetermined | CHN | (Zhang et al., 2008a, Zhang et al., 2008b) |
| Antituberculosis | Caribenols A and B (60, 61)/soft coral | Terpenoide | M. tuberculosis inhibition | 63 and 128 μg/mL+ | Undetermined | USA | (Wei et al., 2007a, Wei et al., 2007b) |
| Antituberculosis | Parguesterols A and B (62, 63)/sponge | Triterpenoidf | M. tuberculosis inhibition | 7.8 and 11.2 μg/mL+ | Undetermined | USA | (Wei et al., 2007a, Wei et al., 2007b) |
| Antituberculosis | Spiculoic acids (64, 65, 66)/sponge | Polyketided | M. tuberculosis inhibition | 50 μg/mL+++ | Undetermined | ESP, FRA | (Berrue et al., 2007) |
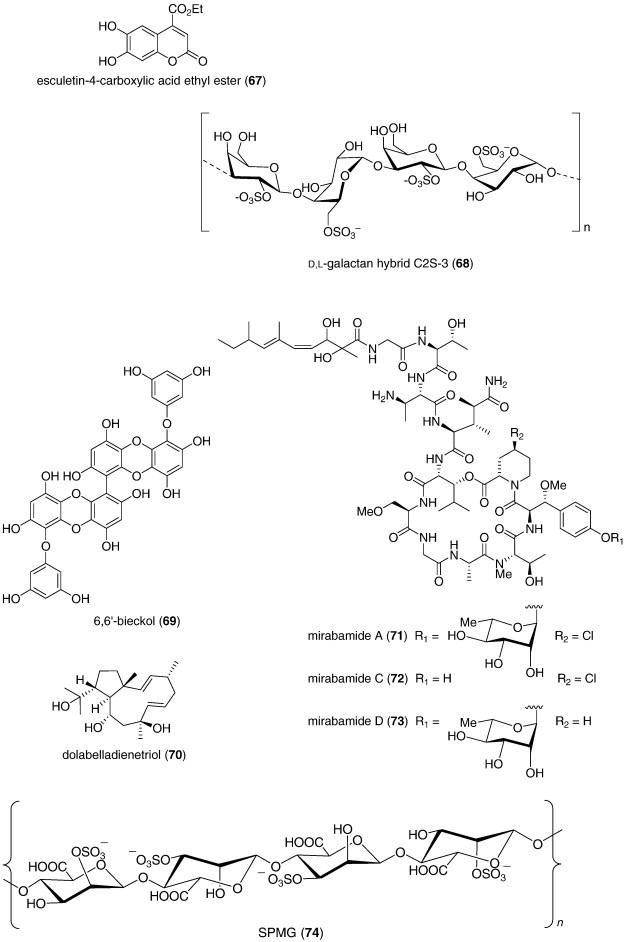
| Antiviral | Esculetin ethyl ester (67)/sponge | Polyketided | SARS-Corona virus viral protease 3CL inhibition | 46 μM | Undetermined | BRA, CAN | (de Lira et al., 2007) |
| Antiviral | Cryptonemia crenulata galactan (68)/alga | Polysaccharideg | Dengue type 2 inhibition | 0.8–16 μg/mL | Inhibition of viral binding and cell penetration | ARG, BRA | (Talarico et al., 2007) |
| Antiviral | 6,6′-Bieckol (69)/alga | Shikimate | Inhibition of HIV-1 infection | 1.07–1.72 μM | Viral p24 antigen production and reverse transcriptase inhibition | CHN, S. KOR | (Artan et al., 2008) |
| Antiviral | Dolabelladienetriol (70)/alga | Terpenoide | Inhibition of HIV-1 replication | 8.4 μM | Noncompetitive inhibition of reverse transcriptase | BRA | (Cirne-Santos et al., 2008) |
| Antiviral | Mirabamides A, C and D (71, 72, 73)/sponge | Peptidef | Inhibition of HIV-1 fusion | 0.041–3.9 μM | Interaction with HIV-1 envelope glycoproteins | NZL, USA | (Plaza et al., 2007) |
| Antiviral | Sulfated SPMG (74)/alga | Polysaccharideg | Inhibition of HIV-1 infection | Inhibition of HIV-1 Tat-induced angiogenesis | CHN | (Lu et al., 2007) |
aOrganism, Kingdom Animalia: polychaeta (Phylum Annelida), rockfish (Phylum Chordata), corals (Phylum Cnidaria), sea cucumber (Phylum Echinodermata), bivalve (Phylum Mollusca), sponge (Phylum Porifera); Kingdom Monera: bacterium (Phylum Cyanobacteria); Kingdom Fungi: fungus; Kingdom Plantae: alga; bIC50: concentration of a compound required for 50% inhibition in vitro; *: estimated IC50, ND: not determined; +MIC: minimum inhibitory concentration; ++MID: minimum inhibitory concentration per disk; +++MIC90: minimum inhibitory concentration; bMMOA: molecular mechanism of action; cCountry: ARG: Argentina; BRA: Brazil; CAN: Canada; CHE: Switzerland; CHN: China; EGY: Egypt; ESP: Spain; FRA: France; DEU: Germany; GBR: United Kingdom; IND: India; ISR: Israel; ITA: Italy; JPN: Japan; NLD: The Netherlands; NZL: New Zealand; PAN: Panama; PAP: Papua New Guinea; PRT: Portugal; S. KOR: South Korea; THAI: Thailand; TUR: Turkey; Chemistry: dpolyketide; eterpene; fnitrogen-containing compound; gpolysaccharide, modified as in the text.
Fig. 1.
Marine compounds with antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis, and antiviral activities.
Table 2.
Marine pharmacology in 2007–8: marine compounds with anti-inflammatory activity, and affecting the immune and nervous systems.
| Drug class | Compound/organisma | Chemistry | Pharmacological activity | IC50b | MMOAc | Countryd | References |
|---|---|---|---|---|---|---|---|
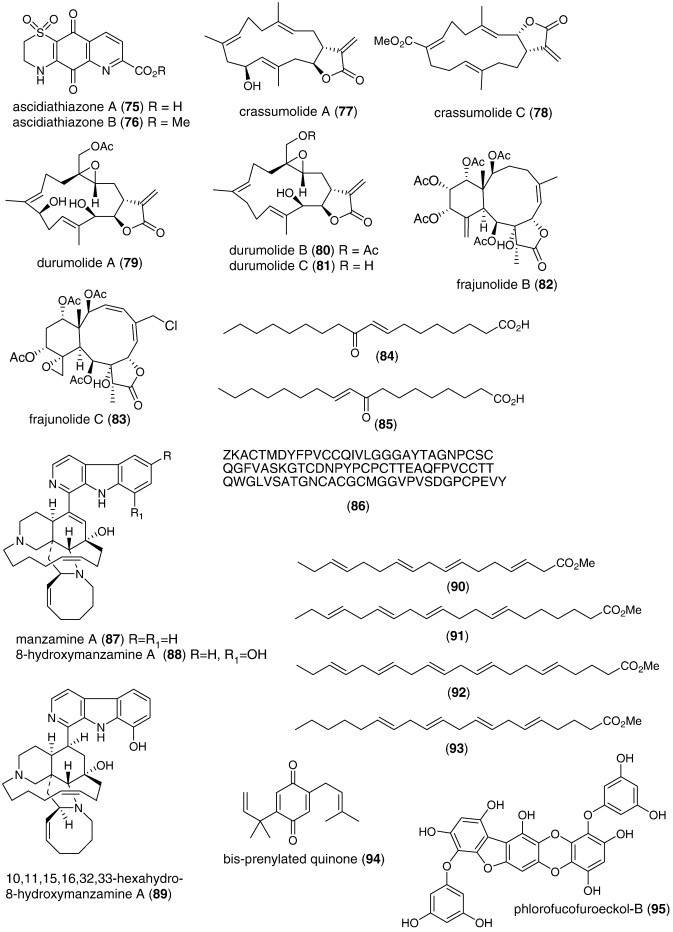
| Anti-inflammatory | Ascidiathiazones A (75) and B (76)/ascidian | Alkaloidg | Human neutrophil free radical inhibition in vitro and in vivo | 0.44–1.55 μM | Superoxide anion inhibition | NZL | (Pearce et al., 2007a) |
| Anti-inflammatory | Crassumolides A and C (77, 78)/soft coral | Terpenoide | Modulation of LPS-activated murine macrophage cell line | < 10 μM | Inducible NOS and COX-2 inhibition | TAIW | (Chao et al., 2008) |
| Anti-inflammatory | Durumolides A-C (79, 80, 81)/soft coral | Terpenoide | Modulation of LPS-activated murine macrophage cell line | < 10 μM | Inducible NOS and COX-2 inhibition | TAIW | (Cheng et al., 2008) |
| Anti-inflammatory | Frajunolides B and C (82, 83)/coral | Terpenoide | Human neutrophil free radical inhibition in vitro | > 10 μg/mL⁎ | Superoxide anion and elastase inhibition | TAIW | (Shen et al., 2007) |
| Anti-inflammatory | Gracilaria verrucosa fatty acids (84, 85)/alga | Polyketided | Modulation of LPS-activated murine macrophages in vitro | < 20 μg/mL⁎ | NO, IL-6 and TNF-α inhibition | S. KOR | (Dang et al., 2008) |
| Anti-inflammatory | Hypnea cervicornis lectin (86)/alga | Peptideg | Antinociception and anti-inflammatory effects in vivo | 0.1–1 mg/kg⁎ | Carbohydrate-binding site interaction | BRA | (Bittencourt Fda S. et al., 2008) |
| Anti-inflammatory | Manzamine (MZA) (87), (–)-8-hydroxy MZA (88), hexahydro-8-hydroxy MZA (89)/sponge | Alkaloidg | Modulation of LPS-activated brain microglia in vitro | 0.25–1.97 μM | TXB2 inhibition | USA | (El Sayed et al., 2008) |
| Anti-inflammatory | ω-3 PUFA (90, 91, 92, 93)/mussel | Polyketided | Human neutrophil lipoxygenase inhibition in vitro | ND | LTB4 and 5-HETE inhibition | AUS | (Treschow et al., 2007) |
| Anti-inflammatory | Perithalia capillaris quinone (94)/alga | Shikimate | Human neutrophil free radical release inhibition in vitro | 2.1 μM | Superoxide anion inhibition | NZL | (Sansom et al., 2007) |
| Anti-inflammatory | PFF-B (95)/alga | Polyketided | Rat basophilic leukemia cell histamine release inhibition | 7.8 μM | Inhibition of β-hexosaminidase release | JPN | (Sugiura et al., 2007) |
| Anti-inflammatory | Plakortide P (54)/sponge | Polyketidee | Modulation of LPS-activated brain microglia in vitro | 0.93 μM | TXB2 inhibition | BRA | (Kossuga et al., 2008) |
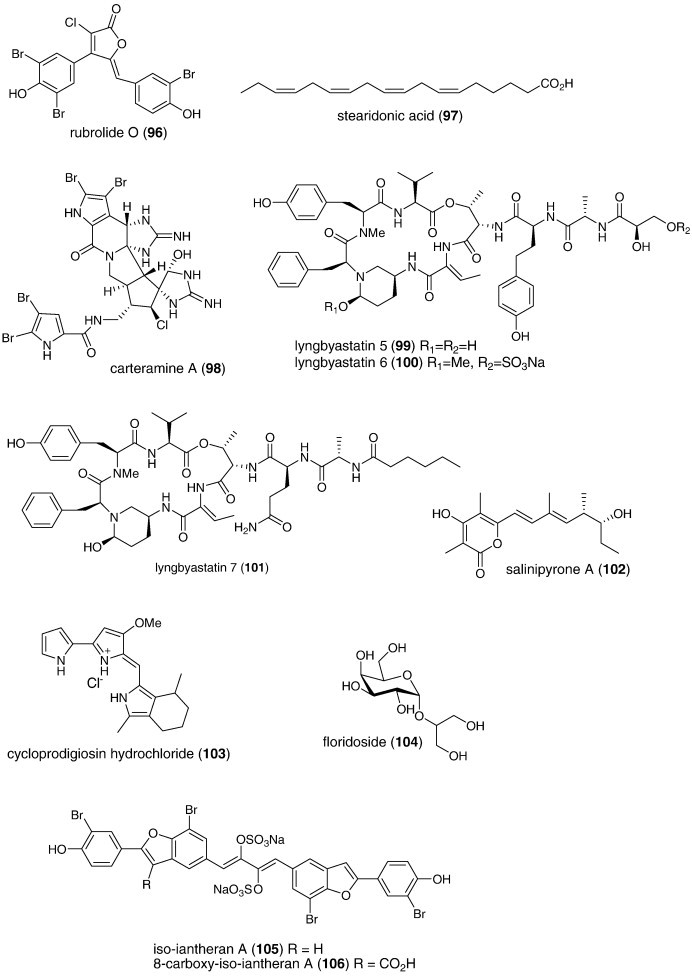
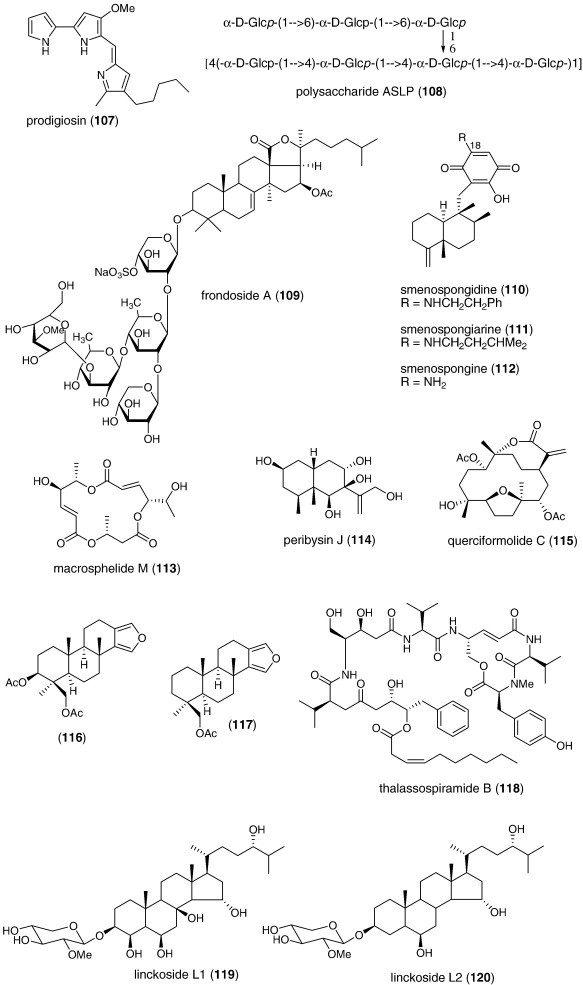
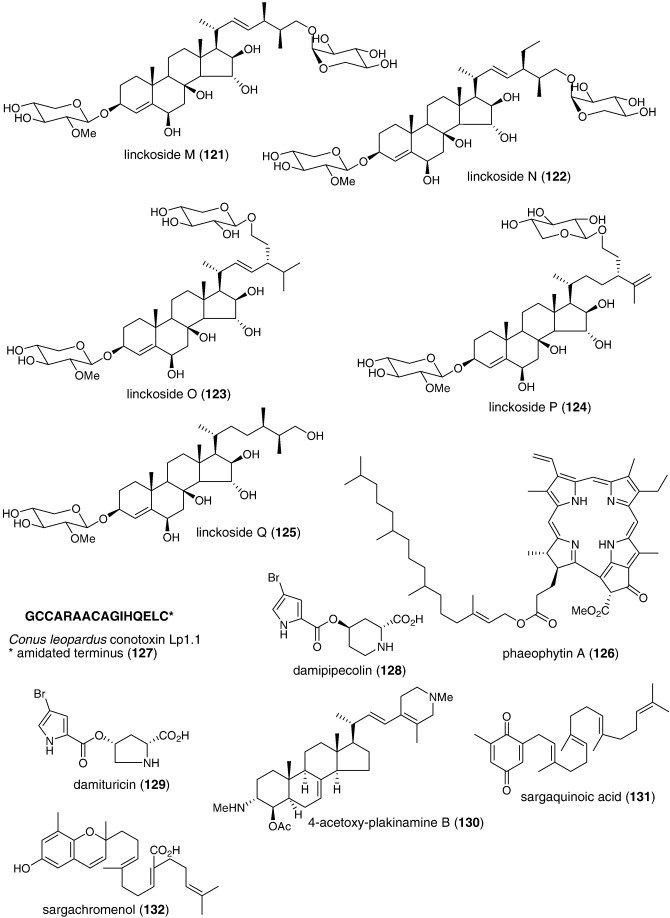
| Anti-inflammatory | Rubrolide O (96)/ascidian | Polyketided | Human neutrophil free radical release inhibition in vitro | 35 μM | Superoxide anion inhibition | NZL | (Pearce et al., 2007b) |
| Anti-inflammatory | Stearidonic (97)/alga | Polyketided | Inhibition of mouse ear inflammation | 160–314 μg/ear | Inhibition of edema, erythema and blood flow | S. KOR, JPN | (Khan et al., 2007) |
| Anti-inflammatory | Carteramine A (98)/sponge | Alkaloidg | Neutrophil chemotaxis inhibition | 5 μM | Undetermined | JPN | (Kobayashi et al., 2007a) |
| Anti-inflammatory | Lyngbyastatins 5–7 (99, 100, 101)/bacterium | Peptideg | Elastase inhibition | 3–10 nM | Undetermined | USA | (Taori et al., 2007) |
| Anti-inflammatory | Salinipyrone A (102)/bacterium | Polyketidee | Mouse splenocyte interleukin-5 inhibition | 10 μg/mL | Undetermined | USA | (Oh et al., 2008) |
| Immune system | Cycloprodigiosin hydrochloride (103)/bacterium | Alkaloidg | Interleukin-8 inhibition | 1 μM | AP-1 transcription factor inhibition | JPN | (Kawauchi et al., 2007) |
| Immune system | Floridoside (104)/alga | Sugarh | Activation of classical complement pathway | 5.9–9.3 μg/mL⁎ | IgM mediated-effect | FRA | (Courtois et al., 2008) |
| Immune system | Iantherans (105, 106)/sponge | Polyketided | Activation of Ca2+-mobilization | 0.48–1.3 μM | Ionotropic P2Y11 receptor activation | DEU, USA | (Greve et al., 2007) |
| Immune system | Prodigiosin (107)/bacterium | Alkaloidg | Macrophage iNOS inhibition | 0.1 μg/mL⁎ | NF-κB transcription factor inhibition | S. KOR | (Huh et al., 2007) |
| Immune system | ASLP (108)/clam | Polysaccharideh | Splenocyte proliferation increase | < 100 μg/mL⁎ | Undetermined | CHN | (He et al., 2007) |
| Immune system | Frondoside A (109)/sea cucumber | Terpenoid glycoside | Lysosomal activity, phagocytosis and ROS activation | 0.1–0.001 μg/mL | Undetermined | RUS, USA | (Aminin et al., 2008) |
| Immune system | Hippospongia sp. quinones (110, 111, 112)/sponge | Terpenoidf | Enhancement of IL-8 release | > 1 μg/mL⁎ | Undetermined | JPN | (Oda et al., 2007) |
| Immune system | Macrosphelide M (113)/fungus | Polyketided | Cell adhesion inhibition | 33.2 μM | Undetermined | JPN | (Yamada et al., 2007) |
| Immune system | Peribysin J (114)/fungus | Terpenoidf | Cell adhesion inhibition | 11.8 μM | Undetermined | JPN | (Yamada et al., 2007) |
| Immune system | Querciformolide C (115)/soft coral | Terpenoidf | Macrophage iNOS and COX-2 inhibition | < 10 μM⁎ | Undetermined | TAIW | (Lu et al., 2008) |
| Immune system | Spongia sp. diterpenoids (116, 117)/sponge | Terpenoidf | Murine spleen cell lysosome activation | > 100 μg/mL⁎ | Undetermined | RUS | (Ponomarenko et al., 2007) |
| Immune system | Thalassospiramide B (118)/bacterium | Peptideg | Interleukin 5 inhibition | 5 μM | Undetermined | USA | (Oh et al., 2007) |
| Nervous system | Linckosides L1 and L2 (119, 120)/sea star | Triterpenoid glycosidef | Induction of neurite outgrowth | 0.3 μM⁎ | Undetermined | ITA, RUS | (Kicha et al., 2007b) |
| Nervous system | Linckosides M–Q (121, 122, 123, 124, 125)/sea star | Triterpenef | Induction of neurite outgrowth | < 10 μM⁎ | Dependent on xylose on side chain | JPN | (Han et al., 2007) |
| Nervous system | Phaeophytin A (126)/alga | Alkaloid/terpenoid | Induction of neurite outgrowth | < 3.9 μM⁎ | MAP kinase activation | JPN | (Ina et al., 2007) |
| Nervous system | Conus leopardus conotoxin Lp1.1 (127)/snail | Peptideg | Seizure and paralysis in goldfish | < 10 μM⁎ | Slow block of α6α3β2 and α3β2 nicotinic receptor | CHN, USA | (Peng et al., 2008) |
| Nervous system | Damipipecolin (128) and damituricin (129)/sponge | Alkaloidg | Inhibition of serotonin receptor binding | 1 μg/mL⁎ | Ca2+ influx inhibition | ITA, DEU | (Aiello et al., 2007) |
| Nervous system | 4-Acetoxy-plakinamine B (130)/sponge | Triterpenoid alkaloidg | Acetylcholinesterase inhibition | 3.75 μM | Mixed-competitive inhibition | THAI | (Langjae et al., 2007) |
| Nervous system | Sargaquinoic acid (131) and sargachromenol (132)/alga | Terpenoidf | Butyrylcholinesterase inhibition | 26 nM | Undetermined | S. KOR | (Choi et al., 2007) |
| Nervous system | SPMG (74)/alga | Polysaccharideh | Neuronal Ca2+-apoptosis inhibition | Decrease in caspase-3 activity | CHN | (Hui et al., 2008) |
aOrganism: Kingdom Animalia: coral (Phylum Cnidaria); ascidian (Phylum Chordata), sea star, cucumber (Phylum Echinodermata); clam, musse, snail (Phylum Mollusca); sponge (Phylum Porifera); Kingdom Fungi: fungus; Kingdom Plantae: alga; Kingdom Monera: bacterium (Phylum Cyanobacteria); bIC50: concentration of a compound required for 50% inhibition; *: apparent IC50; ND: not determined; cMMOA: molecular mechanism of action, NO: nitric oxide; dCountry: AUS: Australia; BRA: Brazil; CHN: China; DEU: Germany; FRA: France; ITA: Italy; JPN: Japan; NZL: New Zealand; RUS: Russia; S. KOR: South Korea; TAIW: Taiwan; THAI: Thailand; Chemistry: epolyketide; fterpene; gnitrogen-containing compound; hpolysaccharide, modified as in the text.
Fig. 2.
Marine compounds with anti-inflammatory activity, and affecting the immune and nervous systems.
Table 3.
Marine pharmacology in 2007–8: marine compounds with miscellaneous mechanisms of action.
| Compound/organisma | Chemistry | Pharmacological activity | IC50b | MMOAc | Countryd | References |
|---|---|---|---|---|---|---|
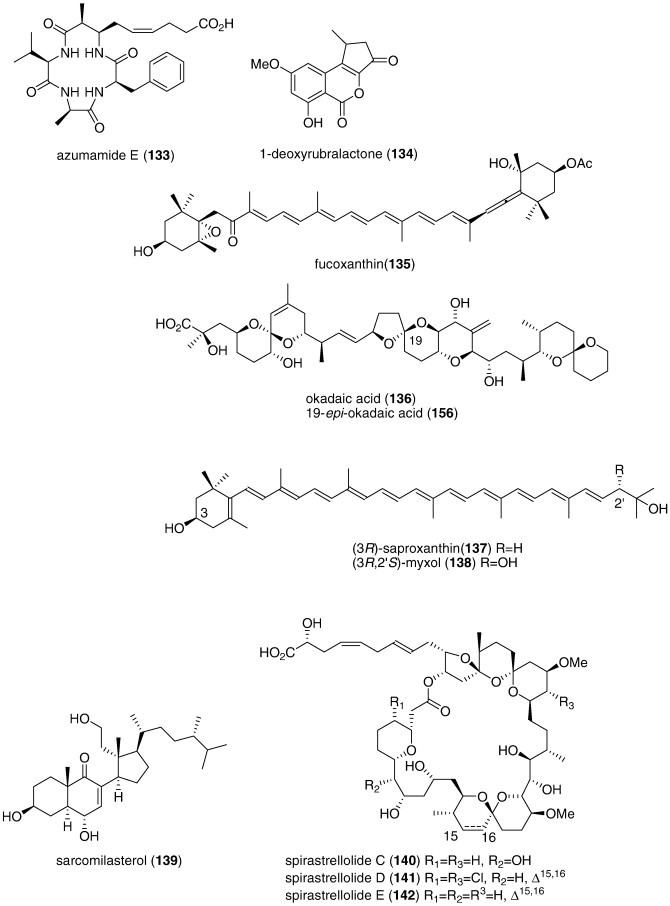
| Azumamide E (133)/sponge | Peptideg | Histone deacetylase inhibition | 50–80 nM | Selective inhibition of isoforms 1, 2, and 3 | ITA | (Maulucci et al., 2007) |
| 1-Deoxyrubralactone (134)/fungus | Shikimate | X and Y DNA polymerase inhibition | 12–60 μM | Specific inhibition of DNA polymerase β and κ | JPN | (Naganuma et al., 2008) |
| Fucoxanthin (135)/alga | Carotenoid | Antioxidant in vitro | 0.14–2.5 mg/mL | Hydroxyl and superoxide radical scavenging | JPN | (Sachindra et al., 2007) |
| Okadaic acid (136)/sponge | Polyketidee | Protein phosphatase 1 and 2A inhibition | 0.96 nM⁎⁎ | Okadaic acid binding to proteins OABP1 and OABP2 | JPN | (Sugiyama et al., 2007) |
| Saproxanthin (137) and myxol (138)/bacterium | Polyketidee | l-Glutamate toxicity inhibition | 3.1–8.1 μM | Lipid peroxidation inhibition | JPN | (Shindo et al., 2007a) |
| Sarcomilasterol (139)/sponge | Triterpenef | Osteoblast growth stimulation | 3 μM | Alkaline phosphatase elevation | S. KOR, VNM | (Van Minh et al., 2007) |
| Spirastrellolides C, D, and E (140, 141, 142)/sponge | Polyketidee | Premature mitosis inhibition | 0.4–0.7 μM | Protein phosphatase 2A inhibition | CAN | (Williams et al., 2007a, Williams et al., 2007b) |
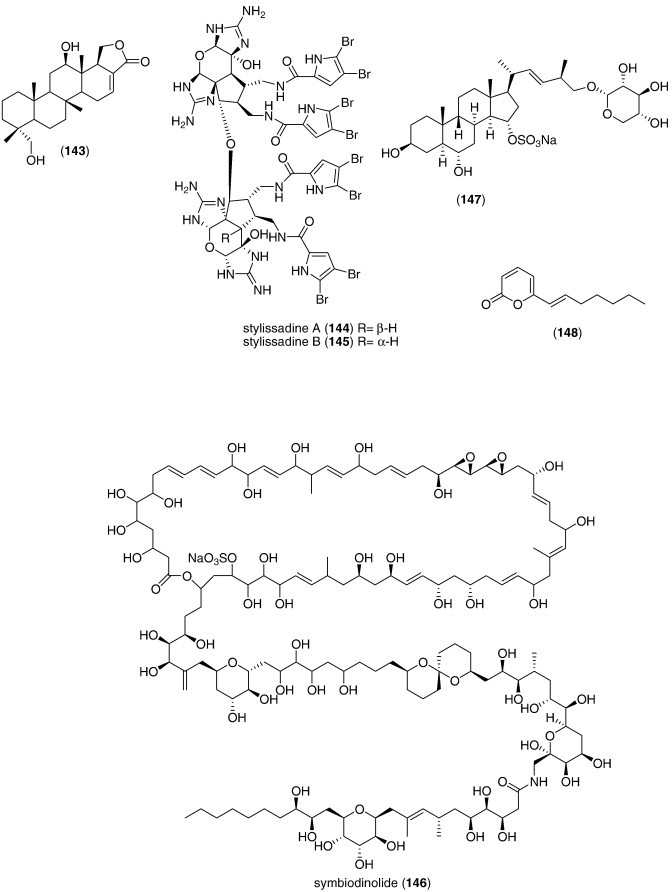
| Spongia sesterterpenoid (143)/sponge | Terpenoidf | Hypercholesterolemia antagonist | 2.4 μM | Farnesoid X-activated receptor coactivator peptide inhibition | S. KOR | (Nam et al., 2007) |
| Stylissadines A and B (144, 145)/sponge | Alkaloidg | Reduction of voltage-dependent Ca2+ entry | 4.5 μM | Irreversible effect requiring lipophilic brominated side chain | DEU | (Bickmeyer et al., 2007) |
| Symbiodinolide (146)/dinoflagellate | Polyketidee | Voltage-dependent N-type Ca2+ channel activation | 7 nM | Cyclooxygenase 1 inhibition | JPN | (Kita et al., 2007) |
| Asterias amurensis saponin (147)/starfish | Triterpenef | Osteoblast cell proliferation | 50 μM⁎ | Undetermined | CHN | (Liu et al., 2008a, Liu et al., 2008b) |
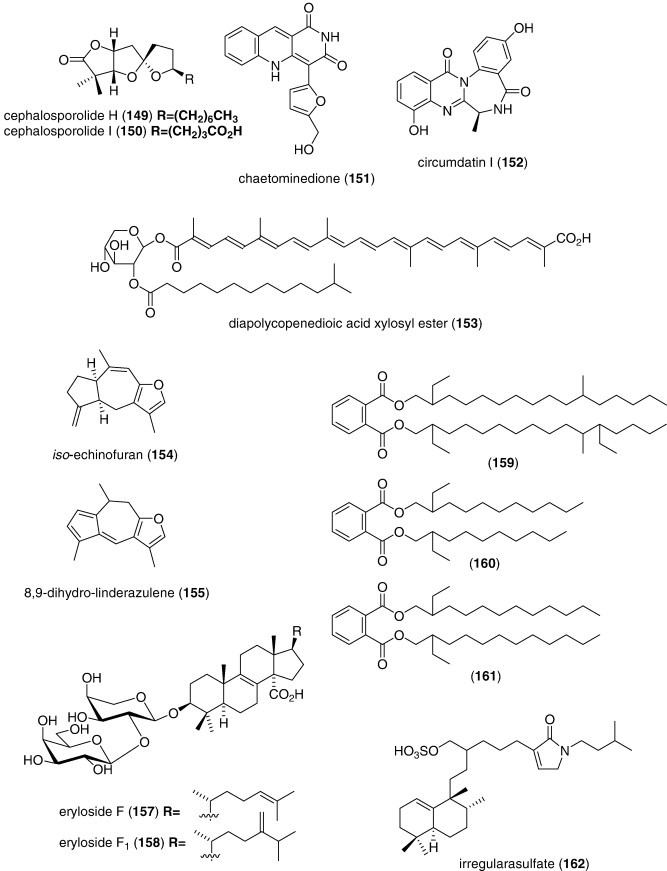
| Botrytis sp. α-pyrone derivative (148)/fungus | Polyketidee | Tyrosinase inhibition | 4.5 μM | Undetermined | CHN, S. KOR | (Zhang et al., 2007) |
| Cephalosporolides H and I (149, 150)/fungus | Polyketidee | Xanthine oxidase and steroid dehydr. inhib. | < 0.29 mM | Undetermined | CHN, DEU | (Li et al., 2007a, Li et al., 2007b) |
| Chaetominedione (151)/fungus | Alkaloidg | p56lck tyrosine kinase inhibition | < 200 μg/mL⁎ | Undetermined | EGY | (Abdel-Lateff A., 2008) |
| Circumdatin I (152)/fungus | Alkaloidg | Ultraviolet A-protecting | 98 μM | Undetermined | S. KOR | (Zhang et al., 2008a, Zhang et al., 2008b) |
| Diapolycopenedioic acid xylosyl ester (153)/bacterium | Terpenoidf | Lipid peroxidation inhibition | 4.6 μM | Undetermined | JPN | (Shindo et al., 2007) |
| Echinogorgia complexa furanosesquiterpenes (154, 155)/soft coral | Terpenoidf | Mitochondrial respiratory chain inhibition | 2.5–4.3 μM | Undetermined | ESP, IND, ITA | (Manzo et al., 2007) |
| 19-epi-Okadaic acid (156)/dinoflagellate | Polyketidee | Protein phosphatase 2A inhibition | 0.47 nM | Undetermined | ESP | (Cruz et al., 2007) |
| Erylosides F and F1 (157, 158)/coral | Terpenoidf | Activation of Ca2+ influx | 100 μg/mL⁎ | Undetermined | ITA, RUS | (Antonov et al., 2007) |
| Hippocampus kuda phthalates (159, 160, 161)/seahorse | Polyketidee | Cathepsin B inhibition | 0.18–.29 mM | Undetermined | CHN, S. KOR | (Li et al., 2008a, Li et al., 2008b, Li et al., 2008c) |
| Irregularasulfate (162)/bacterium | Terpenoidf | Calcineurin inhibition | 59 μM | Undetermined | CAN, NLD, PAP | (Carr et al., 2007) |
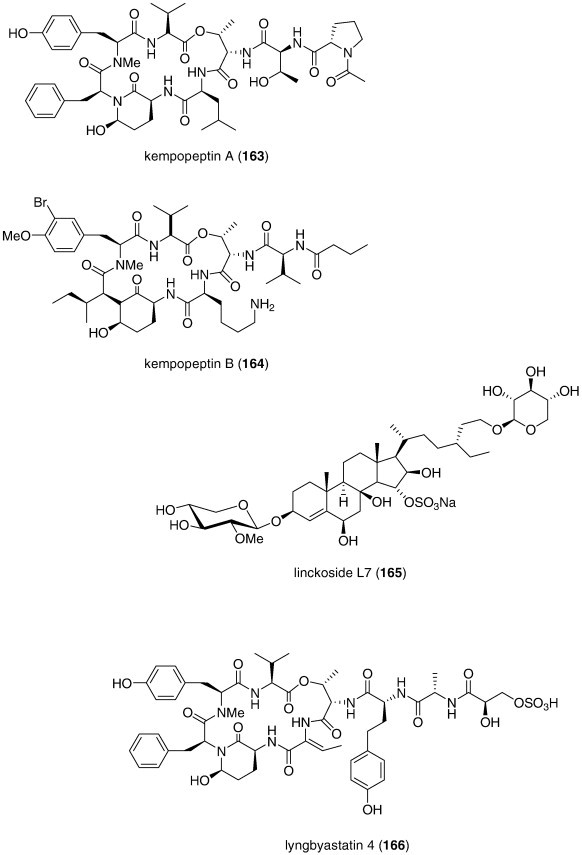
| Kempopeptins A and B (163, 164)/bacterium | Peptideg | Elastase and chymotrypsin inhibition | 0.32–8.4 μM | Undetermined | USA | (Taori et al., 2008) |
| Linckoside L7 (165)/starfish | Triterpenoid glycosidef | Fertilization inhibition | < 25 μg/mL⁎ | Undetermined | RUS | (Kicha et al., 2007a) |
| Lyngbyastatin 4 (166)/bacterium | Peptideg | Elastase and chymotrypsin inhibition | 0.03–0.3 μM | Undetermined | USA | (Matthew et al., 2007) |
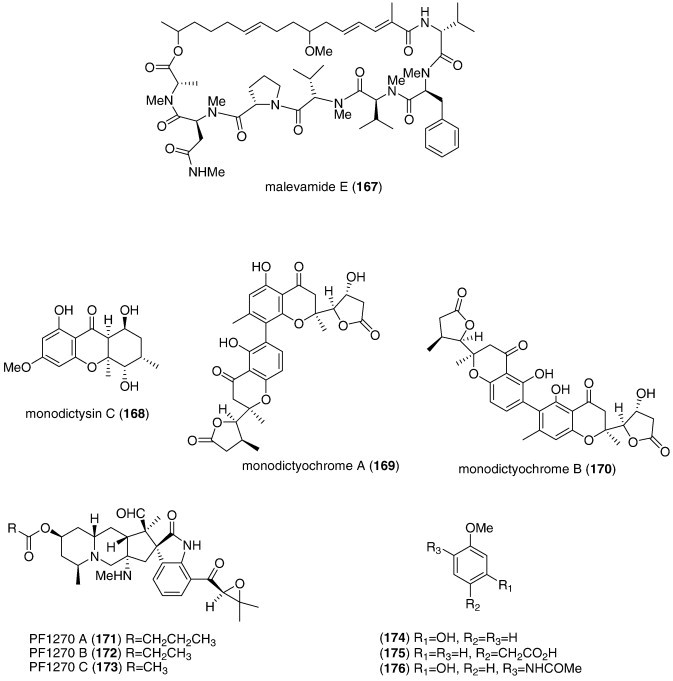
| Malevamide E (167)/bacterium | Peptideg | Extracellular Ca2+ channel inhibition | 9 μM⁎ | Undetermined | USA | (Adams et al., 2008) |
| Monodictysin C (168)/fungus | Shikimate | CYP1A inhibition | 3.0 μM | Undetermined | DEU | (Krick et al., 2007) |
| Monodictyochromes A and B (169, 170)/fungus | Shikimate | CYP1A inhibition | 5.3–7.5 μM | Undetermined | DEU | (Pontius et al., 2008b) |
| Penicillium waksmanii PF1270 A, B, and C (171, 172, 173)/fungus | Alkaloidg | Histamine H3 receptor agonists | 0.12–0.2 μM | Undetermined | JPN | (Kushida et al., 2007) |
| Penicillium sp. anisols (174, 175, 176)/fungus | Polyketidee | CYP3A4 inhibition | 0.4–2 μg/mL | Undetermined | JPN | (El-Beih et al., 2007) |
| Polysiphonia urceolata bromophenols (177, 178, 179, 180)/alga | Polyketidee | DPPH radical scavenging activity | 6.1–8.1 μM | Undetermined | CHN | (Li et al., 2007a, Li et al., 2007b, Li et al., 2008a, Li et al., 2008b, Li et al., 2008c) |
| Pompanopeptin A (181)/bacterium | Peptideg | Trypsin inhibition | 2.4 μM | Undetermined | USA | (Matthew et al., 2008) |
| Purpurone (182)/sponge | Alkaloidg | DPPH radical scavenging activity | 7 μM | Undetermined | CHN, S. KOR, USA | (Liu et al., 2008a, Liu et al., 2008b) |
| Saliniketals A and B (183, 184)/bacterium | Polyketidee | Ornithine decarboxylase induction | 1.9–7.8 μg/mL | Undetermined | USA | (Williams et al., 2007a, Williams et al., 2007b) |
| Sargassum sagamianum monoglyceride (185)/alga | Polyketidee | Phospholipase A2 and COX-2 inhibition | ND | Undetermined | S. KOR | (Chang et al., 2008) |
| Sargassum siliquastrum meroditerpenoids (186, 187, 188, 189, 190, 191)/alga | Terpenoidf | DPPH radical scavenging activity | 0.1–0.31 μg/mL⁎ | Undetermined | S. KOR | (Jung et al., 2008) |
| Symphyocladia latiuscula bromophenols (192, 193, 194, 195)/alga | Polyketidee | DPPH radical scavenging activity | 10.2–24 μM | Undetermined | CHN | (Duan et al., 2007) |
| Tenacibactins C and D (196, 197)/bacterium | PKS/NRPS | Fe-binding (chelating) activity | 110–115 μM | Undetermined | JPN | (Jang et al., 2007a) |
aOrganism, Kingdom Animalia: sea horse (Phylum Chordata), soft corals (Phylum Cnidaria), starfish (Phylum Echinodermata), sponge (Phylum Porifera); Kingdom Chromalveolata: dinoflagellates; Kingdom Fungi: fungus; Kingdom Plantae: alga; Kingdom Monera: bacterium; bIC50: concentration of a compound required for 50% inhibition in vitro; *: estimated IC50; ** Kd: concentration at which 50% of ligand binding sites are occupied; MMOA: molecular mechanism of action ;dCountry: CAN: Canada; CHN: China; EGY: Egypt; ESP: Spain; DEU: Germany; IND: India; ITA: Italy; JPN: Japan; NLD: The Netherlands; PAP: Papua New Guinea; RUS: Russia; S. KOR: South Korea; VNM: Vietnam. Chemistry: epolyketide; fterpene; gnitrogen-containing compound; hpolysaccharide, modified as in the text.
Fig. 3.
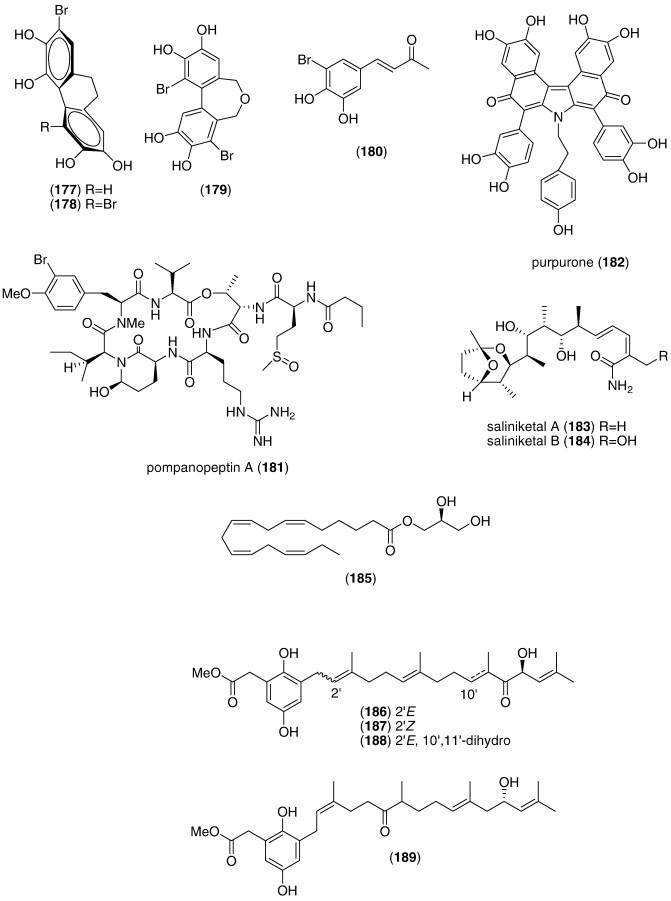
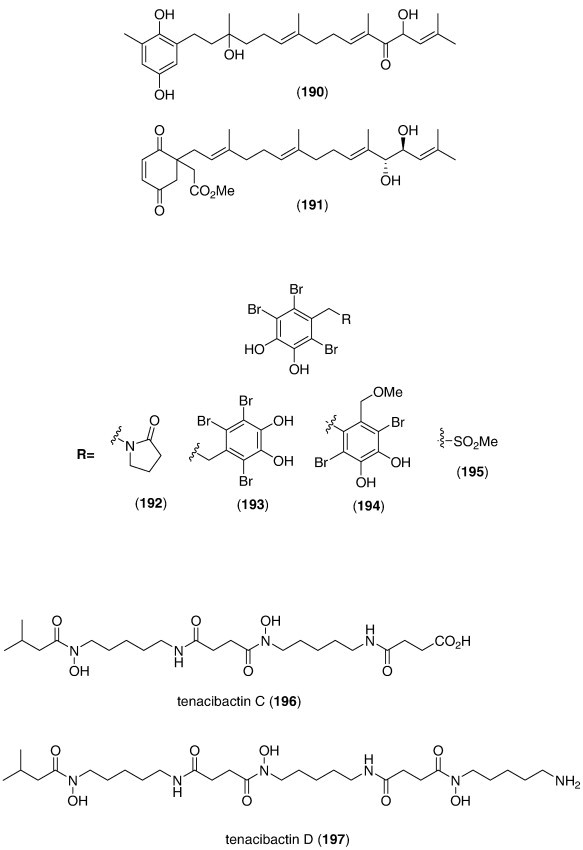
Marine compounds with miscellaneous mechanisms of action.
2. Marine compounds with antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis, and antiviral activities
Table 1 presents preclinical pharmacology reported during 2007–8 on the antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis, and antiviral pharmacology of the marine natural products (1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74) shown in Fig. 1.
2.1. Antibacterial activity
Contributing to the global search for new antimicrobials to combat antibiotic-resistant strains of pathogenic bacteria, marine ecological niches have been described recently as “particularly promising” (Fischbach and Walsh, 2009). During 2007–8, 38 studies reported novel antibacterial marine natural products isolated from marine bacteria, fungi, sponges, worms and fish, a larger effort than the ones reported in previous years (Mayer et al., 2009), and previous reviews of this series.
Only six papers provided detailed mechanism of action studies with marine antimicrobial compounds. Kanoh et al. (2008b) reported the discovery of the novel bioactive spirodioxynaphthalene ascochytatin (1) isolated from cultures of a marine-derived fungus Ascochyta sp. NGB4, which inhibited B. subtilis growth (MID = 0.3 μg/disk) by targeting the function of the bacterial growth regulatory system TCS (YycG/YycF). Kitani et al. (2008) discovered that the 120 kDa acidic glycoprotein l-amino acid oxidase termed SSAP (2), isolated from the rockfish Sebastes schlegelli (Kitani et al., 2007), acted selectively on Gram-negative bacteria (minimum inhibitory concentration (MIC) = 0.078–0.63 μg/mL). While H2O2 was shown to mediate the antibacterial action of SSAP, electron microscopy analysis revealed that SSAP induced cell surface damage and morphological changes in several bacterial species. Lee et al., 2007a, Lee et al., 2007b reported that the 21-residue peptide arenicin-1 (3) isolated from the marine polychaete Arenicola marina exhibited significant antibacterial activity against P. aeruginosa and Staphylococcus aureus (MIC = 2 μg/mL). Interestingly, arenicin-1 induced release of calcein from PE/PG liposomes, thus suggesting that the bacterial cell membrane is the main molecular target of the peptide. Jang et al. (2007c) extended the pharmacology of the alkaloid isoaaptamine (4), isolated from the marine sponge Aaaptos aaptos. Isoaaptamine inhibited sortase A (IC50 = 3.7 μg/mL), an enzyme involved in S. aureus cell wall protein anchoring and virulence, thus potentially providing a novel lead compound “for further development” of potent antibacterials. Lee et al. (2008) isolated seven sesterterpenes (5, 6, 7, 8, 9, 10, 11) from a tropical sponge Dysidea sp. which demonstrated antibacterial activity against B. subtilis (MIC = 1.56–12.5 μg/mL) by inhibiting isocitrate lyase activity, a key enzyme in the glyoxylate cycle which is present in most prokaryotes, lower eukaryotes and plants, but not in vertebrates. Kanoh et al. (2008a) described a new sulfoalkylresorcinol (12) isolated from the marine-derived fungus Zygosporium sp. KNC52, which showed antimicrobial activity against methicillin-resistant S. aureus. The mechanism of action appeared to involve inhibition (IC50 = 12.5 μg/mL) of the in vitro polymerization of FtsZ, a protein which is a structural homolog of eukaryotic tubulin, and that participates in bacterial cell division.
As shown in Table 1, several new marine antibacterials were also reported in 2007–8 (Fig. 1) with MICs less than 10 μg/mL against antibiotic-resistant bacterial strains, although no mechanism of action studies were reported: the alkaloids ambiguine isonitriles H and I (13 14) isolated from a marine cyanobacterium Fischerella sp. (Raveh and Carmeli, 2007); the polyketides ariakemicins A and B (15, 16) isolated from a marine gliding bacterium Rapidithrix sp. (Oku et al., 2008); ayamycin (17) isolated from a bacterium Nocardia sp. ALAA 2000 (El-Gendy et al., 2008a); the alkaloids batzelladines L and M (18, 19) isolated from the Caribbean sponge Monanchora unguifera (Hua et al., 2007); a diphenyl ether (20) isolated from the Indonesian sponge Lamellodysidea herbacea (Hanif et al., 2007); essramycin (21) obtained from the culture broth of a marine Streptomyces sp. isolate Merv8102 (El-Gendy et al., 2008b); a meroditerpene (+)-isojaspic acid (22) isolated from the Papua New Guinean sponge Cacospongia sp. (Rubio et al., 2007); the bisindole pyrroles lynamicins A–D (23, 24, 25, 26) isolated from a novel marine actinomycete Marinispora sp. (McArthur et al., 2008); lipoxazolidinones A and B (27, 28) isolated from a marine actinomycete Marinispora sp. (Macherla et al., 2007); marinopyrrole A (29) isolated from an obligate marine Streptomyces strain (Hughes et al., 2008); a furanosesquiterpene (–)-microcionin-1 (30) isolated from a marine sponge Fasciospongia sp. (Gaspar et al., 2008); a macrolide phomolide B (31) isolated from a fungus Phomopsis sp. (Du et al., 2008); a sargaquinoic acid derivative (32) isolated from the brown alga Sargassum sagamianum (Horie et al., 2008), and tauramamide (33), a lipopeptide isolated from the bacterium Brevibacillus laterosporus PNG276 (Desjardine et al., 2007).
Furthermore during this period, several novel marine metabolites with moderate antimicrobial activity (MIC or IC50 ranging from 10 to 50 μg/mL, or 10 to 50 μM, respectively), were also reported, but their weaker antibacterial activity precluded their inclusion in either Table 1 or Fig. 1: acetylmajapolene A (MIC = 20 μg/disk) (Vairappan et al., 2008), callophycoic acids A, B, G and H (MIC = 16–63.9 μg/mL) (Lane et al., 2007); corallidictyals A, B, C and D (MIC less than 20 μg/mL) (Grube et al., 2007); (S)-(+)-curcuphenol analogs (IC50 = 34–44 μM) (Gul et al., 2007); cyclomarazines A and B (MIC = 13–18 μg/mL) (Schultz et al., 2008); dehydroxychlorofusarielin B (MIC = 62.5 μg/mL) (Nguyen et al., 2007), nodosol (MIC = 16 μg/mL) (Kontiza et al., 2008); paeciloxanthone (MIC = 40 μg/disk) (Wen et al., 2008), palmitoleic acid (IC50 = 10–20 μM) (Desbois et al., 2008); puupehenone-metabolites (MIC = 8–16 μg/mL) (Ciavatta et al., 2007); Tripalea clavaria C-secosteroids (MIC = 25 μg/disk) (Rodriguez Brasco et al., 2007), shishididemniols A and B (MIC = 20 μg/disk) (Kobayashi et al., 2007b), and zafrin (MIC = 50–125 μg/mL) (Uzair et al., 2008).
Noteworthy were reports of novel marine antimicrobial peptides: dicentracin, a new component of the moronecidin family isolated from head kidney leukocytes from the sea bass Dicentrarchus labrax (Salerno et al., 2007); hepcidins, three novel antimicrobial peptides isolated from the tilapia Oreochromis mossambicus, with MICs (50–100 μg/mL) against Listeria monocytogenes, S. aureus, and Enterococcus faecium (Huang et al., 2007); scygonadin, a novel anionic antimicrobial peptide from the seminal plasma of the mud crab, Scylla serrata (Wang et al., 2007a, Wang et al., 2007b), and tunichromes, small dehydrodopamine-containing peptides found in hemocyte cells of the ascidian Ascidia nigra that are capable of crosslinking proteins in vitro (Cai et al., 2008).
2.2. Anticoagulant activity
Four articles published during 2007–8, reported anticoagulant marine natural products isolated from algae and clams, a number very similar to that reported in our previous review (Mayer et al., 2009), and other reviews of this marine pharmacology series.
Jung et al. (2007b) characterized a novel 7.7 kDa anticoagulant polypeptide termed TGAP with a partial sequence (34) from the muscle protein of the South Korean bivalve Tegillarca granosa. The anticoagulant polypeptide, which demonstrated low in vitro cytotoxicity to venous endothelial cells, specifically inhibited the blood coagulation factor Va, as well as the molecular interaction between factor IIa and factor Va in a concentration-dependent manner (IC50 = 77.9 nM), thus resulting in prolonged prothrombin time. The same research group (Jung et al., 2007a) extended the anticoagulant pharmacology of a sulfated polysaccharide from the brown alga E. cava. The sulfated algal polysaccharide potently inhibited the biological activity of human blood coagulation factors IIa, VIIa and Xa in the presence of the glycoprotein antithrombin III in a dose-dependent manner (KD = 15.1, 45.0 and 65.0 nM, respectively). Yoon et al. (2007) reported the purification of a complex and heterogeneous sulfated fucan from the brown alga Laminaria cichorioides. The purified polysaccharide had potent anticoagulant activity which resulted from enhancement of thrombin inhibition by heparin cofactor II, within the same concentration range as the clinically used heparin. Mao et al. (2008) described two sulfated polysaccharides WF1 (870 kDa) and WF3 (70 kDa) from the marine green alga Monostroma nitidum which demonstrated high anticoagulant activities. Interestingly, both polysaccharides inhibited thrombin as well as potentiated antithrombin III-mediated inhibition of coagulation factor Xa.
2.3. Antifungal activity
Ten studies during 2007–8 reported on the antifungal activity of several novel marine natural products isolated from marine algae, bacteria, sponges and sea cucumbers, a decrease from our last review (Mayer et al., 2009), and previous reviews of this series.
As shown in Table 1, only one report extended the molecular pharmacology of novel antifungal marine metabolites. Lee et al., 2007a, Lee et al., 2007b discovered that three bromophenols (35, 36, 37) isolated from the red alga Odonthalia corymbifera potently inhibited isocitrate lyase (ICL) (IC50 = 2.0–2.8 μM), an enzyme that is part of the glyoxylate cycle which is expressed during host infection by diverse pathogenic fungi. Although the investigators studied the effect of the algal bromophenols on the rice fungal pathogen Magnaporthe grisea, it is noteworthy that ICL has also been observed to be upregulated in Mycobacterium tuberculosis-infected human macrophages, and that Candida albicans requires ICL to be fully virulent to human hosts.
Furthermore, several marine natural products showed significant antifungal activity (i.e. MICs that were either less than 10 μg/mL, 10 μM, or 10 μg/disk) (Table 1 and Fig. 1; 38, 39, 40, 41, 42, 43, 44), although no mechanism of action studies were reported in the published articles: callipeltins J and K (38, 39), MIC = 1 μM (D'Auria et al., 2007), the triterpene glycoside holothurin B (40), MIC = 1.56 μg/mL (Kumar et al., 2007), the macrolide neopeltolide (41), MIC = 0.62 μg/mL (Wright et al., 2007), the cyclopeptide pedein A (42), MIC = 0.6–1.6 μg/mL (Kunze et al., 2008), and pseudoceratins A and B (43, 44), MIC = 6.5–8.0 μg/disk (Jang et al., 2007b). Hopefully, future studies on the molecular pharmacology of these marine compounds will elucidate their mechanisms of action.
Finally, several novel structurally characterized marine molecules isolated from sponges demonstrated MICs or IC50s greater than 10 μg/mL or 10 μM, and therefore, because of the reported weaker antifungal activity, they have been excluded from Table 1 and Fig. 1: eurysterols A and B (MIC = 15.6–62.5 μg/mL) (Boonlarppradab and Faulkner, 2007); nagelamides M and N (MIC = 33.3 μg/mL) (Kubota et al., 2008), nortetillapyrone (MIC = 31–62 μg/mL) (Wattanadilok et al., 2007), and Tydemania expeditionis triterpenoids (IC50 = 26–55 μM) (Jiang et al., 2008). These marine compounds may yet provide additional pharmacological leads in the ongoing global search for clinically useful antifungal agents.
2.4. Antimalarial, antiprotozoal, and antituberculosis activity
As shown in Table 1, during 2007–8 fourteen studies reported novel findings on the antimalarial, antiprotozoal and antituberculosis pharmacology of structurally characterized marine natural products, an increase from our previous review (Mayer et al., 2009), and previous reviews of this series.
As shown in Table 1, nine marine molecules were shown during 2007–8 to possess significant antimalarial activity, although mechanism of action studies were reported for only two compounds. Tasdemir et al. (2007) reported that (E)-oroidin (45) and (E)-oroidin TFA salt (46) isolated from the Turkish marine sponge Agelas oroides potently inhibited cultures of multidrug resistant K1 strain of Plasmodium falciparum (IC50 = 3.9 and 7.9 μg/mL, respectively) with concomitant inhibition of FabI (enoyl-ACP reductase), a key enzyme of the type II fatty acid synthase cascade (IC50 = 0.3 and 5.0 μg/mL, respectively). Further studies revealed that oroidin free base appeared to bind to “the enzyme–substrate complex or enzyme–cofactor complex” by an uncompetitive mechanism, providing further pharmacological characterization of the “first antimalarial marine natural product that targets P. falciparum FabI”.
Additional antimalarial activity was reported for seven marine compounds. Two reports were contributed during 2007 by the Panama International Cooperative Biodiversity Groups project: McPhail et al. (2007) isolated a novel linear lipopeptide dragomabin (47) from the cyanobacterium Lyngbya majuscula with moderate antimalarial activity (IC50 = 6.0 μM), and significant differential toxicity between the malarial parasite and mammalian cells. Linington et al. (2007) discovered that the new cyclic hexapeptides venturamides A and B (48, 49) isolated from a Panamanian marine cyanobacterium Oscillatoria sp., demonstrated significant in vitro antiplasmodial activity against the W2 chloroquinone-resistant strain of the parasite (IC50 = 5.6–8.2 μM), with mild cytotoxicity to mammalian cells, the first “example of the identification of cyanobacterial peptides with selective antimalarial activity”. Kasettrathat et al. (2008) proved that a new tetronic acid, nodulisporacid A (50), from a marine-derived fungus Nodulisporium sp. CRIF1 isolated from a Thai soft coral, was moderately antiplasmodial (IC50 = 1–10 μM) against chloroquine-resistant P. falciparum strain 94. Na et al. (2008) identified a new marine Streptomyces sp. H668 polyether (51) from Hawaii that showed in vitro antimalarial activity (IC50 = 0.1–0.2 μg/mL) against both P. falciparum chloroquine-susceptible (D6) and -resistant (W2) clones, with minimal cytotoxicity towards mammalian cells. Clark et al. (2008) found that a novel acylproline derivative, tumonoic acid I (52) from the Papua New Guinean marine cyanobacterium Blennothrix cantharidosmum, showed moderate antimalarial activity against P. falciparum (IC50 = 2 μM). Pontius et al. (2008a) isolated a heterocyclic-substituted xanthone, chaetoxanthone B (53) from cultures of a marine-derived fungus Chaetomium sp. that showed selective activity towards P. falciparum K1 strain (IC50 = 0.5 μg/mL).
Four marine compounds were reported to possess antiprotozoal activity. Kossuga et al. (2008) re-isolated the previously reported plakortide P (54) from the marine sponge Plakortis angulospiculatus. The compound displayed selective effects against both Leishmania chagasi (IC50 = 0.5–1.9 μg/mL) and Trypanosoma cruzi (IC50 = 2.3 μg/mL), with low concomitant hemolytic and cytotoxic activities towards human macrophages. Reportedly, the mechanism of action towards intracellular L. chagasi did not appear to involve nitric oxide. Simmons et al. (2008) found that two novel lipopeptides viridamides A and B (55, 56) were nearly “equipotent” in inhibiting both Leishmania mexicana (IC50 = 1.1 μM) and T. cruzi (IC50 = 1.5 μM). Besides the antimalarial activity, Pontius et al. (2008a) discovered that chaetoxanthone B (53) had selective effects towards T. cruzi (IC50 = 1.5 μg/mL), with minimal cytotoxicity towards rat skeletal L6 myoblasts (IC50 = 47 μg/mL).
Ten new marine compounds were reported in the global search for novel antituberculosis agents, a considerable increase from our previous reviews (Mayer et al., 2009), and previous reviews of this series.
Ospina et al. (2007) isolated a bioactive oxapolycyclic diterpene bipinnapterolide B (57) from the Colombian gorgonian coral Pseudopterogorgia bipinnata which weakly inhibited growth of M. tuberculosis H37Rv (66% inhibition at 128 μg/mL). Zhang et al., 2008a, Zhang et al., 2008b identified two new dimeric naphtha-γ-pyrones 8′-O-demethylnigerone (58) and 8′-O-demethylisonigerone (59) from the marine-derived fungus Aspergillus carbonarius which showed weak antimycobacterial activity against M. tuberculosis (H37Rv, MIC = 43 and 21.5 μM, respectively). Interestingly, the presence of conjugated C=C–C=O bonds in the pyrane ring appeared to be crucial for antifungal activity. As a result of a continued investigation of the Caribbean sea whip Pseudopterogorgia elisabethae, Wei et al. (2007a) reported that the novel tricarbocyclic norditerpenes caribenols A (60) and B (61) weakly inhibited M. tuberculosis (H37Rv, MIC = 128 and 63 μg/mL, respectively). Furthermore, Wei et al. (2007b) discovered two novel ring B abeo-sterols parguesterols A (62) and B (63) in the Caribbean sponge Svenzea zeai, which inhibited M. tuberculosis (H37Rv, MIC = 7.8 and 11.2 μg/mL, respectively). Hopefully future information on the selectivity index of these two compounds will provide additional information to support the notion that they might “constitute important lead structures for the development of novel tuberculosis drugs due to their strong activity, specificity, and low toxicity”. Berrue et al. (2007) noted that several bioactive polyketides, 24-norisospiculoic acid A (64), dinorspiculoic acid A (65), and norspiculoic acid A (66) from the Caribbean sponge Plakortis zyggompha also inhibited M. tuberculosis (H37Rv, MIC99 = 50 μg/mL). Although all of these studies demonstrate that marine terpenes and polyketides constitute potentially novel antituberculosis leads, they unfortunately did not provide detailed mechanism of action pharmacology at the time of their publication.
2.5. Antiviral activity
As shown in Table 1, three reports were published on the antiviral pharmacology of novel marine natural products against severe acute respiratory syndrome (SARS) Corona virus, herpes simplex, and dengue virus during 2007–8. de Lira et al. (2007) discovered that the new esculetin-4-carboxylic acid ethyl ester (67) from the Brazilian marine sponge Axinella cf. corrugata inhibited the SARS 3CL protease (IC50 = 46 μM). This is a potentially significant finding because the 3CL protease is a “high profile target” in SARS drug development as it appears to be involved in the release of replicative viral proteins as well as the RNA polymerase. Talarico et al. (2007) reported that a d,l-galactan hybrid C2S-3 (68) isolated from the Brazilian marine alga Cryptonemia crenulata showed potent antiviral activity against three clinical strains of dengue virus serotype 2 (IC50 = 0.8–16 μg/mL), together with low cytotoxicity. Further mechanistic work determined that C2S-3 appeared to be a “promising DENV-2 entry inhibitor”. Mandal et al. (2008) described a sulfated xylomannan isolated from the Indian red seaweed Scinaia hatei which inhibited HSV-1 and HSV-2 (IC50 = 0.5–1.4 μg/mL), with low cytotoxicity, probably interfering with the HSV-1 multiplication cycle. Interestingly the “very good antiviral activity against the wide spectrum of HSV strains tested” suggested this compound might be a good candidate for further preclinical research.
Five reports contributed preclinical pharmacology of marine compounds active against the human immunodeficiency virus type-1 (HIV-1), the causative agent of the acquired immunodeficiency disease syndrome (AIDS), an increase from our previous reviews (Mayer et al., 2009), and previous reviews of this series. Artan et al. (2008) reported that the phlorglucinol derivative 6, 6′-bieckol (69) isolated from the brown alga E. cava inhibited HIV-induced syncytia formation (IC50 = 1.72 μM), viral p24 antigen production (IC50 = 1.26 μM), and the activity of the HIV reverse transcriptase (IC50 = 1.07 μM), with “no cytotoxicity” at concentrations that inhibited HIV replication “almost completely”. Cirne-Santos et al. (2008) extended the molecular pharmacology of the diterpene dolabelladienetriol (70) isolated from the marine brown alga Dictyota pfaffii. The dolabellane diterpene blocked both synthesis and integration of the HIV-1 provirus by noncompetitively inhibiting the reverse transcriptase enzyme (Ki = 7.2 μM). The investigators proposed dolabelladienetriol (70) as a “potential new agent for anti-HIV-1 therapy”. Plaza et al. (2007) described three new depsipeptides mirabamides A, C and D (71, 72, 73) isolated from the sponge Siliquariaspongia mirabilis that potently inhibited both HIV-1 in neutralization (IC50 = 0.14–0.19 μM) and HIV-1 envelope-mediated cell fusion (IC50 = 0.14–3.9 μM), suggesting these compounds act at an early stage of HIV-1 cell infection, “presumably through interactions with HIV-1 envelope proteins”. Lu et al. (2007) added a “novel mechanistic profiling” of the previously reported sulfated polymannuroguluronate (SPMG) (74), a polysaccharide with an average molecular weight of 8.0 kDa isolated from the brown alga Laminaria japonica, that has been reported to be in Phase II clinical trials in China as an anti-AIDS drug candidate. SPMG appeared to eliminate the viral gene product known as transactivator of transcription protein (Tat)-induced signal transduction as well as angiogenesis in AIDS-associated Kaposi's sarcoma cells. Furthermore, in 2008, Hui et al. (2008) demonstrated that SPMG appeared to show a neuroprotective effect because it decreased apoptosis caused by Tat-stimulated calcium overload in PC12 neuronal cells, thus suggesting SPMG might warrant further clinical studies in HIV-associated dementia.
3. Marine compounds with anti-inflammatory effects and affecting the immune and nervous systems
Table 2 summarizes the preclinical pharmacological research completed during 2007–8 with the marine compounds (75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132) shown in Fig. 2.
3.1. Anti-inflammatory marine compounds
The anti-inflammatory pharmacology of marine compounds reported during 2007–8 showed a considerable increase from our previous review (Mayer et al., 2009), and previous reviews of this series. Several marine natural products were shown in preclinical pharmacological studies to target arachidonic metabolism in neutrophils and macrophages. Pearce et al. (2007a) reported two new tricyclic alkaloids ascidiathiazone A (75) and B (76) isolated from a New Zealand ascidian Aplidium species that affected superoxide production by human neutrophils in vitro (IC50 = 0.44–1.55 μM), as well as murine peritoneal neutrophis ex vivo, with concomitant “low or nonexistent” liver and renal toxicity, thus suggesting that these two compounds might become “potential anti-inflammatory pharmaceutical” leads. Chao et al. (2008) identified the new cembranolides crassumolides A and C (77, 78) from the soft coral Lobophytum crassum which inhibited expression of iNOS and COX-2 (apparent IC50 less than 10 μM). Similarly, from the same university, Cheng et al. (2008) found that new cembranolides from the soft coral Lobophytum duru, durumolides A–C (79, 80, 81) inhibited both the iNOS and COX-2 proteins in LPS-activated RAW 264.7 cells in vitro (apparent IC50 less than 10 μM), suggesting that the α-methylene-γ-lactone moiety of these compounds was necessary for the observed activity. Shen et al. (2007) showed that new briarane-type diterpenoids frajunolides B and C (82, 83), isolated from the Taiwanese gorgonian Junceella fragilis, significantly inhibited superoxide anion and elastase generation from human neutrophils in vitro (apparent IC50 greater than 10 μg/mL). Dang et al. (2008) demonstrated that the previously described fatty acids (84, 85) from the South Korean marine red alga Gracilaria verrucosa inhibited release by LPS-activated murine macrophages of the inflammatory nitric oxide, tumor necrosis factor α and interleukin-6 (apparent IC50 less than 20 μg/mL), thus revealing that the conjugated enone moiety played a critical role in the anti-inflammatory activity observed in vitro. Bittencourt et al. (2008) contributed novel preclinical pharmacology on a previously reported 90 amino acid polypeptide (86) isolated from the red alga Hypnea cervicornis. The mucin-binding agglutinin, which was extensively characterized with several in vivo models of nociception and inflammation, probably exerted its anti-inflammatory activity (apparent IC50 = 0.1–1 mg/kg) via interaction of the polypeptide with the lectin carbohydrate-binding site, although the exact mechanism of action remains undetermined. El Sayed et al. (2008) demonstrated that manzamine A (87), (−)-8-hydroxymanzamine A (88), and hexahydro-8-hydroxymanzamine A (89) potently inhibited TXB2 generation (IC50 = 0.25, less than 0.1, and 1.97 μM, respectively) in brain microglia. These findings provide further support to the notion that manzamine alkaloids appear to be “viable anti-inflammatory leads” for the preclinical pharmaceutical development of agents to modulate both activated brain microglia and eicosanoid generation. Treschow et al. (2007) investigated four novel ω-3 polyunsaturated fatty acids (90, 91, 92, 93) from the New Zealand green-lipped mussel Perna canaliculus with an in vitro bioassay that used stimulated human neutrophil 5-lipoxygenase, demonstrating that the putative anti-inflammatory potential of these compounds might be related to inhibition of leukotriene and prostaglandin metabolite production. Sansom et al. (2007) reported a bis-prenylated quinone (94) from the New Zealand brown alga Perithalia capillaris which inhibited superoxide anion production in human neutrophils (IC50 = 2.1 μM) in vitro, with low toxicity. An explanation as to why the authors decided not to investigate the quinone “as an anti-inflammatory lead” remains unclear, although it was strongly cytotoxic towards human leukemia cells (IC50 = 0.34 μM). Sugiura et al. (2007) characterized a novel anti-allergic phlorotannin phlorofucofuroeckol B (PFF-B) (95) from the edible brown alga Eisenia arborea. PFF-B, inhibited β-hexosaminidase release from rat basophilic leukemia cells (IC50 = 7.8 μM) in vitro, thus showing higher potency than Tranilast (Rizaben®) (IC50 = 46.6 μM), a pharmaceutical agent used for treatment of allergic disorders such as asthma, allergic rhinitis and atopic dermatitis in Japan and South Korea. Kossuga et al. (2008) demonstrated that the polyketide plakortide P (54) isolated from the Brazilian sponge P. angulospiculatus, potently inhibited thromboxane B2 release (IC50 = 0.93 μM) from activated rat brain microglia, thus extending the preclinical pharmacology of this compound, which besides being antiparasitic as discussed earlier in this review, also appears to be a potentially “novel antineuroinflammatory agent”. Pearce et al. (2007b) examined a halogenated furanone rubrolide O (96) isolated from a New Zealand ascidian Synoicum n. sp., which inhibited superoxide anion production in human neutrophils (IC50 = 35 μM) in vitro with low toxicity. Although rubrolides have been reported to be cytotoxic and antibacterial, the authors proposed that the anti-inflammatory activity for these tunicate metabolites though moderate was “unprecedented”. Khan et al. (2007) identified a ω-3 polyunsaturated fatty acid stearidonic acid (97) from the South Korean brown seaweed Undaria pinnatifida, which was shown to be very active against PMA-induced mouse ear inflammation symptoms, edema, erythema and blood flows (IC50 = 160, 314 and 235 μg/per ear, respectively). The in vivo data compared well with indomethacin which was used as a positive control (IC50 = 90, 172, 179 μg/per ear, respectively), and thus supported claims that this “seaweed can be used as a remedy for inflammation-related symptoms”. Kobayashi et al. (2007a) discovered a novel dimeric oroidin derivative carteramine A (98) in the marine sponge Stylissa carteri, and showed that it inhibited neutrophil chemotaxis (IC50 = 5 μM). The authors suggested that because carteramine A has no structural resemblance to known compounds that inhibit neutrophil chemotaxis, their finding provides a “novel platform to develop a new class of anti-inflammatory agents”. Taori et al. (2007) identified three new analogues of dolastatin 13, lyngbyastatins 5–7 (99, 100, 101), isolated from marine cyanobacteria Lyngbya spp. from South Florida. Although the three compounds selectively and potently inhibited porcine pancreatic elastase (IC50 = 3–10 nM), suggesting a potential therapeutic use in pathophysiological conditions where elastase overactivity is involved, no mechanism of action studies were reported at the time. Oh et al. (2008) purified a novel polyketide salinipyrone A (102) from the marine actinomycete Salinispora pacifica, which moderately inhibited interleukin-5 (IC50 = 10 μg/mL) in a mouse splenocyte model of allergic inflammation, with low cell cytotoxicity.
3.2. Marine compounds affecting the immune system
The pharmacology of the marine compounds reporting activity on the immune system during 2007–8 showed a considerable increase from our previous review (Mayer et al., 2009), and previous reviews of this series.
Kawauchi et al. (2007) investigated the red pigment cycloprodigiosin hydrochloride (103) isolated from the marine bacterium Pseudoalteromonas denitrificans. Interestingly, the compound suppressed activator protein 1 (AP-1) (apparent IC50 = 1 μM), and downstream gene expression of interleukin 8, a chemokine involved in the innate immune system. Courtois et al. (2008) assessed the effect of the known compound floridoside (104), isolated from the French red alga Mastocarpus stellatus on the complement system. Floridoside was observed to potently activate the classical complement pathway (apparent IC50 = 5.9–9.3 μg/mL) by recruiting immunoglobulin M (IgM), suggesting that the compound might be an “important step in the development of a potent new anticomplementary agent” useful in therapy aimed at addressing complement depletion. Greve et al. (2007) reported that the novel iso-iantheran A and 8-carboxy-iso-iantheran A (105, 106) purified from the Australian marine sponge Ianthella quadrangulata demonstrated agonist activity at P2Y11 receptors (IC50 = 1.29 and 0.48 μM, respectively). This finding represents an interesting contribution to ionotropic purine receptor P2Y11 pharmacology, because these receptors have been shown to affect the maturation and differentiation of dendritic cells. Huh et al. (2007) extended the pharmacology of prodigiosin (107) isolated from the marine bacterium Hahella chejuencis collected in South Korea. Prodigiosin inhibited iNOS mRNA expression and NO production (apparent IC50 = 0.1 μg/mL) by a mechanism that involved inhibition of NF-κB and p38 MAPK and JNK phosphorylation. He et al. (2007) characterized the pharmacology of a water soluble polysaccharide (108) isolated from the mollusc Arca subcrenata Lischke, a popular Chinese seafood, and named it ASLP. ASLP stimulated mouse spleen lymphocyte proliferation in a concentration-dependent manner (apparent IC50 less than 100 μg/mL), and with the “branches of ASLP” being required for the immunomodulatory bioactivity. Aminin et al. (2008) described the immunostimulant activity of frondoside A (109), a triterpene glycoside isolated from the sea cucumber Cucumaria frondosa. The glycoside was shown to stimulate lysosomal activity and phagocytosis in mouse macrophages, as well as reactive oxygen species formation. Oda et al. (2007) provided novel information on the molecular mechanisms affected by smenospongidine, smenospongiarine and smenospongine (110, 111, 112), sesquiterpene quinones previously isolated from a Palauan marine sponge Hippospongia sp. The observation that at 10 μg/mL, these compounds promoted interleukin-8 release, a member of the C–X–C chemokine superfamily which is involved in tumor progression and metastasis, suggested that “the functional group at C-18” might play a yet undetermined role in the observed results. Yamada et al. (2007) isolated the novel macrosphelide M (113) and peribysin J (114) from Periconia byssoides, a fungal strain discovered from the sea hare Aplysia kurodai. Significantly, both fungal metabolites inhibited the adhesion of human promyelocytic leukemia HL-60 cells to human umbilical vein endothelial cells (IC50 = 33.2 and 11.8 μM, respectively) more potently than herbimycin A (IC50 = 38 μM). The latter compound, a benzochinoid ansamycin antibiotic isolated from Streptomyces sp. was used as a positive control in these studies. Lu et al. (2008) contributed a novel cembranolide querciformolide C (115) from the soft coral Sinularia querciformis, which significantly inhibited the activation of the iNOS and COX-2 enzymes (apparent IC50 less than 10 μM) in LPS-activated murine macrophages in vitro. Ponomarenko et al. (2007) discovered that new diterpenoids (116, 117) isolated from a Northern Cook Islands marine sponge Spongia (Heterofibria) sp. moderately activated murine splenocytes lysosomes (apparent IC50 greater than 100 μg/mL). Oh et al. (2007) characterized a novel cyclic peptide thalassospiramide B (118) isolated from a marine bacterium Thalassospira sp., which showed immunosuppressive activity in an interleukin-5 (IL-5) inhibition assay (IC50 = 5 μM); an interesting finding because IL-5 is expressed both in eosinophils and mast cells in asthmatic patients.
3.3. Marine compounds affecting the nervous system
Pharmacological studies with marine compounds affecting the nervous system involved three areas of neuropharmacology: the stimulation of neurogenesis, the targeting of receptors, and other miscellaneous activities on the nervous system.
Marine natural products reported to be neuritogenic, might be used to treat damaged neuronal cells, and potentially neurodegenerative diseases. As shown in Table 2, compounds (119, 120, 121, 122, 123, 124, 125, 126) isolated from sea stars and a brown alga were observed to enhance the neuritogenic properties of nerve growth factor (NGF), a compound that has a critical role in differentiation, survival, and neuronal regeneration.
Kicha et al. (2007b) contributed two new steroid glycosides, linckosides L1 (119) and L2 (120) isolated from the Vietnamese blue sea star Linckia laevigata. All three glycosides (apparent IC50 = 0.3 μM) showed synergistic effects on NGF-induced (2 ng/mL) neurite outgrowth of murine neuroblastoma C-1300 cells. Han et al. (2007) contributed the novel steroid glycosides linckosides M–Q (121, 122, 123, 124, 125) from the Japanese sea star L. laevigata. Linckoside P (124) induced neurite outgrowth in 55% of rat pheochromocytoma PC12 cells at 10 μM in the presence of nerve growth factor (NGF), while linckosides M–O appeared less active (21–32%), suggesting the importance of “the xylose on a side chain”. Ina et al. (2007) characterized pheophytin A (126) purified from the Japanese brown alga Sargassum fulvellum as a novel neurodifferentiation compound. Pheophytin A at 3.9 μg/mL was observed to synergize with NGF (50 ng/mL) in promoting neurite outgrowth in rat pheochromocytoma PC12 cells by a mechanism that appeared to involve activation of mitogen-activated protein kinase signaling.
As shown in Table 2, during 2007–8, three marine compounds (127, 128, 129) were reported to target receptors in the nervous system. Peng et al. (2008) reported on the preclinical pharmacology of the α4/7-conotoxin Lp1.1 (127) originally isolated from the marine cone snail Conus leopardus. The conotoxin induced seizure and paralysis in goldfish, and selectively yet reversibly inhibited acetylcholine-evoked currents in Xenopus oocytes expressing rat α6α3β2 and α3β2 nicotinic receptors (apparent IC50 less than 10 μM). Aiello et al. (2007) characterized two novel bromopyrrole alkaloids damipipecolin (128) and damituricin (129) isolated from the Mediterranean sponge Axinella damicornis that were observed to modulate serotonin receptor activity in vitro. Although damipipecolin inhibited Ca2+ entry in neurons (apparent IC50 = 1 μg/mL), the serotonin receptor subtype involved in the mechanism of action remains undetermined.
Finally, as shown in Table 2, during 2007–8, additional marine compounds (74 and 130, 131, 132) were reported to have miscellaneous effects on components of the nervous system. Contributing to the ongoing search for acetylcholinesterase inhibitors useful in the treatment of Alzheimer's disease, Langjae et al. (2007) reported a new stigmastane-type steroidal alkaloid 4-acetoxy-plakinamine B (130), isolated from a Thai marine sponge Corticium sp. that significantly inhibited acetylcholinesterase (IC50 = 3.75 μM). Compound 130 was reported to be the “first marine-derived acetylcholinesterase-inhibiting steroidal alkaloid” with a mechanism of action involving an unusual mixed-competitive mode of inhibition. Choi et al. (2007) extended the molecular pharmacology of two known meroditerpenes, sargaquinoic acid (131) and sargachromenol (132) isolated from the brown alga S. sagamianum. Sargaquinoic acid potently inhibited butyrylcholinesterase (IC50 = 26 nM), a novel target for the treatment of Alzheimer's disease, with potency comparable to or greater than anticholinesterases in current clinical use. Hui et al. (2008) further characterized the neuroprotective effects of the polysaccharide sulfated polymannuroguluronate (SPMG) (74), noted earlier as currently being in Phase II clinical trials in China as an anti-AIDS drug candidate. SPMG appeared to decrease the Tat-stimulated calcium overload in PC12 neuronal cells as well as concomitant apoptosis-signaling cascades, thus demonstrating a potential for further clinical development as a therapeutic intervention in HIV-associated dementia.
4. Marine compounds with miscellaneous mechanisms of action
Table 3 lists 65 marine compounds with miscellaneous pharmacological mechanisms of action, and their respective structures (133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197) are presented in Fig. 3. Because during 2007–8 additional pharmacological data were unavailable, it was not possible to assign these marine compounds to a particular drug class as was possible with the compounds included in Table 1, Table 2.
Table 3 shows 14 marine natural products that the peer-reviewed literature reported with a pharmacological activity, an IC50, and a molecular mechanism of action affected by the respective compound: azumamide E (133), 1-deoxyrubralactone (134), fucoxanthin (135), okadaic acid (136), saproxanthin and myxol (137, 138), sarcomilasterol (139), spirastrellolides C, D, and E (140, 141, 142), Spongia sesterterpenoid (143), stylissadines A and B (144, 145) and symbiodinolide (146).
In contrast, although a pharmacological activity was described, and an IC50 for inhibition of an enzyme or receptor determined, detailed molecular mechanism of action studies were unavailable at the time of publication for the following 51 marine compounds included in Table 3: Asterias amurensis saponin (147), Botrytis sp. α-pyrone derivative (148), cephalosporolides H and I (149, 150), chaetominedione (151), circumdatin I (152), diapolycopenedioic acid xylosyl ester (153), Echinogorgia complexa furanosesquiterpenes (154, 155), 19-epi-okadaic acid (156), erylosides F and F1 (157, 158), Hippocampus kuda phthalates (159, 160, 161), irregularasulfate (162), kempopeptins A and B (163, 164), linckoside L7 (165), lyngbyastatin 4 (166), malevamide E (167), monodictysin C (168), monodictyochromes A and B (169, 170), Penicillium waksmanii PF1270 A, B, and C (171, 172, 173), Penicillium sp. anisols (174, 175, 176), Polysiphonia urceolata bromophenols (177, 178, 179, 180), pompanopeptin A (181), purpurone (182), saliniketals A and B (183, 184), S. sagamianum monoglyceride (185), Sargassum siliquastrum meroditerpenoids (186, 187, 188, 189, 190, 191), Symphyocladia latiuscula bromophenols (192, 193, 194, 195), and tenacibactins C and D (196, 197).
5. Reviews on marine pharmacology
Several reviews covering both general and specific subject areas of marine pharmacology were published during 2007–8: (a) general marine pharmacology: marine natural products with an emphasis on source organisms and relevant biological activities (Blunt et al., 2007, Blunt et al., 2008); the value of natural products to future pharmaceutical discovery (Baker et al., 2007); the structures and bioactivities of secondary metabolites from cyanobacteria (Gademann and Portmann, 2008); bioactive natural products from marine cyanobacteria for drug discovery (Tan L.T., 2007); bioactive compounds from fungi in the South China Sea (Pan et al., 2008); biochemical and pharmacological functions of β-carboline alkaloids (Cao et al., 2007); pharmacological properties of lamellarin alkaloids (Kluza et al., 2008); biological activity of brown seaweed fucoidans (Kusaykin et al., 2008, Li et al., 2008a); potential pharmacological uses of the marine green alga polysaccharide ulvan (Lahaye and Robic, 2007); pharmacological properties of marine bis- and tris-indole alkaloids (Gupta et al., 2007); (b) antimicrobial marine pharmacology: a renaissance of genomics in antibacterial discovery from actinomycetes (Baltz R.H., 2008); mining marine genomics for novel drug discovery from phytosymbionts of marine invertebrates (Dunlap et al., 2007); (c) anticoagulant and cardiovascular pharmacology: structure, biology, evolution and medical importance of sulfated fucans and galactans as potential antithrombotic compounds (Pomin and Mourao, 2008); (d) antituberculosis, antimalarial and antifungal marine pharmacology: natural product growth inhibitors of Mycobacterium tuberculosis (Copp and Pearce, 2007); new advances in novel marine fatty acids as antimalarials, antimycobacterial and antifungal agents (Carballeira N.M., 2008); depsipeptides from microorganisms as a new class of antimalarials (Fotie and Morgan, 2008); the manzamine alkaloids for the control of malaria and tuberculosis (Hamann M.T., 2007); (e) immuno- and anti-inflammatory marine pharmacology: chemistry and biology of anti-inflammatory marine natural products (Abad et al., 2008); marine natural products as targeted modulators of the transcription factor NF-κB (Folmer et al., 2008); glycolipids as immunostimulating agents (Wu et al., 2008); (f) nervous system marine pharmacology: marine-derived drugs in neurology (Martinez A., 2007); neuritogenic gangliosides from marine echinoderms (Higuchi et al., 2007); (g) miscellaneous molecular targets: enzyme inhibitors from marine invertebrates (Nakao and Fusetani, 2007); natural products as aromatase inhibitors (Balunas et al., 2008); biology of the aeruginosin family of serine protease inhibitors (Ersmark et al., 2008); and marine natural products affecting membrane and intracellular processes, and ion channels (Folmer et al., 2007).
6. Conclusion
Six years after the approval of the marine compound ziconotide (Prialt®) by the U.S. Food and Drug Administration (Williams et al., 2008), the global marine preclinical pharmaceutical pipeline remains very active. The marine pharmaceutical clinical pipeline is available at http://marinepharmacology.midwestern.edu/clinDev.htm and has recently been reviewed (Mayer et al., 2010).
The current marine preclinical pipeline review contributes to the annual review series which was initiated in 1998 (Mayer and Lehmann, 2000, Mayer and Hamann, 2002, Mayer and Hamann, 2004, Mayer and Hamann, 2005, Mayer et al., 2007, Mayer et al., 2009) and reveals the breadth of preclinical pharmacological research during 2007–8, which resulted from the global effort of natural products chemists and pharmacologists from Argentina, Australia, Brazil, Canada, China, Egypt, France, Germany, India, Israel, Italy, Japan, the Netherlands, New Zealand, Panama, Papua New Guinea, Portugal, Russia, South Korea, Spain, Switzerland, Taiwan, Thailand, Turkey, United Kingdom, Vietnam, and the United States. Thus, it appears that the marine preclinical pharmaceutical pipeline continues to contribute novel pharmacological lead compounds that will probably enable a future expansion of the marine clinical pharmaceutical pipeline, which currently consists of 13 compounds in Phase I, II and III of clinical development (Mayer et al., 2010).
Acknowledgements
This review was made possible with financial support from Midwestern University to AMSM; NIH-SC1 Award (grant 1SC1GM086271-01A1) of the University of Puerto Rico to ADR; and FAPESP grant 05/60175-2 (São Paulo, Brazil) to RGSB. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Assistance with extensive searches of the 2007–8 marine pharmacology literature in PubMed, Marinlit, Current Contents® and Chemical Abstracts®, as well as article retrieval by library staff members, and students from the Chicago College of Pharmacy, Midwestern University, are gratefully acknowledged. The authors are especially thankful to Ms. Mary Hall for the careful review of the manuscript.
References
- Abad M.J., Bedoya L.M., Bermejo P. Natural marine anti-inflammatory products. Mini Rev. Med. Chem. 2008;8:740–754. doi: 10.2174/138955708784912148. [DOI] [PubMed] [Google Scholar]
- Abdel-Lateff A. Chaetominedione, a new tyrosine kinase inhibitor isolated from the algicolous marine fungus Chaetomium sp. Tetrahedron Lett. 2008;49:6398–6400. [Google Scholar]
- Adams B., Porzgen P., Pittman E., Yoshida W.Y., Westenburg H.E., Horgen F.D. Isolation and structure determination of malevamide E, a dolastatin 14 analogue, from the marine cyanobacterium Symploca laete-viridis. J. Nat. Prod. 2008;71:750–754. doi: 10.1021/np070346o. [DOI] [PubMed] [Google Scholar]
- Ahn G., Hwang I., Park E., Kim J., Jeon Y.J., Lee J., Park J.W., Jee Y. Immunomodulatory effects of an enzymatic extract from Ecklonia cava on murine splenocytes. Mar. Biotechnol. (NY) 2008;10:278–289. doi: 10.1007/s10126-007-9062-9. [DOI] [PubMed] [Google Scholar]
- Aiello A., Fattorusso E., Giordano A., Menna M., Müller W.E., Perovic-Ottstadt S., Schroder H.C. Damipipecolin and damituricin, novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella damicornis. Bioorg. Med. Chem. 2007;15:5877–5887. doi: 10.1016/j.bmc.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Aminin D.L., Agafonova I.G., Kalinin V.I., Silchenko A.S., Avilov S.A., Stonik V.A., Collin P.D., Woodward C. Immunomodulatory properties of frondoside A, a major triterpene glycoside from the North Atlantic commercially harvested sea cucumber Cucumaria frondosa. J. Med. Food. 2008;11:443–453. doi: 10.1089/jmf.2007.0530. [DOI] [PubMed] [Google Scholar]
- Antonov A.S., Kalinovsky A.I., Stonik V.A., Afiyatullov S.S., Aminin D.L., Dmitrenok P.S., Mollo E., Cimino G. Isolation and structures of erylosides from the Caribbean sponge Erylus formosus. J. Nat. Prod. 2007;70:169–178. doi: 10.1021/np060364q. [DOI] [PubMed] [Google Scholar]
- Artan M., Li Y., Karadeniz F., Lee S.H., Kim M.M., Kim S.K. Anti-HIV-1 activity of phloroglucinol derivative, 6, 6′-bieckol, from Ecklonia cava. Bioorg. Med. Chem. 2008;16:7921–7926. doi: 10.1016/j.bmc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Assreuy A.M., Gomes D.M., da Silva M.S., Torres V.M., Siqueira R.C., Pires A.F., Criddle D.N., de Alencar N.M., Cavada B.S., Sampaio A.H., Farias W.R. Biological effects of a sulfated-polysaccharide isolated from the marine red alga Champia feldmannii. Biol. Pharm. Bull. 2008;31:691–695. doi: 10.1248/bpb.31.691. [DOI] [PubMed] [Google Scholar]
- Baker D.D., Chu M., Oza U., Rajgarhia V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007;24:1225–1244. doi: 10.1039/b602241n. [DOI] [PubMed] [Google Scholar]
- Baltz R.H. Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 2008;8:557–563. doi: 10.1016/j.coph.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Balunas M.J., Su B., Brueggemeier R.W., Kinghorn A.D. Natural products as aromatase inhibitors. Anticancer Agents Med. Chem. 2008;8:646–682. [PMC free article] [PubMed] [Google Scholar]
- Berrue F., Thomas O.P., Laville R., Prado S., Golebiowski J., Fernandez R., Amade P. The marine sponge Plakortis zyggompha: a source of original bioactive polyketides. Tetrahedron. 2007;63:2328–2334. [Google Scholar]
- Bickmeyer U., Grube A., Klings K.W., Kock M. Disturbance of voltage-induced cellular calcium entry by marine dimeric and tetrameric pyrrole–imidazole alkaloids. Toxicon. 2007;50:490–497. doi: 10.1016/j.toxicon.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Bittencourt F.S., Figueiredo J.G., Mota M.R., Bezerra C.C., Silvestre P.P., Vale M.R., Nascimento K.S., Sampaio A.H., Nagano C.S., Saker-Sampaio S., Farias W.R., Cavada B.S., Assreuy A.M., de Alencar N.M. Antinociceptive and anti-inflammatory effects of a mucin-binding agglutinin isolated from the red marine alga Hypnea cervicornis. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377:139–148. doi: 10.1007/s00210-008-0262-2. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Hu W.P., Munro M.H., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2007;24:31–86. doi: 10.1039/b603047p. [DOI] [PubMed] [Google Scholar]
- Blunt J.W., Copp B.R., Hu W.P., Munro M.H., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2008;25:35–94. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- Boonlarppradab C., Faulkner D.J. Eurysterols A and B, cytotoxic and antifungal steroidal sulfates from a marine sponge of the genus Euryspongia. J. Nat. Prod. 2007;70:846–848. doi: 10.1021/np060472c. [DOI] [PubMed] [Google Scholar]
- Bredholt H., Fjaervik E., Johnsen G., Zotchev S.B. Actinomycetes from sediments in the Trondheim fjord, Norway: diversity and biological activity. Mar. Drugs. 2008;6:12–24. [PMC free article] [PubMed] [Google Scholar]
- Cai M., Sugumaran M., Robinson W.E. The crosslinking and antimicrobial properties of tunichrome. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;151:110–117. doi: 10.1016/j.cbpb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Cao R., Peng W., Wang Z., Xu A. β-Carboline alkaloids: biochemical and pharmacological functions [Review] Curr. Med. Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- Carballeira N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008;47:50–61. doi: 10.1016/j.plipres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr G., Raszek M., van Soest R., Matainaho T., Shopik M., Holmes C.F., Andersen R.J. Protein phosphatase inhibitors isolated from Spongia irregularis collected in Papua New Guinea. J. Nat. Prod. 2007;70:1812–1815. doi: 10.1021/np0702887. [DOI] [PubMed] [Google Scholar]
- Chang H.W., Jang K.H., Lee D., Kang H.R., Kim T.Y., Lee B.H., Choi B.W., Kim S., Shin J. Monoglycerides from the brown alga Sargassum sagamianum: Isolation, synthesis, and biological activity. Bioorg. Med. Chem. Lett. 2008;18:3589–3592. doi: 10.1016/j.bmcl.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Chao C.H., Wen Z.H., Wu Y.C., Yeh H.C., Sheu J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008;71:1819–1824. doi: 10.1021/np8004584. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay K., Ghosh T., Pujol C.A., Carlucci M.J., Damonte E.B., Ray B. Polysaccharides from Gracilaria corticata: sulfation, chemical characterization and anti-HSV activities. Int. J. Biol. Macromol. 2008;43:346–351. doi: 10.1016/j.ijbiomac.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Cheng S., Wen Z., Chiou S., Hsu C., Wang S., Dai C., Chiang M.Y., Duh C. Durumolides A–E, anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron. 2008;64:9698–9704. [Google Scholar]
- Choi B.W., Ryu G., Park S.H., Kim E.S., Shin J., Roh S.S., Shin H.C., Lee B.H. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: lead compounds for Alzheimer's disease therapy. Phytother. Res. 2007;21:423–426. doi: 10.1002/ptr.2090. [DOI] [PubMed] [Google Scholar]
- Ciavatta M.L., Gresa M.P.L., Gavagnin M., Romero V., Melck D., Manzo E., Guo Y.W., van Soest R., Cimino G. Studies on puupehenone-metabolites of a Dysidea sp.: structure and biological activity. Tetrahedron. 2007;63:1380–1384. [Google Scholar]
- Cirne-Santos C.C., Souza T.M., Teixeira V.L., Fontes C.F., Rebello M.A., Castello-Branco L.R., Abreu C.M., Tanuri A., Frugulhetti I.C., Bou-Habib D.C. The dolabellane diterpene dolabelladienetriol is a typical noncompetitive inhibitor of HIV-1 reverse transcriptase enzyme. Antiviral Res. 2008;77:64–71. doi: 10.1016/j.antiviral.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Clark B.R., Engene N., Teasdale M.E., Rowley D.C., Matainaho T., Valeriote F.A., Gerwick W.H. Natural products chemistry and taxonomy of the marine cyanobacterium Blennothrix cantharidosmum. J. Nat. Prod. 2008;71:1530–1537. doi: 10.1021/np800088a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp B.R., Pearce A.N. Natural product growth inhibitors of Mycobacterium tuberculosis. Nat. Prod. Rep. 2007;24:278–297. doi: 10.1039/b513520f. [DOI] [PubMed] [Google Scholar]
- Courtois A., Simon-Colin C., Boisset C., Berthou C., Deslandes E., Guezennec J., Bordron A. Floridoside extracted from the red alga Mastocarpus stellatus is a potent activator of the classical complement pathway. Mar. Drugs. 2008;6:407–417. doi: 10.3390/md20080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz P.G., Daranas A.H., Fernandez J.J., Norte M. 19-epi-okadaic acid, a novel protein phosphatase inhibitor with enhanced selectivity. Org. Lett. 2007;9:3045–3048. doi: 10.1021/ol071099i. [DOI] [PubMed] [Google Scholar]
- D'Auria M.V., Sepe V., D'Orsi R., Bellotta F., Debitus C., Zampella A. Isolation and structural elucidation of callipeltins J-M: antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron. 2007;63:131–140. [Google Scholar]
- Dang H.T., Lee H.J., Yoo E.S., Shinde P.B., Lee Y.M., Hong J., Kim D.K., Jung J.H. Anti-inflammatory constituents of the red alga Gracilaria verrucosa and their synthetic analogues. J. Nat. Prod. 2008;71:232–240. doi: 10.1021/np070452q. [DOI] [PubMed] [Google Scholar]
- Das P., Mukherjee S., Sen R. Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J. Appl. Microbiol. 2008;104:1675–1684. doi: 10.1111/j.1365-2672.2007.03701.x. [DOI] [PubMed] [Google Scholar]
- de Lira S.P., Seleghim M.H., Williams D.E., Marion F., Hamill P., Jean F., Andersen R.J., Hajdu E., Berlinck R.G. A SARS-coronovirus 3CL protease inhibitor isolated from the marine sponge Axinella cf. corrugata: structure elucidation and synthesis. J. Braz. Chem. Soc. 2007;18:440–443. [Google Scholar]
- Desbois A.P., Lebl T., Yan L., Smith V.J. Isolation and structural characterization of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2008;81:755–764. doi: 10.1007/s00253-008-1714-9. [DOI] [PubMed] [Google Scholar]
- Desjardine K., Pereira A., Wright H., Matainaho T., Kelly M., Andersen R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brevibacillus laterosporus isolated from a marine habitat: structure elucidation and synthesis. J. Nat. Prod. 2007;70:1850–1853. doi: 10.1021/np070209r. [DOI] [PubMed] [Google Scholar]
- Devi K.P., Suganthy N., Kesika P., Pandian S.K. Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008;8:38. doi: 10.1186/1472-6882-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Lu C., Li Y., Zheng Z., Su W., Shen Y. Three new antimicrobial metabolites of Phomopsis sp. J. Antibiot. (Tokyo) 2008;61:250–253. doi: 10.1038/ja.2008.37. [DOI] [PubMed] [Google Scholar]
- Duan X.J., Li X.M., Wang B.G. Highly brominated mono- and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007;70:1210–1213. doi: 10.1021/np070061b. [DOI] [PubMed] [Google Scholar]
- Dunlap W.C., Battershill C.N., Liptrot C.H., Cobb R.E., Bourne D.G., Jaspars M., Long P.F., Newman D.J. Biomedicinals from the phytosymbionts of marine invertebrates: a molecular approach. Methods. 2007;42:358–376. doi: 10.1016/j.ymeth.2007.03.001. [DOI] [PubMed] [Google Scholar]
- El Sayed K.A., Yousaf M., Labadie G., Kumar G.M., Franzblau S., Mayer A.M.S., Avery M., Hamann M.T. Semisynthetic studies on the manzamine alkaloids. J. Nat. Prod. 2008;71:300–308. doi: 10.1021/np0703702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Beih A.A., Kato H., Tsukamoto S., Ohta T. CYP3A4 inhibitors isolated from a marine derived fungus Penicillium species. J. Nat. Med. 2007;61:175–177. [Google Scholar]
- El-Gendy M.M., Shaaban M., Shaaban K.A., El-Bondkly A.M., Laatsch H. Essramycin: a first triazolopyrimidine antibiotic isolated from nature. J. Antibiot. (Tokyo) 2008;61:149–157. doi: 10.1038/ja.2008.124. [DOI] [PubMed] [Google Scholar]
- El-Gendy M.M., Hawas U.W., Jaspars M. Novel bioactive metabolites from a marine derived bacterium Nocardia sp. ALAA 2000. J. Antibiot. (Tokyo) 2008;61:379–386. doi: 10.1038/ja.2008.53. [DOI] [PubMed] [Google Scholar]
- Ersmark K., Del Valle J.R., Hanessian S. Chemistry and biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed Engl. 2008;47:1202–1223. doi: 10.1002/anie.200605219. [DOI] [PubMed] [Google Scholar]
- Fischbach M.A., Walsh C.T. Antibiotics for emerging pathogens. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer F., Houssen W.E., Scott R.H., Jaspars M. Biomedical research tools from the seabed. Curr. Opin. Drug Discov. Dev. 2007;10:145–152. [PubMed] [Google Scholar]
- Folmer F., Jaspars M., Dicato M., Diederich M. Marine natural products as targeted modulators of the transcription factor NF-κB. Biochem. Pharmacol. 2008;75:603–617. doi: 10.1016/j.bcp.2007.07.044. [DOI] [PubMed] [Google Scholar]
- Fotie J., Morgan R.E. Depsipeptides from microorganisms: a new class of antimalarials. Mini Rev. Med. Chem. 2008;8:1088–1094. doi: 10.2174/138955708785909916. [DOI] [PubMed] [Google Scholar]
- Freile-Pelegrin Y., Robledo D., Chan-Bacab M.J., Ortega-Morales B.O. Antileishmanial properties of tropical marine algae extracts. Fitoterapia. 2008;79:374–377. doi: 10.1016/j.fitote.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Gademann K., Portmann C. Secondary metabolites from cyanobacteria: complex structures and powerful bioactivities. Curr. Org. Chem. 2008;12:326–341. [Google Scholar]
- Gaspar H., Santos S., Carbone M., Rodrigues A.S., Rodrigues A.I., Uriz M.J., Savluchinske Feio S.M., Melck D., Humanes M., Gavagnin M. Isomeric furanosesquiterpenes from the Portuguese marine sponge Fasciospongia sp. J. Nat. Prod. 2008;71:2049–2052. doi: 10.1021/np800346c. [DOI] [PubMed] [Google Scholar]
- Gowda N.M., Goswami U., Khan M.I. T-antigen binding lectin with antibacterial activity from marine invertebrate, sea cucumber (Holothuria scabra): possible involvement in differential recognition of bacteria. J. Invertebr. Pathol. 2008;99:141–145. doi: 10.1016/j.jip.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Greve H., Meis S., Kassack M.U., Kehraus S., Krick A., Wright A.D., Konig G.M. New iantherans from the marine sponge Ianthella quadrangulata: novel agonists of the P2Y(11) receptor. J. Med. Chem. 2007;50:5600–5607. doi: 10.1021/jm070043r. [DOI] [PubMed] [Google Scholar]
- Grube A., Assmann M., Lichte E., Sasse F., Pawlik J.R., Kock M. Bioactive metabolites from the Caribbean sponge Aka coralliphagum. J. Nat. Prod. 2007;70:504–509. doi: 10.1021/np0603018. [DOI] [PubMed] [Google Scholar]
- Gul W., Hammond N.L., Yousaf M., Peng J., Holley A., Hamann M.T. Chemical transformation and biological studies of marine sesquiterpene (S)-(+)-curcuphenol and its analogs. Biochim. Biophys. Acta. 2007;1770:1513–1519. doi: 10.1016/j.bbagen.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta L., Talwar A., Chauhan P.M. Bis- and tris-indole alkaloids from marine organisms: new leads for drug discovery. Curr. Med. Chem. 2007;14:1789–1803. doi: 10.2174/092986707781058904. [DOI] [PubMed] [Google Scholar]
- Hamann M.T. The manzamines as an example of the unique structural classes available for the discovery and optimization of infectious disease controls based on marine natural products. Curr. Pharm. Des. 2007;13:653–660. doi: 10.2174/138161207780162818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Qi J., Ojika M. Linckosides M–Q: neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. J. Nat. Med. 2007;61:138–145. [Google Scholar]
- Hanif N., Tanaka J., Setiawan A., Trianto A., de Voogd N.J., Murni A., Tanaka C., Higa T. Polybrominated diphenyl ethers from the Indonesian sponge Lamellodysidea herbacea. J. Nat. Prod. 2007;70:432–435. doi: 10.1021/np0605081. [DOI] [PubMed] [Google Scholar]
- He Y., Liu C., Chen Y., Ji A., Shen Z., Xi T., Yao Q. Isolation and structural characterization of a novel polysaccharide prepared from Arca subcrenata Lischke. J. Biosci. Bioeng. 2007;104:111–116. doi: 10.1263/jbb.104.111. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Inagaki M., Yamada K., Miyamoto T. Biologically active gangliosides from echinoderms. J. Nat. Med. 2007;61:367–370. [Google Scholar]
- Horie S., Tsutsumi S., Takada Y., Kimura J. Antibacterial quinone metabolite from the brown alga, Sargassum sagamianum. Bull. Chem. Soc. Jpn. 2008;81:1125–1130. [Google Scholar]
- Hua H.M., Peng J., Dunbar D.C., Schinazi R.F., de Castro Andrews A.G., Cuevas C., Garcia-Fernandez L.F., Kelly M., Hamann M.T. Batzelladine alkaloids from the Caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron. 2007;63:11179–11188. [Google Scholar]
- Huang P.H., Chen J.Y., Kuo C.M. Three different hepcidins from tilapia, Oreochromis mossambicus: analysis of their expressions and biological functions. Mol. Immunol. 2007;44:1922–1934. doi: 10.1016/j.molimm.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Hughes C.C., Prieto-Davo A., Jensen P.R., Fenical W. The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org. Lett. 2008;10:629–631. doi: 10.1021/ol702952n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J.E., Yim J.H., Lee H.K., Moon E.Y., Rhee D.K., Pyo S. Prodigiosin isolated from Hahella chejuensis suppresses lipopolysaccharide-induced NO production by inhibiting p38 MAPK, JNK and NF-kappaB activation in murine peritoneal macrophages. Int. Immunopharmacol. 2007;7:1825–1833. doi: 10.1016/j.intimp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Hui B., Li J., Geng M.Y. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome drug candidate, decreased vulnerability of PC12 cells to human immunodeficiency virus tat protein through attenuating calcium overload. J. Neurosci. Res. 2008;86:1169–1177. doi: 10.1002/jnr.21566. [DOI] [PubMed] [Google Scholar]
- Ina A., Hayashi K., Nozaki H., Kamei Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007;25:63–68. doi: 10.1016/j.ijdevneu.2006.09.323. [DOI] [PubMed] [Google Scholar]
- Jang J.H., Kanoh K., Adachi K., Matsuda S., Shizuri Y. Tenacibactins A–D, hydroxamate siderophores from a marine-derived bacterium, Tenacibaculum sp. A4K-17. J. Nat. Prod. 2007;70:563–566. doi: 10.1021/np060502b. [DOI] [PubMed] [Google Scholar]
- Jang J.H., van Soest R.W., Fusetani N., Matsunaga S. Pseudoceratins A and B, antifungal bicyclic bromotyrosine-derived metabolites from the marine sponge Pseudoceratina purpurea. J. Org. Chem. 2007;72:1211–1217. doi: 10.1021/jo062010+. [DOI] [PubMed] [Google Scholar]
- Jang K.H., Chung S.C., Shin J., Lee S.H., Kim T.I., Lee H.S., Oh K.B. Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorg. Med. Chem. Lett. 2007;17:5366–5369. doi: 10.1016/j.bmcl.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Jiang R.W., Lane A.L., Mylacraine L., Hardcastle K.I., Fairchild C.R., Aalbersberg W., Hay M.E., Kubanek J. Structures and absolute configurations of sulfate-conjugated triterpenoids including an antifungal chemical defense of the green macroalga Tydemania expeditionis. J. Nat. Prod. 2008;71:1616–1619. doi: 10.1021/np800307h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W.K., Athukorala Y., Cha S.H., Lee C.H., Vasanthan T., Choi K.S., Yoo S.H., Kim S.K., Jeon Y.J. Sulfated polysaccharide purified from Ecklonia cava accelerates antithrombin III-mediated plasma proteinase inhibition. J. Appl. Phycol. 2007;19:425–430. [Google Scholar]
- Jung W.K., Jo H.Y., Qian Z.J., Jeong Y.J., Park S.G., Choi I.W., Kim S.K. A novel anticoagulant protein with high affinity to blood coagulation factor Va from Tegillarca granosa. J. Biochem. Mol. Biol. 2007;40:832–838. doi: 10.5483/bmbrep.2007.40.5.832. [DOI] [PubMed] [Google Scholar]
- Jung M., Jang K.H., Kim B., Lee B.H., Choi B.W., Oh K.B., Shin J. Meroditerpenoids from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2008;71:1714–1719. doi: 10.1021/np800321y. [DOI] [PubMed] [Google Scholar]
- Kanoh K., Adachi K., Matsuda S., Shizuri Y., Yasumoto K., Kusumi T., Okumura K., Kirikae T. New Sulfoalkylresorcinol from marine-derived fungus, Zygosporium sp. KNC52. J. Antibiot. (Tokyo) 2008;61:192–194. doi: 10.1038/ja.2008.29. [DOI] [PubMed] [Google Scholar]
- Kanoh K., Okada A., Adachi K., Imagawa H., Nishizawa M., Matsuda S., Shizuri Y., Utsumi R. Ascochytatin, a novel bioactive spirodioxynaphthalene metabolite produced by the marine-derived fungus, Ascochyta sp. NGB4. J. Antibiot. (Tokyo) 2008;61:142–148. doi: 10.1038/ja.2008.123. [DOI] [PubMed] [Google Scholar]
- Karabay-Yavasoglu N.U., Sukatar A., Ozdemir G., Horzum Z. Antimicrobial activity of volatile components and various extracts of the red alga Jania rubens. Phytother. Res. 2007;21:153–156. doi: 10.1002/ptr.2045. [DOI] [PubMed] [Google Scholar]
- Kasettrathat C., Ngamrojanavanich N., Wiyakrutta S., Mahidol C., Ruchirawat S., Kittakoop P. Cytotoxic and antiplasmodial substances from marine-derived fungi, Nodulisporium sp. and CRI247-01. Phytochemistry. 2008;69:2621–2626. doi: 10.1016/j.phytochem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Kawauchi K., Tobiume K., Kaneko S., Kaneshiro K., Okamoto S., Ueda E., Kamata H., Moriyama Y., Hirata H. Suppression of AP-1 activity by cycloprodigiosin hydrochloride. Biol. Pharm. Bull. 2007;30:1792–1795. doi: 10.1248/bpb.30.1792. [DOI] [PubMed] [Google Scholar]
- Khan M.N., Cho J.Y., Lee M.C., Kang J.Y., Park N.G., Fujii H., Hong Y.K. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J. Agric. Food Chem. 2007;55:6984–6988. doi: 10.1021/jf071791s. [DOI] [PubMed] [Google Scholar]
- Kicha A.A., Ivanchina N.V., Kalinovskii A.I., Dmitrenok P.S., Sokolova E.V., Agafonova I.G. Sulfated steroid glycosides from the Vietnamese starfish Linckia laevigata. Chem. Nat. Cmpd. 2007;43:76–80. [Google Scholar]
- Kicha A.A., Ivanchina N.V., Kalinovsky A.I., Dmitrenok P.S., Palyanova N.V., Pankova T.M., Starostina M.V., Gavagnin M., Stonik V.A. New neuritogenic teroid glycosides from the Vietnamese starfish Linckia laevigata. Nat. Prod. Commun. 2007;2:41–46. [Google Scholar]
- Kita M., Ohishi N., Konishi K., Kondo M., Koyama T., Kitamura M., Yamada K., Uemura D. Symbiodinolide, a novel polyol macrolide that activates N-type Ca2+ channel, from the symbiotic marine dinoflagellate Symbiodinium sp. Tetrahedron. 2007;63:6241–6251. [Google Scholar]
- Kitani Y., Tsukamoto C., Zhang G., Nagai H., Ishida M., Ishizaki S., Shimakura K., Shiomi K., Nagashima Y. Identification of an antibacterial protein as l-amino acid oxidase in the skin mucus of rockfish Sebastes schlegeli. FEBS J. 2007;274:125–136. doi: 10.1111/j.1742-4658.2006.05570.x. [DOI] [PubMed] [Google Scholar]
- Kitani Y., Kikuchi N., Zhang G., Ishizaki S., Shimakura K., Shiomi K., Nagashima Y. Antibacterial action of l-amino acid oxidase from the skin mucus of rockfish Sebastes schlegelii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;149:394–400. doi: 10.1016/j.cbpb.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Kluza J., Marchetti P., Bailly C. Lamellarin alkaloids: structure and pharmacological properties. In: Fattorusso E., Taglialatela-Scafati O., editors. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Wiley-VCH Verlag & Co.; Weinheim: 2008. pp. 171–187. [Google Scholar]
- Kobayashi H., Kitamura K., Nagai K., Nakao Y., Fusetani N., van Soest R.W.M., Matsunaga S. Carteramine A, an inhibitor of neutrophil chemotaxis, from the marine sponge Stylissa carteri. Tetrahedron Lett. 2007;48:2127–2129. [Google Scholar]
- Kobayashi H., Ohashi J., Fujita T., Iwashita T., Nakao Y., Matsunaga S., Fusetani N. Complete structure elucidation of shishididemniols, complex lipids with tyramine-derived tether and two serinol units, from a marine tunicate of the family Didemnidae. J. Org. Chem. 2007;72:1218–1225. doi: 10.1021/jo062013m. [DOI] [PubMed] [Google Scholar]
- Kontiza L., Stavri M., Zloh M., Vagias C., Gibbons S., Roussis V. New metabolites with antibacterial activity from the marine angiosperm Cymodocea nodosa. Tetrahedron. 2008;64:1696–1702. [Google Scholar]
- Kossuga M.H., Nascimento A.M., Reimao J.Q., Tempone A.G., Taniwaki N.N., Veloso K., Ferreira A.G., Cavalcanti B.C., Pessoa C., Moraes M.O., Mayer A.M., Hajdu E., Berlinck R.G. Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J. Nat. Prod. 2008;71:334–339. doi: 10.1021/np0705256. [DOI] [PubMed] [Google Scholar]
- Krick A., Kehraus S., Gerhauser C., Klimo K., Nieger M., Maier A., Fiebig H.H., Atodiresei I., Raabe G., Fleischhauer J., Konig G.M. Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 2007;70:353–360. doi: 10.1021/np060505o. [DOI] [PubMed] [Google Scholar]
- Kubota T., Araki A., Ito J., Mikami Y., Fromont J., Kobayashi J. Nagelamides M and N, new bromopyrrole alkaloids from sponge Agelas species. Tetrahedron. 2008;64:10810–10813. doi: 10.1021/ol8003904. [DOI] [PubMed] [Google Scholar]
- Kumar R., Chaturvedi A.K., Shukla P.K., Lakshmi V. Antifungal activity in triterpene glycosides from the sea cucumber Actinopyga lecanora. Bioorg. Med. Chem. Lett. 2007;17:4387–4391. doi: 10.1016/j.bmcl.2006.12.052. [DOI] [PubMed] [Google Scholar]
- Kunze B., Bohlendorf B., Reichenbach H., Hofle G. Pedein A and B: production, isolation, structure elucidation and biological properties of new antifungal cyclopeptides from Chondromyces pediculatus (Myxobacteria) J. Antibiot. (Tokyo) 2008;61:18–26. doi: 10.1038/ja.2008.104. [DOI] [PubMed] [Google Scholar]
- Kusaykin M., Bakunina I., Sova V., Ermakova S., Kuznetsova T., Besednova N., Zaporozhets T., Zvyagintseva T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008;3:904–915. doi: 10.1002/biot.200700054. [DOI] [PubMed] [Google Scholar]
- Kushida N., Watanabe N., Okuda T., Yokoyama F., Gyobu Y., Yaguchi T. PF1270A, B and C, novel histamine H3 receptor ligands produced by Penicillium waksmanii PF1270. J. Antibiot. (Tokyo) 2007;60:667–673. doi: 10.1038/ja.2007.85. [DOI] [PubMed] [Google Scholar]
- Lahaye M., Robic A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 2007;8:1765–1774. doi: 10.1021/bm061185q. [DOI] [PubMed] [Google Scholar]
- Lane A.L., Stout E.P., Hay M.E., Prusak A.C., Hardcastle K., Fairchild C.R., Franzblau S.G., Le Roch K., Prudhomme J., Aalbersberg W., Kubanek J. Callophycoic acids and callophycols from the Fijian red alga Callophycus serratus. J. Org. Chem. 2007;72:7343–7351. doi: 10.1021/jo071210y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langjae R., Bussarawit S., Yuenyongsawad S., Ingkaninan K., Plubrukarn A. Acetylcholinesterase-inhibiting steroidal alkaloid from the sponge Corticium sp. Steroids. 2007;72:682–685. doi: 10.1016/j.steroids.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Lee H.S., Lee T.H., Lee J.H., Chae C.S., Chung S.C., Shin D.S., Shin J., Oh K.B. Inhibition of the pathogenicity of Magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga Odonthalia corymbifera. J. Agric. Food Chem. 2007;55:6923–6928. doi: 10.1021/jf071125r. [DOI] [PubMed] [Google Scholar]
- Lee J.U., Kang D.I., Zhu W.L., Shin S.Y., Hahm K.S., Kim Y. Solution structures and biological functions of the antimicrobial peptide, arenicin-1, and its linear derivative. Biopolymers. 2007;88:208–216. doi: 10.1002/bip.20700. [DOI] [PubMed] [Google Scholar]
- Lee D., Shin J., Yoon K.M., Kim T., Lee S.H., Lee H.S., Oh K.B. Inhibition of Candida albicans isocitrate lyase activity by sesterterpene sulfates from the tropical sponge Dysidea sp. Bioorg. Med. Chem. Lett. 2008;18:5377–5380. doi: 10.1016/j.bmcl.2008.09.059. [DOI] [PubMed] [Google Scholar]
- Li K., Li X.M., Ji N.Y., Wang B.G. Natural bromophenols from the marine red alga Polysiphonia urceolata (Rhodomelaceae): structural elucidation and DPPH radical-scavenging activity. Bioorg. Med. Chem. 2007;15:6627–6631. doi: 10.1016/j.bmc.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Li X., Yao Y., Zheng Y., Sattler I., Lin W. Cephalosporolides H and I, Two Novel Lactones from a Marine-Derived Fungus, Penicillium sp. Arch. Pharm. Res. 2007;30:812–815. doi: 10.1007/BF02978829. [DOI] [PubMed] [Google Scholar]
- Li B., Lu F., Wei X., Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Li X.M., Ji N.Y., Wang B.G. Bromophenols from the marine red alga Polysiphonia urceolata with DPPH radical scavenging activity. J. Nat. Prod. 2008;71:28–30. doi: 10.1021/np070281p. [DOI] [PubMed] [Google Scholar]
- Li Y., Qian Z.J., Kim S.K. Cathepsin B inhibitory activities of three new phthalate derivatives isolated from seahorse, Hippocampus Kuda Bleeler. Bioorg. Med. Chem. Lett. 2008;18:6130–6134. doi: 10.1016/j.bmcl.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Linington R.G., Gonzalez J., Urena L.D., Romero L.I., Ortega-Barria E., Gerwick W.H. Venturamides A and B: antimalarial constituents of the Panamanian marine cyanobacterium Oscillatoria sp. J. Nat. Prod. 2007;70:397–401. doi: 10.1021/np0605790. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Li J.K., Zhang D.W., Zhang J.C., Wang N.L., Cai G.P., Yao X.S. Two new steroidal compounds from starfish Asterias amurensis Lutken. J. Asian Nat. Prod. Res. 2008;10:521–529. doi: 10.1080/10286020801966674. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ji H., Dong J., Zhang S., Lee K.J., Matthew S. Antioxidant alkaloid from the South China Sea marine sponge Iotrochota sp. Z. Naturforsch. C. 2008;63:636–638. doi: 10.1515/znc-2008-9-1003. [DOI] [PubMed] [Google Scholar]
- Lu C.X., Li J., Sun Y.X., Qi X., Wang Q.J., Xin X.L., Geng M.Y. Sulfated polymannuroguluronate, a novel anti-AIDS drug candidate, inhibits HIV-1 Tat-induced angiogenesis in Kaposi's sarcoma cells. Biochem. Pharmacol. 2007;74:1330–1339. doi: 10.1016/j.bcp.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Lu Y., Huang C.Y., Lin Y.F., Wen Z.H., Su J.H., Kuo Y.H., Chiang M.Y., Sheu J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008;71:1754–1759. doi: 10.1021/np8003563. [DOI] [PubMed] [Google Scholar]
- Macherla V.R., Liu J., Sunga M., White D.J., Grodberg J., Teisan S., Lam K.S., Potts B.C. Lipoxazolidinones A, B, and C: antibacterial 4-oxazolidinones from a marine actinomycete isolated from a Guam marine sediment. J. Nat. Prod. 2007;70:1454–1457. doi: 10.1021/np0702032. [DOI] [PubMed] [Google Scholar]
- Mandal P., Pujol C.A., Carlucci M.J., Chattopadhyay K., Damonte E.B., Ray B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry. 2008;69:2193–2199. doi: 10.1016/j.phytochem.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Manzo E., Ciavatta M.L., Gresa M.P.L., Gavagnin M., Villani G., Naik C.G., Cimino G. New bioactive hydrogenated linderazulene-derivatives from the gorgonian Echinogorgia complexa. Tetrahedron Lett. 2007;48:2569–2571. [Google Scholar]
- Mao W., Fang F., Li H., Qi X., Sun H., Chen Y., Guo S. Heparinoid-active two sulfated polysaccharides isolated from marine green algae Monostroma nitidum. Carbohydr. Polym. 2008;74:834–839. [Google Scholar]
- Martinez A. Marine-derived drugs in neurology. Curr. Opin. Investig. Drugs. 2007;8:525–530. [PubMed] [Google Scholar]
- Martins R.F., Ramos M.F., Herfindal L., Sousa J.A., Skaerven K., Vasconcelos V.M. Antimicrobial and cytotoxic assessment of marine cyanobacteria — Synechocystis and Synechococcus. Mar. Drugs. 2008;6:1–11. [PMC free article] [PubMed] [Google Scholar]
- Matthew S., Ross C., Rocca J.R., Paul V.J., Luesch H. Lyngbyastatin 4, a dolastatin 13 analogue with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2007;70:124–127. doi: 10.1021/np060471k. [DOI] [PubMed] [Google Scholar]
- Matthew S., Ross C., Paul V., Luesch H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron. 2008;64:4081–4089. [Google Scholar]
- Maulucci N., Chini M.G., Di Micco S., Izzo I., Cafaro E., Russo A., Gallinari P., Paolini C., Nardi M.C., Casapullo A., Riccio R., Bifulco G., De Riccardis F. Molecular insights into azumamide E histone deacetylases inhibitory activity. J. Am. Chem. Soc. 2007;129:3007–3012. doi: 10.1021/ja0686256. [DOI] [PubMed] [Google Scholar]
- Mayer A.M.S. Marine Pharmacology in 1998 Antitumor and Cytotoxic Compounds. The Pharmacologist. 1999;41:159–164. [Google Scholar]
- Mayer A.M.S., Gustafson K.R. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int. J. Cancer. 2003;105:291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- Mayer A.M.S., Gustafson K.R. Marine pharmacology in 2001–2: antitumour and cytotoxic compounds. Eur. J. Cancer. 2004;40:2676–2704. doi: 10.1016/j.ejca.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Mayer A.M.S., Gustafson K.R. Marine pharmacology in 2003–2004: anti-tumour and cytotoxic compounds. Eur. J. Cancer. 2006;42:2241–2270. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Mayer A.M.S., Gustafson K.R. Marine pharmacology in 2005–2006: antitumour and cytotoxic compounds. Eur. J. Cancer. 2008;44:2357–2387. doi: 10.1016/j.ejca.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.M.S., Hamann M.T. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities;affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2002;132:315–339. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- Mayer A.M.S., Hamann M.T. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. (NY) 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.M.S., Hamann M.T. Marine pharmacology in 2001–2002: marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005;140:265–286. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.M.S., Lehmann V.K.B. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. The Pharmacologist. 2000;42:62–69. [Google Scholar]
- Mayer A.M.S., Lehmann V.K.B. Marine pharmacology in 1999: antitumor and cytotoxic compounds. Anticancer Res. 2001;21:2489–2500. [PubMed] [Google Scholar]
- Mayer A.M.S., Rodriguez A.D., Berlinck R.G., Hamann M.T. Marine pharmacology in 2003–4: marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;145:553–581. doi: 10.1016/j.cbpc.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.M.S., Rodriguez A.D., Berlinck R.G., Hamann M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta. 2009;1790:283–308. doi: 10.1016/j.bbagen.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.M.S., Glaser K.B., Cuevas C., Jacobs R.S., Kem W., Little R.D., McIntosh J.M., Newman D.J., Potts B.C., Shuster D.E. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol. Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- McArthur K.A., Mitchell S.S., Tsueng G., Rheingold A., White D.J., Grodberg J., Lam K.S., Potts B.C. Lynamicins A–E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J. Nat. Prod. 2008;71:1732–1737. doi: 10.1021/np800286d. [DOI] [PubMed] [Google Scholar]
- McPhail K.L., Correa J., Linington R.G., Gonzalez J., Ortega-Barria E., Capson T.L., Gerwick W.H. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007;70:984–988. doi: 10.1021/np0700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moo-Puc R., Robledo D., Freile-Pelegrin Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2008;120:92–97. doi: 10.1016/j.jep.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Na M., Meujo D.A.F., Kevin D., Hamann M.T., Anderson M., Hill R.T. A new antimalarial polyether from a marine Streptomyces sp. H668. Tetrahedron Lett. 2008;49:6282–6285. doi: 10.1016/j.tetlet.2008.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma M., Nishida M., Kuramochi K., Sugawara F., Yoshida H., Mizushina Y. 1-deoxyrubralactone, a novel specific inhibitor of families X and Y of eukaryotic DNA polymerases from a fungal strain derived from sea algae. Bioorg. Med. Chem. 2008;16:2939–2944. doi: 10.1016/j.bmc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- Nair R., Chabhadiya R., Chanda S. Marine algae: screening for a potent antibacterial agent. J. Herb. Pharmacother. 2007;7:73–86. [PubMed] [Google Scholar]
- Nakao Y., Fusetani N. Enzyme inhibitors from marine invertebrates. J. Nat. Prod. 2007;70:689–710. doi: 10.1021/np060600x. [DOI] [PubMed] [Google Scholar]
- Nam S.J., Ko H., Ju M.K., Hwang H., Chin J., Ham J., Lee B., Lee J., Won D.H., Choi H., Ko J., Shin K., Oh T., Kim S., Rho J.R., Kang H. Scalarane sesterterpenes from a marine sponge of the genus Spongia and their FXR antagonistic activity. J. Nat. Prod. 2007;70:1691–1695. doi: 10.1021/np070024k. [DOI] [PubMed] [Google Scholar]
- Nguyen H.P., Zhang D., Lee U., Kang J.S., Choi H.D., Son B.W. Dehydroxychlorofusarielin B, an antibacterial polyoxygenated decalin derivative from the marine-derived fungus Aspergillus sp. J. Nat. Prod. 2007;70:1188–1190. doi: 10.1021/np060552g. [DOI] [PubMed] [Google Scholar]
- Oda T., Wang W., Fujita A., Mochizuki M., Ukai K., Namikoshi M. Promotion of IL-8 production in PMA-stimulated HL-60 cells by sesquiterpene quinones from a marine sponge, Hippospongia sp. J. Nat. Med. 2007;61:434–437. [Google Scholar]
- Oh D.C., Strangman W.K., Kauffman C.A., Jensen P.R., Fenical W. Thalassospiramides A and B, immunosuppressive peptides from the marine bacterium Thalassospira sp. Org. Lett. 2007;9:1525–1528. doi: 10.1021/ol070294u. [DOI] [PubMed] [Google Scholar]
- Oh D.C., Gontang E.A., Kauffman C.A., Jensen P.R., Fenical W. Salinipyrones and pacificanones, mixed-precursor polyketides from the marine actinomycete Salinispora pacifica. J. Nat. Prod. 2008;71:570–575. doi: 10.1021/np0705155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku N., Adachi K., Matsuda S., Kasai H., Takatsuki A., Shizuri Y. Ariakemicins A and B, novel polyketide–peptide antibiotics from a marine gliding bacterium of the genus Rapidithrix. Org. Lett. 2008;10:2481–2484. doi: 10.1021/ol8007292. [DOI] [PubMed] [Google Scholar]
- Ospina C.A., Rodriguez A.D., Zhao H., Raptis R.G. Bipinnapterolide B, a bioactive oxapolycyclic diterpene from the Colombian gorgonian coral Pseudopterogorgia bipinnata. Tetrahedron Lett. 2007;48:7520–7523. [Google Scholar]
- Pan J.H., Jones E.B., She Z.G., Pang J.Y., Lin Y.C. Review of bioactive compounds from fungi in the South China Sea. Bot. Mar. 2008;51:179–190. [Google Scholar]
- Pearce A.N., Chia E.W., Berridge M.V., Clark G.R., Harper J.L., Larsen L., Maas E.W., Page M.J., Perry N.B., Webb V.L., Copp B.R. Anti-inflammatory thiazine alkaloids isolated from the New Zealand ascidian Aplidium sp.: inhibitors of the neutrophil respiratory burst in a model of gouty arthritis. J. Nat. Prod. 2007;70:936–940. doi: 10.1021/np060626o. [DOI] [PubMed] [Google Scholar]
- Pearce A.N., Chia E.W., Berridge M.V., Maas E.W., Page M.J., Webb V.L., Harper J.L., Copp B.R. E/Z-rubrolide O, an anti-inflammatory halogenated furanone from the New Zealand ascidian Synoicum n. sp. J. Nat. Prod. 2007;70:111–113. doi: 10.1021/np060188l. [DOI] [PubMed] [Google Scholar]
- Peng C., Han Y., Sanders T., Chew G., Liu J., Hawrot E., Chi C., Wang C. alpha4/7-conotoxin Lp1.1 is a novel antagonist of neuronal nicotinic acetylcholine receptors. Peptides. 2008;29:1700–1707. doi: 10.1016/j.peptides.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza A., Gustchina E., Baker H.L., Kelly M., Bewley C.A. Mirabamides A–D, depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J. Nat. Prod. 2007;70:1753–1760. doi: 10.1021/np070306k. [DOI] [PubMed] [Google Scholar]
- Pomin V.H., Mourao P.A. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology. 2008;18:1016–1027. doi: 10.1093/glycob/cwn085. [DOI] [PubMed] [Google Scholar]
- Ponomarenko L.P., Kalinovsky A.I., Afiyatullov S.S., Pushilin M.A., Gerasimenko A.V., Krasokhin V.B., Stonik V.A. Spongian diterpenoids from the sponge Spongia (Heterofibria) sp. J. Nat. Prod. 2007;70:1110–1113. doi: 10.1021/np070068t. [DOI] [PubMed] [Google Scholar]
- Pontius A., Krick A., Kehraus S., Brun R., Konig G.M. Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J. Nat. Prod. 2008;71:1579–1584. doi: 10.1021/np800294q. [DOI] [PubMed] [Google Scholar]
- Pontius A., Krick A., Mesry R., Kehraus S., Foegen S.E., Muller M., Klimo K., Gerhauser C., Konig G.M. Monodictyochromes A and B, dimeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 2008;71:1793–1799. doi: 10.1021/np800392w. [DOI] [PubMed] [Google Scholar]
- Pushpamali W.A., Nikapitiya C., De Zoysa M., Whang I., Kim S.J., Lee J. Isolation and purification of an anticoagulant from fermented red seaweed Lomentaria catenata. Carbohydr. Polym. 2008;73:274–279. [Google Scholar]
- Rasmussen H.E., Blobaum K.R., Park Y.K., Ehlers S.J., Lu F., Lee J.Y. Lipid extract of Nostoc commune Var. sphaeroides Kutzing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells. J. Nutr. 2008;138:476–481. doi: 10.1093/jn/138.3.476. [DOI] [PubMed] [Google Scholar]
- Raveh A., Carmeli S. Antimicrobial ambiguines from the cyanobacterium Fischerella sp. collected in Israel. J. Nat. Prod. 2007;70:196–201. doi: 10.1021/np060495r. [DOI] [PubMed] [Google Scholar]
- Rodriguez Brasco M.F., Genzano G.N., Palermo J.A. New C-secosteroids from the gorgonian Tripalea clavaria. Steroids. 2007;72:908–913. doi: 10.1016/j.steroids.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Rubio B.K., van Soest R.W., Crews P. Extending the record of meroditerpenes from Cacospongia marine sponges. J. Nat. Prod. 2007;70:628–631. doi: 10.1021/np060633c. [DOI] [PubMed] [Google Scholar]
- Sachindra N.M., Sato E., Maeda H., Hosokawa M., Niwano Y., Kohno M., Miyashita K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007;55:8516–8522. doi: 10.1021/jf071848a. [DOI] [PubMed] [Google Scholar]
- Salerno G., Parrinello N., Roch P., Cammarata M. cDNA sequence and tissue expression of an antimicrobial peptide, dicentracin; a new component of the moronecidin family isolated from head kidney leukocytes of sea bass, Dicentrarchus labrax. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;146:521–529. doi: 10.1016/j.cbpb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Sansom C.E., Larsen L., Perry N.B., Berridge M.V., Chia E.W., Harper J.L., Webb V.L. An antiproliferative bis-prenylated quinone from the New Zealand brown alga Perithalia capillaris. J. Nat. Prod. 2007;70:2042–2044. doi: 10.1021/np070436t. [DOI] [PubMed] [Google Scholar]
- Saravanakumar D.E.M., Folb P.I., Campbell B.W., Smith P. Antimycobacterial activity of the red alga Polysiphonia virgata. Pharm. Biol. 2008;46:254–260. [Google Scholar]
- Schmitz F.J., Bowden B.F., Toth S.I. Antitumor and cytotoxic compounds from marine organisms. In: Attaway D.H., Zaborsky O.R., editors. vol. 1. Plenum Press; New York: 1993. pp. 197–308. (Marine Biotechnology, Pharmceutical and Bioactive Natural Products). [Google Scholar]
- Schultz A.W., Oh D.C., Carney J.R., Williamson R.T., Udwary D.W., Jensen P.R., Gould S.J., Fenical W., Moore B.S. Biosynthesis and structures of cyclomarins and cyclomarazines, prenylated cyclic peptides of marine actinobacterial origin. J. Am. Chem. Soc. 2008;130:4507–4516. doi: 10.1021/ja711188x. [DOI] [PubMed] [Google Scholar]
- Shen Y.C., Chen Y.H., Hwang T.L., Guh J.H., Khalil A.T. Four new briarane diterpenoids from the gorgonian coral Junceella fragilis. Helv. Chim. Acta. 2007;90:1391–1398. [Google Scholar]
- Shindo K., Mikami K., Tamesada E., Takaichi S., Adachi K., Misawa N., Maoka T. Diapolycopenedioic acid xylosyl ester, a novel glyco-C30-carotenoic acid produced by a new marine bacterium Rubritalea squalenifaciens. Tetrahedron Lett. 2007;48:2725–2727. [Google Scholar]
- Simmons T.L., Engene N., Urena L.D., Romero L.I., Ortega-Barria E., Gerwick L., Gerwick W.H. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigro-viridis. J. Nat. Prod. 2008;71:1544–1550. doi: 10.1021/np800110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y., Matsuda K., Yamada Y., Nishikawa M., Shioya K., Katsuzaki H., Imai K., Amano H. Anti-allergic phlorotannins from the edible brown alga, Eisenia arborea. Food Sci. Technol. Res. 2007;13:54–60. doi: 10.1271/bbb.60417. [DOI] [PubMed] [Google Scholar]
- Sugiyama N., Konoki K., Tachibana K. Isolation and characterization of okadaic acid binding proteins from the marine sponge Halichondria okadai. Biochemistry. 2007;46:11410–11420. doi: 10.1021/bi700490n. [DOI] [PubMed] [Google Scholar]
- Tadesse M., Gulliksen B., Strom M.B., Styrvold O.B., Haug T. Screening for antibacterial and antifungal activities in marine benthic invertebrates from northern Norway. J. Invertebr. Pathol. 2008;99:286–293. doi: 10.1016/j.jip.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Talarico L.B., Duarte M.E., Zibetti R.G., Noseda M.D., Damonte E.B. An algal-derived DL-galactan hybrid is an efficient preventing agent for in vitro dengue virus infection. Planta Med. 2007;73:1464–1468. doi: 10.1055/s-2007-990241. [DOI] [PubMed] [Google Scholar]
- Tan L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68:954–979. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Taori K., Matthew S., Rocca J.R., Paul V.J., Luesch H. Lyngbyastatins 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007;70:1593–1600. doi: 10.1021/np0702436. [DOI] [PubMed] [Google Scholar]
- Taori K., Paul V.J., Luesch H. Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 2008;71:1625–1629. doi: 10.1021/np8002172. [DOI] [PubMed] [Google Scholar]
- Tasdemir D., Topaloglu B., Perozzo R., Brun R., O'Neill R., Carballeira N.M., Zhang X., Tonge P.J., Linden A., Ruedi P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg. Med. Chem. 2007;15:6834–6845. doi: 10.1016/j.bmc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Treschow A.P., Hodges L.D., Wright P.F., Wynne P.M., Kalafatis N., Macrides T.A. Novel anti-inflammatory omega-3 PUFAs from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;147:645–656. doi: 10.1016/j.cbpb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Uzair B., Ahmed N., Ahmad V.U., Mohammad F.V., Edwards D.H. The isolation, purification and biological activity of a novel antibacterial compound produced by Pseudomonas stutzeri. FEMS Microbiol. Lett. 2008;279:243–250. doi: 10.1111/j.1574-6968.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Vairappan C.S., Suzuki M., Ishii T., Okino T., Abe T., Masuda M. Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry. 2008;69:2490–2494. doi: 10.1016/j.phytochem.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Van Minh C., Cuong N.X., Tuan T.A., Choi E.M., Kim Y.H., Kiem P.V. A new 9, 11-secosterol from the Vietnamese sea soft coral, Sarcophyton mililatensis, increases the function of osteoblastic MC3T3-E1 cells. Nat. Prod. Commun. 2007;2:1095–1100. [Google Scholar]
- Wang K.J., Huang W.S., Yang M., Chen H.Y., Bo J., Li S.J., Wang G.Z. A male-specific expression gene, encodes a novel anionic antimicrobial peptide, scygonadin, in Scylla serrata. Mol. Immunol. 2007;44:1961–1968. doi: 10.1016/j.molimm.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Wang S.C., Bligh S.W., Shi S.S., Wang Z.T., Hu Z.B., Crowder J., Branford-White C., Vella C. Structural features and anti-HIV-1 activity of novel polysaccharides from red algae Grateloupia longifolia and Grateloupia filicina. Int. J. Biol. Macromol. 2007;41:369–375. doi: 10.1016/j.ijbiomac.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Wattanadilok R., Sawangwong P., Rodrigues C., Cidade H., Pinto M., Pinto E., Silva A., Kijjoa A. Antifungal activity evaluation of the constituents of Haliclona baeri and Haliclona cymaeformis, collected from the Gulf of Thailand. Mar. Drugs. 2007;5:40–51. doi: 10.3390/md502040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Rodriguez I.I., Rodriguez A.D., Barnes C.L. Caribenols A and B, sea whip derived norditerpenes with novel tricarbocyclic skeletons. J. Org. Chem. 2007;72:7386–7389. doi: 10.1021/jo070649n. [DOI] [PubMed] [Google Scholar]
- Wei X., Rodriguez A.D., Wang Y., Franzblau S.G. Novel ring B abeo-sterols as growth inhibitors of Mycobacterium tuberculosis isolated from a Caribbean Sea sponge, Svenzea zeai. Tetrahedron Lett. 2007;48:8851–8854. [Google Scholar]
- Wen L., Lin Y.C., She Z.G., Du D.S., Chan W.L., Zheng Z.H. Paeciloxanthone, a new cytotoxic xanthone from the marine mangrove fungus Paecilomyces sp. (Tree1–7). J. Asian Nat. Prod. Res. 2008;10:133–137. doi: 10.1080/10286020701273783. [DOI] [PubMed] [Google Scholar]
- Williams D.E., Keyzers R.A., Warabi K., Desjardine K., Riffell J.L., Roberge M., Andersen R.J. Spirastrellolides C to G: macrolides obtained from the marine sponge Spirastrella coccinea. J. Org. Chem. 2007;72:9842–9845. doi: 10.1021/jo7018174. [DOI] [PubMed] [Google Scholar]
- Williams P.G., Asolkar R.N., Kondratyuk T., Pezzuto J.M., Jensen P.R., Fenical W. Saliniketals A and B, bicyclic polyketides from the marine actinomycete Salinispora arenicola. J. Nat. Prod. 2007;70:83–88. doi: 10.1021/np0604580. [DOI] [PubMed] [Google Scholar]
- Williams J.A., Day M., Heavner J.E. Ziconotide: an update and review. Expert Opin. Pharmacother. 2008;9:1575–1583. doi: 10.1517/14656566.9.9.1575. [DOI] [PubMed] [Google Scholar]
- Wright A.E., Botelho J.C., Guzman E., Harmody D., Linley P., McCarthy P.J., Pitts T.P., Pomponi S.A., Reed J.K. Neopeltolide, a macrolide from a lithistid sponge of the family Neopeltidae. J. Nat. Prod. 2007;70:412–416. doi: 10.1021/np060597h. [DOI] [PubMed] [Google Scholar]
- Wu D., Fujio M., Wong C.H. Glycolipids as immunostimulating agents. Bioorg. Med. Chem. 2008;16:1073–1083. doi: 10.1016/j.bmc.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Miao L., Li X.C., Xiao X., Qian P.Y. Antibacterial and antilarval activity of deep-sea bacteria from sediments of the West Pacific Ocean. Biofouling. 2007;23:131–137. doi: 10.1080/08927010701219323. [DOI] [PubMed] [Google Scholar]
- Yamada T., Minoura K., Tanaka R., Numata A. Cell-adhesion inhibitors produced by sea hare-derived Periconia sp. III. Absolute stereostructures of peribysins J and macrosphelide M. J. Antibiot. (Tokyo) 2007;60:370–375. [Google Scholar]
- Yoon S.J., Pyun Y.R., Hwang J.K., Mourao P.A. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohydr. Res. 2007;342:2326–2330. doi: 10.1016/j.carres.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Zhang D., Li X., Kang J.S., Choi H.D., Son B.W. A new α-pyrone derivative, 6-[(E)-hept-1-enyl]-α-pyrone, with tyrosinase inhibitory activity from a marine isolate of the fungus Botrytis. Bull. Korean Chem. Soc. 2007;28:887–888. [Google Scholar]
- Zhang D., Yang X., Kang J.S., Choi H.D., Son B.W. Circumdatin I, a new ultraviolet-A protecting benzodiazepine alkaloid from a marine isolate of the fungus Exophiala. J. Antibiot. (Tokyo) 2008;61:40–42. doi: 10.1038/ja.2008.108. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ling S., Fang Y., Zhu T., Gu Q., Zhu W.M. Isolation, structure elucidation, and antimycobacterial properties of dimeric naphtho-γ-pyrones from the marine-derived fungus Aspergillus carbonarius. Chem. Biodivers. 2008;5:93–100. doi: 10.1002/cbdv.200890017. [DOI] [PubMed] [Google Scholar]


